Abstract
Background
Contemporary data are lacking on the prognostic importance of heart failure (HF) after myocardial infarction (MI). We evaluated the prognostic impact of HF post MI according to preserved/reduced ejection fraction (EF) and the timing of its occurrence.
Methods and Results
All Olmsted County, Minnesota residents (n=2,596) with incident MI diagnosed in 1990-2010 and no prior HF were followed through March 2013. Cox models were used to examine (1) the hazard ratios (HRs) for death associated with HF type and timing; and (2) secular trends in survival by HF status. During a mean follow-up of 7.6 years, there were 1116 deaths, 634 in the 902 patients who developed HF (70%), and 482 in the 1694 patients who did not develop HF (28%). After adjustment for age and sex, HF as a time-dependent variable was strongly associated with mortality (HR=3.31, 95% confidence interval [CI]: 2.93-3.75), particularly from cardiovascular causes (HR=4.20, 95% CI: 3.50-5.03). Further adjustment for MI severity and comorbidity, acute treatment, and recurrent MI moderately attenuated these associations (HR=2.49 and 2.94 for all-cause and cardiovascular mortality, respectively). Mortality did not differ by EF, but was higher for delayed- vs. early-onset HF (p for heterogeneity=0.002). The age- and sex-adjusted 5-year survival estimates in 2001-2010 vs. 1990-2000 were 82% and 81% among HF-free and 61% and 54% among HF patients, respectively (p for heterogeneity of trends=0.05).
Conclusions
HF markedly increases the risk of death after MI. This excess risk is similar regardless of EF but greater for delayed- vs. early-onset HF. Mortality after MI declined over time, primarily as a result of improved HF survival.
Keywords: myocardial infarction, heart failure, mortality, ejection fraction, epidemiology, trends, cohort studies, secondary prevention
Heart failure (HF) is common after acute myocardial infarction (MI),1-4 which is considered to be one of its major precursors,5-7 and has been associated with excess mortality.4, 8-15 The magnitude of this excess risk was reported to be unchanged during the 1980s and 1990s.16, 17 However, important changes in the epidemiology of MI have taken place over the last decades, characterized by an increased proportion of non-ST-segment-elevation MI, improved acute treatment and secondary prevention measures, reduced short-term case fatality rates and an increasing burden of morbidity and mortality from non-cardiovascular causes.18-22 These have likely influenced the already complex and multifaceted association between HF after MI and mortality. Changes in the epidemiology of HF after MI occurred as well, with a decline in its incidence3, 15, 23 and a change in the case mix according to left ventricular dysfunction, characterized by an increasing proportion of HF cases presenting with preserved ejection fraction (EF),3 for which treatment benefits are less established.24, 25 These complex changes in key determinants of the incidence and prognosis of HF after MI point to the need to evaluate its current prognostic role. Indeed, previous estimates based on well-defined clinical cohorts are now outdated4, 8, 9, 12, 13, 16 because they do not reflect the aforementioned changes in the epidemiology of MI, and HF complicating MI, in the population. More recent studies have not applied standardized methods of MI and HF ascertainment11, 14 or were limited to HF developing during the index MI hospitalization only.10, 15 Including HF after hospital discharge is important, however, as evidence suggests that these cases face poor prognosis.8, 11, 14 Hence, existing results are predictably conflicting, with mortality hazard ratios (HRs) associated with incident HF ranging from less than twofold4, 15 to more than tenfold.8 Most importantly, reports classifying HF by reduced (HFrEF) or preserved (HFpEF) EF are rare, and no data are available evaluating the association with cause-specific death. This is critical in light of the reported shift in deaths after HF toward non-cardiovascular causes, particularly among patients with HFpEF.26
The purpose of this study, using a population-based approach with robust, standardized methods of MI and HF ascertainment, was to determine (1) the impact of HF complicating MI on all-cause and cause-specific mortality; (2) whether these associations differ according to EF and timing of HF onset after MI; and (3) changes over a 20-year study period (1990-2010) in relative and absolute survival by HF status.
Methods
Study Design and Setting
This research was conducted in Olmsted County, Minnesota, a location ideally suited for epidemiological studies because of its relative isolation from other urban centers and because comprehensive medical records from all sources of care for the local population are indexed and linked via the Rochester Epidemiology Project.27 As virtually all Olmsted County residents are represented in this system, this data source provides a nearly complete enumeration of the source population for many decades.28 Following approval as minimal risk study by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards, a follow-up study was carried out utilizing the above-mentioned resources. All persons included in the study provided authorization for use of their medical records for research.
Cohort Identification and Validation
Residents admitted to Olmsted County hospitals with possible MI from 1990 to 2010 were identified using methods previously described.19 Briefly, all events with International Classification of Diseases, 9th revision (ICD-9) code 410 (acute MI) were reviewed. In addition, events with code 411 (other ischemic heart disease) were reviewed in a 50% random sample until 1998, a 10% random sample from 1999 to 2002, and a 100% sample from 2003 to 2010. Additional codes were not included because of their low yield.
MIs were validated using standard epidemiologic criteria.19 Patients diagnosed with MI prior to 1990 were excluded so that only incident (first-ever) cases were included. The diagnosis of MI was verified based on the presence of two of the following: cardiac pain, elevated biomarkers, and electrocardiogram (ECG) changes. Biomarkers used in clinical practice included creatine kinase (CK) and CK-MB until 2000 and troponin thereafter. However, CK-MB was measured until the end of the period as part of a surveillance study. Troponin T, CK, and CK-MB were measured with a sandwich electrochemiluminescence immunoassay on the Elecsys 2010 (Roche Diagnostics Corp, Indianapolis, IN) in the Laboratories of the Department of Medicine and Pathology at Mayo Clinic.
Main Exposure Measure
The primary exposure was incident HF. Participants were followed through March 2013 using their complete inpatient and outpatient medical records in the community from index MI date to HF incidence, death, or the most recent clinical contact. Participants diagnosed with HF by ICD-9 code 428 were identified. Abstractors then reviewed records to validate HF using the Framingham criteria. These criteria require the presence of at least 2 major criteria, or 1 major criterion in addition to 2 minor criteria, to confirm HF.29 This approach was applied previously, showing minimal missing data and excellent inter-observer agreement.30
The type of HF was defined according to echocardiographic measurement as HFrEF (EF<50%) and HFpEF (EF≥50%). EF was measured using an approach that was recently described.31 The EF measurement that was closest to the HF diagnosis (applying a predefined maximum period of 60 days) was recorded for each participant; the median (25th, 75th percentile) time from EF measurement to HF was −1 (−2, 0) days and did not change over the study period (p=0.07). The cutoff of 50% to define preserved/reduced EF was selected according to the guidelines.32 Time of HF onset was classified as “early-onset” (≤3 days after MI) and “delayed-onset” (>3 days), based on median length of hospital stay after MI during the 2000s.
