Abstract
Purpose/Objective(s)
To compare long-term disease control and overall survival between children treated with proton and photon radiotherapy (RT) for standard risk medulloblastoma.
Methods and Materials
This multi-institution cohort study includes 88 children treated with chemotherapy and proton (n=45) or photon (n=43) RT between 2000 and 2009. Overall survival (OS), recurrence-free survival (RFS) and patterns of failure were compared among the two cohorts.
Results
Median (range) age at diagnosis was 6 yrs (3 - 21) for proton pts vs. 8 yrs (3 -19) for photon pts (p=0.011). Cohorts were similar with respect to gender, histology, extent of surgical resection, craniospinal (CSI) RT dose, total RT dose, whether the RT boost was delivered to the posterior fossa (PF) or tumor bed (TB), time from surgery to RT start, or total duration of RT. RT consisted of a median (range) CSI dose of 23.4 Gy (18 - 27) and a boost of 30.6 Gy (27 - 37.8). Median (95% CI) f/up time is 6.2 yrs (5.1 – 6.6) for proton pts vs. 7.0 yrs (5.8 – 8.9) for photon pts. There was no significant difference in RFS or OS between pts treated with proton vs. photon RT: 6 yr RFS 78.8% vs. 76.5% (p=0.948) and 6 yr OS 82.0 vs. 87.6% (p=0.285). On multivariable analysis, there was a trend for longer RFS with female gender (p=0.058) and higher CSI dose (p=0.096), and for longer OS with female gender (p=0.093). Patterns of failure were similar among the two cohorts (p=0.908).
Conclusions
Disease control with proton and photon radiotherapy appears equivalent for standard risk medulloblastoma.
Introduction
Medulloblastoma is the second most common pediatric brain tumor, with an estimated 500 new cases diagnosed annually in the United States.[1,2] Given the propensity of medulloblastoma to disseminate throughout the neuroaxis, craniospinal irradiation (CSI) plays a critical role in providing long-term disease control. With standard multi-modality management including maximal safe resection followed by CSI, involved field or posterior fossa radiation boost, and chemotherapy, survival rates for standard risk patients approximate 85%[3,4]. Though survival rates are good, multi-modality treatment does not come without significant risk of long-term treatment related morbidity.
Proton radiation therapy (PRT) is becoming increasingly utilized for patients with medulloblastoma in an effort to reduce treatment related sequelae[5]. Multiple dosimetric studies have demonstrated that PRT delivers equivalent target volume coverage while significantly sparing the normal tissues anterior to the vertebral bodies, such as the heart, lungs, thyroid gland, lives and kidneys, and reducing the dose received to critical intracranial structures, such as the cochlea, hypothalamic pituitary axis and temporal lobes, when compared with either conventional or intensity modulated photon radiation (XRT)[6-8]. Based on this dosimetric advantage, proton radiation is generally expected to provide equivalent tumor control while reducing the late effects of radiation. However, any potential differences in treatment efficacy and/or toxicity that may result from small differences in RBE have not been well studied. This has led some to question whether PRT could potentially lead to an increased risk of tumor recurrence in medulloblastoma [9,10]. Both prospective and retrospective single institution proton therapy series report promising clinical outcomes with PRT [11,12], though data directly comparing long-term disease control among patients treated with proton and photon therapy are lacking.
The purpose of this analysis is to compare overall survival, progression free survival, and patterns of failure among two modern case-matched cohorts of children with standard risk medulloblastoma treated with proton and photon radiation therapy.
Methods
Patient Selection
This multi-institutional cohort study includes children with standard risk medulloblastoma treated with PRT at XXXXXXXXXXXXX XXXXXXX XXXXXXXX (XXX) or XRT at XXXXX XXXXXXXXXX between 2000 and 2009. Standard risk patients met the following criteria for inclusion: age >3 years old at diagnosis, <1.5 cm2 residual disease after surgery, and M0 disease based on MRI of the spine and cerebrospinal fluid cytology examination. Patients treated at XXX were prospectively in enrolled in the Phase II study XXXXXXXXXXX[11]. Concurrent enrollment in Children's Oncology Group (COG) or other protocols was allowed. Institutional review board approval at both institutions was approved for this analysis.
