Abstract
The contexts for action may be only transiently visible, accessible, and relevant. The corticobasal ganglia (BG) circuit addresses these demands by allowing the right motor plans to drive action at the right times, via a BG-mediated gate on motor representations. A long-standing hypothesis posits these same circuits are replicated in more rostral brain regions to support gating of cognitive representations. Key evidence now supports the prediction that BG can act as a gate on the input to working memory, as a gate on its output, and as a means of reallocating working memory representations rendered irrelevant by recent events. These discoveries validate key tenets of many computational models, circumscribe motor and cognitive models of recurrent cortical dynamics alone, and identify novel directions for research on the mechanisms of higher-level cognition.
Introduction
The world is rich with information, much of it only transiently available to the senses. And yet, an animal must leverage a small, but crucial, fraction of this input in order to provide a context for its behavior. Working memory is a central adaptation to confront this problem, selecting behaviorally relevant information, maintaining it in time, and referencing it when appropriate in order to make decisions about how to act in the world. Indeed, the elaborated working memory system of higher primates partly underlies their distinguishing intelligence and flexible behavior.
Working memory is capacity limited. Measures of capacity predict individual differences in cognitive ability, including scholastic aptitude, intelligence, and aging-related cognitive change [1,2]. Moreover, changes in working memory capacity accompany neurological and psychiatric disease [3] and may underlie behavioral and cognitive deficits associated with these disorders [4]. However, just as the world is dynamic, so is the working memory system adapted to address these dynamics. Thus, control processes are required in order to rapidly and selectively store information in memory (input control), to rapidly and selectively deploy subsets of that information for use in behavior (output control), and to selectively eliminate an obsolete representation from memory when its predicted utility declines (reallocation). Such control functions would seem to be crucial for strategically making use of capacity-limited working memory. And indeed, though less understood, individual differences in these control processes could be equally or even more important than the size of a static capacity for intellectual ability.
Though still in its early stages, the last few years have yielded rapid advances in our understanding of how the brain solves the input, output, and allocation control problems facing working memory. These experiments have associated all three functions with interactions between frontal and basal ganglia systems. Below, we review this work to outline an account of how the brain manages working memory.
From motor control to cognitive control
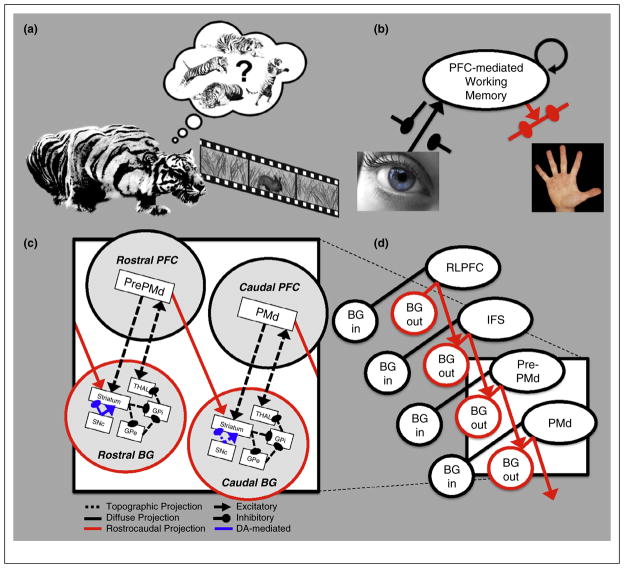
There is a clear parallel between the problems addressed by working memory control processes and the fundamental challenges faced by an animal’s motor system. Consider the task of hunting for dinner. For example, a predator must program motor actions on the basis of transiently observed information about prey (input control); maintain these programs until the time is right, enacting only the most appropriate motor program at that time (output control); and finally, refrain from perseveratively considering outdated motor programs, should the prey escape (reallocation; Figure 1a). Thus, demands on selective encoding, maintenance, utilization, and clearing of information face a variety of species.
Figure 1.
Theoretical overview. (a) All behaving animals must be capable of selecting useful motor actions at the right times. A long-standing hypothesis [11] holds that the same frontostriatal mechanisms supporting this kind of action selection might also support higher-order cognitive functions. (b) Frontostriatal mechanisms can implement a gate to select useful but transient information for rapid storage in working memory, as well as a gate to select of information from working memory to inform motor planning [6,10,13]. (c) Models involving rostral to caudal nesting of corticostriatal input and output gating loops have been shown to solve abstract, multiply contingent action problems [18] as well as forms of Bayesian inference [22••] and symbolic referencing [23••]. A key feature of these models is the presence of a ‘diagonal’ rostrocaudal projection (red arrows) allowing rostral areas to modulate the striatal input to more caudal basal ganglia; one implemented model is shown here. (d) Multiple such frontostriatal circuits are thought to exist, each modulated in a top-down manner by more rostral circuits (PMd by pre-PMd; pre-PMd by the inferior frontal sulcus [IFS]; and IFS by the rostrolateral prefrontal cortex [RLPFC]). The diagonal rostrocaudal projections are thought to be particularly important for modulating output gating mechanisms (‘BG out’) as opposed to input gating mechanisms (‘BG in’).