Outcome Measures
The primary outcomes were time to all-cause and cause-specific death. Using the medical record, follow-up began at the time of the index MI and continued through March 2013. In addition to death noted in clinical care, the Mayo Clinic registration office records obituaries and local death notices, and death data are obtained quarterly from the State of Minnesota Department of Vital and Health Statistics. Information on the date of death and its underlying cause was obtained, through which deaths were classified as cardiovascular (ICD-9 390-459) and non-cardiovascular.33
Additional Clinical Data
The medical record was reviewed to ascertain cardiovascular risk factors, comorbidities, MI characteristics, and acute treatment variables at the index date or at the closest time before hospital admission. Smoking was classified as current versus non-current smoking. Body mass index (BMI, kg/m2;) was calculated using the current weight and earliest adult height. Clinical definitions were used to assess hypertension, diabetes mellitus, and hyperlipidemia. Overall comorbidity burden was assessed by the Charlson comorbidity index.34 The Modification of Diet in Renal Disease equation was used to estimate glomerular filtration rate, with less than 60 mL/min per 1.73 m2 regarded as impaired renal function.35 MI presentation according to ST-segment-elevation, Q-wave and anterior location was determined, as well as Killip class. The latter was assessed within 24 hours of the index MI and analyzed as a categorical variable (class >1 vs. class 1). Revascularization procedures during the index hospitalization included coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI). Recurrent MI (occurrence and date) was recorded on the basis of clinical diagnoses.
Statistical Analysis
Baseline characteristics, overall and by HF status during follow-up are presented as mean and standard deviations for continuous variables and as frequencies for categorical variables. Death rates with person-time denominators were calculated for HF and HF-free categories and compared with Fisher’s exact test. Person-time at risk for the HF-free category was accumulated from the index MI until HF diagnosis, death, or end of follow-up. For the HF category, person-time at risk was accumulated from HF validation date until death or end of follow-up. Cox proportional hazards models were constructed to estimate the HRs and 95% confidence intervals (CIs) for all-cause, cardiovascular, and non-cardiovascular disease mortality associated with HF. HF was modeled as a time-dependent variable, allowing subjects to transfer from one exposure group to another during follow-up. Initial adjustment was made for age (as a linear term) and sex (“base model”). Subsequently, Charlson comorbidity index, Killip class, PCI, and recurrent MI (modeled as a time-dependent covariate) were further adjusted for (“multivariable-adjusted model”). The selection of variables for the multivariable model was based on the percent change in the age- and sex-adjusted regression coefficient for HF (regressed on time to death) upon inclusion of individual candidate confounding variables, applying a 5% threshold.36, 37 Models were repeated with HF defined according to type (HFrEF vs. HFpEF) and timing (early- vs. delayed-onset HF), with the same set of covariates used to enhance comparability across analyses. The proportional hazards assumption was tested by examining the Schoenfeld residuals (applying the cox.zph function in R), with no violations detected.
Temporal trends in the association between HF and mortality (overall and by cardiovascular/non-cardiovascular causes) were assessed using Cox models, adjusting for the aforementioned sets of covariates. Four groups were defined according to year of entry into the cohort (1990-2000 vs. 2001-2010) and HF status (modeled as a time-dependent variable). Both HR and absolute risk reduction estimates were calculated, with the direct adjustment method used for the latter.
EF was missing in 19% of the cases, necessitating multiple imputations.38 Five datasets were created with missing values replaced by imputed values based on a model incorporating various demographic and clinical variables and an indicator for HF along with the cumulative baseline hazard of HF approximated by the Nelson-Aalen estimator.39 The results of these datasets were then combined using Rubin’s rules.38 Analyses were performed using SAS statistical software, version 9.3 (SAS Institute Inc., Cary, North Carolina) and R, version 2.14.0 (The R Foundation for Statistical Computing). Heterogeneity tests for differences across strata40, 41 were done with WINPEPI, version 11.23.42
Results
Between January 1990 and December 2010, 2,943 residents of Olmsted County, MN, were hospitalized with first MI, representing the entire experience of a community. Among these, 347 patients had a history of prior HF and were excluded leaving 2,596 participants in the present study (mean age, 67 years; 60% men).
During a mean (SD) follow-up of 7.6 (5.8) years (19,814 person-years overall), 902 patients developed HF (425 [47%] within 3 days; 563 [62%] HFrEF), 535 experienced a recurrent MI (which occurred on the same day or preceded HF in 127 patients [14% of HF cases]), and 1,116 died. A total of 477 patients developed delayed-onset HF (>3 days after MI) at a mean (SD) of 4.6 (5.0) years (median [25th-75th percentiles], 3.0 [0.2-7.7] years). The proportion of HFrEF was 68% in early-onset HF and 58% in delayed-onset HF patients. The incidence rate of HF per 100 person-years was 5.8 (9.0 when restricting follow-up to 5 years post-MI). The incidence rates per 100 person-years for HFrEF and HFpEF were 3.6 and 2.2, respectively (5.8 and 3.2 when restricting follow-up to 5 years post-MI, respectively). Baseline characteristics of the patients at the time of the index MI by HF status and features during follow-up are presented in Table 1. On average, patients who developed HF after MI were older and more likely to be female, hypertensive and diabetic. They also presented with higher Killip class and more comorbidities, were more likely to have anterior MI, and less likely to undergo PCI compared with their HF-free counterparts. Among HF cases, patients with HFrEF were more likely to be male; they also presented with more comorbidities and were more likely to have anterior MI compared with patients with HFpEF. Patients with early-onset HF presented with more comorbidities, higher Killip class and were more likely to have anterior MI compared with patients with delayed-onset HF.
Table 1.
