Abstract
BACKGROUND CONTEXT
Recent advanced studies have demonstrated that cytokines and extracellular matrix (ECM) could trigger various types of neural differentiation. However, the efficacy of differentiation and in vivo transplantation has not yet thoroughly been investigated.
PURPOSE
To highlight the current understanding of the effects of ECM on neural differentiation of human bone marrow-derived multipotent progenitor cells (MPCs), regarding state-of-art cure for the animal with acute spinal cord injury (SCI), and explore future treatments aimed at neural repair.
STUDY DESIGN
A selective overview of the literature pertaining to the neural differentiation of the MSCs and experimental animals aimed at improved repair of SCI.
METHODS
Extracellular matrix proteins, tenascin-cytotactin (TN-C), tenascin-restrictin (TN-R), and chondroitin sulfate (CS), with the cytokines, nerve growth factor (NGF)/brain-derived neurotrophic factor (BDNF)/retinoic acid (RA) (NBR), were incorporated to induce transdifferentiation of human MPCs. Cells were treated with NBR for 7 days, and then TN-C, TN-R, or CS was added for 2 days. The medium was changed every 2 days. Twenty-four animals were randomly assigned to four groups with six animals in each group: one experimental and three controls. Animals received two (bilateral) injections of vehicle, MPCs, NBR-induced MPCs, or NBR/TN-C-induced MPCs into the lesion sites after SCI. Functional assessment was measured using the Basso, Beattie, and Bresnahan locomotor rating score. Data were analyzed using analysis of variance followed by Student-Newman-Keuls (SNK) post hoc tests.
RESULTS
Results showed that MPCs with the transdifferentiation of human MPCs to neurons were associated with increased messenger-RNA (mRNA) expression of neuronal markers including nestin, microtubule-associated protein (MAP) 2, glial fibrillary acidic protein, βIII tubulin, and NGF. Greater amounts of neuronal morphology appeared in cultures incorporated with TN-C and TN-R than those with CS. The addition of TN-C enhanced mRNA expressions of MAP2, βIII tubulin, and NGF, whereas TN-R did not significantly change. Conversely, CS exposure decreased MAP2, βIII tubulin, and NGF expressions. The TN-C-treated MSCs significantly and functionally repaired SCI-induced rats at Day 42. Present results indicate that ECM components, such as tenascins and CS in addition to cytokines, may play functional roles in regulating neurogenesis by human MPCs.
CONCLUSIONS
These findings suggest that the combined use of TN-C, NBR, and human MPCs offers a new feasible method for nerve repair.
Keywords: Chondroitin sulfate, Extracellular matrix, Human multipotent progenitor cells, Neurogenesis, Tenascin-cytotactin, Tenascin-restrictin
Introduction
Classically, mature neurons were thought to lack the capacity to proliferate or respond to either acute or chronic pathologic changes. However, in 1992, Reynolds and Weiss [1] isolated multipotential stem cells from the striatum of the adult mouse brain and succeeded in differentiating these cells into neurons and astrocytes in vitro. This raised the possibility of repairing central nervous system neurons. However, subsequent work demonstrated that only very few sites, such as the hippocampus and ventricular and subventrical zones [2,3], can recruit neural stem cells, which generally appear in the resting state and do not significantly aid in repairing severe neural defects. Exogenic or allogenic progenitor cells are required to serve as seed cells for the repair of neural lesions. Of these candidates, adult bone marrow-derived multipotent progenitor cells (MPCs) were especially attractive as bone marrow harvesting is associated with fewer ethical debates than the use of embryonic cell sources. Moreover, it has been long established that the MPCs are multipotent and act as precursors of various mesoderm-type cells such as osteoblasts, chondrocytes, and adipocytes in cultivation [4].
Several attempts have been made to induce neuronal transdifferentiation from mammalian and human MPCs. Intravenous transplantation of labeled MPCs resulted in the wide distribution and differentiation of these cells in bone marrow, muscles, spleen, kidneys, lungs, liver, endothelium, and brain tissues, which demonstrated the transdifferentiation potential of the MPCs [5–7]. Neuroprogenitor cells were triggered in the brain from mouse bone marrow [8] and rat hematopoietic stem cells [9]. Transdifferentiation of MPCs to neural lineages was reported to be spontaneously induced after removing the basic fibroblast growth factor and epidermal growth factor signals [10–12]; however neural differentiation can be promoted using neurotrophic-3 (NT-3), platelet-derived growth factor, nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), retinoic acid (RA), sonic hedgehog, and α-secretase-cleaved fragment of the amyloid precursor protein [13,14].
The extracellular matrix (ECM) has been suggested to modulate the differentiation of embryonic stem cells [15]. For example, Type IV collagen, laminins, and fibronectin were shown to guide neurite extension on differentiated neuron cells [16–19]. Conversely, exposure to chondroitin sulfate (CS) resulted in the inhibition of axon outgrowth [20]. Another ECM molecule, tenascin restrictin (TN-R), was also shown to induce the formation of microprocesses along neurites and enlarged growth cones of chick tectal neurons [21,22]. Tenascin-cytotactin (TN-C), highly expressed in the subventricular zone, generates a stem cell “niche” that acts to orchestrate neural stem cell development in mice [23,24]. Among transdifferentiation studies, most have been conducted with animal cells [25–28] and some with human MPCs [29,30].
Previous study reported that the local transplantation of hNSC improved the functional recovery of animals with spinal cord injury (SCI) [31]. The MPC was also considered a candidate for SCI [32,33] Advantages of MPCs used in the repair of SCI include the ease of isolation, low immunogenicity, a potential increase of cell proliferation, and some degree of differentiation. However, there is still a clinical demand to achieve more complete neuronal differentiation using a human in vitro study. The purpose of the present research was to investigate the ECM effects on neuronal transdifferentiation of human bone marrow-derived MPCs and confirm its potential to regenerate the spinal cord. The effects of TN-R, TN-C, or CS together with cytokines including NGF/BDNF/RA (NBR) were evaluated using immunocytochemistry and a reverse transcriptase polymerase chain reaction (RT-PCR) in vitro, followed by a cell therapy in rats after an SCI.
Materials and methods
Subjects
Consenting bone marrow donors were selected from the patients admitted to the Orthopedic Section of Taipei Municipal Chung-Hsin Hospital (Taipei, Taiwan). None had endocrine disease or was receiving hormone replacement therapy. Bone marrow was obtained from a femur fracture site by proximal femur aspiration during surgical treatment procedures.