This similarity motivates the search for neural solutions that might also be shared across species. Indeed, recent phylogenetic analyses show that the basal ganglia (BG) has been highly conserved evolutionarily — all its major structures preserved since their debut in an unknown ancestor common to all vertebrates [5]. This conservation of structure may attest to the BG’s efficacy in solving the action selection problems faced by many species.
One way to describe the dynamics of this selection function is as a gate that regulates the passage of information from one neural circuit to another [6], such as in the case of motor selection, between thalamus and motor cortex. Theoretical models posit that motor gating occurs via the opposing circuit-level effects of the two classes of medium spiny neurons of the striatum: Go and NoGo cells. The net effect of D1-receptor - expressing Go cells is to ‘open the gate’ by facilitating recurrent thalamocortical information flow, whereas D2-receptor-expressing NoGo cells ‘close the gate’ by blocking thalamocortical information flow. By this scheme, a planned motor action represented cortically might trigger the activation of Go cells via a corticostriatal projection, in turn facilitating a projection from thalamus to the primary motor neurons responsible for enacting specific movements. At the same time, alternative action plans would trigger NoGo cells and so would have negligible thalamocortical influence.
A variety of recent evidence has offered novel support for this framework. Go and NoGo cells are coactive when animals are motorically active, but not quiescent [7], in particular when action sequences are being initiated [8] — all consistent with a role for these cells in gating for action selection as opposed to a more general pro-kinetic vs. anti-kinetic dichotomy between Go and NoGo cells. Further evidence for this framework has recently been provided by optogenetic techniques [9••]. Transgenic mice expressing light-activated ion channels in putative Go and NoGo cells chose between one of the two ports after the onset of a cue. Light-induced firing of Go cells led to an increase in contralateral movements, whereas light-induced firing of NoGo cells led to an decrease in contralateral movements. The effect of stimulation was greatest when the value of the two potential actions was closely matched (as estimated by a computational model), suggesting stimulation was capable of mimicking a small shift in their relative value. Moreover, this stimulation was effective only when delivered simultaneously with the cue, consistent with a particular influence of action value during action selection.
As discussed below, these BG-mediated gating mechanisms may extend beyond the selection of motor actions and into the more abstract domains of working memory [10] (Figure 1b) and cognitive control (Figure 1c); where they can be used to solve analogous problems of selection and updating. Indeed, the known anatomy of parallel motor, frontal, and prefrontal basal ganglia-thalamocortical circuits hints at analogous computation (Figure 1d) [11]. And, a variety of computational models have demonstrated the feasibility of such an architecture for solving complex working memory control problems [6,10, 12–21,22••,23••]. However, only recently have animal and human behavioral, neuropsychological, pharmacological, PET and fMRI studies provided direct functional evidence for multiple BG gating dynamics in WM and their importance for higher thought and action.
Input gating of working memory
Gating dynamics provide a powerful solution to the input control problem for working memory [6,10,12]. When useful information becomes available in the environment, the gate is open and working memory is updated with this useful information. Otherwise, the gate is closed and irrelevant information is kept from needlessly occupying capacity.
Several computational models of working memory have achieved this gating dynamic using corticostriatal mechanisms analogous to those described for the motor system. Just as a cortically represented motor action could cause Go cells to fire via corticostriatal projections, thereby facilitating thalamic-motoneuron information flow for movement programming (as described above), a cortically represented stimulus could also cause Go cells to fire, again via corticostriatal projections, and thereby facilitate thalamic-prefrontal information flow for working memory updating. By contrast, distracting sensory representations would trigger NoGo cells and so would have negligible thalamoprefrontal influence. By this scheme, updating is favored (and stable maintenance prevented) by input to Go cells, whereas updating is prevented (and stable maintenance favored) by input to NoGo cells. Thus, the Go/NoGo system is a potent means of circumventing stability/flexibility tradeoffs that plague single-component systems.
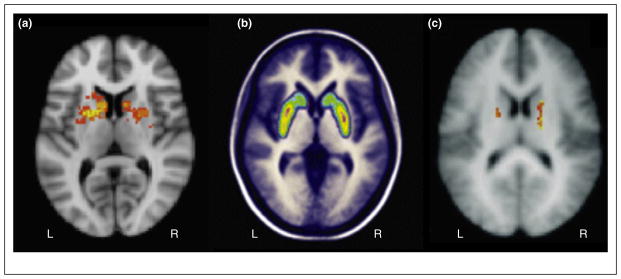
Several features of this and related striatal input gating models are supported by human neuroscience evidence. First, there is evidence that D1-expressing Go cells support the rapid updating of information in working memory. Striatal activation in fMRI, thought to be driven primarily by D1 receptor activation [24] is a common observation during working memory tasks that require updating (Figure 2a). Training of updating transfers to other tasks involving overlapping striatal BOLD responses [25]; this transfer is accompanied by alterations in the striatal hemodynamic response to updating challenges [26] and results in increased striatal dopamine receptor binding [27] (Figure 2b) as assessed via PET. Shifting the striatal balance toward Go firing (via blockade of D2 receptors with haloperidol) also enhances working memory updating [28]. Second, there is evidence that D2-expressing NoGo cells act to limit the rapid updating of information in working memory. For example, the ‘attentional blink’ is more pronounced among individuals with enhanced D2/D3 receptor binding in the BG [29•] (Figure 2c). Likewise, the depletion of central dopamine due to Parkinson’s disease counterintuitively enhances resistance to distraction in these patients, while producing deficits in the updating of working memory [30]. In summary, a variety of recent evidence strongly implicates BG-mediated input gating in working memory updating.
