Abstract
Background
The American Heart Association Cardiovascular Health (CVH) score is inversely associated with cardiovascular disease, but its relations to cardiac remodeling traits and to heart failure (HF) incidence have not been examined.
Methods and Results
A 14-point score was constructed for each participant based on the presence of poor, intermediate or ideal status on each of the 7 CVH metrics (ideal score=14). We related the CVH score to echocardiographic traits cross-sectionally, and to HF incidence prospectively in the Framingham Offspring Study. In age- and sex-adjusted models, a higher CVH score was associated with lower left ventricular (LV) mass, LV wall thickness, LV diastolic dimension, and left atrial dimension (p<0.01 for all; N=2392, mean age 58 years, 56% women), and with a 12-15% lower odds of prevalent LV concentric remodeling and concentric hypertrophy, respectively (p<0.0001 for both). On follow-up (mean 12.3 years), 188 incident HF events were observed in 3201 participants (mean age 59 years, 53% women). In age- and sex-adjusted Cox proportional hazards models, the CVH score was inversely associated with HF incidence (hazards ratio [HR] per 1-point higher CVH score 0.77, 95% CI 0.72-0.83). This association was partially attenuated upon adjustment for LV mass and interim myocardial infarction (HR 0.84, 95% CI 0.76-0.93) and was consistent for HF with preserved and reduced ejection fractions.
Conclusions
In our community-based sample, comprised predominantly of middle-aged white individuals of European descent, better CVH was associated with lower HF incidence, in part due to a lower prevalence of adverse cardiac remodeling.
Keywords: American Heart Association, prevention, heart failure, remodeling, lifestyle
It is estimated that almost six million Americans are currently living with heart failure (HF), a number that is projected to exceed 8 million by 2030.1 As a result of the high prevalence and substantial morbidity and mortality associated with HF, the financial burden to the healthcare system is enormous, with an estimated annual cost of $31 billion in the United States.1 Accordingly, preventing HF is integral to improving population health and reducing financial costs, which is reflected by the emphasis on prevention in recent HF practice guidelines.2, 3
The primary modifiable risk factors for HF include high blood pressure, diabetes, obesity, and smoking.4, 5 Recently, investigators from the Physicians Health Study I and Women's Health Initiative have also demonstrated associations of lifestyle factors including healthy diet, physical activity, and moderate alcohol intake with a reduced incidence of HF in the community.6, 7 Cardiovascular risk factors and unhealthy behaviors have been theorized to contribute to HF pathogenesis by promoting atherosclerotic vascular disease, myocardial infarction (MI), and adverse structural remodeling. These changes in cardiac structure and function, corresponding to stage B of the American Heart Association/American College of Cardiology (AHA/ACC) HF Stages, are best characterized by increases in left ventricular (LV) mass (LVM), left atrial dimension (LAD) and LV geometric patterns indicative of adverse remodeling (i.e., concentric remodeling, concentric and eccentric hypertrophy). Each of the aforementioned echocardiographic traits and LV geometry patterns has been related to incident HF in community-based cohorts.8-11
In 2010 the American Heart Association (AHA) announced the goals of improving cardiovascular health (CVH) by 20%, and reducing deaths from cardiovascular disease (CVD) and stroke by 20% by the year 2020.12 In order to achieve these objectives, the AHA has promoted a set of seven behaviors and risk factor values (Life's Simple 7™) including: abstinence from smoking, ideal body mass index (BMI), regular physical activity, healthy diet, low untreated serum total cholesterol concentrations, optimal blood pressure and absence of diabetes.12 Together, these components comprise CVH, which is inversely associated with the risk of CVD and overall mortality in multiple reports.13-16 However, the relations between CVH and HF have not been systematically assessed. We therefore hypothesized that CVH is inversely associated with the risk of incident HF, and that this association is partly attributable to the inverse association of CVH with the prevalence of adverse cardiac remodeling.
Methods
Study Sample
The descriptions of the history, design, and methodology of the Framingham Offspring Study (FOS) have been reported elsewhere.17 For this investigation, 3532 FOS participants attending the sixth examination cycle (1995-1998) were considered for inclusion, and two distinct samples were evaluated for analysis of echocardiographic traits and incidence of HF, respectively, as detailed below. At the sixth examination cycle (referred to as baseline for the present investigation), all attendees underwent a medical history, anthropometry, a cardiovascular-targeted physical examination, electrocardiography, echocardiography and phlebotomy for measurement of standard cardiovascular risk factors at the Framingham Heart Study (FHS) clinic. Of 3532 attendees, we initially excluded participants for serum creatinine concentration ≥2 mg/dL (n=15), body mass index (BMI) <18.5 kg/m2 (n=62), missing components of the CVH score (n=219), or outlier laboratory values (n=1), yielding a base sample of 3235 participants. To evaluate the association between CVH and echocardiographic indices of cardiac remodeling, additional exclusions were made from the base sample for missing echocardiographic measurements (n=843), resulting in a sample of 2392 participants (sample 1). For evaluating the association between CVH and incident HF, we excluded from the base sample people with prevalent HF (n=30) and non-available follow-up time (n=4), resulting in a sample of 3201 participants (sample 2); this sample was larger because availability of echocardiographic measurements was not required for relating CVH score to incident HF. The Boston University Medical Center Institutional Review Board approved all study protocols. Written informed consent was provided by all participants.