Treatment
All patients underwent maximal safe resection of the primary tumor followed by CSI and involved field (IF) or posterior fossa (PF) RT boost and chemotherapy. Chemotherapy was most often administered adjuvantly and consisted of vincristine, cisplatin, cyclophosphamide and/or lomustine delivered on or per current COG protocols, though some patients received pre-irradiation chemotherapy. Patients underwent either prone and/or supine CT simulation for RT planning. The CSI target volume included the entire subarachnoid volume, nerve roots, and the whole vertebral body in skeletally immature patients (as assessed by age, height, and bone age). PRT was delivered with three-dimensional conformal (3DC) PRT and dose was prescribed in gray relative biological equivalents (Gy(RBE)) using the RBE value of 1.1. XRT was delivered with either 3DC XRT or intensity modulated radiation therapy (IMRT)[13]. The most common CSI dose was 23.4 Gy (range18 – 27 Gy). A CSI dose of 18 Gy was used exclusively in patients concurrently enrolled on COG protocol with the exception of one 3 year old patient treated with protons whose parents insisted the lower CSI dose be used and were fully informed of the risks. At the discretion of the treating radiation oncologist, 27 Gy CSI was used in one patient with anaplastic histology, and 26.4 Gy CSI was used in one patient treated at 1.2 Gy per fraction twice daily, All other patients received 23.4 Gy CSI at 1.8 Gy per fraction followed by an IF or PF boost to a cumulative dose of 54-55.8 Gy. IF or PF boost was used at the discretion of the treating radiation oncologist for those patients not concurrently enrolled on COG protocol. Following completion of radiation, patients were followed with serial MRIs of the brain and spine and physical exams, typically every 3-4 months for the first 2 years, every 6 months for years 3-4 and annually thereafter.
Outcome Variables
The primary endpoints for this study were overall survival (OS), recurrence free survival (RFS) and patterns of failure. OS and RFS were defined from time of diagnosis until time of death from any cause or time of first medulloblastoma tumor recurrence, respectively, or were censored at last patient follow-up. Medulloblastoma tumor recurrence was defined by MRI of the brain or spine with or without pathologic confirmation and was categorized by location as focal failure in the posterior fossa, supratentorial brain, or spine, or diffuse/leptomeningeal dissemination.
Statistical Analysis
Patient's characteristics were summarized and compared between those treated with PRT or XRT by Wilcoxon rank-sum test for numerical covariates and chi-square test or Fisher's exact test for categorical covariates, where appropriate. Covariates included patient age, gender, histology, whether surgery was gross total resection (GTR) or <1.5 cm2 residual remained, year of diagnosis, CSI dose, total RT dose, location of RT boost, time interval from surgery to RT, and duration of RT. Survival functions were estimated by the Kaplan-Meier method, and the log-rank test was used to assess the difference in OS and RFS between patients stratified by RT type[14]. Univariate and multivariable survival analyses were carried out using the Cox proportional hazards model[15]. The proportional hazards assumption was also checked with a Kolmogorov-type supremum test. The best predictive models of OS and RFS were identified using a backward variable selection method with an alpha level of removal of 0.1. RT type was forced in the model, and the models were stratified by diagnosis year.
To further adjust for any patient differences between the two RT types, propensity score matching was performed, in which patients treated with proton RT were matched in a 1:1 ratio to those treated with photon RT according to gender, age, date of diagnosis, histology, location of RT boost, and RT CSI dose, using a greedy algorithm with the nearest available pair matching method on estimated propensity score. Survival outcomes were again assessed by Cox proportional hazards models among this matched subset. All analyses are done using SAS 9.3 (SAS Institute, Inc., Cary, North Carolina) and R package version 3.1.2 (The R Foundation for Statistical Computing) with a significant level of 0.05.