Figure 2.
Basal ganglia (BG) contributions to working memory input control. (a) A meta-analysis of over 8000 studies, carried out with Neurosynth’s python package [53], reveals that studies with abstracts including the term ‘updating’ are significantly more likely to report a BOLD response in the bilateral BG (Z = 2.58–7.03, FDR to p < .05; among other regions, not shown). (b) Binding of the competitive dopamine agonist raclopride within the BG is decreased during an updating task (letter memory), relative to a control task (Stroop). (c) Individual differences in D2 binding affinity within the bilateral BG predict individual differences in the rapid updating of working memory (as assessed by the attentional blink), uniquely throughout the brain (even at a liberal threshold of p < .01). (b,c) adapted from [27,29•] respectively.
It is important to note that BG-mediated gating is unlikely to be the only mechanism by which working memory is updated. For example, dopaminergic projections might directly ‘toggle’ prefrontal ensembles from a labile state to a more stable one, and hence act as a second kind of gating mechanism [21]. Indeed, high-resolution fMRI reveals the dopaminergic midbrain reliably responds to demands on working memory updating [31•] even after the requisite gating policies have been acquired — a finding not required by BG-mediated gating models, and broadly consistent with direct dopaminergic gating of PFC. However, there remain some challenges for this kind of account, most notably in cases where updating would be selective. Dopaminergic projections into PFC are diffuse and may not have the necessary spatial specificity for selective updating of distinct representations [32]. Selective updating by dopaminergic input might occur temporally instead (e.g. via phase-tuned or frequency-tuned signals), but the prefrontal dopamine response may also lack the temporal resolution required by this scheme [33] (unlike BG output to thalamus [34••]). Thus, while dopamine clearly has effects in PFC (perhaps largely via effects on the gain of neuronal ensembles), the spatial-coarseness and temporal-coarseness of prefrontal dopaminergic afferents might render those projections ineffective for selective working memory updating. Nonetheless, people are capable of simultaneously updating the entirety of working memory [35]; diffuse dopaminergic neuromodulation might be well adapted for such ‘global updates’ (but see [36,37]).
Output gating of working memory
According to the prevailing top-down ‘biased competition’ model of prefrontal function, information residing in working memory actively biases behavior. However, not all information in working memory needs to be relevant at the same time, and indeed might cross-talk or mutually interfere if mere maintenance yielded an obligatory biasing influence. Clearly, the capacity to ‘single out’ or select relevant representations stored within working memory is adaptive [38]. Behavioral evidence indicates that humans are capable of selecting information from within working memory [39].
One possibility is that BG-mediated gating mechanisms for selecting actions might also be extended for selecting the outputs of working memory. In fact, the analogy between the BG’s role in action selection and its potential role in selecting working memory output is straightforward. Premotor areas gating the output of primary motor neurons requires similar rostral-to-caudal frontostriatal projections as required for more abstract representations in working memory to influence premotor planning. In other words, higher-order plans can select motor plans via rostral corticostriatal circuits, just as motor plans can select individual movements via caudal ones.
Hierarchical, rostrocaudal neural architectures have recently been argued to support the performance of complex tasks involving conditional rules [40,41,42••,43–45,46•]. A priori, output gating is an advantageous scheme in frontostriatal hierarchies of this kind. Unlike hierarchical input gating, hierarchical output gating allows subordinate regions to proceed with their own input and reallocation policies until (or unless) higher-order regions identify an important context or conditionality. Only at that point would higher-order regions impinge on the function of lower-order regions by biasing their output toward a contextually appropriate subset of candidates. Computational models have demonstrated the feasibility of this corticostriatal output-gating architecture for solving hierarchical tasks [18,22••,42••], and at least one such model has been supported by data from fMRI [42••]. Moreover, human diffusion tractography confirms a prediction motivated by this model — namely, that any given area of striatum is more likely to also receive projections from frontal areas more rostral, rather than caudal, to its primary input source [47].
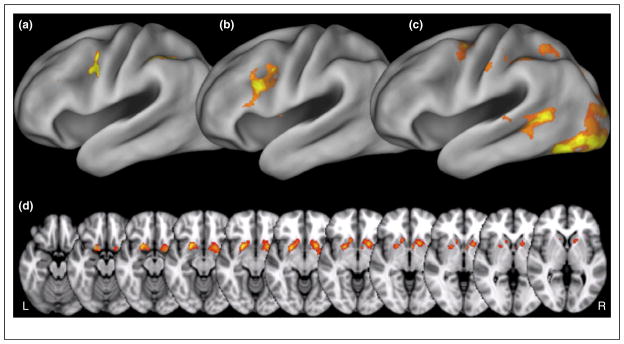
Though a variety of computational modeling thus indicates that corticostriatal circuits can support output gating, empirical studies have only begun to test the function of this hypothesized system. We recently confirmed the differential importance of output gating in hierarchical control [48••]. Our task used three sequentially presented and completely reorderable stimuli: two ‘item’ stimuli and a ‘context’ stimulus that specified which of the two items would be relevant for responses. The core logic was straightforward: when the context appears first, it can be used to drive selective input gating of only the relevant subsequent item into working memory; however, when context appeared last, it could only be used for selectively output gating the relevant item out of all those seen. All trials showed sustained recruitment of a relatively caudal sector of frontal cortex (the dorsal pre-motor cortex, or PMd), but a somewhat more rostral area (the pre-PMd) transiently increased its recruitment specifically when context was provided last, and was therefore implicated output gating (Figure 3a). An overlapping region of the pre-PMd also increased its coupling with the BG in the same conditions (Figure 3b). These two dynamics in pre-PMd each predicted a distinct kind of individual difference during selective output gating alone: whereas bilateral prePMd recruitment predicted the mean efficiency of responses during selective output gating, its bilateral coupling with BG predicted response variability, as expected of a stochastic BG-mediated output gate.