Isolation, cultivation, and identification of MPCs
Multipotent progenitor cells were isolated and expanded from human bone marrow as described previously [34]. They were mixed with sodium-heparin and diluted with five volumes of phosphate-buffered saline. The cell suspension was fractionated on a Percoll gradient (40% initial density, GE Healthcare, Piscataway, NJ, USA). The MPC-enriched interface fraction was collected and cultured in Dulbecco modified Eagle medium and 1 g/mL glucose (DMEM/LG, D5523 Sigma, St. Louis, MO, USA), 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL fungizone under normal conditions. Before differentiation, the identity of MPCs was confirmed by negative staining with markers of hemapoietic precursor cells, CD14 and CD34, and positive staining with the nonhematopoietic markers, CD44, CD73, CD90w, and CD105. The medium was changed every 4 days.
Differentiation of MPCs
Neuronal induction was modified as previously described [35]. Dulbecco modified Eagle medium/F12 incorporated with NGF (20 ng/mL, Chemicon, Billerica, MA, USA), brain-derived growth factor (Chemicon, 10 ng/mL), all-trans RA (Sigma, 2 μM), ascorbic acid 2-phosphate (Sigma, 0.1 mM), and antibiotics was used. Tenascin-cytotactin (Chemicon, 0.5 μg/mL), TN-R (Chemicon, 0.5 μg/mL), and CS-proteoglycan (Chemicon, 0.5 μg/mL) were added to determine their regulatory effects on neurotrophin-induced MPC neuronal differentiation. Cells were treated with neuronal differentiation medium for 7 days, then TN-C, TN-R, or CS were added for 2 days. The medium was changed every 2 days.
Antibodies
Primary antibodies (Abs) directed against the immature neuronal markers, nestin (Chemicon, 1:500) and βIII tubulin (Chemicon, 1:500), and the mature neuronal markers, microtubule-associated protein (MAP) 2 (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA), neuroglial marker glial fibrillary acidic protein (GFAP) (Abcam, 1:500), and NGF (Chemicon, 1: 500), were used for immunocytochemistry. Secondary Abs, FITC-conjugated goat anti-mouse immunoglobulin G (IgG) (AP124; 1:500), rhodamine-conjugated goat anti-rabbit IgG (AP132R; 1:500), Fluorescein isothiocyanate (FITC)-conjugated donkey anti-mouse IgG (AP192F; 1:500), and rhodamine-conjugated goat anti-rabbit IgG (AP187R; 1:500) were observed using immunofluorescence microscope (X71; Olympus, Tokyo, Japan). 4′, 6-diamidino-2-phenylindole (DAPI) (Sigma, 1 mg/mL) staining was used in all cytochemical studies to identify cell nuclei.
RNA extraction and semiquantitative RT-PCR
Total RNA harvested from monolayer cultures was extracted using the TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and subjected to RT followed by PCR amplification of specifically expressed genes. Reverse transcription was performed with SuperScript III (Invitrogen Life Technologies) and an Oligo d(T)12–18 primer. RNA was added to a final volume of a 21-ml solution containing 10 mM Deoxyribonucleotide (dNTP) mix, 10× RT buffer, 25 mM MgCl2, 0.1 M Dithiothreitol (DTT), RNase inhibitor, and RNase H. Six micrograms of the RT product was used for the PCR amplification in a final volume of 50 mL, containing 2.5 mM dNTP, 25 mM MgCl2, upstream/downstream primers (Table), and Taq DNA polymerase (Invitrogen Life Technologies). Following an initial denaturation at 95°C for 5 minutes, DNA was amplified in the Touchgene Gradient (Techne, Cambridge, UK) using 35 cycles of 1 minute at 94°C for denaturation and extension at 72°C for 1 minute. This was followed by a final extension at 72°C for 5 minutes. The annealing temperatures differed from 55°C to 68°C depending on the specific genes. Polymerase chain reaction products were then run on 1% agarose gels (Agarose I; AMRESCO, Solon, OH, USA) and visualized with ethidium bromide staining. Images were analyzed using FloGel-I (Fluorescent Gel Image System, TOP BIO, Taipei, Taiwan), and glyceraldehyde 3-phosphate dehydrogenase was used as an internal control. The primer sets were as shown in Table.
Table.
Primer sets
| Specific genes | Primer sequences (5′→3′) | Product size (bp) |
|---|---|---|
| Nestin | P1 CAGCTGGCGCACCTCAAGATG P2 AGGGAAGTTGGGCTCAGGACTGG |
209 |
| MAP2 | P1 CCATTTGCAACAGGAAGACAC P2 CAGCTCAAATGCTTTGCAACTAT |
428 |
| GFAP | P1 GTGGGCAGGTGGGAGCTTGATTCT P2 CTGGGGCGGCCTGGTAGACA |
387 |
| βIII tubulin | P1 GAGCGGGATCAGCGTCTACTA P2 GTCGCAGTTTTCACACTCCT |
264 |
| NGF | P1 CAGGACTCACAGGAGCAAGC P2 GCCTTCCTGCTGAGCACACA |
360 |
| GAPDH | P1 GCTCTCCAGAACATCATCCCTGCC P2 CGTTGTCATACCAGGAAATGFGCTT |
346 |
MAP2, microtubule-associated protein 2; GFAP, glial fibrillary acidic protein; NGF, nerve growth factor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Animals
Twenty-four female Sprague-Dawley rats, 8 weeks old, were purchased from the BioLASCO Animal Center (BioL-ASCO Taiwan, Taipei, Taiwan) and used in this study. After arrival in the laboratory, animals were housed in the Animal Center of Taipei Medical University that has a 12:12 hours light/dark cycle (with lights on at 07:00 hours) and controlled temperature (19°C ~21°C) and humidity (50% ~60%). Animals had free access to food and water ad libitum. We followed the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals when conducting this study. All procedures for surgery and animal care were approved by the University Laboratory Animal Care and Use Committee of Taipei Medical University.
Rat SCI model
Spinal cord injury was induced at T10 based on our published protocols with minor modifications [36,37]. In brief, a laminectomy was performed at T10 and SCI was induced by 200 kdynes delivered by an IH Spinal Cord Impactor (Precision Systems and Instrumentation, LLC, Fairfax Station, VA, USA). Each injection was delivered in 3 minutes, and the needle was left in place for another 2 minutes. Muscles around the lesion site were sutured with 5 to 0 absorbable sutures, and the incision in the skin was closed with wound clips. After SCI surgery, the urinary bladder of SCI rats was expressed twice a day until the bladder was small or empty. Cefazolin (50 mg/Kg) was administered subcutaneously twice a day for 10 days. The locomotor behavior was evaluated using the Basso, Beattie, and Bresnahan (BBB) scoring system, which determined that a minimum of six subjects in each group were required to blindly detect a three-point difference. The BBB score of SCI rats was recorded before SCI surgery and 1, 4, 7, 10, 14, 21, 28, 35, and 42 days after SCI based on a published protocol [38].
