Abstract
Although it is well established that Bipolar Disorder (BD) is characterized by excessive positive emotionality, the cognitive and neural processes that underlie such responses are unclear. We addressed this issue by examining the role that an emotion regulatory process called self-distancing plays in two potentially different BD phenotypes—BD with vs. without a history of psychosis—and healthy individuals. Participants reflected on a positive autobiographical memory and then rated their level of spontaneous self-distancing. Neurophysiological activity was continuously monitored using electroencephalogram. As predicted, participants with BD who have a history of psychosis spontaneously self-distanced less and displayed greater neurophysiological signs of positive emotional reactivity compared to the other two groups. These findings shed light on the cognitive and neural mechanisms underlying excessive positive emotionality in BD. They also suggest that individuals with BD who have a history of psychosis may represent a distinct clinical phenotype characterized by dysfunctional emotion regulation.
Keywords: Bipolar Disorder, self-distancing, emotion regulation, psychosis history
The core clinical features for Bipolar Disorder (BD) involve difficulties regulating positive emotions, including periods of mania characterized by persistent and abnormally elevated positive mood associated with significant impairment (American Psychiatric Association, 2013; Angst, Stassen, Clayton, & Angst, 2002). Empirical models of BD suggest that the inability to effectively regulate positive emotions may play a critical role in the onset and maintenance of BD (Gruber, Eidelman, Johnson, Smith, & Harvey, 2011; Johnson, 2005). Yet, surprisingly few studies have examined the cognitive and neurophysiological mechanisms associated with positive emotion regulation in BD using carefully controlled laboratory paradigms.
The current research explored this issue in two ways. First, using a combination of self-report and neural measures, it examined whether individuals with BD differ from healthy individuals in their tendency to spontaneously engage in a widely studied emotion regulatory process referred to as self-distancing (Ayduk & Kross, 2010a; Kross, 2009; Kross & Ayduk, 2011), when they reflect on positive autobiographical memories. Second, it examined whether a history of psychosis influences the ability of people with BD to regulate positive emotions spontaneously—in particular, their ability to spontaneously self-distance while reflecting on positive autobiographical memories.
Self-Distancing as an Emotion Regulatory Process
Converging evidence indicates that people can reflect on emotional memories from different vantage points, which directly influence the type of information that becomes accessible and the degree of emotional reactivity they display (Kross, Ayduk, & Mischel, 2005; Nigro & Neisser, 1983; Robinson & Swanson, 1993). For example, when people reflect on emotional memories, they typically adopt a self-immersed perspective in which they visualize their experience happening all over again through their own eyes. However, it is also possible for people to analyze their experiences by adopting a self-distanced perspective in which they see themselves in the event from afar, akin to a “fly on the wall” peering down on the scene.
A number of recent studies performed with healthy children (Kross, Duckworth, Ayduk, Tsukayama, & Mischel, 2011), adult (Kross, 2009; Kross & Ayduk, 2011), sub-clinical (Kross & Ayduk, 2009; Wisco & Nolen-Hoeksema, 2011), and clinical (Kross, Gard, Deldin, Clifton, & Ayduk, 2012) populations indicate that people who adopt a self-distanced (vs. self-immersed) perspective—either because they are instructed to do so in the context of an experiment or because they spontaneously engage in this process—are buffered against the harmful consequences of analyzing negative experiences. That is, they display lower levels of negative emotional and physiological reactivity, and engage less in maladaptive rumination (for reviews, see Ayduk & Kross, 2010a; Kross, 2009; Kross & Ayduk, 2011).
Recent work has extended these findings by examining the role that self-distancing plays in positive emotion regulation. In one line of work, Verduyn and colleagues (2012) used experience sampling methods with an unselected sample of young adults to examine the relationship between spontaneous self-distancing and daily positive mood intensity and duration. Complementing prior research on self-distancing and negative emotion regulation, they found that reflecting on daily positive events from a self-distanced perspective led to shorter and less intense positive mood episodes. More germane to the present study, Gruber and colleagues (2009) found that individuals with BD are capable of reflecting on positive emotional experiences from a self-distanced perspective when they are instructed to do so. Moreover, they demonstrated that doing so led to reductions in both self-report and physiological positive emotional reactivity. Importantly, in this study the self-distancing manipulation led to equivalent reductions in positive emotional reactivity for both people with BD and healthy individuals, suggesting that self-distancing may play an equally effective role for facilitating positive emotion regulation in both groups.