Baseline Characteristics by Heart Failure Status, Type and Timing after Myocardial Infarction
| Characteristic | Missing, n |
Overall (n=2596) |
HF Status at Follow-up (n=2596) |
HF Type (n=902) |
HF Timing (n=902) |
|||
|---|---|---|---|---|---|---|---|---|
| Did not Develop HF (n=1694) |
Developed HF (n=902) |
Reduced EF (n=563) |
Preserved EF (n=339) |
Early-onset (n=425) |
Delayed - onset (n=477) |
|||
| Age, years | - | 66.5 (14.5) * | 63.1 (14.2) * | 73.0 (12.8) * | 72.8 (13.1) * | 73.4 (12.3) * | 73.9 (13.7) * | 72.3 (11.9) * |
| Male | - | 1566 (60) | 1128 (67) | 438 (49) | 299 (53) | 139 (41) | 202 (48) | 236 (50) |
| BMI, kg/m2 | 2 | 28.4 (5.9) * | 28.5 (6.0) * | 28.3 (6.1) * | 28.1 (5.8) * | 28.7 (6.5) * | 27.5 (6.0) * | 29.0 (6.1) * |
| Hypertension | - | 1571 (61) | 926 (55) | 645 (72) | 387 (69) | 258 (76) | 297 (70) | 348 (73) |
| Hyperlipidemia | 1 | 1351 (52) | 906 (56) | 445 (49) | 261 (46) | 184 (54) | 200 (47) | 245 (52) |
| Current smoking | 3 | 670 (26) | 464 (27) | 206 (23) | 133 (24) | 73 (21) | 98 (23) | 108 (23) |
| Diabetes mellitus | - | 534 (21) | 285 (17) | 249 (28) | 161 (29) | 88 (26) | 125 (29) | 124 (26) |
| Charlson comorbidity index |
1 | 1 (0, 2) † | 0 (0, 2) † | 1 (0, 3) † | 2 (0, 3) † | 1 (0, 3) † | 2 (0, 3) † | 1 (0, 3) † |
| Killip >1 | 30 | 646 (25) | 245 (15) | 401 (45) | 261 (47) | 140 (42) | 294 (70) | 107 (23) |
| ST-elevation MI | 42 | 824 (32) | 546 (33) | 278 (31) | 186 (34) | 92 (28) | 141 (33) | 137 (29) |
| Anterior MI | 42 | 907 (36) | 528 (32) | 379 (43) | 269 (48) | 110 (33) | 216 (51) | 163 (35) |
| Q-wave MI | 175 | 1305 (54) | 787 (50) | 518 (61) | 353 (66) | 165 (52) | 265 (65) | 253 (57) |
| PCI | - | 1402 (54) | 1026 (61) | 376 (42) | 233 (41) | 143 (42) | 162 (38) | 214 (45) |
| CABG | - | 239 (9) | 133 (8) | 106 (12) | 66 (12) | 40 (12) | 50 (12) | 56 (12) |
| Atrial fibrillation | 16 | 280 (11) | 117 (7) | 163 (18) | 101 (18) | 62 (18) | 96 (23) | 67 (14) |
| Estimated GFR, <60 mL/min per 1.73 m2 |
17 | 1203 (47) | 644 (38) | 559 (62) | 347 (62) | 212 (63) | 280 (66) | 279 (59) |
BMI = body mass index; CABG = coronary artery bypass graft surgery; GFR = glomerular filtration rate; HF = heart failure; MI = myocardial infarction; PCI = percutaneous coronary intervention; SD = standard deviation. Values expressed as n (%) unless otherwise noted.
Value expressed as mean (SD).
Value expressed as median (25th, 75th percentiles).
More than half (n=634, 57%) of the deaths during follow-up occurred among patients with preceding HF. The incidence densities of mortality per 1,000 person-years were 150 and 31 among patients with and without HF, respectively (p<0.001). After adjusting for age and sex, HF as a time-dependent variable was strongly associated with all-cause mortality (HR=3.31; 95% CI: 2.93-3.75, compared with HF-free status). Mortality did not differ by HF type (HR=3.45 for HFrEF vs. 3.07 for HFpEF, p for heterogeneity=0.31), but was substantially higher for delayed- than for early-onset HF (HR=4.02 vs. 2.81, respectively, p for heterogeneity=0.001). Further adjustment for indicators of MI severity and comorbidity burden, acute intervention, and recurrent MI moderately attenuated the HRs (2.49 overall; 2.55 for HFrEF vs. 2.37 for HFpEF [p for heterogeneity=0.56]; 2.93 for delayed-onset vs. 2.03 for early-onset HF [p for heterogeneity=0.002]) (Table 2). Approximately 50% of the deaths were ascribed to cardiovascular causes (541 of a total of 1,075 deaths classified). The HF-mortality association was stronger for cardiovascular than for non-cardiovascular causes. Patterns seen in the associations between HF type and between HF timing were similar to those observed for all-cause mortality (Table 2).
Table 2.
Mortality Associated with Heart Failure after Myocardial Infarction Modeled as a Time-Dependent Variable
| Hazard Ratio (95% Confidence Interval) | ||||||
|---|---|---|---|---|---|---|
| All HF | HF Type | HF Timing | ||||
| Did Not Develop HF (n=1694) |
Developed HF (n=902) |
Reduced EF (n=563) |
Preserved EF (n=339) |
Early-onset (n=425) |
Delayed -onset (n=477) |
|
| All-cause Mortality | ||||||
| Number of Events | 482 | 634 | 412 | 222 | 299 | 335 |
| Basic Adjusted Model* | 1.0 (referent) | 3.31 (2.93-3.75) | 3.45 (3.00-3.96) | 3.07 (2.58-3.66) | 2.81 (2.42-3.26) | 4.02 (3.46-4.66) |
| Multivariable-adjusted Model† | 1.0 (referent) | 2.49 (2.18-2.85) | 2.55 (2.19-2.97) | 2.37 (1.96-2.87) | 2.03 (1.72-2.41) | 2.93 (2.51-3.42) |
| Cardiovascular Mortality | ||||||
| Number of Events | 206 | 335 | 228 | 107 | 168 | 167 |
| Basic Adjusted Model* | 1.0 (referent) | 4.20 (3.50-5.03) | 4.51 (3.71-5.49) | 3.62 (2.82-4.66) | 3.43 (2.78-4.29) | 5.66 (4.53-7.07) |
| Multivariable-adjusted Model† | 1.0 (referent) | 2.94 (2.41-3.58) | 3.09 (2.49-3.84) | 2.65 (2.02-3.49) | 2.30 (1.81-2.92) | 3.74 (2.97-4.71) |
| Non-Cardiovascular Mortality | ||||||
| Number of Events | 250 | 284 | 176 | 108 | 128 | 156 |
| Basic Adjusted Model* | 1.0 (referent) | 2.71 (2.27-3.24) | 2.74 (2.24-3.35) | 2.66 (2.08-3.41) | 2.35 (1.89-2.92) | 3.13 (2.53-3.87) |
| Multivariable-adjusted Model† | 1.0 (referent) | 2.10 (1.74-2.55) | 2.09 (1.68-2.60) | 2.12 (1.64-2.74) | 1.74 (1.35-2.26) | 2.40 (1.94-2.98) |
EF = ejection fraction; HF = heart failure; MI = myocardial infarction. The cause for 41 deaths was undetermined.