Results
Patient Population
Patient demographics and clinical characteristics are listed in Table 1. Photon patients were slightly older at diagnosis (median age 8.2 years (range 3.4 – 19.5) vs. 6.2 years (range 3.3 – 21.9) (p=0.011) and were more often diagnosed prior to 2005 (46.5% vs. 20%, p=0.008) than proton patients. All other patient characteristics, including gender, histology, extent of surgical resection, CSI RT dose, total RT dose, whether the RT boost was delivered to PF or IF, time from surgery to RT start, or total duration of RT were similar among the two cohorts.
Table 1. Patient Characteristics.
| RT type | |||
|---|---|---|---|
|
|
|||
| Covariate | Proton Therapy (N=45) | Photon Therapy (N=43) | P-value* |
|
| |||
| Age at Diagnosis, Median (Range) | 6.2 (3.3 - 21.9) | 8.2 (3.4 - 19.5) | 0.011 |
|
| |||
| Gender | |||
| Female | 20 (44.4) | 14 (32.6) | 0.252 |
| Male | 25 (55.6) | 29 (67.4) | |
|
| |||
| Date of Diagnosis | |||
| 2000-2004 | 9 (20) | 20 (46.5) | 0.008 |
| 2005-2009 | 36 (80) | 23 (53.5) | |
|
| |||
| Histology | |||
| Classic | 34 (75.6) | 37 (86) | 0.326 |
| Anaplastic | 6 (13.3) | 3 (7) | |
| Other | 5 (11.1) | 3 (7) | |
|
| |||
| Residual disease after surgery | |||
| <1.5 cm2 | 5 (11.1) | 1 (2.3) | 0.203 |
| None/GTR | 40 (88.9) | 42 (97.7) | |
|
| |||
| Location of RT boost | |||
| Tumor Bed (TB) | 28 (62.2) | 22 (53.7) | 0.361 |
| Posterior Fossa (PF) | 13 (28.9) | 11 (26.8) | |
| PF > TB | 4 (8.9) | 8 (19.5) | |
|
| |||
| RT CSI dose, Median (Range) | 23.4 (18 - 27) | 23.4 (18 - 26.4) | |
| 18 Gy | 3 (6.7) | 8 (18.6) | 0.129 |
| 23.4 Gy | 41 (91.1) | 34 (79.1) | |
| 26.4-27 Gy | 1 (2.2) | 1 (2.3) | |
|
| |||
| Total Dose to Primary | |||
| 54-55.8 Gy | 45 (100) | 42 (97.7) | 0.489 |
| >55.8 Gy | 0 | 1 (2.3) | |
|
| |||
| Surgery to RT interval (days), Median (Range) | 31 (22 - 219) | 29 (11 - 60) | 0.645 |
|
| |||
| Treatment length (Months), Median (Range) | 1.42 (1.29 - 1.55) | 1.39 (0.89 - 20.6) | 0.623 |
Data are presented as number of patients (%) or median (range).
P-value is calculated by Wilcoxon rank-sum test for numerical covariates; and chi-square test or Fisher's exact test for categorical covariates, where appropriate.
Overall Survival
The median (95% CI) follow-up time is 6.2 yrs (5.1 – 6.6) for the proton cohort and 7.0 yrs (5.8 – 8.9) for the photon cohort. There was no significant difference in OS between the two cohorts (p=0.285, Figure 1). The estimated 6-year OS rates were similar for proton and photon patients; 82.0% (95 % CI 65.4-91.1) and 87.6% (95% CI 72.7-94.7), respectively. On univariate analysis, there was a trend for longer OS with female gender (p=0.073) and age > 7 years at diagnosis (p=0.085). There was no significant difference in OS between the two cohorts on multivariable analysis (Table 2), though the trend for improved OS with female gender persisted (p=0.093). Further analysis among the 1:1 matched sample of 25 proton patients and 25 photon patients, confirmed no significant difference in OS, with a HR for proton vs. photon patients of 1.0 (95% CI: 0.14-7.10; p=1.00).