AHA Cardiovascular Health Score
A Cardiovascular Health Score (CVH score) was constructed for each participant by assigning a score of 0 (poor status), 1 (intermediate status) or 2 (ideal status) for each of the 7 metrics of CVH (Supplementary Table 1). The CVH score was obtained by summing these values and it can thus vary from a minimum of 0 (consistent with poor CVH) to a maximum of 14 (indicating ideal CVH). Resting blood pressure, BMI, cholesterol, fasting blood glucose, and self-reported smoking status were measured at the FHS clinic. The physical activity index was calculated as the sum of the reported time spent performing specific physical activities during a 24-hour period, as previously described.16, 18 The highest quartile of this index was used to define ideal physical activity, and values higher than the median but less than the top quartile were used to define intermediate status. Dietary quality was assessed using a food frequency questionnaire (Supplementary Table 1). In agreement with previous investigations, a score of ≥2 was considered to be ideal status, 1 was considered intermediate status, and 0 was considered poor status.14, 19
Echocardiographic Measurements
All attendees at the sixth FOS examination cycle (1995-1998) underwent comprehensive two-dimensional transthoracic echocardiography with Doppler color flow imaging as described previously.20 Echocardiograms were read by a sonographer or a cardiologist who was blinded to clinical information. M-mode measurements, averaged over ≥ 3 cardiac cycles, were obtained from digitized images using the leading edge-to-leading edge technique for LAD, end-diastolic LV septal wall thickness, posterior wall thickness, and LV diameter at the end of diastole (LVDD) and systole (LVDS). Fractional shortening (FS) was calculated as: [(LVDD-LVDS)/LVDD]×100, LV wall thickness (LVWT) was calculated by summing LV septal wall thickness and posterior wall thickness in end-diastole, and relative wall thickness (RWT) was obtained by dividing LVWT by LVDD. We calculated LVM using the formula: LVM=0.8{1.04[(LVDD + posterior wall thickness+ septal wall thickness)3 – (LVDD)3]} + 0.6, based on American Society of Echocardiography (ASE) guidelines.21 LV hypertrophy (LVH) was determined by indexing the LVM to body surface area and values >115 g/m2 for men and >95 g/m2 for women were considered abnormal.21 LV systolic dysfunction (LVSD) was defined by FS <29% or by mild or greater reduction in ejection fraction based on qualitative assessment. Using ASE cut-points for normal LVM (≤115 g/m2 for men and ≤95 g/m2 for women) and the normal RWT cut-point of ≤0.42,21 geometric patterns were defined as: normal geometry (normal LVM and RWT), concentric remodeling (normal LVM and increased RWT), concentric hypertrophy (increased LVM and RWT) and eccentric hypertrophy (increased LVM and normal RWT), Supplementary Table 2.
Outcome Events
Participants were followed continuously throughout the study period for the incidence of cardiovascular disease events including HF, which was defined using the FHS criteria.22 In brief, ascertainment of HF requires the presence of two major or one major and two minor criteria; major criteria include paroxysmal nocturnal dyspnea or orthopnea, increased venous pressure, distended neck veins, rales, cardiomegaly by radiograph, pulmonary edema, a third heart sound, hepatojugular reflux, and weight loss on diuretic therapy. The presence of ankle edema, nocturnal cough, hepatomegaly, dyspnea on exertion, pleural effusion, decrease in vital capacity and tachycardia comprise the minor criteria. Medical records were obtained for all physician visits and hospitalizations related to cardiovascular disease, and events were adjudicated by a review committee of three FHS physicians. The date of onset of HF was determined by the earliest onset of symptoms, or the date of hospitalization or clinic visit. In secondary analyses, we assessed the associations between CVH score and HF subtype after categorizing HF events as HF with reduced ejection fraction (HFREF) if the ejection fraction was <45% and HF with preserved ejection fraction (HFPEF) if the ejection fraction was ≥45% based on echocardiographic reports from the index hospitalization.
Statistical Analysis
In both samples, the predictor of interest was the CVH score, which was modeled as a continuous variable in all primary analyses. Using sample 1, we examined the cross-sectional relations of the CVH score with: A) the following echocardiographic indices: LVM, LVDD, LVWT, LAD, FS in age- and sex-adjusted linear regression models; B) the presence of LVSD and LVH in age- and sex-adjusted logistic regression models (both binary variables); and C) the presence of four LV geometric patterns (normal geometry, concentric remodeling, concentric hypertrophy and eccentric hypertrophy) in age- and sex-adjusted multinomial logistic regression models using generalized logits treating those with normal geometry as the referent group.
Using sample 2, we evaluated the prospective association between the CVH score and HF incidence using age- and sex-adjusted Cox proportional hazards regression models, after confirming that the assumption of proportionality of hazards was met. In secondary analyses, we examined the relations of CVH score with the incidence of HFPEF and HFREF (separate analyses for each). Fine-Gray proportional hazards models were used to account for competing risks, including the HF subtype not modeled, and indeterminate HF events (i.e., HF with unavailable EF). We examined penalized cubic spines to assess for potential non-linearity of the associations of the CVH score with HF, and with each of the HF subtypes.