Taken together, these studies highlight the role that self-distancing plays in allowing normal healthy and clinical populations to reflect adaptively on intense positive and negative emotional experiences in ways that minimize emotional reactivity and duration. However, they leave open an important question concerning whether people with BD display heightened positive emotional reactivity because they fail to spontaneously self-distance sufficiently when reflecting on positive memories. The first goal of this research was to address this issue.
Psychosis and Emotion Regulation in BD
The second goal of this work was to examine whether a history of psychosis influences the tendency of individuals with BD to spontaneously self-distance. Researchers have increasingly begun to examine the role that psychosis history plays in BD to enhance our understanding of the heterogeneity of this disorder in terms of symptom severity, clinical course, and other illness characteristics such as age of onset and comorbidity (Aminoff et al., 2013; Delgado & Chaves, 2013; Mazzarini et al., 2010; Ryu, Song, Hab, Ha, & Cho, 2012).
Approximately 50–70% of people with BD experience psychotic symptoms at some point during their lifetime (Goodwin & Jamison, 1990; Keck et al., 2003). Recent work suggests that those with (vs. without) psychotic symptoms are genetically distinguishable (Pearlson et al., 1995; Potash et al., 2003), and are characterized by more severe illness courses, including earlier illness onset (Bellivier, Golmard, Henry, Leboyer, & Schurhoff, 2001), greater lifetime frequency of manic episodes (Tohen et al., 2003), and slower illness recovery times (Brockington, Hiller, Francis, Helzer, & Wainwright, 1983).
Emerging evidence also suggests that a history of psychosis in BD is linked to a range of neuropsychological deficits that are related to BD, including impaired executive functioning (Glahn et al., 2007), learning (Martinez-Aran et al., 2008), visual processing (Albus et al., 1996), and memory recall (Simonsen et al., 2011) (see Bora, Yücel, & Pantelis, 2010 for a meta-analysis on this topic). With respect to emotion regulation in BD, this is especially important as known deficits in cognitive functioning (especially executive functioning) impact emotion regulation (Gyurak, Goodkind, Kramer, Miller, & Levenson, 2012; Gyurak et al., 2009).
Despite these findings, relatively little is known about how a history of psychosis influences emotional processing in BD. One recent study (Anticevic et al., 2013) found that individuals with BD show fronto-limbic dysfunction, indexed by reduced connectivity within medial prefrontal cortex (mPFC) and its connectivity abnormalities with limbic structures, which is likely to be responsible for emotion dysregulation in BD (Phillips, Ladouceur, & Drevets, 2008). Importantly, this effect was driven by those with a history of psychosis, suggesting that these individuals may be more vulnerable to emotion dysregulation than those without a history of psychosis. Yet, no research that we are aware of has directly compared these two BD subgroups in their positive emotion regulation capacity or examined the psychological mechanisms that underlie differences in the way they process emotional information.
Our second goal was to address these issues by exploring whether a history of psychosis influences people’s ability to regulate positive emotions via self-distancing. In particular, we explored whether a history of psychosis impairs the capacity of individuals with BD to spontaneously self-distance while reflecting on positive memories compared to those without a history of psychosis and healthy individuals.
Research Overview
We addressed these issues by recruiting individuals with BD I, both with (n = 25) and without (n = 16) a history of psychosis, and healthy control participants (HC; n = 20). To induce positive emotions, we asked participants to reflect on a positive autobiographical memory. We used idiosyncratic memories because the imagery component of emotional memories tend to amplify manic responses in people with BD (Holmes, Geddes, Colom, & Goodwin, 2008; Holmes & Mathews, 2010). We thus expected that asking participants to reflect on positive emotional memories would simulate more closely the types of emotional experiences that are associated with BD in everyday life. While participants reflected on their positive memories, we continuously monitored their brain activity via electroencephalogram (EEG). At the end of the study, we asked participants to rate (a) the extent to which they spontaneously self-distanced while reflecting on their positive memories, and (b) their current level of positive emotions.