Experimental design
Animals were anesthetized with intraperitoneal injections of Zoletil 50 (50 mg/mL/Kg, Virbac Laboratories, Carros, France) and Rompun (xylazine, 20 mg/mL/Kg, Bayer AG, Leverkusen, Germany). Animals were randomly assigned to four groups: Group I (SCI+V group), Group II (SCI+MPC group), Group III (SCI+MPC+NBR group), and Group IV (SCI+MPC+NBR+TN-C group). In Group I, animals received SCI and two (bilateral) injections of vehicle, DMEM only (1.5 mL each), into the lesion site (SCI+V group, n=6). Animals in Group II received SCI, immediately followed by two injections of MPCs (1.5 mL each of 105 cells/mL) into the lesion site (SCI+MPC group, n=5). One animal of Group II died in the experiment. In Group III, animals received SCI, immediately followed by two injections of MPCs (1.5 mL each of 105 cells/mL), which were incubated with neuron-differentiation factors, into the lesion site (SCI+MPC+NBR group, n=6). In Group IV, we induced SCI in animals and then injected MPCs (1.5 mL each of 105 cells/mL), which were cultured with neuron-differentiation factors and TN-C, into the lesion site (SCI+MPC+NBR+TN-C group, n=6). Two different intraspinal injections were bilaterally made at one-third to the rostral and caudal edge of the injured spinal cord.
Histologic evaluation and quantitative analysis of SCI repair
After the final behavioral test, animals were anesthetized with the same anesthetics described previously and sacrificed by a transcardial perfusion with 0.9% saline solution, followed by a 4% paraformaldehyde solution (0.1 M) (Sigma-Aldrich, St. Louis, MO, USA). The spinal cord was removed and postfixed in a 4% paraformaldehyde solution overnight. Then, the spinal cord was transferred to 20% sucrose for cryoprotection until the cord sank. Next, the spinal cord was sectioned on a cryostat at a thickness of 10 mm and mounted onto gelatin-coated slides. One in every six spinal sections was counterstained with thionin. For each section, the remaining tissue in the 3 mm lesion center was traced and measured using the morphometric AxioVision imaging software 4.0 (Carl Zeiss, Inc., Jena, Germany). The total volume of the remaining tissue was calculated by the measured area × 10 μm (the thickness of the spinal section). Because one in every six spinal sections was selected for the measurement of the remaining tissue, the total volume of the remaining tissue in the 3 mm lesion center was calculated by the measured volume of the remaining tissue multiplied by six.
Statistical analyses
The RT-PCR data of MPCs induced by NGF, BDNF, and RA (NBR) with and without CS, TN-C, and TN-R were analyzed using at least three samples and counted for each of the two groups. Statistical computations were performed using Student t test.
Data regarding locomotor behavior were analyzed by repeated-measures analysis of variance (ANOVA), followed by Student-Newman-Keuls (SNK) post hoc tests. Comparison of the remaining tissue in the lesion center among different treatments was analyzed by a one-way ANOVA, followed by SNK post hoc tests. All data are expressed as the mean±standard error of the mean. An alpha level of 0.05 was used for all statistical analyses.
Results
Multipotent progenitor cells derived from human bone marrow were subjected to conditions described previously [35], and over 60 doublings were negative for markers of hematopoietic precursor cells, that is, CD14 and CD34, and positive for nonhematopoietic precursors including CD44, CD73, CD90w, and CD105 (Fig. 1). These findings were consistent with several previous studies. Multipotent progenitor cells appeared as large fibroblast-like cells [39,40].
Fig. 1.
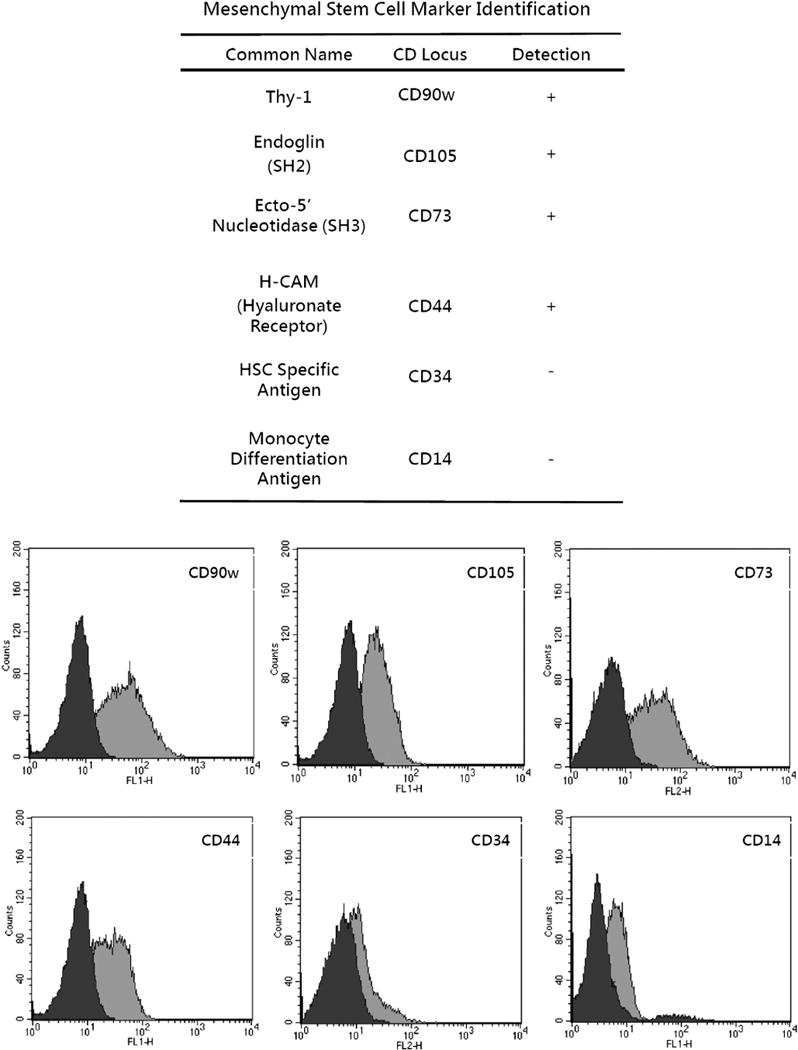
Multipotent progenitor cell membrane marker identification. HSC, hematopoietic stem cell.