In further secondary analyses, the CVH score was modeled in categories with grouping of 0-7, 8-9, 10-14 (to provide approximate tertiles), treating the lower CVH tertile (scores of 0-7) as the referent. We also analyzed the CVH score as a 7-point score with each of the 7 AHA metrics considered as a binary variable. Sensitivity analyses were performed after removing the hypertension metric from the CVH score, thus constructing a score with a maximum value of 12-points. Statistical significance was assessed based on a two-sided p-value of <0.05. The SAS Software version 9.3 (Cary, NC) was used for all analyses. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
The baseline characteristics of the larger sample (sample 2) are shown in Table 1. Participants were mostly middle-aged and overweight with a high prevalence of hypertension. Ideal CVH was present in a very small proportion of participants (Figure 1). Characteristics were similar in sample 1 (Supplementary Table 3, Supplementary Figure 1).
Table 1. Clinical characteristics of study sample 2.
| Characteristics | Women (N=1703) | Men (N=1498) |
|---|---|---|
| Age, years | 58.8±9.6 | 59.2±9.6 |
| Follow-up time, years | 12.6±2.6 | 12.0±3.3 |
| Body mass index, kg/m2 | 27.4±5.7 | 28.7±4.4 |
| Total cholesterol, mg/dl | 212.0±38.3 | 198.3±36.0 |
| HDL cholesterol, mg/dl | 57.9±16.0 | 43.3±11.9 |
| Lipid lowering medication (%) | 10.9 | 16.3 |
| Fasting glucose, mg/dL | 100.2±26.7 | 107.5±28.3 |
| Diabetes (%) | 7.6 | 11.9 |
| Diabetes medication (%) | 3.8 | 6.5 |
| Systolic blood pressure, mm Hg | 127.0±20.1 | 129.7±17.0 |
| Diastolic blood pressure, mm Hg | 73.9±9.3 | 77.4±9.4 |
| Hypertensive (%) | 66.8 | 77.4 |
| Antihypertensive medication (%) | 25.0 | 30.6 |
| Smokers (%) | 14.9 | 14.2 |
| Diet score | 1.7±0.9 | 1.4±0.9 |
| Physical activity score | 33.8±5.1 | 36.4±7.3 |
| Biomarkers*, median (Q1, Q3) | ||
| B-type natriuretic peptide, pg/mL | 9.8 (4.0, 19.6) | 6.3 (4.0, 15.9) |
| Urine albumin-to-Cr ratio, μg/mg | 8.5 (3.5, 17.3) | 4.8 (2.0, 10.6) |
| CVH score (mean±SD) | 8.7±2.2 | 8.4±2.0 |
HDL indicates high-density lipoprotein; Q1, quartile 1; Q3, quartile 3; Cr, creatinine; CVH, cardiovascular health.
Sample size for the biomarker evaluation was 2046.
Figure 1.
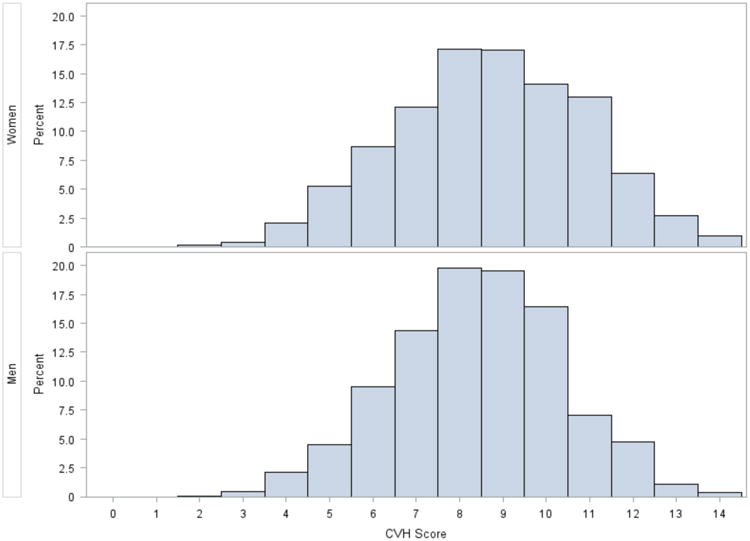
Distribution of CVH scores in sample 2.
Association Between CVH Score and Echocardiographic Traits
In cross-sectional analyses, statistically significant associations between the CVH score and several echocardiographic measures were observed (Table 2). LVM was inversely associated with CVH score, in concordance with its two primary components: LVWT and LVDD. LAD also demonstrated an inverse association with the CVH score. No statistically significant associations were observed between the CVH score and FS, LVSD or LVH.
Table 2. Age- and sex-adjusted associations between CVH score and echocardiographic measurements.
| Echocardiographic Measurement | Beta Coefficient (95% CI) | p-value |
|---|---|---|
| LV mass, gm | -3.88 (-4.59, -3.17) | <0.0001 |
| LV wall thickness, mm | -0.27 (-0.31, -0.23) | <0.0001 |
| LV diastolic dimension, mm | -0.15 (-0.23, -0.06) | 0.001 |
| Left atrial dimension, mm | -0.40 (-0.49, -0.30) | <0.0001 |
| Fractional shortening, % | 0.00 (0.00, 0.00) | 0.56 |
| Odds Ratio (95% CI) | p-value | |
| Left ventricular systolic dysfunction | 0.94 (0.86, 1.03) | 0.16 |
| Left ventricular hypertrophy | 0.96 (0.92-1.00) | 0.07 |
Beta coefficient values represent the mean change in echocardiographic measurement per 1-pointhigher CVH score.