Figure 3.
Output gating and reallocation. (a) A transient BOLD response is elicited in the dorsal pre-premotor cortex (pre-PMd) by demands on selective output gating. Individual differences in the recruitment of this area and its right hemisphere homologue uniquely predict the mean efficiency of selective output gating, as assessed in behavior. (b) A partially overlapping region also in the vicinity of the pre-PMd shows a differential increase in coupling with the BG during output gating. Individual differences in this coupling uniquely predict behavioral estimates of stochastic variability during selective output gating. (c) BOLD in a more caudal sector of frontal cortex, the PMd, tracks trial to trial changes in the predicted utility of information (as estimated from a reinforcement learning model), but only when it is specified as relevant by a higher-order contextual stimulus. (d) By contrast, when contexts specify information in working memory as irrelevant, predicted utility is differentially tracked by the BOLD response in the bilateral ventral BG.
Working memory content control: the case of reallocation
The rapidly developing literature on working memory input and output control has been strongly guided by the numerous models to posit that BG-mediated gating processes may address these problems. Unfortunately, computational models differ widely in how they treat a third kind of control problem. How is working memory reallocated when already-stored information is later revealed to be irrelevant? By some accounts, an active removal process is necessary; by others, passive decay could be sufficient [49]. Finally, a third class of models posit that irrelevant representations will tend to linger until (or unless) they are overwritten with new information, such as by input gating mechanisms [6,10,15,23••]. All such accounts lead to the prediction that the utility of information in WM for future behavior is tracked in some way. Given its established role in action value coding, the BG is again an a priori candidate for this function.
We recently found evidence consistent with this hypothesis [50••]. We analyzed trials of our reorderable working memory task where context appeared in the middle position, between the presentation of the two lower-level items. When this ‘context middle’ stimulus rendered the preceding lower-level item irrelevant, we observed a large benefit to behavioral performance when sufficient time followed presentation of the context. This benefit was much larger than that seen in any other condition — as though subjects required time to reallocate working memory capacity occupied by the irrelevant item. This result parallels others (see [50••]) demonstrating a sluggish time course for WM reallocation, with irrelevant information impacting behavior even 1.5 s later.
We predicted that this slowing could occur because to-be-removed items were nonetheless predicted to have utility, even though they were specified as irrelevant by the contextual stimulus. To test this counterintuitive prediction, we adapted a simple reinforcement learning model to track the likelihood that each item, regardless of the context in which it was presented, would in fact be associated with the correct answer. Learning rates in this model were fit to reaction times in our behavioral task, and from this, we predicted a function of trial-to-trial predicted utility of irrelevant items. This timecourse correlated with activation in ventral striatum in a separate fMRI experiment. By contrast, the model-based estimates of the utility of relevant items were tracked by recruitment in frontal, not striatal regions (Figure 3c,d).
These results motivate the inclusion of BG-mediated mechanisms in models of WM reallocation [51] and other WM control processes. They also reaffirm the dichotomous stability vs. flexibility functions sometimes ascribed to frontal vs. striatal regions in the service of working memory, as well as the opposing actions of dopamine on these two areas. One intriguing possibility consistent with these results is that BG-mediated gating mechanisms might be capable of ‘vetoing’ the clearance of information from working memory, analogous to the motoric preservation induced by stimulation of the ventral striatum [52].
Conclusions
Working memory contends with the complexity of the real world via a set of control processes that select what items to maintain, which maintained items to use, and the priority of items within memory. Many of these demands are analogous to those faced in movement selection by the motor system. Accordingly, frontostriatal mechanisms for motor selection might be elaborated in more rostral frontostriatal circuits and used for more abstract working memory operations. This long-held hypothesis has now been subjected to empirical tests. Abundant evidence supports a role for BG-mediated input gating mechanisms during working memory updating. In addition, there is now emerging evidence for BG-mediated mechanisms during selection from working memory and in tracking the predicted utility of items within working memory. Both of these latter functions may be crucial in supporting more sophisticated forms of planning and thought. And though many unanswered questions remain (Box 1), these new discoveries represent a major success story for the use of neurocomputational modeling to inform the cognitive neuroscience of how working memory might actually work, in the brain.
Box 1. Open questions.
How do gating dynamics develop across the lifespan [54•,55], and could they underpin age-related shifts in modes of cognitive control [56,57]?
What is the pharmacology and neurogenetics of working memory output control and reallocation?
Might BG-mediated gating enable frontal cortex to implement Bayesian inference [22••] and symbolic referencing [23••] (see also [58])?
