Fibrinogen is a 340 kDa glycoprotein that circulates in healthy humans at 2–4 mg/mL; however, fibrinogen is an acute phase protein synthesized in the liver, and its circulating levels can exceed 7 mg/mL during acute inflammation. Elevated fibrinogen levels are associated with increased risk of incident cardiovascular disease (CVD).1,2 Healthy mice infused with unfractionated human fibrinogen and subjected to FeCl3-mediated carotid artery injury have a shortened time to vessel occlusion and increased resistance of thrombi to acute thrombolysis, suggesting elevated fibrinogen independently contributes to thrombosis.3,4
Fibrinogen is composed of two sets of three polypeptide chains: Aα, Bβ, and γ. Alternative splicing of the γA chain leads to synthesis of a γ′ chain containing a unique 20-amino acid sequence at the C-terminus. Between 8–15% of circulating fibrinogen in healthy individuals contains a γ′ chain (γA/γ′). Cross-sectional and retrospective studies have associated elevated circulating levels of the γA/γ′ isoform with increased incidence of coronary artery disease5, myocardial infarction6, and ischemic stroke7–9. The observation that some patients have an increased γ′-to-total fibrinogen ratio7–11 suggests γA/γ′ fibrinogen is not simply a biomarker for increased total fibrinogen. Together with data from in vitro studies demonstrating clots formed from purified γA/γ′ fibrinogen are composed of abnormally-structured fibers and are highly resistant to fibrinolysis12–15, these observations have led to the notion that γA/γ′ fibrinogen is an etiologic risk factor for CVD.
In this issue of Arteriosclerosis, Thrombosis, and Vascular Biology, Appiah and colleagues report a large prospective study examining the association of plasma γ′ fibrinogen levels with incident CVD endpoints.16 Their unadjusted analysis shows a positive association of γ′ fibrinogen with incident coronary heart disease, ischemic stroke, peripheral artery disease, heart failure, and CVD deaths. However, adjustment for established CVD risk factors and levels of plasma fibrinogen and C-reactive protein (CRP) as a biomarker for inflammation abolished the associations with coronary heart disease and ischemic stroke, and sharply attenuated the significance of the association with heart failure and peripheral artery disease. In contrast to previous studies, Appiah et al. conclude that γ′ fibrinogen levels reflect an inflammatory process that accompanies, and may promote, CVD, but that γ′ fibrinogen does not independently contribute to CVD.16 Strengths of their analysis include the large number of subjects and its prospective design which are directly responsive to prior calls for this type of study.17,18 Limitations include drift in measurements of γ′ fibrinogen over time and the fact that γ′ fibrinogen and CRP measurements were made from samples collected at separate visits.
Given these conclusions, what is the role of γ′ fibrinogen in vivo? In addition to its prothrombotic characteristics, fibrinogen has critical anticoagulant functions by adsorbing thrombin during clotting (known as “antithrombin I” activity).17 Afibrinogenemic patients have elevated markers of coagulation activation and experience acute thrombosis19,20. Notably, repletion of afibrinogenemic plasma with γA/γ′ fibrinogen is more effective than γA/γA fibrinogen at reducing thrombin generation.21 This effect has been attributed to the ability of γ′ fibrinogen to support high affinity non-substrate binding of thrombin.22–24 Although fibrin-bound thrombin resists heparin-catalyzed inactivation by antithrombin III24, its activity towards its endogenous substrates is also reduced. Accordingly, in vitro studies show that presence of γA/γ′ fibrinogen reduces thrombin-mediated activation of cofactors VIII25 and V26, and increases plasma sensitivity to activated protein C27. Consequently, the net contribution of γ′ fibrinogen to coagulation in vivo – either pro- or antithrombotic – is difficult to predict.
Muthard et al.28 recently found that γ′ fibrin(ogen) reduces thrombin-mediated clot growth at venous, but not arterial shear rates, suggesting the contributions of γ′ fibrinogen are mediated by the vascular bed. Observations from animal models of venous and arterial thrombosis are consistent with this premise. Data from venous thrombosis models demonstrate a net antithrombotic effect of γ′ fibrinogen: 1) transgenic expression of the human γ′ chain reduces venous thrombus volume in mice that are heterozygous for the factor V Leiden mutation29, and 2) infusion of an 18-amino acid peptide mimicking the γ′ chain C-terminus (γ′ 410–427) reduces fibrin formation in an arteriovenous shunt in baboons25. In contrast, in an arterial thrombosis model, mice infused with γA/γA fibrinogen have a shorter time to artery occlusion than control mice, but mice infused with γA/γ′ fibrinogen do not.4 This finding is notable since mice infused with γA/γ′ fibrinogen have lower circulating levels of thrombin-antithrombin complexes than either control mice or γA/γA fibrinogen-infused mice.4 Thus, it appears that in this model, the antithrombin I activity of γA/γ′ fibrinogen mitigates, but does not overcome, any procoagulant effects of this molecule. Together with findings from Appiah et al.16, these data suggest the net effect of γ′ fibrinogen during arterial thrombosis is neutral (Figure).