Regulation of NGF/BDNF/RA-induced neuronal differentiation of MPCs by CS, TN-C, and TN-R
To observe possible neural progenitors and immature and mature differentiation, MPCs were cultivated with NBR neural differentiation medium for 7 days. Progressive transformation to neural lineage cells coincided with the loss of the flat, fibroblastic cell morphology, characteristic of the undifferentiated MPCs. Multipotent progenitor cells showed no immunoreactivity of the neuron markers, nestin, βIII tubulin, or MAP2, when cultured with only DMEM (Fig. 2A, F). The appearance of neuronal-like morphologies in differentiating MPCs was supported by the immunocytochemical expression of neuronal proteins, such as nestin, neural/stem cell marker (green); βIII tubulin, immature neuronal marker (red); and MAP2, mature neuronal marker (red) (Fig. 2B, G). We further determined the regulatory effects of ECM components such as CS, TN-C, and TN-R on cytokine NGF/BDNF/RA-induced neuronal differentiation of MPCs. After incorporation with CS, MPCs exhibited decreased levels of nestin, MAP 2, and βIII tubulin (Fig. 2C, H). After adding TN-C or TN-R, cells showed increased immunocytochemistry with Abs to the immature neuronal marker, βIII tubulin, and mature neuronal marker, MAP2, but not the progenitor marker, nestin (Fig. 2D, E, I, J). Moreover, when adding TN-C, MPCs seemed to exhibit significantly increased expressions of MAP2 and βIII tubulin compared with the addition of TN-R. The RT-PCR demonstrated parallel expressions of nestin, βIII tubulin, and MAP 2 with those in the immunocytochemical results (Figs. 2K, L).
Fig. 2.
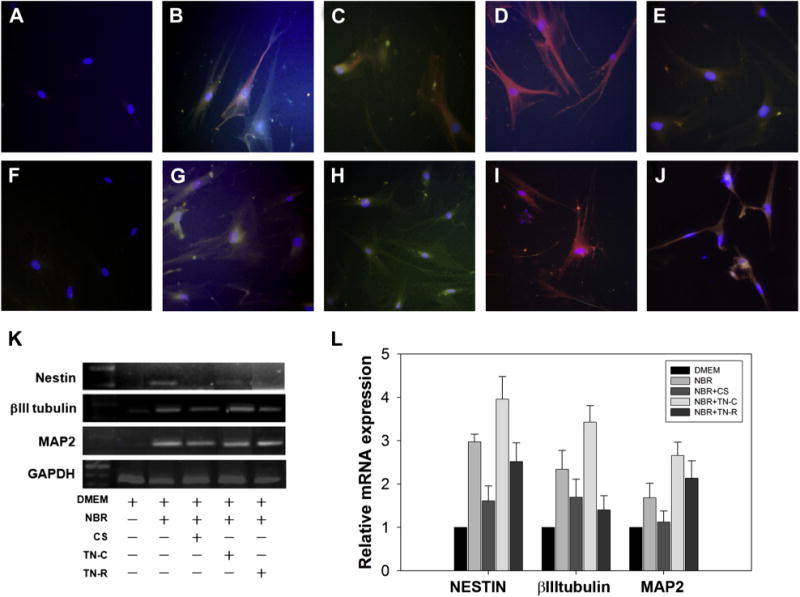
Expressions of the neural stem/progenitor marker, nestin, the immature neuronal marker, βIII tubulin, and the mature neuronal marker, MAP2. Immunocytochemical detection of neural proteins in multipotent progenitor cells (MPCs). Undifferentiated bone marrow-derived MPCs appeared as large, flat, fibroblast-like cells. (A, F) Four days’ expansion of MPCs seeded at a density of 1 ×105 showed no immunoreactivity of the neuron markers, nestin (green), βIII tubulin (red), or MAP2 (red). (B, G) Within 7 days after adding nerve growth factor (NGF; 20 ng/mL), brain-derived neurotropic factor (BDNF; 10 ng/mL), and retinoic acid (RA; 2 μM), MPCs differentiated in the presence of NGF, BDNF, and RA showed positive staining for the neuronal proteins, nestin (green), βIII tubulin (red), and MAP2 (red). (C, H) After 6 days of incorporation with CS-proteoglycan, MPCs exhibited decreased levels of nestin, MAP2, and βIII tubulin. (D, I, E, J) When TN-C or TN-R was added, cells showed an increase of immunocytochemistry with antibodies to late-stage neuronal markers, βIII tubulin and MAP2, but not nestin. However, TN-C produced more significant expression than TN-R did. Nuclei were stained by DAPI (blue) in all panels. (K) A reverse transcriptase polymerase chain reaction (RT-PCR) for nestin, βIII tubulin, and MAP2 showed similar expressions. (L) For quantification and analysis of variance statistical analysis (n=3, error bars represents standard error of the mean) of the RT-PCR results, nestin, βIII tubulin, and MAP2 were calculated as a ratio to GAPDH. MAP2, microtubule-associated protein 2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; DMEM, Dulbecco modified Eagle medium; NBR, NGF/BDNF/RA; CS, chondroitin sulphate; TN-C, tenascin cytotactin; TN-R, tenascin restrictin; mRNA, messenger-RNA.
Regulation of NGF/BDNF/RA-induced neuroglial differentiation of MPCs by CS-PG, TN-C, and TN-R
No immunoreactivity of neuron markers, including the neuroglial marker, GFAP, the immature neuronal marker, βIII tubulin, or the mature neuronal marker, MAP2, was detected after 4 days’ expansion of MPCs (Fig. 3A, F). After 7 days of cultivation with NBR medium, the majority of induced MPCs exhibited morphologies consistent with neurons. The transdifferentiation of MPCs to neurons was associated with increased immunoreactivity of GFAP, MAP2, and βIII tubulin (Fig. 3B, G). Six days after adding TN-C or TN-R, cells showed increased immunocytochemistry with Abs to MAP2 and βIII tubulin. However, the neuroglial marker, GFAP, was also downregulated (Fig. 3D, E, I, J). Moreover, after incorporation of CS, MPCs exhibited decreased levels of GFAP, MAP2, and βIII tubulin (Fig. 3C, H). With quantification and ANOVA statistical analyses (n=3, error bars represents standard error of the mean) of the RT-PCR results, the transdifferentiation of MPCs to neurons was associated with increased messenger-RNA expression of neuronal markers including nestin, MAP2, GFAP, and βIII tubulin that showed parallel results to the immunohistochemical analysis (Fig. 3K, L).
Fig. 3.