Adjusted for age and sex.
Further adjusted for Charlson comorbidity index, Killip class, percutaneous coronary intervention, and recurrent MI (modeled as a time-dependent covariate).
Changes in patient characteristics and acute management occurred between 1990-2000 and 2001-2010 including lower Killip class, more comorbidities, and greater utilization of reperfusion/revascularization therapy. Among HF cases, patients in the more recent era were older and had a worse cardiovascular profile, but were more likely to undergo PCI than HF patients in the earlier era (Table 3). The risk of recurrent MI during follow-up declined, as did the risk of incident HF (both p<0.001 from the log-rank test). The incidence rates of HF per 100 person-years (truncating follow-up at 5 years) were 10.2 in 1990-2000 and 7.9 in 2001-2010 (p=0.001). Among HF patients, the proportion of HFrEF decreased, whereas the median time from index MI to HF diagnosis remained unchanged (Table 3). A summary of deaths within 2 years after the MI stratified by HF status and time period, overall and by HF type and timing, is provided in Table 4. To include all follow-up on patients and account for censoring, age- and sex-adjusted survival estimates were calculated for the two time periods and compared. The age- and sex-adjusted 5-year survival estimates (95% CIs) in 2001-2010 vs. 1990-2000 were 82% (80-84%) and 81% (79-83%) among HF-free subjects, compared with 61% (57-64%) and 54% (51-57%) among incident HF patients, respectively (p for heterogeneity in trends=0.05) (Figure 1). This translates into an absolute risk reduction estimate from 1990-2000 to 2001-2010 of 1.3 deaths per 100 patients (95% CI: −1.5-4.2) for HF-free subjects compared with 6.5 deaths per 100 patients (95% CI: 2.1-10.9) for HF cases, adjusted for age and sex. Further adjustment for Charlson comorbidity index, Killip class, PCI, and recurrent MI (modeled as a time-dependent covariate) did not appreciably change the results. In relative terms, the age- and sex-adjusted HR for mortality in 2001-2010 vs. 1990-2000 was 0.77 (95% CI: 0.65-0.92). Multivariable-adjusted all-cause and cause-specific mortality HRs according to index MI period and HF status are shown in Figure 2. A temporal decline in all-cause mortality risk was evident after HF, whereas no reduction was observed among HF-free subjects. The improved survival after HF was primarily attributable to cardiovascular causes. For HF-free subjects, improvement over time in cardiovascular survival was offset by greater mortality risk from non-cardiovascular causes. The proportion of HFrEF out of all HF cases decreased between 1990-2000 and 2001-2010 (67% to 56%, p=0.001). A temporal decline was observed for all-cause mortality in patients with HFpEF, driven by a 50% reduction in cardiovascular mortality. No statistically significant reduction was observed in mortality for patients with HFrEF. Regarding trends by timing of HF onset, the proportion of early-onset HF out of all HF cases diagnosed within 5 years after MI did not change between 1990-2000 and 2001-2010 (58% and 59%, respectively, p=0.99). Similarly, among delayed-onset HF cases diagnosed within 5 years after MI, no significant differences in the time from index MI to HF diagnosis were detected (mean [SD], 522 [621] vs. 455 [555] days in 1990-2000 and 2001-2010, respectively, p=0.33). There was a substantial decline over calendar year in all-cause mortality associated with early-onset HF (HR=0.63; 95% CI: 0.50-0.81, adjusted for age, sex, Charlson comorbidity index, Killip class, PCI, and recurrent MI), whereas no decline was evident for delayed-onset HF (HR=1.02; 95% CI: 0.79-1.31). The temporal decline in mortality in patients with early-onset HF was primarily attributable to a reduction in cardiovascular mortality (adjusted HR=0.58; 95% CI: 0.42-0.80), whereas less of a reduction was observed in non-cardiovascular mortality (adjusted HR=0.71; 95% CI: 0.49-1.02). For all temporal trends analyses, similar trends were observed with year of index MI modeled as a continuous variable, indicating a linear temporal trend.
Table 3.
Changes in Baseline Characteristics from 1990-2000 to 2001-2010 by Heart Failure Status at Follow-up
| Characteristic | Overall (n=2596) |
HF Status at Follow-up |
||||
|---|---|---|---|---|---|---|
| Developed HF (n=902) |
Did not Develop HF (n=1694) |
|||||
| 1990-2000 (n=1211) |
2001-2010 (n=1385) |
1990-2000 (n=541) |
2001-2010 (n=361) |
1990-2000 (n=670) |
2001-2010 (n=1024) |
|
| Age, years | 67.0 (14.2) * | 66.2 (14.8) * | 72.2 (12.6) * | 74.2 (13.0) * | 62.7 (14.0) * | 63.3 (14.3) * |
| Male | 689 (57) | 877 (63) | 255 (47) | 183 (51) | 434 (65) | 694 (68) |
| BMI, kg/m2 | 27.9 (5.7) * | 28.9 (6.0) * | 28.2 (6.0) * | 28.5 (6.3) * | 27.7 (5.5) * | 29.0 (5.9) * |
| Hypertension | 657 (54) | 914 (66) | 357 (66) | 288 (80) | 300 (45) | 626 (61) |
| Hyperlipidemia | 468 (39) | 883 (64) | 210 (39) | 235 (65) | 258 (39) | 648 (63) |
| Current smoking | 350 (29) | 320 (23) | 128 (24) | 78 (22) | 222 (33) | 242 (24) |
| Diabetes mellitus | 229 (19) | 305 (22) | 138 (26) | 111 (31) | 91 (14) | 194 (19) |
| Charlson comorbidity index | 1 (0, 2) † | 1 (0, 2) † | 1 (0, 2) † | 2 (1, 4) † | 0 (0, 1) † | 1 (0, 2) † |
| Killip >1 | 349 (29) | 297 (22) | 228 (43) | 173 (49) | 121 (18) | 124 (12) |
| ST-elevation MI | 458 (39) | 366 (27) | 188 (36) | 90 (25) | 270 (42) | 276 (27) |
| Anterior MI | 444 (38) | 463 (34) | 218 (41) | 161 (45) | 226 (35) | 302 (30) |
| Q-wave MI | 638 (56) | 667 (52) | 306 (60) | 212 (63) | 332 (53) | 455 (48) |
| PCI | 530 (44) | 872 (63) | 200 (37) | 176 (49) | 330 (49) | 696 (68) |
| CABG | 124 (10) | 115 (8) | 63 (12) | 43 (12) | 61 (9) | 72 (7) |
| Atrial fibrillation | 145 (12) | 135 (10) | 95 (18) | 68 (19) | 50 (8) | 67 (7) |
| Estimated GFR, <60 mL/min per 1.73 m2 |
627 (52) | 576 (42) | 339 (63) | 220 (61) | 288 (44) | 356 (35) |
| HF Characteristics | ||||||
| HFrEF | — | — | 363 (67) | 200 (55) | — | — |
| Time from index MI, days ‡ | — | — | 2 (0, 64) † | 2 (0, 65) † | — | — |
BMI = body mass index; CABG = coronary artery bypass graft surgery; GFR = glomerular filtration rate; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; MI = myocardial infarction; PCI = percutaneous coronary intervention; SD = standard deviation.