Figure. Model illustrating the procoagulant and antithrombotic functions of γ′ fibrinogen.
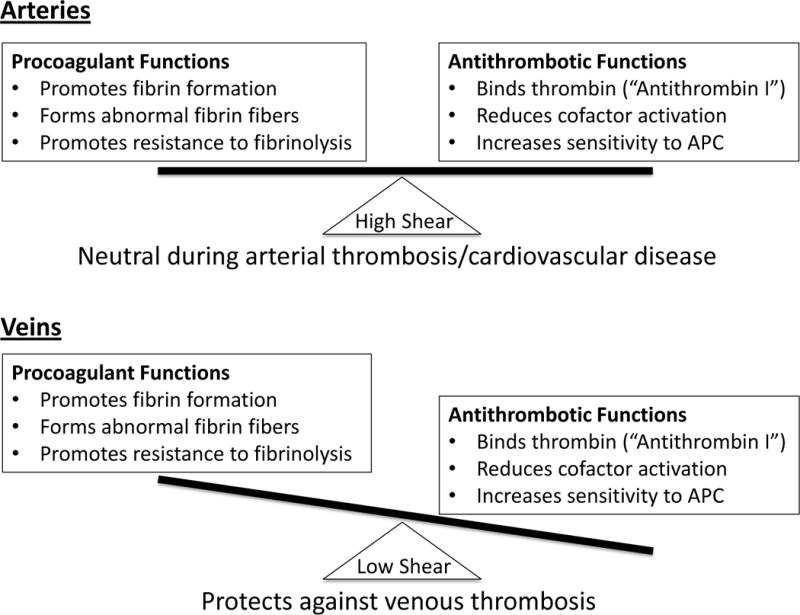
During thrombosis in high arterial shear (top), the antithrombotic activity of γ′ fibrinogen is not sufficient to overcome the prothrombotic properties of this molecule, resulting in a net neutral effect. In low shear in venous circulation (bottom), the thrombin-binding ability of γA/γ′ fibrinogen reduce cofactor activation and increase sensitivity to APC, outweighing its procoagulant contributions and reducing venous thrombosis risk.
If γA/γ′ fibrinogen does not influence CVD outcomes, why are its levels increased in CVD patients? CVD is a proinflammatory pathology associated with elevated levels of fibrinogen, and the proinflammatory cytokine interleukin-6 preferentially up-regulates hepatocyte production of γA/γ′ fibrinogen versus γA/γA fibrinogen.30 Accordingly, γA/γ′ fibrinogen may be increased to down-regulate inflammation-induced prothrombotic activity in certain situations. Notably, reduced γA/γ′ fibrinogen levels and γ′-to-total fibrinogen ratio are associated with increased risk of venous thromboembolism31 and thrombotic microangiopathy32, related pathologies that are also associated with vascular inflammation. These findings are consistent with observations from the animal studies.25,29 Thus, increased γA/γ′ levels associated with CVD may simply reflect its common etiology with venous disease, in which this molecule has a protective, antithrombotic role. To this end, it is curious that elevated γ′ fibrinogen appears differentially associated with the different CVD outcomes studied and remains significantly associated with the broad category of CVD deaths, even after adjustment for CVD risk factors, fibrinogen, and CRP. A greater understanding of the common and unique pathophysiologic mechanisms associated with these pathologies may shed light on this issue.
Acknowledgments
Sources for Funding: A.S. Wolberg is supported by a research grant from the National Institutes of Health (R56HL094740).