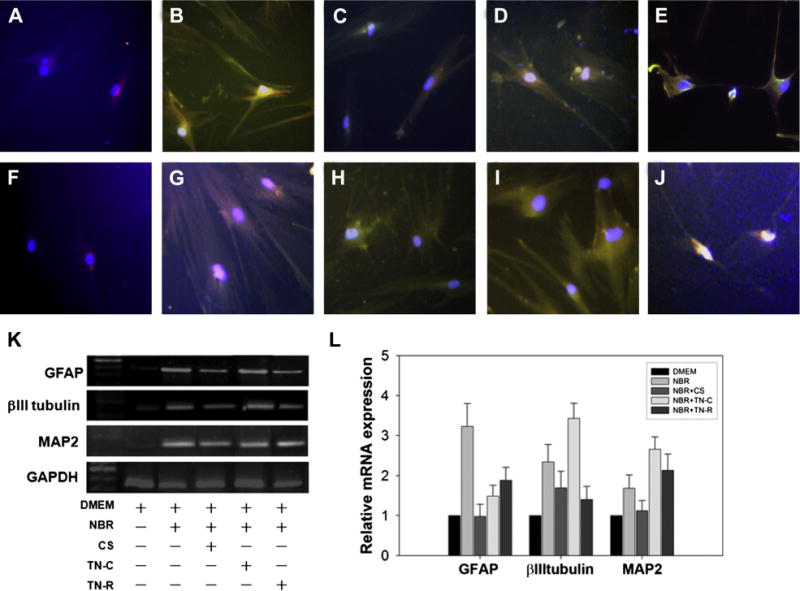
Expression of the neuroglial marker, GFAP, the immature neuronal marker, βIII tubulin, and the mature neuronal marker, MAP2. Immunocytochemical detection of neural proteins in multipotent progenitor cells (MPCs). Undifferentiated bone marrow derived-MPCs appeared as large, flat, fibroblast-like cells. (A, F) Four days’ expansion of MPCs seeded at a density of 1 ×105 showed no immunoreactivity of the neuron markers, GFAP (green), MAP2 (red), or βIII tubulin (red). (B,G) Within 7 days after adding nerve growth factor (NGF; 20 ng/mL), brain-derived neurotropic factor (BDNF; 10 ng/mL), and retinoic acid (RA; 2 μM), MPCs differentiated in the presence of BDNF, NGF, and RA and showed increased levels of the neural proteins, nestin (green), GFAP (green), MAP2 (red), and βIII tubulin (red). (C, H) After 6 days of incorporation with CS-proteoglycan, MPCs exhibited decreased levels of GFAP, MAP2, and βIII tubulin. After adding TN-C or TN-R, cells showed an increase of immunocytochemistry with antibodies to the late-stage neuronal markers, MAP2 and βIII tubulin. (D, E, I, J) However, the neuroglial marker, GFAP, was downregulated. Nuclei were stained by DAPI (blue) in all panels. (K) A reverse transcriptase polymerase chain reaction (RT-PCR) for GFAP, βIII tubulin, and MAP2 showed similar expression levels. (L) For quantification and analysis of variance statistical analysis (n=3, error bars represents standard error of the mean) of the RT-PCR results, GFAP, βIII tubulin, and MAP2 were calculated as a ratio to GAPDH. DAPI, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein; MAP2, microtubule-associated protein 2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; DMEM, Dulbecco modified Eagle medium; NBR, NGF/BDNF/RA; CS, chondroitin sulphate; TN-C, tenascin cytotactin; TN-R, tenascin restrictin; mRNA, messenger-RNA.
Repair of rat SCI by MPC implantation
To determine the effects of MPC-pretreatment on ECM in an animal model, we implanted MPCs preincubated with NBR/TN-C into injured spinal cords, which significantly promoted the functional recovery of SCI rats. Repeated-measures ANOVA showed a significant testing day effect (F [9, 171]=623.480, p<.0001) and an interaction effect (F (27, 171)=1.874, p=.0088) on the locomotor behavior of SCI rats measured by the BBB score. The SNK post hoc tests further revealed that SCI rats in Group IV that received implantation of MPCs incubated with NBR/TN-C showed a significantly increased BBB score compared with SCI rats with vehicle treatment (Group I) at 14, 21, 28, 35, and 42 days after SCI (p<.05 for Day 14 and p<.01 for Days 21, 28, 35, and 42) (Fig. 4).
Fig. 4.
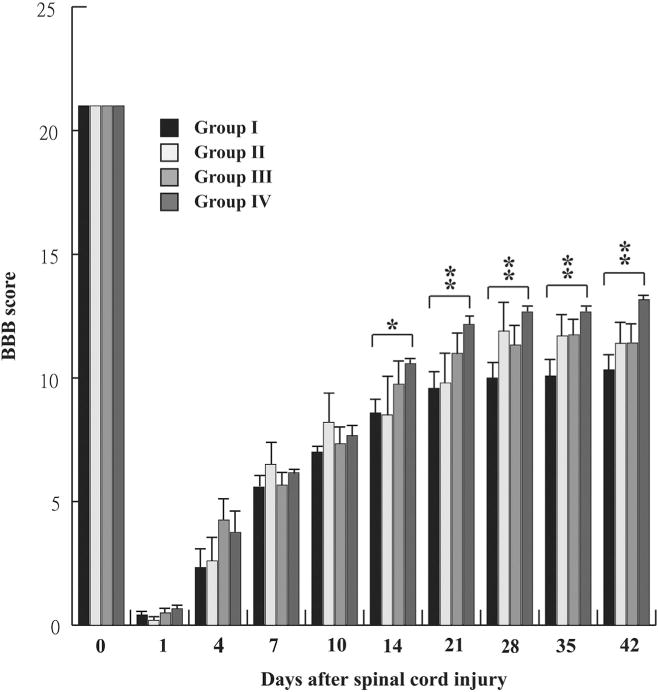
The BBB score of rats with spinal cord injury (SCI) receiving different treatments. A repeated-measures analysis of variance, followed by SNK post hoc tests revealed that SCI rats (Group IV) receiving implantation of multipotent progenitor cells incubated with nerve growth factor/brain-derived neurotropic factor/retinoic acid and tenascin cytotactin showed significantly improved functional recovery measured by the BBB score compared with SCI rats receiving vehicle treatment (Group I) 14, 21, 28, 35, and 42 days after SCI (p<.05 for Day 14 and p<.01 for Days 21, 28, 35, and 42). Respective animal numbers for Groups I, II, III, and IV were 6, 5, 6, and 6. *p<.05; **p<.01 compared with SCI rats with vehicle treatment in Group I. BBB, Basso, Beattie, and Bresnahan. SNK, Student-Newman-Keuls.