How do BG contribute to the time-varying, high-dimensional cortical representations highlighted in the cortex-centric recurrent network models of motor [59] and cognitive [60•,61•] control?
Are motor and cognitive corticostriatal circuits distinct truly isomorphic save their rostrocaudal locus, and evolutionary history (for at least one exception, see [62])?
How might BG-mediated gating dynamics illuminate classic cognitive phenomena like the psychological refractory period, the focus of attention, and object-based encoding in visual working memory [63••,64,65••], independent of expectancy violations [65••]?
Acknowledgments
This work was supported by awards from the National Institute of Neurological Disease and Stroke (R01 NS065046), the Alfred P. Sloan Foundation BR2011-010, and the James S. McDonnell Foundation 220020332. We also thank Michael Frank, Thomas Hazy, Seth Herd, Randy O’Reilly, and members of the Badre Lab for many valuable discussions on these topics.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Cowan N. Working Memory Capacity. Hove, East Sussex: Psychology Press; 2005. [Google Scholar]
- 2.Fukuda K, Vogel E, Mayr U, Awh E. Quantity, not quality: the relationship between fluid intelligence and working memory capacity. Psychon Bull Rev. 2010;17:673–679. doi: 10.3758/17.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbey AK, Colom R, Paul EJ, Grafman J. Architecture of fluid intelligence and working memory revealed by lesion mapping. Brain Struct Funct. 2013:1–10. doi: 10.1007/s00429-013-0512-z. [DOI] [PubMed] [Google Scholar]
- 4.Johnson MK, McMahon RP, Robinson BM, Harvey AN, Hahn B, Leonard CJ, et al. The relationship between working memory capacity and broad measures of cognitive ability in healthy adults and people with schizophrenia. Neuropsychology. 2013;27:220–229. doi: 10.1037/a0032060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephenson-Jones M, Samuelsson E, Ericsson J, Robertson B, Grillner S. Evolutionary conservation of the basal ganglia as a common vertebrate mechanism for action selection. Curr Biol. 2011;21:1081–1091. doi: 10.1016/j.cub.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Frank MJ, Loughry B, O’Reilly RC. Interactions between the frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci. 2001;1:137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- 7.Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin X, Tecuapetla F, Costa RM. Basal ganglia subcircuits distinctively encode the parsing and concatenation of action sequences. Nat Neurosci. 2014;17:423–430. doi: 10.1038/nn.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Tai LH, Lee AM, Benavidez N, Bonci A, Wilbrecht L. Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nat Neurosci. 2012;15:1281–1289. doi: 10.1038/nn.3188. A variety of work associated striatum with the assignment of value to action. Here, optogenetic striatal stimulation increases or decreases response vigor, and biases responses towards or away from particular actions (respectively for D1 and D2 stimulation), but only when stimulation is provided immediately before choice, and primarily when the value of the two choices is fairly well-matched. The results are consistent with a role for striatum in gating the output of the motor system on the basis of action value, selecting the most utile actions in a given context. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazy TE, Frank MJ, O’Reilly RC. Towards an executive without a homunculus: computational models of the prefrontal cortex/ basal ganglia system. Philos Trans R Soc B. 2007;362:1601–1613. doi: 10.1098/rstb.2007.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia–thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1989;85:119–146. [PubMed] [Google Scholar]
- 12.Hochreiter S, Schmidhuber J. Long short-term memory. Neural Comput. 1997;9:1735–1780. doi: 10.1162/neco.1997.9.8.1735. [DOI] [PubMed] [Google Scholar]
- 13.Brown JW, Bullock D, Grossberg S. How laminar frontal cortex and basal ganglia circuits interact to control planned and reactive saccades. Neural Netw. 2004;17:471–510. doi: 10.1016/j.neunet.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Rougier NP, Noelle DC, Braver TS, Cohen JD, O’Reilly RC. Prefrontal cortex and flexible cognitive control: rules without symbols. Proc Natl Acad Sci U S A. 2005;102:7338–7343. doi: 10.1073/pnas.0502455102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chatham CH, Herd SA, Brant AM, Hazy TH, Miyake A, O’Reilly RC, Friedman NP. From an executive network to executive control: a computational model of the n-back task. J Cogn Neurosci. 2011;23:3598–3619. doi: 10.1162/jocn_a_00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kriete T, Noelle DC. Generalisation benefits of output gating in a model of prefrontal cortex. Cogn Sci. 2011;23:119–129. [Google Scholar]
- 17.Eliasmith C, Stewart TC, Choo X, Bekolay T, DeWolf T, Tang C, Rasmussen D. A large-scale model of the functioning brain. Science. 2012;338:1202–1205. doi: 10.1126/science.1225266. [DOI] [PubMed] [Google Scholar]
- 18.Frank MJ, Badre D. Mechanisms of hierarchical reinforcement learning in corticostriatal circuits. 1. Computational analysis. Cereb Cortex. 2012;22:509–526. doi: 10.1093/cercor/bhr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang TR, Hazy TE, Herd SA, O’Reilly RC. Assembling old tricks for new tasks: a neural model of instructional learning and control. J Cogn Neurosci. 2013;25:843–851. doi: 10.1162/jocn_a_00365. [DOI] [PubMed] [Google Scholar]
- 20.Wiecki TV, Frank MJ. A computational model of inhibitory control in frontal cortex and basal ganglia. Psychol Rev. 2013;120:329. doi: 10.1037/a0031542. [DOI] [PubMed] [Google Scholar]
- 21.Braver TS, Cohen JD. Dopamine, cognitive control, and schizophrenia: the gating model. Prog Brain Res. 1999;121:327–350. doi: 10.1016/s0079-6123(08)63082-4. [DOI] [PubMed] [Google Scholar]