References
- 1.Danesh J, Lewington S, Thompson SG, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 2.Wilhelmsen L, Svardsudd K, Korsan-Bengsten K, Larsson B, Welin L, Tibblin G. Fibrinogen as a risk factor for stroke and myocardial infarction. N Engl J Med. 1984;311:501–505. doi: 10.1056/NEJM198408233110804. [DOI] [PubMed] [Google Scholar]
- 3.Machlus KR, Cardenas JC, Church FC, Wolberg AS. Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood. 2011;117:4953–4963. doi: 10.1182/blood-2010-11-316885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walton BL, Getz TM, Bergmeier W, Lin FC, Uitte de Willige S, Wolberg AS. The fibrinogen gammaA/gamma′ isoform does not promote acute arterial thrombosis in mice. J Thromb Haemost. 2014;12:680–689. doi: 10.1111/jth.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovely RS, Falls LA, Al-Mondhiry HA, Chambers CE, Sexton GJ, Ni H, Farrell DH. Association of gammaA/gamma′ fibrinogen levels and coronary artery disease. Thromb Haemost. 2002;88:26–31. [PubMed] [Google Scholar]
- 6.Mannila MN, Lovely RS, Kazmierczak SC, Eriksson P, Samnegard A, Farrell DH, Hamsten A, Silveira A. Elevated plasma fibrinogen gamma′ concentration is associated with myocardial infarction: effects of variation in fibrinogen genes and environmental factors. J Thromb Haemost. 2007;5:766–773. doi: 10.1111/j.1538-7836.2007.02406.x. [DOI] [PubMed] [Google Scholar]
- 7.Cheung EY, Uitte de Willige S, Vos HL, Leebeek FW, Dippel DW, Bertina RM, de Maat MP. Fibrinogen gamma′ in ischemic stroke: a case-control study. Stroke. 2008;39:1033–1035. doi: 10.1161/STROKEAHA.107.495499. [DOI] [PubMed] [Google Scholar]
- 8.Cheung EY, Vos HL, Kruip MJ, den Hertog HM, Jukema JW, de Maat MP. Elevated fibrinogen gamma′ ratio is associated with cardiovascular diseases and acute phase reaction but not with clinical outcome. Blood. 2009;114:4603–4604. doi: 10.1182/blood-2009-08-236240. author reply 4604–4605. [DOI] [PubMed] [Google Scholar]
- 9.van den Herik EG, Cheung EY, de Lau LM, den Hertog HM, Leebeek FW, Dippel DW, Koudstaal PJ, de Maat MP. gamma′/total fibrinogen ratio is associated with short-term outcome in ischaemic stroke. Thromb Haemost. 2011;105:430–434. doi: 10.1160/TH10-09-0569. [DOI] [PubMed] [Google Scholar]
- 10.Drouet L, Paolucci F, Pasqualini N, Laprade M, Ripoll L, Mazoyer E, Bal dit Sollier C, Vanhove N. Plasma gamma′/gamma fibrinogen ratio, a marker of arterial thrombotic activity: a new potential cardiovascular risk factor? Blood Coagul Fibrinolysis. 1999;10(Suppl 1):S35–39. [PubMed] [Google Scholar]
- 11.Alexander KS, Madden TE, Farrell DH. Association between gamma′ fibrinogen levels and inflammation. Thromb Haemost. 2011;105:605–609. doi: 10.1160/TH10-09-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falls LA, Farrell DH. Resistance of gammaA/gamma′ fibrin clots to fibrinolysis. J Biol Chem. 1997;272:14251–14256. doi: 10.1074/jbc.272.22.14251. [DOI] [PubMed] [Google Scholar]
- 13.Cooper A, Standeven K, Ariens R. Fibrinogen gamma-chain splice variant gamma′ alters fibrin formation and structure. Blood. 2003;102:535–540. doi: 10.1182/blood-2002-10-3150. [DOI] [PubMed] [Google Scholar]
- 14.Collet JP, Nagaswami C, Farrell DH, Montalescot G, Weisel JW. Influence of gamma′ fibrinogen splice variant on fibrin physical properties and fibrinolysis rate. Arterioscler Thromb Vasc Biol. 2004;24:382–386. doi: 10.1161/01.ATV.0000109748.77727.3e. [DOI] [PubMed] [Google Scholar]
- 15.Siebenlist KR, Mosesson MW, Hernandez I, Bush LA, Di Cera E, Shainoff JR, Di Orio JP, Stojanovic L. Studies on the basis for the properties of fibrin produced from fibrinogen-containing gamma′ chains. Blood. 2005;106:2730–2736. doi: 10.1182/blood-2005-01-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appiah D, Schreiner PJ, MacLehose RF, Folsom AR. Association of Plasma gamma′ Fibrinogen With Incident Cardiovascular Disease: The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb Vasc Biol. 2015 doi: 10.1161/ATVBAHA.115.306284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uitte de Willige S, Standeven KF, Philippou H, Ariëns RA. The pleiotropic role of the fibrinogen gamma′ chain in hemostasis. Blood. 2009;114:3994–4001. doi: 10.1182/blood-2009-05-217968. [DOI] [PubMed] [Google Scholar]