Values expressed as n (% of non-missing values) unless otherwise noted.
Value expressed as mean (SD).
Value expressed as median (25th, 75th percentiles).
Follow-up restricted to 5 years after MI (385 patients developed HF from 1990-2000; 342 patients developed HF from 2001-2010).
Table 4.
Number of deaths* within 2 years of MI stratified by time period.
| 1990-2000 | 2001-2010 | ||||
|---|---|---|---|---|---|
| N | Deaths within 2 Years, N %) |
N | Deaths within 2 Years, N %) |
||
| All HF | Did Not Develop HF |
670 | 96 (14.3) | 1024 | 96 (9.4) |
| Developed HF | 541 | 127 (23.5) | 361 | 84 (23.3) | |
| HF Type | Reduced EF | 363 | 94 (26.0) | 200† | 50 (25.1) |
| Preserved EF | 178 | 33 (18.3) | 161‡ | 34 (21.0) | |
| HF Timing | Early-Onset | 225 | 102 (45.3) | 200† | 51 (25.5) |
| Delayed -Onset | 316 | 25 (7.9) | 16 ‡ | 33 (20.5) | |
These results do not take into account the censoring of some subjects.
These are not the same persons.
These are not the same persons.
Figure 1.
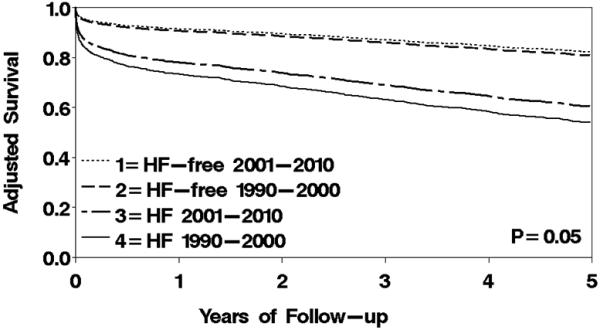
Temporal trends in age- and sex-adjusted survival by HF status after MI. Using the direct adjustment method, the figure describes survival during 5 years of follow-up across 4 categories: HF-free, 1990-2000; HF-free, 2001-2010; HF, 1990-2000; and HF, 2001-2010. HF was modeled as a time-dependent variable with the counting process approach.
Figure 2.
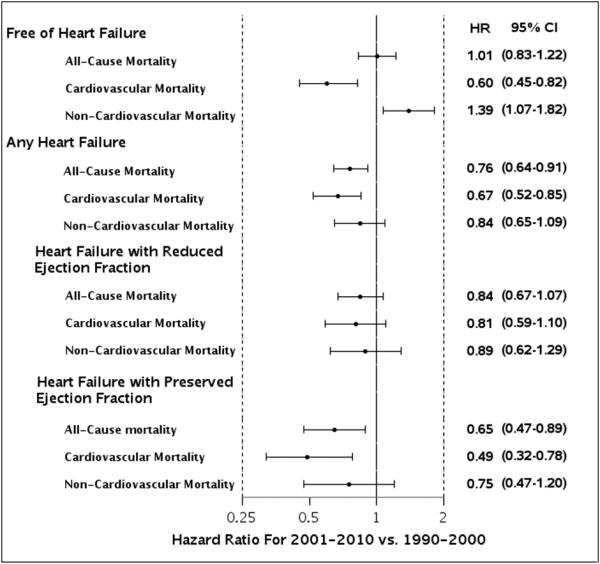
Temporal trends in adjusted survival (overall and by cardiovascular and non-cardiovascular causes) by HF status after MI and by type of HF (HFpEF vs. HFrEF). Results are presented as HR (95% CI). Models were adjusted for age, sex, Charlson comorbidity index, Killip class, percutaneous coronary intervention, and recurrent MI (modeled as a time-dependent covariate).
Discussion
Summary of Findings
This population-based cohort study provides contemporary quantification of the survival impact of HF complicating MI. After MI, HF strongly increases the risk of all-cause death, cardiovascular death and non-cardiovascular death independently of key confounders including MI severity, comorbidity, and acute treatment. Patients with HFrEF and HFpEF share a similar prognosis, whereas HF developing more than 3 days after MI confers a worse prognosis than HF occurring concurrently with the index MI or shortly after.
Using data spanning over 20 years we demonstrated herein, with strong evidence, an improvement in survival after MI. Nevertheless, survival varied considerably by HF status. From 1990-2000 to 2001-2010, the adjusted absolute risk reduction estimates (fewer deaths per 100 patients) at 5 years of follow-up were 7 and 1 in MI patients with and without HF, respectively. Among the latter group, some improvement over time in cardiovascular survival was offset by a greater risk of non-cardiovascular death, resulting in an overall plateau. In contrast, a sizable decline was evident among patients with HF, primarily due to a large decrease in the risk of cardiovascular death. Over time, the proportion of HFpEF increased, with its prognosis improving more clearly than that of HFrEF. The mortality trends diverged markedly between early-onset and delayed-onset HF, with a considerable decline in the former category and none in the latter.