Odds ratios represent the odds of having the echocardiographic trait for each 1-point higher CVH score versus not having the trait..
Association Between CVH Score and Geometric Patterns of Cardiac Remodeling
Normal LV geometry was present in 1283 participants (54%), concentric remodeling in 486 (20%), eccentric hypertrophy in 352 (15%) and concentric hypertrophy in 271 (11%). Each one-point higher CVH score was associated with a 12 and 15% lower odds of concentric remodeling and concentric hypertrophy, respectively, compared to the normal geometric pattern (Table 3). When the CVH score was analyzed according to categories, the higher CVH category (score 10-14) had 42% and 54% lower odds of concentric remodeling and concentric hypertrophy respectively, when compared to the lower CVH category (score 0-7; p<0.01 for both), Supplementary Table 4. We did not observe a statistically significant association between the CVH score (continuous or categorical) and the odds of eccentric hypertrophy.
Table 3. Age- and sex-adjusted association between CVH score and LV geometric patterns.
| LV Geometric Pattern | OR (95% CI) | p-value |
|---|---|---|
| Concentric remodeling | 0.88 (0.83, 0.93) | <0.0001 |
| Concentric hypertrophy | 0.85 (0.79, 0.91) | <0.0001 |
| Eccentric hypertrophy | 0.97 (0.92, 1.03) | 0.31 |
OR indicates odds ratio.
Values represent the odds of having the geometric pattern for each 1-point higher CVH score compared to the normal geometric pattern.
Association Between CVH Score and HF Incidence
A total of 3201 participants were followed for up to 16 years (mean 12.3 years) and 188 individuals developed HF (5.9%). The CVH score was inversely associated with incident HF (Figure 2). For each one-point higher CVH score, we observed a 23% lower risk of HF, adjusting for age and sex (Table 4). When the CVH score was modeled as a categorical variable, the HF hazards ratio for CVH scores 8-9 and 10-14 was 45% and 66% lower, respectively as compared with scores of 0-7 (Supplementary Table 5). The effect of CVH score on incident HF was partially attenuated when further adjusted for LVM and interim myocardial infarction (MI; Table 4 models 2-4), which occurred in 250 participants (7.8%). This partial attenuation is reflected by a 35% change in the parameter estimate corresponding to the CVH score (β = -0.26 in the model adjusted for age and sex as compared to β =-0.17 in the model adjusted for age, sex, interim MI and LVM). Further adjustments for B-type natriuretic peptide (BNP) and urine albumin-to-creatinine ratio (UACR) did not result in additional attenuation (Table 4, model 5).
Figure 2.
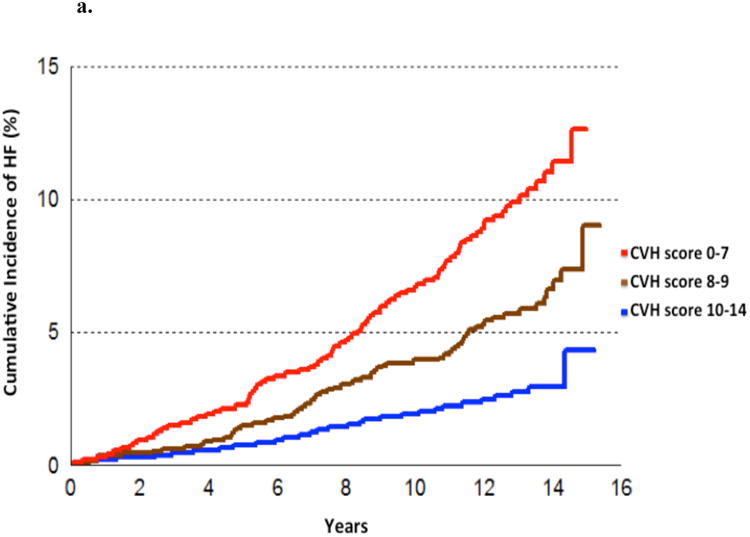
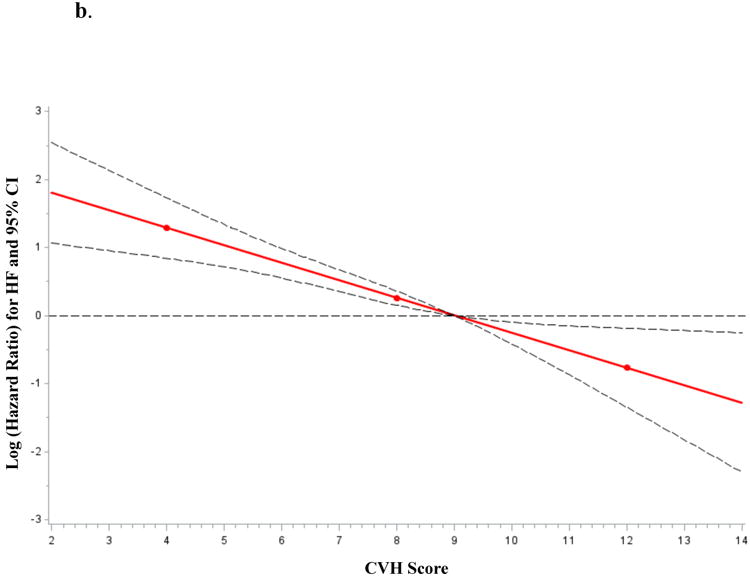
a. Cumulative incidence of HF according to CVH score (modeled as categories).
b. Age-, and sex-adjusted association between CVH score (modeled as a continuous variable) and HF incidence.