Emerging evidence suggests that increased EEG activity in frontal regions of the left (vs. right) hemisphere reflects both a trait and a state-level propensity to engage a stimulus, including heightened positive emotional reactivity (for review, see Coan & Allen, 2004). Importantly, Harmon-Jones and colleagues (2008) found that individuals with BD exhibit increased relative left frontal cortical activation to positive challenges (i.e., performing a challenging task to obtain reward vs. performing the same task to avoid punishment), thereby demonstrating their greater approach motivation to positive stimuli compared to healthy controls. Thus, we focused on relative left frontal activity in this study as a neurophysiological index of positive emotional reactivity.
Method
Participants
Forty-one individuals diagnosed with BD I (BD; 24 women; Mage = 41.49, SDage = 10.23) and 20 healthy controls with no lifetime DSM-IV Axis I diagnosis (HC; 10 women; Mage = 37.10, SDage = 13.23) participated in this study. They were compensated for $15/hour. All participants were right-handed and had normal or corrected-normal vision.
Participants were a subset of those who were recruited for a larger longitudinal study, who provided written consent to participate in future studies. They were recruited through an outpatient specialty psychiatry clinic, an inpatient psychiatric unit, and community advertisements on the web, in the newspaper, on the radio and on billboards. The Diagnostic Interview for Genetic Studies (DIGS; Nurnberger et al., 1994) was administered to confirm BD diagnosis, and no current or past DSM-IV Axis I psychiatric diagnosis of healthy controls. Final diagnoses were determined through a best estimate process, which two psychiatrists and clinical psychologists performed using clinical interviews and, when available, medical record data.1
To evaluate mood state at the time of participation, depressive and manic symptoms were assessed with the Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) and the Altman Self-Rating Mania Scale (ARSM; Altman, Davis, Hedekar, & Peterson, 1997), respectively. For the BD group, 18 (43.9%) were euthymic (BDI < 14; ASRM < 6), nine (22.0%) were hypomanic/manic (BDI < 14; ASRM ≥ 6), eight (19.5%) were depressed (BDI ≥ 14; ASRM < 6), and two (4.9%) were mixed (BDI ≥14; ASRM ≥ 6). The mood state of four BD participants (9.8%) was unknown since two of them did not complete both scales; two additional participants did not complete the ASRM. All healthy controls scored below the clinical cutoffs on both the BDI (< 14; M = .55, SD = 1.23) and ASRM (< 6; M = 1.20, SD = 1.96).
The average age at onset of BD was 17.63 years (SD = 7.50); the average illness duration was 23.85 years (SD = 12.47). Thirty-seven BD participants (90.2%) were taking at least one psychotropic medication (M = 1.98, SD = 1.11), including mood stabilizer (63.4%), antidepressants (61.0%), antipsychotics (39.0%), and lithium (34.1%). As common in individuals with BD, 14 (34.1 %) had at least one additional current comorbid Axis I disorder (M = .41, SD = .59), including panic disorder (12.2%), agoraphobia (2.4%), social phobia (9.8%), specific phobia (4.9%), obsessive-compulsive disorder (4.9%), post-traumatic stress disorder (2.4%), and attention deficit-hyperactivity disorder (2.4%), but for these participants, the primary diagnosis was confirmed as BD I (Di Nardo, Moras, Barlow, Rapee, & Brown, 1993).
Among the BD group, 22 (58.5%) had at least one current and/or past diagnosis of substance abuse or dependence (M = 1.28, SD = 1.56), including abuse or dependence of alcohol (39.0%), cannabis (36.6%), cocaine (9.7%), opiate (9.7%), sedative (7.3%), stimulant (2.4%), and others (17.1%). One healthy control participant (5%) had a past diagnosis of nicotine dependence. Five BD participants (12.2%) and one healthy control participant (5%) were current smokers.