Interpretation of Study Findings
A number of studies have shown an association between HF complicating MI and mortality.4, 8-16, 23 Yet, because many of these studies were based on cohorts assembled during the 1980s and 1990s,4, 8, 9, 12, 13, 15, 16 the relevance of their findings to contemporary practice is questionable. Indeed, remarkable changes were documented during the past decades in the epidemiology and management of MI that dramatically affected clinical presentation, treatment, and outcomes.19, 20, 22 These advances probably had a beneficial impact on the incidence of HF and deaths attributable to HF complicating MI.11 Moreover, the increasing use of more sensitive biomarkers has resulted in detection of smaller MIs,19, 22 likely contributing to reduced risk of subsequent HF3, 43 and potentially to decreased severity of HF and improved prognosis.11 In a previous study of the cohort analyzed herein,3 we observed a notable decline in the incidence of HF after first MI between 1990 and 2010. Stratified by type, this decline was limited to HFrEF, with no detectable change in the rate of HFpEF, resulting in a change in the case mix of HF. Stratified by timing of occurrence, a decline in incidence was shown in both early-onset and delayed-onset HF, with temporal changes in MI presentation and acute management affecting mostly the former patient group. To this end, however, no change in the survival impact of HF after MI was observed during the 1980s and 1990s,16, 17 whereas mixed trends were reported between 1998 and 2010 in a study involving Medicare beneficiaries.23 Yet, the latter study did not distinguish incident from prevalent HF cases and, like many other recent studies on this topic,4, 10, 11, 14 used administrative data and did not apply standardized methods of MI and HF ascertainment. As such, its data may have uncertain validity due to evolving coding practices44, 45 and incomplete capture of HF cases because of the shift of care toward outpatient settings (which typically involve less severe cases).46
The present study demonstrates a strong association between HF after MI and mortality, but also suggests a decline in this association from 1990-2010. Moreover, HF after MI was also associated with non-cardiovascular death over the entire study period. The precise mechanism for the latter association is yet to be determined, but may involve frailty, an age-related syndrome of increasing vulnerability and decreasing resistance to stressors, which was shown to be both overrepresented in HF patients and predictive of death 47. It was previously proposed that HF after MI not only increases mortality, but also augments the associated risk of other prognostic factors such as cancer, diabetes mellitus, and chronic renal failure.4 Interestingly, we have recently linked incident HF to subsequent cancer risk in a prospective cohort of patients with MI.48 Regardless of the mechanisms involved in the latter association, an increasing body of evidence supports the concept of HF as a sentinel condition which might reflect end-stage chronic diseases.4 Indeed, the present report, which is in line with previous findings,4, 11 shows that most deaths among MI patients occur in the context of a preceding HF. Importantly, we found the survival gains over the past two decades to be primarily attributable to HF cases, compared with no major change in HF-free MI survival. This extends the findings of McManus et al.,15 examining in-hospital survival after MI. The improved survival of patients with HFpEF over the study period is intriguing considering the lack of specific effective treatment for this syndrome. This raises the question about the relative importance of secondary prevention versus therapy of HF. In this context, a recent Scandinavian study suggested that an observed temporal improvement in short-term survival of post-MI HF patients was only partly attributable to changes in interventional and pharmacological treatment.49
One can argue that earlier detection of HF, resulting perhaps from increased awareness, may result in the appearance of prolonged survival, akin to “lead time bias”. This is an unlikely explanation, however, for the diverging temporal trends in the survival of patients with and without HF, as most cases of HF are diagnosed soon after MI,3, 11 and the proportion of patients with early-onset HF did not change over the study period as reported herein.
The categorization of “early” vs. “delayed” HF is by necessity arbitrary and the Framingham criteria were designed to evaluate HF in a chronic situation. The time difference between the echocardiogram and the clinical diagnosis could impact the categorization of the type of HF. Hence, we cannot exclude some degree of misclassification of the diagnosis or categorization of HF. However, there was no statistically significant difference for the time from EF measurement to HF over the study period such that it is unlikely that misclassification would be differential over time. As our study focuses on secular trends, it is unlikely that such putative misclassification would bias our results.
The reasons for the divergence in mortality trends in patients with early-onset and delayed-onset HF are yet to be determined and may include greater treatment opportunities for HF developing in direct relation to the MI. Different mechanisms according to HF timing are also important in this regard. Conceptually, early-onset HF following MI reflects extensive myocardial damage and is thus related to infarct characteristics including location and size, and time to reperfusion. In contrast, delayed -onset HF has been linked to other mechanisms such as progressive remodeling, recurrent MI, and subclinical ischemia.8 As most patients with incident HF in this cohort did not experience a recurrent MI, remodeling is more likely to play a role as the underlying mechanism of delayed -onset HF.
After MI, it is often assumed that systolic dysfunction is the typical HF presentation.7 Nonetheless, we have recently shown a temporal change in the case mix of HF after MI, with an increasing proportion of HfpEF.3 The worse survival associated with HFrEF compared with HFpEF after MI10, 12 could be hypothesized to attenuate the strength of an association between HF after MI and mortality over time. However, herein, the prognosis of HFrEF and HFpEF was similar and, unlike previous reports, EF measurements were not limited to those obtained at the index MI date.
Limitations and Strengths
Some limitations should be acknowledged in interpreting these data. These results emanate from a single community and thus may not be applicable to other populations. Yet, comparisons of previous population-based studies of various chronic diseases in Olmsted County with those from other communities in the United States indicate the results for the population of this area can be extrapolated to a large part of the population of the country.50 While HF was validated with the use of Framingham criteria, no data were available on its severity. Also, it is possible that heightened surveillance during the index MI hospitalization could contribute to a higher diagnosis rate of HF during this time. Echocardiograms were missing in 19% of the HF cases, necessitating the use of multiple imputations in the analysis of HF type. The lack of routine data on prognostic factors and interventions at the time of HF, and on secondary prevention measures afterwards, precludes assessment of the relative importance of secondary prevention versus therapy in HF survival. Changes in clinical practice, healthcare policy and recording of relevant variables over time should be considered when interpreting the results of secular trend analyses.