Figure 1.
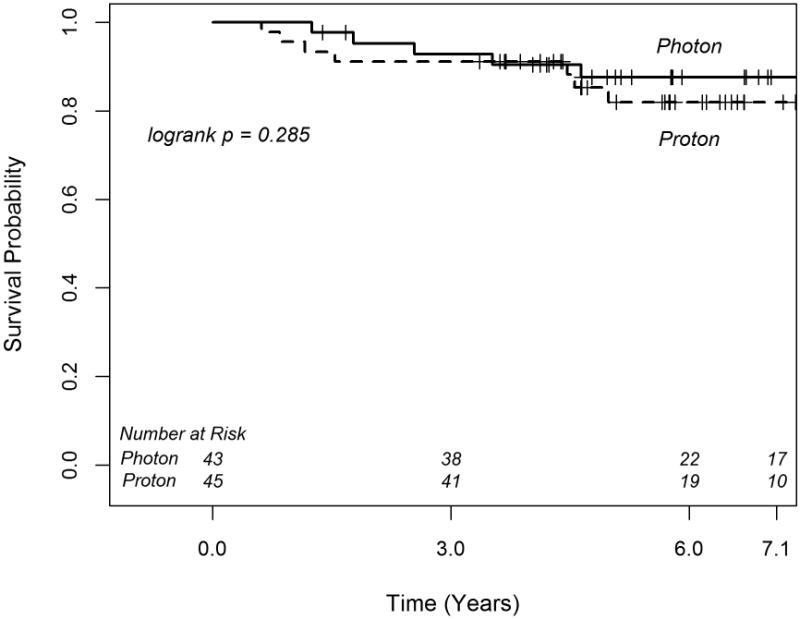
Kaplan-Meier curves of overall survival for medulloblastoma patients treated with photon and proton radiotherapy.
Table 2. Multivariable Analysis of Overall Survival and Recurrence Free Survival.
| Covariate | Hazard Ratio(95% CI) | P-value |
|---|---|---|
| Overall Survival | ||
| Proton vs. Photon RT | 2.17 (0.66-7.16) | 0.201 |
| Male vs. Female | 3.66 (0.80-16.67) | 0.093 |
| Recurrence Free Survival | ||
| Proton vs. Photon RT | 1.31 (0.50-3.41) | 0.584 |
| Male vs. Female | 2.92 (0.96-8.83) | 0.058 |
| RT CSI dose | 0.83 (0.66-1.03) | 0.096 |
Of 88, 88 observations are used in the multivariable COX proportional hazards model. Backward variable selection method with an alpha level of removal of .1 was used. The model was stratified by Date of Diagnosis; RT type was forced in the model.
The following variables were removed from the model: Age at Diagnosis, Location of RT boost, Histology, Residual disease after surgery, days from surgery to RT, RT treatment length, Date of Diagnosis, RT CSI dose (for RFS).
Recurrence Free Survival and Patterns of Failure
There was no significant difference in RFS between proton and photon patients (p=0.948, Figure 2). The estimated 6-year RFS rates were similar for proton and photon patients; 78.8% (95% CI 63.0-89.0) and 76.5% (95% CI 60.6-86.6), respectively. On univariate analysis, there was a trend for longer RFS among female patients (p=0.059). This trend remained marginally significant on multivariable analysis (Table 2). Additionally on multivariable analysis, there was a trend for reduced risk of recurrence with greater CSI dose (p=0.096, Table 2). Out of 77 patients treated to ≥ 23.4 Gy CSI, 16 patients (20.8%) experienced tumor recurrence, while 4 of 11 patients (36.4%) treated to 18 Gy CSI experienced tumor recurrence, and all 4 recurred in the spine or as leptomeningeal disease. Further analysis of RFS using the 1:1 matched sample, again confirmed no significant difference in RFS among the proton vs. photon treated cohorts, with a HR of 1.0 (95% CI: 0.20-4.95; p=1.00).