The BD group was further categorized into those with (i.e., psychotic BD; n = 25; 61.0%) or without (i.e., non-psychotic BD; n = 16; 39.0%) a history of psychosis based on the criterion used in the vast majority of the current literature on BD (e.g., Bora et al., 2010; Glahn et al., 2007; Savitz, van der Merwe, Stein, Solms, & Ramesar, 2009). Specifically, the lifetime history of experiencing psychosis such as hallucinations, delusions, or grossly disorganized thoughts or behaviors was assessed through the structured diagnostic interview (DIGS) and review of medical records when available, and confirmed through the best estimate process by two doctoral level clinicians. When the best estimators were not certain about the presence of psychosis history, we categorized participants based on their self-reported experience of psychotic symptoms assessed during the DIGS interview. There were three such BD participants who endorsed experiencing psychotic symptoms. These participants were included in the psychotic BD group for further analyses.2 Among the 25 BD participants with a history of psychosis, 14 (56.0%) experienced hallucinations, 19 (76.0%) experienced delusions, and two (8.0%) experienced grossly disorganized thoughts and behaviors. None of the BD participants had chronic psychosis or psychosis outside of two weeks of each mood episode.
BD participants with and without a history of psychosis did not differ in any of their clinical and health characteristics, including their age at onset of BD, illness duration, number of medications, comorbidity, past or current diagnoses of substance abuse/dependence, and smoking status, ps > .28, except that those with a history of psychosis were less depressed than those without a history of psychosis (BDI scores: 7.71 vs. 13.47), F(1, 37) = 7.16, p < .05, ηp2 = .16. The subgroups did not differ in their manic symptoms (ASRM scores: 4.40 vs. 2.93), F(1, 35) = 1.58, p = .22. Table 1 illustrates demographic variables and clinical and health characteristics of the study participants.
Table 1.
Demographic variables, current mood state, and clinical and health characteristics of study participants
| Psychotic BD (n = 25)
|
Non-psychotic BD (n = 16)
|
HC (n = 20)
|
Group Difference
|
|||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F or X2 statistics | p-value | |
| Demographic variables | ||||||||
| Age | 39.48a | 11.31 | 44.63a | 7.56 | 37.10b | 13.23 | F(2, 58) = 2.07 | .07 |
| Gender (n = females) | 15a | 9a | 10a | χ2(2) = .45 | .80 | |||
| Education (years) | 16.00a | 2.11 | 14.87a | 1.92 | 16.35a | 2.68 | F(2, 56) = 1.93 | .16 |
| Parental education (years) | 14.68a | 2.94 | 15.31a | 2.80 | 15.85a | 3.22 | F(2, 58) = .85 | .43 |
|
| ||||||||
| Current mood state | ||||||||
| Beck Depression Inventory | 7.71a | 6.38 | 13.47b | 6.79 | .55c | 1.23 | F(2, 56) = 25.50 | < .001 |
| Altman Self-Rating Mania Scale | 4.41a | 3.94 | 2.93a | 2.74 | 1.20b | 1.96 | F(2, 54) = 5.79 | < .01 |
| Mood based subgroups (n) | ||||||||
| Euthymic | 11a | 7a | 20b | χ2(2) = 15.45 | < .001 | |||
| Depressed | 3a | 7b | 0c | χ2(2) = 13.28 | = .001 | |||
| Hypomanic/Manic | 9a | 2a | 0b | χ2(2) = 11.72 | < .01 | |||
| Mixed | 1a | 1a | 0a | χ2(2) = 1.24 | .54 | |||
| Missing | 3a | 1a | 0a | χ2(2) = 2.62 | .27 | |||
|
| ||||||||
| Clinical and health characteristics | ||||||||
| Substance abuse/dependence (n) | ||||||||
| Past diagnosis | 13a | 7a | 1b | χ2(2) = 12.58 | < .01 | |||
| Current diagnosis | 6a | 6a | 0b | χ2(2) = 9.01 | < .05 | |||
| Smoking status (n = smokers) | 2a | 3a | 1a | χ2(2) = 2.26 | .32 | |||
| Age at onset of BD | 17.32a | 7.72 | 18.13a | 7.37 | NA | F(1, 39) = .11 | .74 | |
| Illness duration | 22.16a | 14.17 | 26.50a | 9.00 | NA | F(1, 39) = 1.19 | .28 | |
| Comorbidity (n) | 9a | 5a | NA | χ2(1) = .07 | .79 | |||
| Medication use (n) | 21a | 16a | NA | χ2(1) = 2.84 | .09 | |||
| Psychotic experiences (n) | ||||||||
| None | 0 | 16 | NA | |||||
| During depressed episode | 1 | 0 | NA | |||||
| During mania episode | 12 | 0 | NA | |||||
| During mixed episode | 9 | 0 | NA | |||||
| Uncertain when it happened | 3 | 0 | NA | |||||
Note. Numbers that share subscripts are not significantly different from one another.