- 22••.Collins AGE, Frank MJ. Cognitive control over learning: creating, clustering and generalizing task-set structure. Psychol Rev. 2013;120:190–229. doi: 10.1037/a0030852. A hierarchical model in which rostral prefrontal areas modulate the output gating of more caudal frontal cortex successfully predicts human performance in two novel tasks, and is shown to approximate a form of nonparametric Bayesian inference. In particular, the model shows how abstract task sets could be flexibly created and more concrete stimulus–response associations clustered within them. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Kriete T, Noelle DC, Cohen JD, O’Reilly RC. Indirection and symbol-like processing in the prefrontal cortex and basal ganglia. Proc Natl Acad Sci U S A. 2013;110:16390–16395. doi: 10.1073/pnas.1303547110. While output gating alleviates some of the generalizations deficits present in neural networks that utilize only input gating, further advantages to generalization are conferred by a qualitatively distinct kind of hierarchical modulation known as indirection. In this scheme, the gated representations act as ‘pointers’ to the address of other representations that should be gated. This model makes intriguing functional and microarchitectural predictions that should be tested in future work. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knutson B, Gibbs SE. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl) 2007;191:813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- 25.Dahlin E, Neely AS, Larsson A, Bäckman L, Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008;320:1510–1512. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- 26.Kühn S, Schmiedek F, Noack H, Wenger E, Bodammer NC, Lindenberger U, Lövden M. The dynamics of change in striatal activity following updating training. Hum Brain Mapp. 2013;34:1530–1541. doi: 10.1002/hbm.22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bäckman L, Nyberg L, Soveri A, Johansson J, Andersson M, Dahlin E, Neely AS, Virta J, Laine M, Rinne JO. Effects of working-memory training on striatal dopamine release. Science. 2011;333:718. doi: 10.1126/science.1204978. [DOI] [PubMed] [Google Scholar]
- 28.Frank MJ, O’Reilly RC. A mechanistic account of striatal dopamine function in human cognition: psychopharmacological studies with cabergoline and haloperidol. Behav Neurosci. 2006;120:497–517. doi: 10.1037/0735-7044.120.3.497. [DOI] [PubMed] [Google Scholar]
- 29•.Slagter HA, Tomer R, Christian BT, Fox AS, Colzato LS, King CR, Davidson RJ. PET evidence for a role for striatal dopamine in the attentional blink: functional implications. J Cogn Neurosci. 2012;24:1932–1940. doi: 10.1162/jocn_a_00255. When two targets in a rapid visual stream are separated by a 100–500 ms interval, the second target is often missed entirely — as though working memory could not be updated quickly enough. This ‘attentional blink’ is more pronounced among individuals with enhanced D2-like receptor binding in the BG, as assessed via PET, thus demonstrating novel support for BG-mediated input gating models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cools R, Miyakawa A, Sheridan M, D’Esposito M. Enhanced frontal function in Parkinson’s disease. Brain. 2010;133:225–233. doi: 10.1093/brain/awp301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.D’Ardenne K, Eshel N, Luka J, Lenartowicz A, Nystrom LE, Cohen JD. Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proc Natl Acad Sci U S A. 2012;109:19900–19909. doi: 10.1073/pnas.1116727109. Among many interesting findings, the authors use high-resolution fMRI to show increased recruitment of the SNc and VTA during the updating of working memory with important contextual information. This result is consistent with the prediction from gating models that dopaminergic mechanisms may play a particularly important role during working memory updating. Moreover, the result sits most naturally with the midbrain-mediated gating models, in which direct dopaminergic projections from the midbrain to the prefrontal cortex play an important role in input gating even after gating policies have been acquired. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yetnikoff L, Lavezzi HN, Reichard RA, Zahm DS. An update on the connections of the ventral mesencephalic dopaminergic complex. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.04.010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacob SN, Ott T, Nieder A. Dopamine regulates two classes of primate prefrontal neurons that represent sensory signals. J Neurosci. 2013;33:13724–13734. doi: 10.1523/JNEUROSCI.0210-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34••.Goldberg JH, Farries MA, Fee MS. Basal ganglia output to the thalamus: still a paradox. Trends Neurosci. 2013;36:695–705. doi: 10.1016/j.tins.2013.09.001. This review describes evidence that BG-thalamic dynamics can take any of three modes, depending on the level of thalamic drive. Gating dynamics dominate at intermediate levels of glutamatergic input to thalamus. At higher levels, thalamus is modulated by BG inter-spike intervals (ISIs) with sub-millisecond precision — a prerequisite for mechanisms capable of gating representations into, or out of, different phases of a cortical oscillation. Intriguingly, the effect of each BG ISI is also history-independent. This means that a single BG-thalamic synapse could, over several BG ISIs, be understood as equivalent to as many parallel and independent synaptic gates operating simultaneously, each being ‘open’ to a different degree. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kessler Y, Meiran N. Two dissociable updating processes in working memory. J Exp Psychol Learn Mem Cogn. 2008;34:1339. doi: 10.1037/a0013078. [DOI] [PubMed] [Google Scholar]