Figure 2.
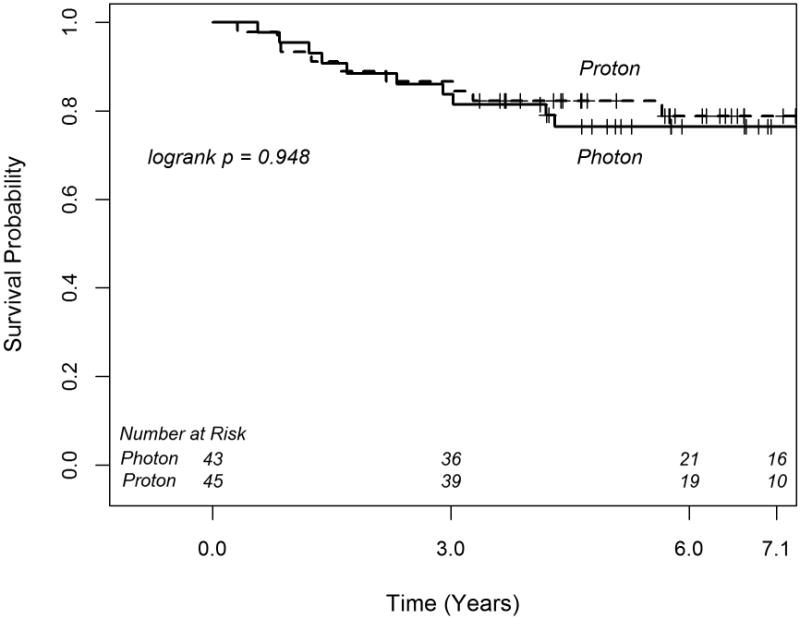
Kaplan-Meier curves of relapse free survival for medulloblastoma patients treated with photon and proton radiotherapy.
In total 10 patients in each cohort experienced a medulloblastoma recurrence. Patterns of failure were similar among the patients treated with proton and photon RT (p=0.908), with the most common site of first failure being diffuse or leptomeningeal disease (Table 3). An additional 3 patients treated with photons developed a second malignancy, including an anaplastic astrocytoma, intracranial desmoid tumor, and thyroid cancer occurring 12.9, 3.7, and 12.7 years after treatment, respectively. No patients treated with protons developed a second malignancy.
Table 3. Patterns of Failure among Proton and Photon Treated Cohorts.
| Characteristic | Proton Therapy (n=45) | Photon Therapy (n=43) | P value* |
|---|---|---|---|
|
| |||
| Total Relapses | 10 (22.2%) | 10 (23.3%) | 0.908 |
|
| |||
| Patterns of Failure | 1.000 | ||
| Diffuse or Leptomeningeal Disease | 5 (50%) | 5 (50%) | |
| Isolated Focal Spine | 2 (20%) | 3 (30%) | |
| Isolated Posterior Fossa | 1 (10%) | 2 (20%) | |
| Isolated Brain, other | 1 (10%) | 0 | |
| Posterior Fossa + Focal Spine | 1 (10%) | 0 | |
P-value is calculated by chi-square test or Fisher's exact test, where appropriate.
Discussion
In this direct comparison of clinical outcomes among matched cohorts of children with standard risk medulloblastoma treated in the modern era with chemotherapy and proton or photon radiotherapy, no difference in patterns of failure, recurrence free survival, or overall survival were found according to radiotherapy modality. Among both cohorts, OS and RFS are similar to national cooperative group study results[3,16]. The predominant site of failure was diffuse or leptomeningeal disease (50%), and isolated posterior fossa failure was rare (10-20%). This pattern of failure is similar to previously published reports from both prospective cooperative group studies and retrospective series, which have reported a disseminated failure rate 64-68%[3,16-18], and an isolated posterior fossa failure rate of 9-15%[19-21].