Histologic evaluation and quantitative analysis of SCI repair
A large defect with minimal reparative tissue was found in the SCI lesions of rats receiving vehicle treatment (Group I) (Fig. 5A). No spindle-shaped MPCs were found. Microglial cells and macrophage/hemosiderin infiltration were noted (Fig. 5B, C). Rats with SCI receiving treatment with MPCs (Group II) and SCI rats receiving implantation of MPCs incubated with NBR (Group III) showed moderate repair with proliferative spinal tissue (Fig. 5D, G). Multipotent progenitor cells in Group III showed more proliferative spinal tissue and differentiated progenitor cells compared with Group II (Fig. E, F, H, J). Rats with SCI receiving treatment with MPCs incubated with NBR/TN-C (Group IV) at a similar section level showed significantly increased proliferation of preserved spinal tissues compared with SCI rats receiving only vehicle treatment (Group I) (Fig. 5A, D, G, J). All groups showed remarkable macrophage/hemosiderin infiltration in the repair areas. Administration of MPCs incubated with NBR/TN-C into the injured spinal cord significantly preserved a greater amount of spinal tissue after SCI. Spindle-shaped MPCs apparently scattered in repair areas. In addition, Group IV spindle-shaped MPCs differentiated well compared with SCI rats receiving treatment with MPCs (Group II) and SCI rats receiving implantation of MPCs incubated with NBR (Group III) (Fig. 5F, J, L). One animal in Group III (SCI+MPCs pretreated with NBR) was excluded from the analysis of the remaining spinal tissue in the lesion center because of a folding of spinal sections in the lesion center during mounting of sections onto slides. One-way ANOVA showed a significant difference in the remaining spinal tissue in the lesion center among SCI rats with different treatments (F [3, 18]=3.644, p=.0326). The SNK post hoc tests showed that SCI rats in Group IV receiving implantation of MPCs incubated with NBR/TN-C had a greater amount of preserved spinal tissue in the lesion center than did SCI rats with vehicle treatment (Group I) (p<.05) (Fig. 5M).
Fig. 5.
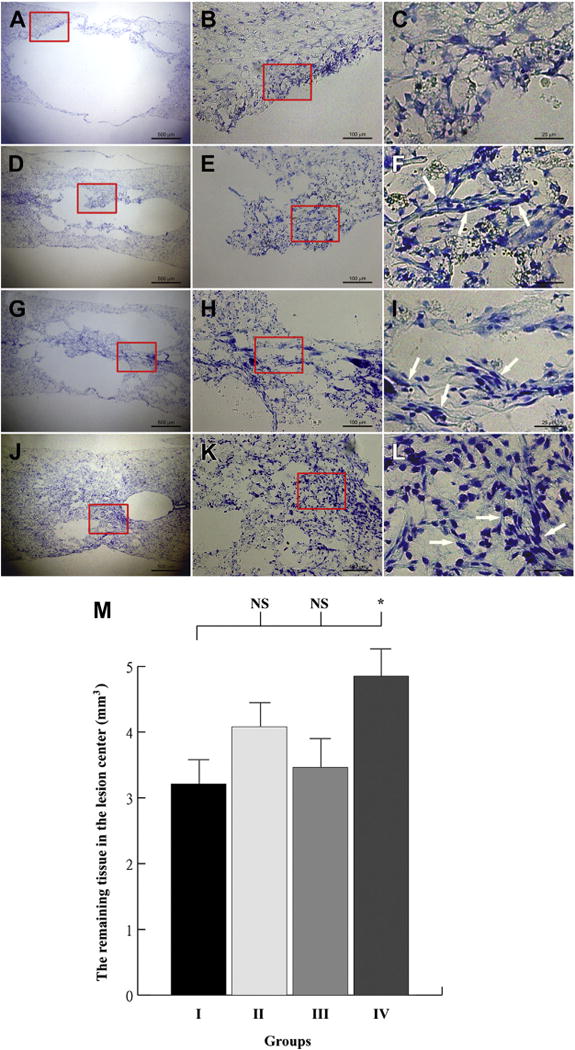
Histologic evaluation and quantitative analysis of spinal tissue in lesions of rats with spinal cord injury (SCI). The representative pictures show the spinal lesions of (A–C) SCI rat receiving vehicle treatment (Group I), (D–F) SCI rats receiving treatment of multipotent progenitor cells (MPCs) (Group II), (G–I) SCI rat receiving implantation of MPCs incubated with nerve growth factor/brain-derived neurotrophic factor/retinoic acid (NBR) (Group III), and (J–L) SCI rats receiving treatment of MPCs incubated with NBR/tenascin cytotactin (TN-C) (Group IV) at the similar section level. The Group I animals had a large lesion with minimal tissue repair without evidence of stem cell. Macrophage/hemosiderin infiltration was apparent. Group II and III spinal lesions showed a similar moderate lesion. The MPCs in Group III showed more proliferative spinal tissue and differentiated progenitor cells (arrows) compared with Group II. A small lesion with well-differentiated progenitor cells (arrows) and proliferative spinal tissue were found in Group IV. All four groups contained apparent macrophage/hemosiderin infiltration in the repair area. The remaining spinal tissue in the lesion center of SCI rats in four groups is shown in (M). One-way analysis of variance followed by SNK post hoc tests revealed that SCI rats in Group IV receiving treatment of MPCs incubated with NBR/TN-C had more preserved spinal tissue in the lesion center than SCI rats receiving vehicle treatment (Group I). The animal numbers for Groups I, II, III, and IV were 6, 5, 5, and 6, respectively. NS, p>.05; *, p<.05 when compared with SCI rats with vehicle treatment in Group I. The scale bar is (A, D, G, J) 500 μm, (B, E, H, K) 100 μm, (C, F, I, L) 25 μm. SNK, Student-Newman-Keuls. NS, No Significance.
Discussion
In this study, we isolated MPCs from human bone marrow and successfully expanded and identified them in vitro as in previous reports [35,39,41]. Several studies reported a certain degree of neuronal or glial differentiation from bone marrow-derived MPCs in vivo [25,26,35] and in vitro [27,28,42]. Our results are consistent with these studies. Using NGF/BDNF/RA, we induced MPCs to differentiate into cells with a neural phenotype in a similar but not identical manner, as with most animal cells [25–28,35]. However, Park [30] and Crobu [29] have demonstrated that human MPCs can be differentiated into a neural phenotype. Our data also showed a similar result of neural differentiation of human MPCs.
In an in vivo attempt to mimic physiologic condition, we avoided using chemically induced neuronal differentiation by β-mercaptoethanol in the media and only incorporated cytokines. The neurogenic factors, that is, cytokines of NGF, BDNF, and RA, are capable of supporting viability and differentiation as modified from a previous study [35]. The design of neurogenic differentiation was also based on report by Crigler et al. [43] of a human MSC complementary (c) DNA library that revealed expressed transcripts encoding BDNF and β-NGF but not NT-3 or NT-4.