Table 4. Multivariable associations between CVH score and incident HF.
| Model | HR (95% CI) | p-value |
|---|---|---|
| 1. Adjusted for age and sex | 0.77 (0.72, 0.83) | <0.0001 |
| 2. Adjusted for age, sex and LVM | 0.82 (0.74, 0.90) | <0.0001 |
| 3. Adjusted for age, sex and interim MI | 0.80 (0.74, 0.86) | <0.0001 |
| 4. Adjusted for age, sex, LVM and interim MI | 0.84 (0.76, 0.93) | 0.0006 |
| 5. Adjusted for age, sex, LVM, interim MI, log (BNP) and UACR* | 0.83 (0.75, 0.93) | 0.0008 |
HR indicates hazard ratio; BNP, B-type natriuretic peptide; LVM, left ventricular mass; MI, myocardial infarction; UACR, urine albumin-to-creatinine ratio.
Values represent HRs for each 1-point higher CVH score.
Due to unavailability of biomarker measurements in a number of participants, the sample size for this model included 2046 participants. When tested in this smaller sample, the results for the other models were unchanged.
Association Between CVH Score and HF Subtype
Of the 188 participants with incident HF, 85 (45.2%) developed HFPEF, 89 (47.3%) developed HFREF and in 14 (7.5%) subjects the HF subtype was indeterminate due to an unavailable EF. Using Fine-Gray Proportional Hazards models to adjust for competing risks, the relations of the CVH score with incident HF did not vary significantly by HF subtype (Table 5 and Supplementary Figure 2). When adjusted for interim MI, the relation between the CVH score and HFPEF remained essentially unchanged (β = -0.22 for model adjusted for age and sex as compared to β =-0.23 in the model adjusted for age, sex and interim MI) whereas the association of CVH score and incident HFREF was mildly attenuated (β = -0.27 for model adjusted for age and sex as compared to β = -0.21 in the model adjusted for age, sex and interim MI).
Table 5. Associations between CVH score and HFPEF and HFREF.
| Model | HR (95% CI) | p-value |
|---|---|---|
| HFPEF | ||
| a. Adjusted for age and sex | 0.80 (0.72-0.89) | <0.0001 |
| b. Adjusted for age, sex and interim MI | 0.80 (0.71-0.90) | <0.0001 |
| HFREF | ||
| a. Adjusted for age and sex | 0.77 (0.69-0.85) | <0.0001 |
| b. Adjusted for age, sex and interim MI | 0.81 (0.73-0.90) | <0.0001 |
HR indicates hazard ratio; HFPEF, heart failure with preserved ejection fraction; HFREF, heart failure with reduced ejection fraction
Values represent HRs for each 1-point higher CVH score.
In secondary analyses, the CVH score was modeled in categories and as a 7-point score without notable differences from these findings (data not shown). Additionally, we performed a sensitivity analyses after removing the hypertension metric from the CVH score (thus creating a 12-point CVH score) and the results were essentially unchanged (Supplementary Tables 6-9).
Discussion
Principal Findings
The principal findings of our report are twofold. First, CVH was inversely associated with the prevalence of several echocardiographic traits and with concentric forms of LV geometric patterns cross-sectionally. Second, CVH was inversely associated with HF incidence prospectively, a relation that was consistent amongst the HF subtypes of HFPEF and HFREF and was partly attributable to the inverse association of CVH with the presence of adverse LV remodeling.
Comparison with Prior Studies
Ideal CVH was rare in our sample, consistent with findings from other community-based cohorts.13-16, 23, 24 Previous reports have demonstrated associations between CVH and a reduced incidence of cardiovascular outcomes including stroke,15 cardiovascular death14, 23 and the composite of CVD.13, 16, 25, 26 Several studies have demonstrated the beneficial effects of a healthy lifestyle on reducing HF incidence.6, 7, 27 However, these investigations focused primarily on four of the seven components of the CVH score, namely: abstinence from smoking, BMI ≤25 kg/m2, physical activity and healthy diet, with one study also including alcohol consumption. In each of these previous reports, there was a graded and stepwise association between the positive behavior or risk factor value achieved and a reduced risk of HF. Similarly, in our study we found that each point achieved in the CVH score was associated with a lower incidence of HF. Furthermore, we observed that this association was true for both HFPEF and HFREF; prior reports did not examine the association of CVH with HF subtypes.