Despite concerns of some that proton therapy could negatively impact disease control as a result of relative under dosing from a more homogeneous dose distribution or improper RBE weighting[9], the results presented here indicate there is no compromise to tumor control with the use of proton therapy. The association between patterns of failure and linear energy transfer (LET) and RBE distribution with the use of proton therapy for medulloblastoma has previously been evaluated, and no correlation between LET or RBE and recurrence location was found[12]. The similar pattern of failure among the two cohorts in this analysis further indicates that potential dosimetric differences with the use of proton therapy do not impact tumor control. Additional concerns have been raised over the negative impact that referral to an outside center, potentially causing a delay in RT start time, could have on disease control[4]. Though the impact of outside referral was not specifically evaluated in this analysis, no difference in the time interval from surgery to RT or duration of RT was found among the proton and photon treated cohorts.
No significant associations between clinical variables and RFS or OS were found in this analysis, likely resulting from the relative uniformity of the population included and small total patient numbers. While a trend for poor RFS with reduced CSI dose was identified, this association was not statistically significant and should be interpreted with caution as other prognostic variables among patients treated with reduced and higher dose CSI were not specifically evaluated in this analysis. Patients at each institution were allowed enrollment onto the COG clinical trial ACNS 0331 evaluating the use of reduced dose CSI (18 Gy) in standard risk children age 3-7, and results of this trial are awaited to definitively address the impact of reduced dose CSI on tumor control. A trend was also identified between male gender and shorter RFS and OS in this analysis, a finding which has previously been demonstrated in other literature[22-24].
The ability to compare clinical outcomes according to radiotherapy modality is made possible in this analysis by the use of relatively homogeneous patient sample, similar with respect to disease stage and other prognostic variables, and treated in a uniform fashion with chemotherapy, CSI and posterior fossa or involved field boost radiotherapy. Additionally, a great strength of this analysis is the use of two modern cohorts treated in parallel over the same time period from 2000-2009, which allows for a comparison of PRT with modern XRT techniques and further allows for consistent practices in diagnosis, staging and treatment between the two cohorts. While a greater proportion of patients in the proton group were treated after 2004 (80% vs. 54%), treatment approaches remained uniform over this time period and this difference is unlikely to impact the RT modality comparison. Though patients in the proton cohort were slightly younger, the small difference in age (median 6.2 vs. 8.2 years) would not be expected to influence outcomes. Furthermore, when using a more select sample of patients matched one to one according to year of diagnosis, gender, age, CSI dose and RT boost type, the initial results were confirmed, and RFS and OS were found to be equivalent among proton and photon treated patients.
While a greater number of second malignancies were demonstrated in the photon treated cohort (3 vs. none), there is a lack of sufficient follow to adequately compare second malignancy incidence according to RT type in this analysis. Treatment associated sequelae are of great interest when comparing outcomes among patients treated with proton and photon therapy, though this was not the focus of the current analysis. Additional clinical data comparing late effects according to each modality is needed.
In conclusion, the results of this case matched analysis of patients with standard risk medulloblastoma treated with proton and photon radiotherapy demonstrates no difference in patterns of failure, recurrence free survival or overall survival according to RT modality. Disease control with protons and photons appears equivalent.
Summary.
This case-matched, multi-institutional cohort study, compares clinical outcomes of patients with standard risk medulloblastoma treated with modern proton (n=45) and photon (n=43) radiotherapy. Results demonstrate no difference in patterns of failure, recurrence free survival or overall survival according to RT modality.