Our data demonstrated that CS inhibits NBR-induced neuronal differentiation, but enhances neuroglial differentiation of human MPCs. These results are similar to Sirko report of an inhibitory effect of CS on neuronal differentiation, but an increase in neuroglial differentiation of rat neural progenitor cells [44]. Both TN-C and TN-R upregulate the immature neuronal marker, βIII tubulin, and mature neuronal marker, MAP2, but not the progenitor marker, nestin. In contrast, TN-C and TN-R downregulated GFAP, a neuroglial marker. These results indicate progressive maturity toward neuronal differentiation. Our findings are also parallel to the results of study by Song et al. [2], in which rat neural progenitor cells were shown to present neuronal- and glial-specific markers after triggering differentiation in vitro. Collectively, the ECM may play an important role in modulating the fate of neural differentiation.
Nestin is a large intermediate filament protein (class Type VI) expressed in neuroepithelial neuronal precursor stem cells. The expression of nestin decreases with neuronal maturation [45]. Our data also showed an increase in nestin expression, when MPCs were incorporated with NBR in a serum-free condition. Interestingly, nestin expression decreased after treatment with TN-C and TN-R, even though other neuronal markers of βIII tubulin and MAP2 increased in the same period. Trends of nestin in a serum-free condition mimicked normal neuronal differentiation in vivo.
The SCI method in this study followed previously published protocols that are widely used [35,36]. The BBB score has been well defined to evaluate the locomotive recovery after SCI since BBB developed it in 1994 [34]. Our data showed a significantly higher BBB score in SCI rats receiving NBR/TN-C-induced MPCs than SCI rats given the vehicle in all groups on Days 14, 21, 28, 35, and 42. On Day 42, SCI rats receiving implantation of MPCs incubated with NBR/TN-C showed significant repair of preserved spinal tissues compared with SCI rats receiving vehicle treatment. Our data demonstrated that BBB score 13 of Gr IV is significantly different from score 11 of Gr II, III, and score 10 of Gr I. The difference may indicate a clinical relevance. Rats with BBB score 13 appear to have frequent to consistent weight-supported steps and frequent forelimb-hindlimb (FL-HL) coordination, whereas frequent to consistent weight-supported steps with no FL-HL coordination in the groups of BBB score 11, and occasional weight-supported steps with no FL-HL coordination in the group of BBB score 10. The histologic findings further documented the effect of TN-C on locomotive recovery in animals. We found the TN-C group showed more abundant MPC-like cells than those in other groups, although we could not determine whether the MPC-like cell came from donor or from the host. A label-stem cell experiment enables to monitor the MPC proliferation in the host.
In the undifferentiated state, MPCs, in fact, exist in a “pending” state, ready to differentiate in the presence of appropriate tissue-specific signals. Neuron-specific cytokines first trigger signaling to move toward neural differentiation. Subsequently, the ECM modulates the signaling of plasticity to either a neuronal or glial lineage. Garcion et al. [23] demonstrated that TN-C provides an environmental “niche” to facilitate neurosphere development in mice. Most studies have been conducted on either animal cells [25–28] or human MPCs [29,30]. Tenascin-restrictin is one of the smallest members of the tenascin family of ECM glycoproteins. Neurite outgrowth-promoting, cell-binding, and non-permissive segments were identified in TN-R by in vitro assays. Zacharias reported induction of actin-rich micro-processes and branches along neurite shafts in chick embryonic tectal cells [21,22]. Tenascin-restrictin is expressed on the surface of neurons and glial cells. In this study, we first demonstrated that TN-C and TN-R up-modulated the immature neuronal marker, βIII tubulin, and mature neuronal marker, MAP2, but down-modulated the neuroglial marker, GFAP.
In summary, ECM components, such as tenascin and CS, in addition to cytokines, may play a key role in regulating neurogenesis by MPCs. The combined use of TN-C, NBR, and MPCs provides a new method of functional recovery for SCI. Further studies will analyze the synergistic effects of TN-C and TN-R on neuronal differentiation and additional molecular mechanisms underlying the neural phenotypic plasticity of MPCs in the repair of SCI.
Supplementary Material
Acknowledgments
This work was supported by TUNG-TMU-96-01 and Taiwan National Science Council grants (NSC94-2320-B-038-013, NSC96-2320-B-038-022, and NSC100-2314-B-038-011).
Footnotes
FDA device/drug status: Not applicable.
Author disclosures: W-PD: Nothing to disclose. C-CY: Nothing to disclose. L-YY: Nothing to disclose. C-WDC: Nothing to disclose. W-HC: Nothing to disclose. C-BY: Nothing to disclose. Y-HC: Nothing to disclose. W-FTL: Nothing to disclose. PFR: Stock Ownership: Ridge Diagnostics (Stock options of unclear value); Consulting: Kyowa Hakko Kirin (Less than B); Research Support (Investigator Salary, Staff/Materials): Research support from from Takeda (D), Research support from BMS (E).
The disclosure key can be found on the Table of Contents and at www.TheSpineJournalOnline.com.
Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.spinee.2014.04.024.