Mechanisms Underlying the Association of CVH with a Reduction in HF Incidence
There are several plausible pathomechanisms responsible for the increased risk of HF in those with poor CVH. First, poor CVH is known to promote atherosclerotic vascular disease16, 25 and therefore may lead to clinical HF via reductions in ventricular systolic and diastolic function as a result of MI. Although this sequence of events is frequently encountered in clinical practice, the association between the CVH score and HF incidence was only mildly affected by adjusting for interim MI in our study. However, when HF incidence was assessed according to HF subtype, we did observe that the magnitude of association between the CVH score and HFREF was slightly attenuated after adjusting for interim MI, while the association between CVH score and HFPEF was unchanged. This is consistent with prior observations that ischemic heart disease is more closely related to incident HFREF than HFPEF. Certainly subclinical ischemic heart disease resulting in reduced cardiac function without overt MI is another possible mechanism linking incident HF with poor CVH that was not assessed by the present study.
Alternatively, our findings support a second plausible pathomechanism that poor CVH promotes a program of adverse cardiac remodeling that begins as subclinical changes in cardiac structure and function (AHA/ACC stage B heart failure) and eventually progresses to clinical HF (AHA/ACC stage C heart failure). In our study, a lower CVH score was associated with higher LVM and LAD, and higher odds of having concentric remodeling or concentric hypertrophy. These abnormalities are associated with incident HF and may suggest a mechanistic link between the CVH score and HF incidence. This notion is further supported by the attenuation of the association between CVH score and incident HF when adjusted for LVM, suggesting that increased LVM partially attenuates the relation.
Traditional cardiovascular risk factors leading to clinical HF via subclinical remodeling is biologically plausible and, indeed, several of the components of the CVH score have been individually associated with both clinical HF and adverse cardiac remodeling. Increased LVM, for example, is strongly associated with the presence of hypertension, diabetes, obesity, physical inactivity and smoking,28, 29 while left atrial dilatation correlates with increasing age, BMI, elevated systolic BP and the use of antihypertensive medications.30 Concentric geometric patterns of LV remodeling appear to be more related to the presence of hypertension and diabetes, while eccentric patterns may correlate more strongly with elevated BMI.31 Our data appear consistent with these findings, suggesting that the CVH score may be an important correlate of cardiac structure and geometry.
On the other hand, the associations of the CVH score with incident HF remain statistically significant after adjustment for LVM and other important HF predictors such as interim MI, BNP and UACR. These findings suggest that CVH may also be related to HF incidence independently of cardiovascular remodeling and MI. Therefore, a third plausible explanation for the observed association of CVH with HF incidence involves the positive effects of CVH on other organ systems or other components of the cardiovascular system. Endothelial dysfunction and vascular stiffness, for example, are known precursors of HF that may be impacted by CVH. We did not have measures of vascular stiffness at the sixth FOS examination cycle to examine this premise. Further more, the CVH score has been related to biomarkers of select biological pathways (such as B-type natriuretic peptide, GDF-15, inflammation, renin-angiotensin-aldosterone pathway16) and the latter have been implicated in mediating HF risk.32, 33 The CVH score may also be related to non-cardiovascular predictors of HF such as altered lung or renal function.34 A detailed exploration of these possibilities was beyond the scope of our present investigation. These and other novel mechanisms linking CVH with HF incidence are also possible and warrant further investigation in future reports.
Given the powerful effect of CVH score on incident HF, it is perhaps intriguing that relations were not observed between CVH score and FS, LVSD or eccentric hypertrophy. One potential explanation is that ischemic heart disease, which is more likely to lead to changes in ventricular function, LVSD and eccentric remodeling was relatively uncommon in our middle-aged sample. In addition, the biological mechanisms underlying the associations of the CVH score with LV systolic function might be more complex than the mechanisms linking the CVH score with concentric hypertrophy. This notion is supported by data from the Multiethnic Study of Atherosclerosis (MESA) cohort, in which Heckbert et al. reported that ejection fraction increased with higher systolic blood pressure until a critical point (≥180 mmHg) after which it demonstrated an inverse relationship.28 Diastolic blood pressure, in contrast, was inversely associated with ejection fraction throughout its range of values.28, 35 The CVH score does not take into account this level of complexity. For example, two individuals would both be assigned 0 points for the “blood pressure” metric even if one had a blood pressure of 140/90 mmHg and the other had a blood pressure of 190/120 mmHg. Additionally, we used relatively crude measures of LV pump function and not more sophisticated measures of myocardial performance (such as strain rate imaging, diastolic function, torsion and synchronicity, all of which may contribute to HF risk and may be potentially related to the CVH score).
Strengths and Limitations
This study was performed in a large community-based sample under continuous surveillance for clinical outcomes (including HF) over a long period of follow-up. Our findings are consistent with previous reports of the relations between traditional cardiovascular risk factors and HF incidence and extend this knowledge by specifically evaluating the impact of the CVH score on HF incidence, by analyzing the HF subtypes (HFPEF and HFREF) separately, and by examining a wide range of echocardiographic measures of cardiac remodeling.