This study has several strengths. Community-wide studies, which monitor population trends in disease incidence and outcomes, are well-suited to evaluate the prognostic impact of HF after MI. Their data are more generalizable to the broader spectrum of patients seen in day-to-day practice and provide a representative and contemporary picture of the natural history of this clinical syndrome. The comprehensive population-based approach provided by the Rochester Epidemiology Project, along with a rigorous ascertainment of incident MI and the access to complete inpatient and outpatient data in the process of HF validation, offers a unique opportunity to conduct robust surveillance. This surveillance system enables capture of long-term nonfatal clinical events that occur after the initial hospitalization, a distinctive strength that allows the integration of intercurrent clinical events after MI in the prediction of death, which has important implications for risk stratification. Echocardiographic data were routinely obtained allowing the analysis of HFrEF and HFpEF, a crucial element in understanding the contemporary burden of HF complicating MI.
Conclusions and Implications
HF developing after MI is a strong risk factor for all-cause, cardiovascular, and non-cardiovascular mortality. While this finding, based on more contemporary data, supports earlier reports, our data further document and quantify the association and identify more vulnerable subgroups and specific times of higher risk. Furthermore, these data demonstrate important secular trends. The magnitude of the excess risk attributable to HF is similar between HFrEF and HFpEF but greater for delayed -onset than for early-onset HF. Mortality after MI declined over the past two decades, primarily as a result of improved HF survival. However, the survival benefit was limited to early-onset HF. As most deaths after MI still occur in patients who developed HF, future survival gains will likely be achieved through improved treatment strategies among MI patients at risk for HF, specifically enhanced secondary prevention. Such efforts should be deployed to target delayed -onset HF, for which no improvement in prognosis was evident.
Clinical Perspective.
Major changes in the epidemiology of MI have taken place over the last decades and have likely influenced the already complex and multifaceted association between heart failure (HF) after myocardial infarction (MI) and mortality. However, contemporary data on the prognostic importance of HF after MI are lacking. In this population-based cohort of patients with a first-ever MI from 1990-2010, we found that HF was strongly associated with mortality, particularly from cardiovascular causes. This excess risk is similar regardless of EF but greater for late- vs. early-onset HF. Mortality after MI declined over time, primarily as a result of improved HF survival. There are important clinical implications of these data. As most deaths after MI occur in patients who develop HF, future survival gains will most likely be achieved through improved treatment strategies among MI patients at risk for HF. Such efforts should be deployed to target late-onset HF, for which no improvement in prognosis was evident. Furthermore, these data demonstrate important secular trends.
Acknowledgments
Sources of Funding
This study was supported by the National Institutes of Health (R01 HL59205, R01 HL72435, and R01 HL120957), and made possible by the Rochester Epidemiology Project (R01 AG034676 from the National Institute on Aging). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Gerber et al: Mortality Associated With HF After MI
Disclosures
None.
References
- 1.Kostis WJ, Deng Y, Moreyra AE, Pantazopoulos JS, Kostis JB. No decrease in the incidence of heart failure following acute myocardial infarction in the years 1994-2006. Circulation. 2011;124:A17546. [Google Scholar]
- 2.Velagaleti RS, Pencina MJ, Murabito JM, Wang TJ, Parikh NI, D'Agostino RB, Levy D, Kannel WB, Vasan RS. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118:2057–2062. doi: 10.1161/CIRCULATIONAHA.108.784215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerber Y, Weston SA, Berardi C, McNallan SM, Jiang R, Redfield MM, Roger VL. Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. Am J Epidemiol. 2013;178:1272–1280. doi: 10.1093/aje/kwt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC, McAlister FA. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;53:13–20. doi: 10.1016/j.jacc.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 5.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282–289. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 6.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 7.Minicucci MF, Azevedo PS, Polegato BF, Paiva SA, Zornoff LA. Heart failure after myocardial infarction: clinical implications and treatment. Clin Cardiol. 2011;34:410–414. doi: 10.1002/clc.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis EF, Moye LA, Rouleau JL, Sacks FM, Arnold JM, Warnica JW, Flaker GC, Braunwald E, Pfeffer MA. Predictors of late development of heart failure in stable survivors of myocardial infarction: the CARE study. J Am Coll Cardiol. 2003;42:1446–1453. doi: 10.1016/s0735-1097(03)01057-x. [DOI] [PubMed] [Google Scholar]
- 9.Lewis EF, Velazquez EJ, Solomon SD, Hellkamp AS, McMurray JJ, Mathias J, Rouleau JL, Maggioni AP, Swedberg K, Kober L, White H, Dalby AJ, Francis GS, Zannad F, Califf RM, Pfeffer MA. Predictors of the first heart failure hospitalization in patients who are stable survivors of myocardial infarction complicated by pulmonary congestion and/or left ventricular dysfunction: a VALIANT study. Eur Heart J. 2008;29:748–756. doi: 10.1093/eurheartj/ehn062. [DOI] [PubMed] [Google Scholar]
- 10.van Diepen S, Chen AY, Wang TY, Alexander KP, Ezekowitz JA, Peterson ED, Roe MT. Influence of heart failure symptoms and ejection fraction on short- and long-term outcomes for older patients with non-ST-segment elevation myocardial infarction. Am Heart J. 2014;167:267–273. doi: 10.1016/j.ahj.2013.11.005. e261. [DOI] [PubMed] [Google Scholar]
- 11.Hung J, Teng TH, Finn J, Knuiman M, Briffa T, Stewart S, Sanfilippo FM, Ridout S, Hobbs M. Trends from 1996 to 2007 in incidence and mortality outcomes of heart failure after acute myocardial infarction: a population-based study of 20,812 patients with first acute myocardial infarction in Western Australia. J Am Heart Assoc. 2013;2:e000172. doi: 10.1161/JAHA.113.000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moller JE, Brendorp B, Ottesen M, Kober L, Egstrup K, Poulsen SH, Torp-Pedersen C. Congestive heart failure with preserved left ventricular systolic function after acute myocardial infarction: clinical and prognostic implications. Eur J Heart Fail. 2003;5:811–819. doi: 10.1016/s1388-9842(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 13.Steg PG, Dabbous OH, Feldman LJ, Cohen-Solal A, Aumont MC, Lopez-Sendon J, Budaj A, Goldberg RJ, Klein W, Anderson FA., Jr. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE) Circulation. 2004;109:494–499. doi: 10.1161/01.CIR.0000109691.16944.DA. [DOI] [PubMed] [Google Scholar]