- 36.Kessler Y, Oberauer K. Working memory updating latency reflects the cost of switching between maintenance and updating modes of operation. J Exp Psychol Learn Mem Cogn. 2014;40:738. doi: 10.1037/a0035545. [DOI] [PubMed] [Google Scholar]
- 37.Murty VP, Sambataro F, Radulescu E, Altamura M, Iudicello J, Zoltick B, Weinberger DR, Goldberg TE, Mattay VS. Selective updating of working memory content modulates mesocorticostriatal activity. Neuroimage. 2011;57:1264–1272. doi: 10.1016/j.neuroimage.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oberauer K, Souza AS, Druey MD, Gade M. Analogous mechanisms of selection and updating in declarative and procedural working memory: experiments and a computational model. Cogn Psychol. 2013;66:157–211. doi: 10.1016/j.cogpsych.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Oberauer K, Hein L. Attention to information in working memory. Curr Dir Psychol Sci. 2012;21:164–169. [Google Scholar]
- 40.Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 41.Badre D, D’Esposito M. FMRI evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci. 2007;19:1–18. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- 42••.Badre D, Frank MJ. Mechanisms of hierarchical reinforcement learning in corticostriatal circuits. 2. Evidence from fMRI. Cereb Cortex. 2012;22:527–536. doi: 10.1093/cercor/bhr117. A hierarchical model of multiple frontostriatal loops is shown to capture the results of Badre et al. [44]. Critically, the diagonal rostrocaudal projection of the model yielded an adequate fit to observation, as well as an increased rate of learning, only when it connected rostral PFC to the BG areas responsible for output-gating more caudal PFC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Badre D, Hoffman J, Cooney JW, D’Esposito M. Hierarchical cognitive control deficits following damage to the human frontal lobe. Nat Neurosci. 2009;12:515–522. doi: 10.1038/nn.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Badre D, Kayser AS, D’Esposito M. Frontal cortex and the discovery of abstract action rules. Neuron. 2010;66:315–316. doi: 10.1016/j.neuron.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nee DE, Brown JW. Rostral–caudal gradients of abstraction revealed by multivariate pattern analysis of working memory. Neuroimage. 2012;63:1285–1294. doi: 10.1016/j.neuroimage.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Nee DE, Brown JW. Dissociable frontal–striatal and frontal–parietal networks involved in updating hierarchical contexts in working memory. Cereb Cortex. 2013;23:2146–2158. doi: 10.1093/cercor/bhs194. As predicted by BG-mediated input gating models, coupling between the prefrontal cortex and BG increased during working memory updating, relative to maintenance. However, this was only the case when the to-be-updated information was abstract by virtue of being only indirectly relevant for responses. By contrast, updating with more concrete information yielded a change only in prefrontal-parietal coupling. This result challenges gating models to account for why the abstractness of to-be-updated information seems to determine the BG’s involvement in updating. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verstynen T, Badre D, Jarbo K, Schneider W. Microstructural organizational patterns in the human corticostriatal system. J Neurophys. 2012;107:2984–2995. doi: 10.1152/jn.00995.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Chatham CH, Frank MJ, Badre D. Corticostriatal output gating during selection from working memory. Neuron. 2014;81:930–942. doi: 10.1016/j.neuron.2014.01.002. Relative to input gating demands and working memory load, contingent output gating yields increased recruitment of the pre-PMd, a prefrontal area previously implicated in second-order hierarchical control, and increases in its coupling with the caudate, consistent with BG-mediated output gating models. Brain–behavior correlations yield further dissociations consistent with these models. First, mean BOLD in pre-PMd uniquely correlated with mean RT during selection from working memory, as though indexing the efficiency with which that particular selection rule could be implemented. By contrast, stochastic delays in the execution of this rule were uniquely and independently correlated with mean BOLD in the caudate, as well as its coupling with pre-PMd, during selective output gating — as though indexing further delays caused by stochastic closures of an output gate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ecker UK, Lewandowsky S, Oberauer K. Removal of information from working memory: a specific updating process. J Mem Lang. 2014;74:77–90. [Google Scholar]
- 50••.Chatham CH, Badre D. Working memory management and predicted utility. Front Behav Neurosci. 2013;7 doi: 10.3389/fnbeh.2013.00083. The benefit of an increased stimulus–response interval is larger when working memory should be reallocated, as indicated by a contextual stimulus rendering prior information irrelevant. Trial-by-trial fluctuations in this benefit can be predicted by a model that tracks the likelihood each item will be associated with the correct response. These ‘predicted utility’ estimates have variable neural correlates: caudal cortical regions track the predicted utility of information specified as relevant (akin to a graded stability signal), whereas ventral striatal regions track the predicted utility of information specified as irrelevant (akin to a graded flexibility signal). The use of utility prediction error may be a powerful way of applying reinforcement learning mechanisms to tasks lacking overt rewards and explicit feedback. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oberauer K. The focus of attention in working memory — from metaphors to mechanisms. Front Hum Neurosci. 2013:7. doi: 10.3389/fnhum.2013.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jurado-Parras MT, Gruart A, Delgado-García JM. Observational learning in mice can be prevented by medial prefrontal cortex stimulation and enhanced by nucleus accumbens stimulation. Learn Mem. 2012;19:99–106. doi: 10.1101/lm.024760.111. [DOI] [PubMed] [Google Scholar]