References
- 1.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 2.Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nature Neuroscience. 2002;5:438–45. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- 3.Milner R, Edwards G, Streuli C, Ffrench-Constant C. A role in migration for the alpha V beta 1 integrin expressed on oligodendrocyte precursors. J Neurosci. 1996;16:7240–52. doi: 10.1523/JNEUROSCI.16-22-07240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 6.Mezey E, Key S, Vogelsang G, et al. Transplanted bone marrow generates new neurons in human brains. Proc Natl Acad Sci U S A. 2003;100:1364–9. doi: 10.1073/pnas.0336479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mezey E, Chandross KJ, Harta G, et al. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–82. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 8.Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–9. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 9.Kabos P, Ehtesham M, Kabosova A, et al. Generation of neural progenitor cells from whole adult bone marrow. Experimental Neurology. 2002;178:288–93. doi: 10.1006/exnr.2002.8039. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn HG, Winkler J, Kempermann G, et al. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–9. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maric D, Maric I, Chang YH, Barker JL. Prospective cell sorting of embryonic rat neural stem cells and neuronal and glial progenitors reveals selective effects of basic fibroblast growth factor and epidermal growth factor on self-renewal and differentiation. J Neurosci. 2003;23:240–51. doi: 10.1523/JNEUROSCI.23-01-00240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tropepe V, Sibilia M, Ciruna BG, et al. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208:166–88. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- 13.Palmer TD, Markakis EA, Willhoite AR, et al. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–97. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoo JU, Barthel TS, Nishimura K, et al. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745–57. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Czyz J, Wobus A. Embryonic stem cell differentiation: the role of extracellular factors. Differentiation. 2001;68:167–74. doi: 10.1046/j.1432-0436.2001.680404.x. [DOI] [PubMed] [Google Scholar]
- 16.Perris R, Syfrig J, Paulsson M, Bronner-Fraser M. Molecular mechanisms of neural crest cell attachment and migration on types I and IV collagen. J Cell Sci. 1993;106(Pt 4):1357–68. doi: 10.1242/jcs.106.4.1357. [DOI] [PubMed] [Google Scholar]
- 17.Powell SK, Kleinman HK. Neuronal laminins and their cellular receptors. Int J Biochem Cell Biol. 1997;29:401–14. doi: 10.1016/s1357-2725(96)00110-0. [DOI] [PubMed] [Google Scholar]
- 18.Lein PJ, Higgins D, Turner DC, et al. The NC1 domain of type IV collagen promotes axonal growth in sympathetic neurons through interaction with the alpha 1 beta 1 integrin. J Cell Biol. 1991;113:417–28. doi: 10.1083/jcb.113.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali SA, Pappas IS, Parnavelas JG. Collagen type IV promotes the differentiation of neuronal progenitors and inhibits astroglial differentiation in cortical cell cultures. Brain Res Dev Brain Res. 1998;110:31–8. doi: 10.1016/s0165-3806(98)00091-1. [DOI] [PubMed] [Google Scholar]
- 20.Fitch MT, Silver J. Glial cell extracellular matrix: boundaries for axon growth in development and regeneration. Cell Tissue Res. 1997;290:379–84. doi: 10.1007/s004410050944. [DOI] [PubMed] [Google Scholar]
- 21.Zacharias U, Leuschner R, Norenberg U, Rathjen FG. Tenascin-R induces actin-rich microprocesses and branches along neurite shafts. Mol Cell Neurosci. 2002;21:626–33. doi: 10.1006/mcne.2002.1203. [DOI] [PubMed] [Google Scholar]
- 22.Zacharias U, Rauch U. Competition and cooperation between tenascin-R, lecticans and contactin 1 regulate neurite growth and morphology. J Cell Sci. 2006;119:3456–66. doi: 10.1242/jcs.03094. [DOI] [PubMed] [Google Scholar]
- 23.Garcion E, Halilagic A, Faissner A, Ffrench-Constant C. Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development. 2004;131:3423–32. doi: 10.1242/dev.01202. [DOI] [PubMed] [Google Scholar]
- 24.de Chevigny A, Lemasson M, Saghatelyan A, et al. Delayed onset of odor detection in neonatal mice lacking tenascin-C. Mol Cell Neurosci. 2006;32:174–86. doi: 10.1016/j.mcn.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Azizi SA, Stokes D, Augelli BJ, et al. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats–similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998;95:3908–13. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munoz-Elias G, Marcus AJ, Coyne TM, et al. Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long-term survival. J Neurosci. 2004;24:4585–95. doi: 10.1523/JNEUROSCI.5060-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wislet-Gendebien S, Hans G, Leprince P, et al. Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem Cells. 2005;23:392–402. doi: 10.1634/stemcells.2004-0149. [DOI] [PubMed] [Google Scholar]
- 28.Dvornik D, Hohman TC, Basso MD. Aminoguanidine does not inhibit aldose reductase activity in galactosefed rats. J Diabetes Complications. 1996;10:23–30. doi: 10.1016/1056-8727(94)00054-9. [DOI] [PubMed] [Google Scholar]
- 29.Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 30.Crobu F, Latini V, Marongiu MF, et al. Differentiation of single cell derived human mesenchymal stem cells into cells with a neuronal phenotype: RNA and microRNA expression profile. Molecular biology reports. 2012;39:3995–4007. doi: 10.1007/s11033-011-1180-9. [DOI] [PubMed] [Google Scholar]
- 31.Cheng I, Mayle RE, Cox CA, et al. Functional assessment of the acute local and distal transplantation of human neural stem cells after spinal cord injury. Spine J. 2012;12:1040–4. doi: 10.1016/j.spinee.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Richardson SM, Walker RV, Parker S, et al. Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells. 2006;24:707–16. doi: 10.1634/stemcells.2005-0205. [DOI] [PubMed] [Google Scholar]
- 33.Yang H, Wu J, Liu J, et al. Transplanted mesenchymal stem cells with pure fibrinous gelatin-transforming growth factor-beta1 decrease rabbit intervertebral disc degeneration. Spine J. 2010;10:802–10. doi: 10.1016/j.spinee.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 34.Chen CW, Tsai YH, Deng WP, et al. Type I and II collagen regulation of chondrogenic differentiation by mesenchymal progenitor cells. J Orthop Res. 2005;23:446–53. doi: 10.1016/j.orthres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Chen CW, Boiteau RM, Lai WF, et al. sAPPalpha enhances the trans-differentiation of adult bone marrow progenitor cells to neuronal phenotypes. Curr Alzheimer Res. 2006;3:63–70. doi: 10.2174/156720506775697205. [DOI] [PubMed] [Google Scholar]
- 36.Tsai SY, Yang LY, Wu CH, et al. Injury-induced Janus kinase/protein kinase C-dependent phosphorylation of growth-associated protein 43 and signal transducer and activator of transcription 3 for neurite growth in dorsal root ganglion. J Neurosci Res. 2007;85:321–31. doi: 10.1002/jnr.21119. [DOI] [PubMed] [Google Scholar]
- 37.Liu WL, Lee YH, Tsai SY, et al. Methylprednisolone inhibits the expression of glial fibrillary acidic protein and chondroitin sulfate proteoglycans in reactivated astrocytes. Glia. 2008;56:1390–400. doi: 10.1002/glia.20706. [DOI] [PubMed] [Google Scholar]
- 38.Basso DM, Beattie MS, Bresnahan JC, et al. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996;13:343–59. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- 39.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 40.Fekete N, Gadelorge M, Furst D, et al. Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: production process, content and identification of active components. Cytotherapy. 2012;14:540–54. doi: 10.3109/14653249.2012.655420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verfaillie CM. Adult stem cells: assessing the case for pluripotency. Trends Cell Biol. 2002;12:502–8. doi: 10.1016/s0962-8924(02)02386-3. [DOI] [PubMed] [Google Scholar]
- 42.Lei Z, Yongda L, Jun M, et al. Culture and neural differentiation of rat bone marrow mesenchymal stem cells in vitro. Cell Biol Int. 2007;31:916–23. doi: 10.1016/j.cellbi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Crigler L, Robey RC, Asawachaicharn A, et al. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006;198:54–64. doi: 10.1016/j.expneurol.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 44.Sirko S, von Holst A, Wizenmann A, Gotz M, Faissner A. Chondroitin sulfate glycosaminoglycans control proliferation, radial glia cell differentiation and neurogenesis in neural stem/progenitor cells. Development. 2007;134:2727–38. doi: 10.1242/dev.02871. [DOI] [PubMed] [Google Scholar]
- 45.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–95. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