- 14.Kaul P, Ezekowitz JA, Armstrong PW, Leung BK, Savu A, Welsh RC, Quan H, Knudtson ML, McAlister FA. Incidence of heart failure and mortality after acute coronary syndromes. Am Heart J. 2013;165:379–385. doi: 10.1016/j.ahj.2012.12.005. e372. [DOI] [PubMed] [Google Scholar]
- 15.McManus DD, Chinali M, Saczynski JS, Gore JM, Yarzebski J, Spencer FA, Lessard D, Goldberg RJ. 30-year trends in heart failure in patients hospitalized with acute myocardial infarction. Am J Cardiol. 2011;107:353–359. doi: 10.1016/j.amjcard.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spencer FA, Meyer TE, Goldberg RJ, Yarzebski J, Hatton M, Lessard D, Gore JM. Twenty year trends (1975-1995) in the incidence, in-hospital and long-term death rates associated with heart failure complicating acute myocardial infarction: a community-wide perspective. J Am Coll Cardiol. 1999;34:1378–1387. doi: 10.1016/s0735-1097(99)00390-3. [DOI] [PubMed] [Google Scholar]
- 17.Hellermann JP, Jacobsen SJ, Redfield MM, Reeder GS, Weston SA, Roger VL. Heart failure after myocardial infarction: clinical presentation and survival. Eur J Heart Fail. 2005;7:119–125. doi: 10.1016/j.ejheart.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Kostis WJ, Deng Y, Pantazopoulos JS, Moreyra AE, Kostis JB. Trends in mortality of acute myocardial infarction after discharge from the hospital. Circ Cardiovasc Qual Outcomes. 2010;3:581–589. doi: 10.1161/CIRCOUTCOMES.110.957803. [DOI] [PubMed] [Google Scholar]
- 19.Roger VL, Weston SA, Gerber Y, Killian JM, Dunlay SM, Jaffe AS, Bell MR, Kors J, Yawn BP, Jacobsen SJ. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121:863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 21.Gerber Y, Melton LJ, 3rd, Weston SA, Roger VL. Association between myocardial infarction and fractures: an emerging phenomenon. Circulation. 2011;124:297–303. doi: 10.1161/CIRCULATIONAHA.110.007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, Wagenknecht L, Ni H, Folsom AR. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities 1987-2008. Circulation. 2012;125:1848–1857. doi: 10.1161/CIRCULATIONAHA.111.047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Hsieh AF, Dharmarajan K, Masoudi FA, Krumholz HM. National trends in heart failure hospitalization after acute myocardial infarction for Medicare beneficiaries: 1998-2010. Circulation. 2013;128:2577–2584. doi: 10.1161/CIRCULATIONAHA.113.003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jhund PS, McMurray JJ. Heart failure after acute myocardial infarction: a lost battle in the war on heart failure? Circulation. 2008;118:2019–2021. doi: 10.1161/CIRCULATIONAHA.108.813493. [DOI] [PubMed] [Google Scholar]
- 26.Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. 2008;1:91–97. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. New Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 30.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 31.Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012;5:720–726. doi: 10.1161/CIRCHEARTFAILURE.111.966366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 33.Gerber Y, Jacobsen SJ, Frye RL, Weston SA, Killian JM, Roger VL. Secular trends in deaths from cardiovascular diseases: a 25-year community study. Circulation. 2006;113:2285–2292. doi: 10.1161/CIRCULATIONAHA.105.590463. [DOI] [PubMed] [Google Scholar]
- 34.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 35.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 36.Greenland S. Invited commentary: variable selection versus shrinkage in the control of multiple confounders. Am J Epidemiol. 2008;167:523–529. doi: 10.1093/aje/kwm355. discussion 530-521. [DOI] [PubMed] [Google Scholar]
- 37.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 38.Rubin D. Multiple imputations for nonresponse in surveys. John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 39.White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28:1982–1998. doi: 10.1002/sim.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fleiss J. Statistical methods for rates and proportions. John Wiley & Sons; New York: 1981. [Google Scholar]
- 41.Rothman KJ. Modern epidemiology. Little, Brown & Co.; Boston: 1986. [Google Scholar]
- 42.Abramson JH. WINPEPI (PEPI-for-Windows): computer programs for epidemiologists. Epidemiol Perspect Innov. 2004;1:6. doi: 10.1186/1742-5573-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerber Y, Jaffe AS, Weston SA, Jiang R, Roger VL. Prognostic value of cardiac troponin T after myocardial infarction: a contemporary community experience. Mayo Clin Proc. 2012;87:247–254. doi: 10.1016/j.mayocp.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Assaf AR, Lapane KL, McKenney JL, Carleton RA. Possible influence of the prospective payment system on the assignment of discharge diagnoses for coronary heart disease. N Engl J Med. 1993;329:931–935. doi: 10.1056/NEJM199309233291307. [DOI] [PubMed] [Google Scholar]
- 45.Jollis JG, Ancukiewicz M, DeLong ER, Pryor DB, Muhlbaier LH, Mark DB. Discordance of databases designed for claims payment versus clinical information systems. Implications for outcomes research. Ann Intern Med. 1993;119:844–850. doi: 10.7326/0003-4819-119-8-199310150-00011. [DOI] [PubMed] [Google Scholar]
- 46.Ezekowitz JA, Kaul P, Bakal JA, Quan H, McAlister FA. Trends in heart failure care: has the incident diagnosis of heart failure shifted from the hospital to the emergency department and outpatient clinics? Eur J Heart Fail. 2011;13:142–147. doi: 10.1093/eurjhf/hfq185. [DOI] [PubMed] [Google Scholar]
- 47.Cacciatore F, Abete P, Mazzella F, Viati L, Della Morte D, Ambrosio D, Gargiulo G, Testa G, Santis D, Galizia G, Ferrara N, Rengo F. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest. 2005;35:723–730. doi: 10.1111/j.1365-2362.2005.01572.x. [DOI] [PubMed] [Google Scholar]
- 48.Hasin T, Gerber Y, Weston S, Killian J, McNallan S, Cerhan J, Roger V. Heart failure after myocardial infarction is associated with increased risk of cancer. J Am Coll Cardiol. 2014;63 [Google Scholar]
- 49.Gjesing A, Gislason GH, Kober L, Gustav Smith J, Christensen SB, Gustafsson F, Olsen AM, Torp-Pedersen C, Andersson C. Nationwide trends in development of heart failure and mortality after first-time myocardial infarction 1997-2010: A Danish cohort study. Eur J Intern Med. 2014;25:731–738. doi: 10.1016/j.ejim.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