Procedure
The study proceeded in seven phases
Phase 1: Baseline affect
First, participants rated how happy they felt “right now” (1 = very unhappy, 9 = very happy; M = 6.36, SD = 1.34) using the valence subscale of the Self-Assessment Mannequin (SAM; Bradley & Lang, 1995).
Phase 2: Baseline EEG
Next, participants were told that their brain activity would be non-invasively monitored using EEG. After the attachment of the electrodes, baseline EEG was recorded during both an eyes-open and an eyes-closed resting period for 3 min each, with the order of the two counterbalanced across participants (see Physiological Recording and Processing section for more details on EEG recording).
Phase 3: Positive memory reflection task
After the baseline EEG recording, participants completed a positive memory reflection task while we continuously monitored their EEG activity. Following a modified version of established procedures (Ayduk & Kross, 2010b; Grossmann & Kross, 2010), participants were asked to recall an experience from their past in which they felt extreme joy and happiness (Recall Time: M = 34.22s, SD = 68.72). Next, they were asked to reflect on the emotions they experienced during the event for 90s (Reflection1). To allow a wider window of brain responses during the reflection period, we extended the standard reflection period duration by another 90s. During the second reflection phase (Reflection2), participants were asked to continue focusing on the causes and reasons underlying their feelings surrounding the event for another 90s (see Appendix 1 for the instructions).
Appendix 1.
Verbatim instructions used in the positive memory reflection task
| Phase | Instructions |
|---|---|
| Opening | Welcome to the study. This recording that you are listening to has been designed to guide you through this session. The study that you’re about to participate in is about feelings, memory, and language. It focuses on the interaction between emotions and the semantics of sentences. We are especially interested in how language and feelings interact in different people. Throughout the course of this study, we will be asking you questions that have to do with feelings and providing you with instructions regarding how to think about experiences from your past. |
| It is important that you do your best to follow the instructions you receive throughout this study to the best of your ability. Although you may be asked to think about feelings and memories in ways that you are not accustomed to, the validity of our research depends on your cooperation in following the exact instructions you receive as best as you can. | |
| If you have any questions at this point, please signal the experimenter. If not, sit back and listen to the following instructions. Press the space bar to continue. | |
| Recall | We will now ask you to think about a time from your past in which you felt happy. Although people experience a variety of positive and negative events in their lives, there are times when they experience extreme happiness. Times in which they are overwhelmed with joy and positivity. Take a few moments right now to think about times from your past that makes you feel happy when you think about them now. As you do this, try to identify a specific experience that makes you feel overwhelmed with happiness when you think about it now. Although it may be difficult, most people can usually remember at least one incident. Take your time as you try to do this. Press the space bar when you are ready to continue. Now close your eyes. Go back to the time and place of the experience you just recalled and see the scene in your mind’s eye. Take a few moments to do this. When you’re ready to continue press the space bar. |
| Reflection1 (90s) | As you continue to think about this experience, try to understand your feelings. Why did you have those feelings? What were the underlying causes and reasons? Take a few moments to do this. We will continue in 90 seconds. |
| Reflection2 (90s) | Please continue to think about why you experienced the feelings you did during the situation you recalled. What were the underlying causes and reasons? Take a few moments to continue doing this. We will continue with the final phase of the study in 90 seconds. |
Phase 4: Spontaneous self-distancing
Following prior research (Kross et al., in press; Mischowski, Kross, & Bushman, 2012), two items were used to assess spontaneous self-distancing. First, participants rated the extent to which they adopted the perspective of an immersed participant (i.e., “saw the event replay through my own eyes, as if I were right there…”) versus a distanced observer (i.e., “watched the event unfold from the perceptive of an observer, in which I could see myself from afar…”) as they pondered their deepest thoughts and feelings during the task (1 = predominantly immersed participant, 7 = predominantly distanced observer). Next, they rated how far they were from the scene in their mind’s eye during the task (1 = very close, saw it through my own eyes, 7 = very far, saw it as if an observer). These ratings were averaged to create a single self-distancing index (α = .90; M = 3.00, SD = 1.52). Since self-distancing scores were significantly non-normal, [D(61) = .17, p < .001, Kolmogorov-Smirnov test], they were log-transformed.