- 53.Yarkoni T. Neurosynth Core Tools v0.3.1. ZENODO; 2014. [DOI] [Google Scholar]
- 54•.Amso D, Haas S, McShane L, Badre D. Working memory updating and the development of rule-guided behavior. Cognition. 2014;133(1):201–210. doi: 10.1016/j.cognition.2014.06.012. Development confers an increasing capacity to engage in reasoning on the basis of complex hierarchical rules. This study identifies the primary developmental benefit between the ages of 7–10 and 12–30 is linked to the demands on working memory updating that is imposed by these rules, rather than the demands they impose on resolving competition. Socio-economic status was also found to predict greater age-related gains in rule-guided behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Podell JE, Sambataro F, Murty VP, Emery MR, Tong Y, Das S, Goldberg TE, Weinberger DR, Mattay VS. Neurophysiological correlates of age-related changes in working memory updating. Neuroimage. 2012;62:2151–2160. doi: 10.1016/j.neuroimage.2012.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chatham CH, Frank MJ, Munakata Y. Pupillometric and behavioral markers of a developmental shift in the temporal dynamics of cognitive control. Proc Natl Acad Sci U S A. 2009;106:5529–5533. doi: 10.1073/pnas.0810002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn Sci. 2012;16:106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lara AH, Wallis JD. Executive control processes underlying multi-item working memory. Nat Neurosci. 2014;17:876–883. doi: 10.1038/nn.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shenoy KV, Sahani M, Churchland MM. Cortical control of arm movements: a dynamical systems perspective. Annu Rev Neurosci. 2013;36:337–359. doi: 10.1146/annurev-neuro-062111-150509. [DOI] [PubMed] [Google Scholar]
- 60•.Rigotti M, Barak O, Warden MR, Wang XJ, Daw ND, Miller EK, Fusi S. The importance of mixed selectivity in complex cognitive tasks. Nature. 2013;497:585–590. doi: 10.1038/nature12160. Multi-unit activity in monkey prefrontal cortex reveals representations corresponding to all possible combinations of task features — representations far more comprehensive than those that are strictly necessary to solve the animal’s task. Surprisingly, these representations persist even after extensive training, suggesting they reflect an obligatory byproduct of prefrontal function. Though these high-dimensional representations offer considerable computational power in principle, they also confer increased fragility in the presence of task-irrelevant features. An important open question concerns how PFC might gate the task relevant components of its high-dimensional representations. For example, could oscillations be a means of selection among representation, and could such oscillations be controlled via gating interactions with striatum? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Mante V, Sussillo D, Shenoy KV, Newsome WT. Context-dependent computation by recurrent dynamics in prefrontal cortex. Nature. 2013;503:78–84. doi: 10.1038/nature12742. Multi-unit activity throughout monkey prefrontal cortex fails to show any indication of filtering out the features of a random dot stimulus that are irrelevant for the animal’s response. These results may imply that input gating works by influencing more complex aspects of prefrontal representations than merely its information content, for example by influencing its trajectory through a higher-dimensional space. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stelzel C, Fiebach CJ, Cools R, Tafazoli S, D’Esposito M. Dissociable frontostriatal effects of dopamine D2 receptor stimulation on cognitive versus motor flexibility. Cortex. 2013;49:2799–2811. doi: 10.1016/j.cortex.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63••.Gayet S, Paffen CL, Van der Stigchel S. Information matching the content of visual working memory is prioritized for conscious access. Psychol Sci. 2013;24:2472–2480. doi: 10.1177/0956797613495882. Only the relevant feature of an object stored in working memory facilitates the rise of near-threshold stimuli to conscious awareness. Given the evidently obligatory encoding of object features into WM (e.g. Marshall and Bays, 2013), this could imply that much of the selectivity in working memory control is on the output gating side, rather than the input gating side. Similar results have also been found by van Moorselaar et al. [64] [DOI] [PubMed] [Google Scholar]
- 64.van Moorselaar D, Theeuwes J, Olivers CN. In competition for the attentional template: can multiple items within visual working memory guide attention? J Exp Psychol Hum Percept Perform. 2014;40(4):1450–1464. doi: 10.1037/a0036229. [DOI] [PubMed] [Google Scholar]
- 65••.Yu Y, FitzGerald TH, Friston KJ. Working memory and anticipatory set modulate midbrain and putamen activity. J Neurosci. 2013;33:14040–14047. doi: 10.1523/JNEUROSCI.1176-13.2013. Working memory gating is only necessary when conditions have changed — either because new relevant information has appeared (and should therefore be input-gated), or because new response demands are now present (and any newly relevant information should now be output-gated). This is the first study to dissociate the expectancy violations from the gating processes they may induce. Surprisingly, the results showed no differential BG recruitment during updating, when updating could be fully predicted in advance. This result challenges future modeling and empirical work to disentangle the effects of expectancy violations from the gating dynamics that may follow them. [DOI] [PMC free article] [PubMed] [Google Scholar]