However several limitations also merit consideration. Our sample included predominantly white, middle-aged participants of European ancestry with a high prevalence of hypertension, potentially limiting the generalizability of our findings to other age groups and ethnicities with differing risk factor distributions. In addition, the exposure (CVH score) was assessed at a single examination and therefore changes in the score over time could not be assessed. Similarly, the cross-sectional nature of the echocardiographic measurements limits our ability to track changes in the indices over time and may lead to a regression dilution bias, which would be expected to result in an underestimation of the true underlying associations of CVH with echocardiographic traits and with HF. We used M-mode echocardiography rather than 2D measurements and determined left atrial size by diameter rather than volume, both of which are potential limitations. Finally, the definitions used for diet and physical activity were extrapolated from data available from questionnaires completed at the clinical examinations and differ slightly from the AHA metrics.16
Conclusion and Future Directions
Better CVH was associated with a lower risk of HF incidence in our large community-based sample. This association was likely at least partially attributable to a lower prevalence of adverse cardiac remodeling in those with a high CVH score. Further studies are warranted to evaluate the biologic mechanisms underlying the associations between CVH and adverse cardiac remodeling, as well as to assess the role for potential interventions (behavioral and pharmacologic) that might assist in preventing or delaying HF events in higher risk populations. While observational, our findings provide evidence that targeting strategies to improve CVH in the community (using the CVH score as a metric) may potentially reduce the prevalence of subclinical HF stages (Stage B) and perhaps overt HF as well.
Supplementary Material
Clinical Perspective.
Heart failure (HF) is an important public health problem and evidence regarding appropriate tools to promote HF prevention is currently limited. In 2010 the American Heart Association (AHA) articulated seven metrics of cardiovascular health (CVH) with the goal of improving CVH by 20% and as a result reducing cardiovascular disease mortality by 20% by the year 2020. Using data from the Framingham Heart Study, we evaluated whether a score comprised of these seven metrics is associated with cardiac remodeling and HF incidence in the community. In this well-characterized sample of predominantly middle-aged white individuals of European descent, a higher (better) CVH score was associated with lower left ventricular (LV) mass, LV wall thickness, LV diastolic dimension and left atrial dimension, and with lower odds of concentric remodeling patterns in cross-sectional analyses (among 2392 participants with echocardiographic imaging). Over a mean follow-up of approximately 12 years, 188 incident HF events (85 [45.2%] with preserved ejection fraction, 89 [47.3%] with reduced ejection fraction, and 14 [7.5%] with unknown ejection fraction) were observed in 3201 participants. The CVH score was inversely associated with HF incidence with a 23% lower hazard of HF observed per 1-point higher CVH score. The association was consistent among the HF subtypes of preserved versus reduced ejection fractions. These findings suggest that the AHA CVH score might be a valuable tool for public health efforts to promote HF prevention, and that the benefits of improved CVH might be related in part to a lower prevalence of adverse cardiac remodeling.
Acknowledgments
Sources of Funding: This work was supported by National Institute of Health T32-HL007604 (MN), AHA Clinical Research Program award 13CRP14090010 (VX), and by contract NHLBI N01-HC-25195 (RSV).
Footnotes
Disclosures: None.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics C and Stroke Statistics S. Heart disease and stroke statistics-2015 update: a report from the american heart association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, Narula J, Shor ES, Young JB, Hong Y American Heart Association Council on E, Prevention, American Heart Association Council on Clinical C, American Heart Association Council on Cardiovascular N, American Heart Association Council on High Blood Pressure R, Quality of C, Outcomes Research Interdisciplinary Working G, Functional G and Translational Biology Interdisciplinary Working G. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–2565. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL. 2013 ACCF/AHA Guideline for the Management of Heart FailureA Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Dunlay SM, Weston SA, Jacobsen SJ, Roger VL. Risk factors for heart failure: a population-based case-control study. The American Journal of Medicine. 2009;122:1023–1028. doi: 10.1016/j.amjmed.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Archives of Internal Medicine. 2001;161:996–002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 6.Agha G, Loucks EB, Tinker LF, Waring ME, Michaud DS, Foraker RE, Li W, Martin LW, Greenland P, Manson JE, Eaton CB. Healthy Lifestyle and Decreasing Risk of Heart Failure in Women. Journal of the American College of Cardiology. 2014;64:1777–1785. doi: 10.1016/j.jacc.2014.07.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Djoussé L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302:394–400. doi: 10.1001/jama.2009.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The Relationship of Left Ventricular Mass and Geometry to Incident Cardiovascular EventsThe MESA (Multi-Ethnic Study of Atherosclerosis) Study. Journal of the American College of Cardiology. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, Manolio TA, Dries DL, Siscovick DS. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–15. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 10.Takemoto Y, Barnes ME, Seward JB, Lester SJ, Appleton CA, Gersh BJ, Bailey KR, Tsang TS. Usefulness of left atrial volume in predicting first congestive heart failure in patients > or = 65 years of age with well-preserved left ventricular systolic function. Am J Cardiol. 