Acknowledgments
Financial support: Funded in part by the National Cancer Institute, Award number P01CA021239, and the Federal Share of program income earned by Massachusetts General Hospital on C06 CA059267, Proton Therapy Research and Treatment Center. The content are solely the responsibility of the authors and do necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Conflicts of interest: none
Meeting presentation: Presented in oral abstract form at SIOP 2014 in Toronto, Canada
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 2.McNeil DE, Cote TR, Clegg L, Rorke LB. Incidence and trends in pediatric malignancies medulloblastoma/primitive neuroectodermal tumor: A seer update. Surveillance epidemiology and end results. Med Pediatr Oncol. 2002;39:190–194. doi: 10.1002/mpo.10121. [DOI] [PubMed] [Google Scholar]
- 3.Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, Bayer L, LaFond D, Donahue BR, Marymont MH, Muraszko K, Langston J, Sposto R. Phase iii study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 4.Wolden SL, Dunkel IJ, Souweidane MM, Happersett L, Khakoo Y, Schupak K, Lyden D, Leibel SA. Patterns of failure using a conformal radiation therapy tumor bed boost for medulloblastoma. J Clin Oncol. 2003;21:3079–3083. doi: 10.1200/JCO.2003.11.140. [DOI] [PubMed] [Google Scholar]
- 5.Johnstone PA, McMullen KP, Buchsbaum JC, Douglas JG, Helft P. Pediatric csi: Are protons the only ethical approach? Int J Radiat Oncol Biol Phys. 2013;87:228–230. doi: 10.1016/j.ijrobp.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 6.St Clair WH, Adams JA, Bues M, Fullerton BC, La Shell S, Kooy HM, Loeffler JS, Tarbell NJ. Advantage of protons compared to conventional x-ray or imrt in the treatment of a pediatric patient with medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;58:727–734. doi: 10.1016/S0360-3016(03)01574-8. [DOI] [PubMed] [Google Scholar]
- 7.Miralbell R, Lomax A, Russo M. Potential role of proton therapy in the treatment of pediatric medulloblastoma/primitive neuro-ectodermal tumors: Spinal theca irradiation. Int J Radiat Oncol Biol Phys. 1997;38:805–811. doi: 10.1016/s0360-3016(97)00005-9. [DOI] [PubMed] [Google Scholar]
- 8.Lee CT, Bilton SD, Famiglietti RM, Riley BA, Mahajan A, Chang EL, Maor MH, Woo SY, Cox JD, Smith AR. Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: How do protons compare with other conformal techniques? Int J Radiat Oncol Biol Phys. 2005;63:362–372. doi: 10.1016/j.ijrobp.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 9.Jones B, Wilson P, Nagano A, Fenwick J, McKenna G. Dilemmas concerning dose distribution and the influence of relative biological effect in proton beam therapy of medulloblastoma. Br J Radiol. 2012;85:e912–918. doi: 10.1259/bjr/24498486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones B. Patterns of failure after proton therapy in medulloblastoma. Int J Radiat Oncol Biol Phys. 2014;90:25–26. doi: 10.1016/j.ijrobp.2014.05.054. [DOI] [PubMed] [Google Scholar]
- 11.Yock T, Yeap BY, Ebb D, Weyman E, Eaton B, Sherry N, Jones R, MacDonald SM, Pulsifer MB, Tarbell NJ. Pediatric medulloblastoma: Clinical outcomes from a prospective phase II study of proton radiotherapy. J Clin Oncol. 2015 [Google Scholar]
- 12.Sethi RV, Giantsoudi D, Raiford M, Malhi I, Niemierko A, Rapalino O, Caruso P, Yock TI, Tarbell NJ, Paganetti H, MacDonald SM. Patterns of failure after proton therapy in medulloblastoma; linear energy transfer distributions and relative biological effectiveness associations for relapses. Int J Radiat Oncol Biol Phys. 2014;88:655–663. doi: 10.1016/j.ijrobp.2013.11.239. [DOI] [PubMed] [Google Scholar]