- 18.Farrell DH. gamma′ Fibrinogen as a novel marker of thrombotic disease. Clin Chem Lab Med. 2012;50:1903–1909. doi: 10.1515/cclm-2012-0005. [DOI] [PubMed] [Google Scholar]
- 19.Korte W, Feldges A. Increased prothrombin activation in a patient with congenital afibrinogenemia is reversible by fibrinogen substitution. Clin Investig. 1994;72:396–398. doi: 10.1007/BF00252836. [DOI] [PubMed] [Google Scholar]
- 20.Dupuy E, Soria C, Molho P, Zini JM, Rosenstingl S, Laurian C, Bruneval P, Tobelem G. Embolized ischemic lesions of toes in an afibrinogenemic patient: possible relevance to in vivo circulating thrombin. Thromb Res. 2001;102:211–219. doi: 10.1016/s0049-3848(01)00247-x. [DOI] [PubMed] [Google Scholar]
- 21.de Bosch NB, Mosesson MW, Ruiz-Saez A, Echenagucia M, Rodriquez-Lemoin A. Inhibition of thrombin generation in plasma by fibrin formation (antithrombin 1) Thromb Haemost. 2002;88:253–258. [PubMed] [Google Scholar]
- 22.Seegers WH, Nieft M, Loomis EC. Note on the adsorption of thrombin on fibrin. Science. 1945;101:520–521. doi: 10.1126/science.101.2629.520. [DOI] [PubMed] [Google Scholar]
- 23.Meh DA, Siebenlist KR, Brennan SO, Holyst T, Mosesson MW. The amino acid sequence in fibrin responsible for high affinity thrombin binding. Thrombosis and Haemostasis. 2001;85:470–474. [PubMed] [Google Scholar]
- 24.Fredenburgh JC, Stafford AR, Leslie BA, Weitz JI. Bivalent binding to gammaA/gamma′-fibrin engages both exosites of thrombin and protects it from inhibition by the antithrombin-heparin complex. J Biol Chem. 2008;283:2470–2477. doi: 10.1074/jbc.M707710200. [DOI] [PubMed] [Google Scholar]
- 25.Lovely RS, Boshkov LK, Marzec UM, Hanson SR, Farrell DH. Fibrinogen gamma′ chain carboxy terminal peptide selectively inhibits the intrinsic coagulation pathway. Br J Haematol. 2007;139:494–503. doi: 10.1111/j.1365-2141.2007.06825.x. [DOI] [PubMed] [Google Scholar]
- 26.Omarova F, Uitte De Willige S, Ariëns RA, Rosing J, Bertina RM, Castoldi E. Inhibition of thrombin-mediated factor V activation contributes to the anticoagulant activity of fibrinogen gamma′. J Thromb Haemost. 2013;11:1669–1678. doi: 10.1111/jth.12354. [DOI] [PubMed] [Google Scholar]
- 27.Omarova F, Uitte de Willige S, Simioni P, Ariëns RA, Bertina RM, Rosing J, Castoldi E. Fibrinogen gamma′ increases the sensitivity to activated protein C in normal and factor V Leiden plasma. Blood. 2014;124:1531–1538. doi: 10.1182/blood-2014-02-554055. [DOI] [PubMed] [Google Scholar]
- 28.Muthard RW, Welsh JD, Brass LF, Diamond SL. Fibrin, gamma′-fibrinogen, and transclot pressure gradient control hemostatic clot growth during human blood flow over a collagen/tissue factor wound. Arterioscler Thromb Vasc Biol. 2015;35:645–654. doi: 10.1161/ATVBAHA.114.305054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosesson MW, Cooley BC, Hernandez I, Diorio JP, Weiler H. Thrombosis risk modification in transgenic mice containing the human fibrinogen thrombin-binding gamma′ chain sequence. J Thromb Haemost. 2009;7:102–110. doi: 10.1111/j.1538-7836.2008.03213.x. [DOI] [PubMed] [Google Scholar]
- 30.Rein-Smith CM, Anderson NW, Farrell DH. Differential regulation of fibrinogen gamma chain splice isoforms by interleukin-6. Thromb Res. 2013;131:89–93. doi: 10.1016/j.thromres.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uitte de Willige S, de Visser MC, Houwing-Duistermaat JJ, Rosendaal FR, Vos HL, Bertina RM. Genetic variation in the fibrinogen gamma gene increases the risk for deep venous thrombosis by reducing plasma fibrinogen gamma′ levels. Blood. 2005;106:4176–4183. doi: 10.1182/blood-2005-05-2180. [DOI] [PubMed] [Google Scholar]
- 32.Mosesson MW, Hernandez I, Raife TJ, Medved L, Yakovlev S, Simpson-Haidaris PJ, Uitte de Willige S, Bertina RM. Plasma fibrinogen gamma′ chain content in the thrombotic microangiopathy syndrome. J Thromb Haemost. 2007;5:62–69. doi: 10.1111/j.1538-7836.2006.02270.x. [DOI] [PubMed] [Google Scholar]