2005;96:832–6. doi: 10.1016/j.amjcard.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 11.Velagaleti RS, Gona P, Pencina MJ, Aragam J, Wang TJ, Levy D, D'Agostino RB, Lee DS, Kannel WB, Benjamin EJ. Left Ventricular Hypertrophy Patterns and Incidence of Heart Failure With Preserved Versus Reduced Ejection Fraction. The American Journal of Cardiology. 2014;113:117–122. doi: 10.1016/j.amjcard.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD American Heart Association Strategic Planning Task F and Statistics C. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 13.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. Journal of the American College of Cardiology. 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. Jama. 2012;307:1273–1283. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, McClellan WM, Muntner P, Hong Y, Safford MM, Goyal A, Cushman M. Life's Simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke; a journal of cerebral circulation. 2013;44:1909–1914. doi: 10.1161/STROKEAHA.111.000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xanthakis V, Enserro DM, Murabito JM, Polak JF, Wollert KC, Januzzi JL, Wang TJ, Tofler G, Vasan RS. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation. 2014;130:1676–83. doi: 10.1161/CIRCULATIONAHA.114.009273. [DOI] [PubMed] [Google Scholar]
- 17.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 18.Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study. Arch Intern Med. 1979;139:857–61. [PubMed] [Google Scholar]
- 19.Molenaar EA, Massaro JM, Jacques PF, Pou KM, Ellison RC, Hoffmann U, Pencina K, Shadwick SD, Vasan RS, O'Donnell CJ, Fox CS. Association of lifestyle factors with abdominal subcutaneous and visceral adiposity: the Framingham Heart Study. Diabetes Care. 2009;32:505–10. doi: 10.2337/dc08-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xanthakis V, Larson MG, Wollert KC, Aragam J, Cheng S, Ho J, Coglianese E, Levy D, Colucci WS, Michael Felker G, Benjamin EJ, Januzzi JL, Wang TJ, Vasan RS. Association of novel biomarkers of cardiovascular stress with left ventricular hypertrophy and dysfunction: implications for screening. Journal of the American Heart Association. 2013;2:e000399. doi: 10.1161/JAHA.113.000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2015;28:1–39 e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 22.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–6. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 23.Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125:987–995. doi: 10.1161/CIRCULATIONAHA.111.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shay CM, Ning H, Allen NB, Carnethon MR, Chiuve SE, Greenlund KJ, Daviglus ML, Lloyd-Jones DM. Status of cardiovascular health in US adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003-2008. Circulation. 2012;125:45–56. doi: 10.1161/CIRCULATIONAHA.111.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the northern Manhattan study. Circulation. 2012;125:2975–2984. doi: 10.1161/CIRCULATIONAHA.111.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu S, Huang Z, Yang X, Zhou Y, Wang A, Chen L, Zhao H, Ruan C, Wu Y, Xin A, Li K, Jin C, Cai J. Prevalence of ideal cardiovascular health and its relationship with the 4-year cardiovascular events in a northern Chinese industrial city. CirculationCardiovascular quality and outcomes. 2012;5:487–493. doi: 10.1161/CIRCOUTCOMES.111.963694. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Tuomilehto J, Jousilahti P, Antikainen R, Mahonen M, Katzmarzyk PT, Hu G. Lifestyle factors in relation to heart failure among Finnish men and women. Circulation Heart failure. 2011;4:607–12. doi: 10.1161/CIRCHEARTFAILURE.111.962589. [DOI] [PubMed] [Google Scholar]
- 28.Heckbert SR, Post W, Pearson GDN, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imagingThe Multiethnic Study of Atherosclerosis. Journal of the American College of Cardiology. 2006;48:2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lieb W, Xanthakis V, Sullivan LM, Aragam J, Pencina MJ, Larson MG, Benjamin EJ, Vasan RS. Longitudinal tracking of left ventricular mass over the adult life course: clinical correlates of short- and long-term change in the framingham offspring study. Circulation. 2009;119:3085–3092. doi: 10.1161/CIRCULATIONAHA.108.824243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McManus DD, Xanthakis V, Sullivan LM, Zachariah J, Aragam J, Larson MG, Benjamin EJ, Vasan RS. Longitudinal tracking of left atrial diameter over the adult life course: Clinical correlates in the community. Circulation. 2010;121:667–674. doi: 10.1161/CIRCULATIONAHA.109.885806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox ER, Taylor J, Taylor H, Han H, Samdarshi T, Arnett D, Myerson M. Left ventricular geometric patterns in the Jackson cohort of the Atherosclerotic Risk in Communities (ARIC) Study: clinical correlates and influences on systolic and diastolic dysfunction. Am Heart J. 2007;153:238–44. doi: 10.1016/j.ahj.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, Ho JE, Fradley MG, Ghorbani A, Xanthakis V, Kempf T, Benjamin EJ, Levy D, Vasan RS, Januzzi JL. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velagaleti RS, Gona P, Levy D, Aragam J, Larson MG, Tofler GH, Lieb W, Wang TJ, Benjamin EJ, Vasan RS. Relations of biomarkers representing distinct biological pathways to left ventricular geometry. Circulation. 2008;118:2252–8. doi: 10.1161/CIRCULATIONAHA.108.817411. 5p following 2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lam CS, Lyass A, Kraigher-Krainer E, Massaro JM, Lee DS, Ho JE, Levy D, Redfield MM, Pieske BM, Benjamin EJ, Vasan RS. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation. 2011;124:24–30. doi: 10.1161/CIRCULATIONAHA.110.979203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, Benjamin EJ, Vasan RS. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation. 2010;122:570–578. doi: 10.1161/CIRCULATIONAHA.110.937821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