- 13.Pai Panandiker A, Ning H, Likhacheva A, Ullman K, Arora B, Ondos J, Karimpour S, Packer R, Miller R, Citrin D. Craniospinal irradiation with spinal imrt to improve target homogeneity. Int J Radiat Oncol Biol Phys. 2007;68:1402–1409. doi: 10.1016/j.ijrobp.2007.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalbfleisch JD, P RL. Book The statistical analysis of failure time data. New York: John Wiley and Sons; 1980. The statistical analysis of failure time data. Editor, editorˆeditors. [Google Scholar]
- 15.Cox DR. Regression models and life tables. J Royal Stat Society. 1972;B34:187–220. [Google Scholar]
- 16.Packer RJ, Goldwein J, Nicholson HS, Vezina LG, Allen JC, Ris MD, Muraszko K, Rorke LB, Wara WM, Cohen BH, Boyett JM. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A children's cancer group study. J Clin Oncol. 1999;17:2127–2136. doi: 10.1200/JCO.1999.17.7.2127. [DOI] [PubMed] [Google Scholar]
- 17.Taylor RE, Bailey CC, Robinson K, Weston CL, Ellison D, Ironside J, Lucraft H, Gilbertson R, Tait DM, Walker DA, Pizer BL, Imeson J, Lashford LS. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The international society of paediatric oncology/united kingdom children's cancer study group pnet-3 study. J Clin Oncol. 2003;21:1581–1591. doi: 10.1200/JCO.2003.05.116. [DOI] [PubMed] [Google Scholar]
- 18.Skowronska-Gardas A, Chojnacka M, Morawska-Kaczynska M, Perek D, Perek-Polnik M. Patterns of failure in children with medulloblastoma treated with 3d conformal radiotherapy. Radiother Oncol. 2007;84:26–33. doi: 10.1016/j.radonc.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Oyharcabal-Bourden V, Kalifa C, Gentet JC, Frappaz D, Edan C, Chastagner P, Sariban E, Pagnier A, Babin A, Pichon F, Neuenschwander S, Vinchon M, Bours D, Mosseri V, Le Gales C, Ruchoux M, Carrie C, Doz F. Standard-risk medulloblastoma treated by adjuvant chemotherapy followed by reduced-dose craniospinal radiation therapy: A french society of pediatric oncology study. J Clin Oncol. 2005;23:4726–4734. doi: 10.1200/JCO.2005.00.760. [DOI] [PubMed] [Google Scholar]
- 20.Carrie C, Grill J, Figarella-Branger D, Bernier V, Padovani L, Habrand JL, Benhassel M, Mege M, Mahe M, Quetin P, Maire JP, Baron MH, Clavere P, Chapet S, Maingon P, Alapetite C, Claude L, Laprie A, Dussart S. Online quality control, hyperfractionated radiotherapy alone and reduced boost volume for standard risk medulloblastoma: Long-term results of msfop 98. J Clin Oncol. 2009;27:1879–1883. doi: 10.1200/JCO.2008.18.6437. [DOI] [PubMed] [Google Scholar]
- 21.Fukunaga-Johnson N, Lee JH, Sandler HM, Robertson P, McNeil E, Goldwein JW. Patterns of failure following treatment for medulloblastoma: Is it necessary to treat the entire posterior fossa? Int J Radiat Oncol Biol Phys. 1998;42:143–146. doi: 10.1016/s0360-3016(98)00178-3. [DOI] [PubMed] [Google Scholar]
- 22.Chatty EM, Earle KM. Medulloblastoma. A report of 201 cases with emphasis on the relationship of histologic variants to survival. Cancer. 1971;28:977–983. doi: 10.1002/1097-0142(1971)28:4<977::aid-cncr2820280422>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Curran EK, Sainani KL, Le GM, Propp JM, Fisher PG. Gender affects survival for medulloblastoma only in older children and adults: A study from the surveillance epidemiology and end results registry. Pediatr Blood Cancer. 2009;52:60–64. doi: 10.1002/pbc.21832. [DOI] [PubMed] [Google Scholar]
- 24.Tait DM, Thornton-Jones H, Bloom HJ, Lemerle J, Morris-Jones P. Adjuvant chemotherapy for medulloblastoma: The first multi-centre control trial of the international society of paediatric oncology (siop i) Eur J Cancer. 1990;26:464–469. [PubMed] [Google Scholar]