Phase 5: Self-reported positive emotion
Subsequently, participants completed three positive emotion measures. First, participants again rated how happy they felt “right now” using the SAM (1 = very unhappy, 9 = very happy; M = 6.85, SD = 1.38). Second, participants’ global positive affect was assessed with the positive affect (PA) subscale of the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988). Participants rated on a 5-point scale (1 = very slightly or not at all, 5 = extremely) the extent to which they felt 10 positive emotions (e.g., excited, active; α = .85; M = 3.02, SD = .77). Third, following prior work (Ayduk & Kross, 2010b; Grossmann & Kross, 2010), to directly examine emotional reactivity surrounding participants’ recalled experiences, their agreement (1 = strongly disagree, 7 = strongly agree) with the following two items were averaged to create an index of event-specific emotional reactivity: “I re-experienced the emotions I originally felt during the event when I thought about it now,” and “As I think about this event now, my emotions and physical reactions are still intense” (α = .90; M = 4.77, SD = 1.35).
Phase 6: Stream of thoughts
Next, participants were asked to describe in writing the thoughts and feelings that flowed through their mind as they thought about their positive experience during the memory reflection task to examine whether participants recalled qualitatively different types of positive memories or memories that varied in their degree of positive emotional content. Sample essays are presented in Appendix 2. We analyzed the essays in two ways. First, following a modified version of established procedures (Ayduk & Kross, 2010b; Grossmann & Kross, 2010; Kross et al., 2005), the essays were rated by two independent coders who were blind to each participant’ diagnosis in terms of the extent to which positive emotions were described in each essay (0 = not at all, 3 = a great deal). The two coders’ ratings were averaged to create a single index of emotionality (ICC = .78; M = 2.19, SD = 1.00). Second, the essays were also analyzed with the Linguistic Inquiry and Word Count (LIWC; Pennebaker, Booth, & Francis, 2007), which yielded the percentage of positive emotion words participants wrote (e.g., happy, excided; M = 10.82, SD = 15.24).
Appendix 2.
Sample essays about positive memories
| Phase | Instructions |
|---|---|
| Participant A: BD participant with a history of psychosis | I though of my pet Colic and the years of love, devotion, companionship, and being a best friend I got from having him. We were inseparable for years. He was the best pet I ever had and a better companion than some people I knew. He was very intelligent and very protective of me, and loved him so much I could never replace him. |
| Participant B: BD participant without a history of psychosis | How, as a child, I loved staying at our grandparents' farm. I felt much love and good there, I loved being outside with the sheep, pigs and cows. I loved walking to the woods. I loved the outside sights and smells and the chatter of the birds. I loved most in the world my grandma and grandpa who loved me for me and loved me unconditionally. I loved how grandma was patient with craft projects or teaching me to crochet. I loved grandma's cooking. |
| Participant C: Healthy control participant | My thoughts returned to how nice it was to feel loved and accepted by someone I loved. I felt very happy to think that someone who I enjoyed so much would want to spend the rest of his life with me. I felt also confident that I could sustain the relationship because of all the weird interests we shared, and was largely unconcerned that I would get bored over the long term. |
Phase 7: Positive memory characteristics
Previous research indicates that the farther in the past a memory occurred, the more people self-distance and the less emotion they display when they think about it (Nigro & Neisser, 1983; Robinson & Swanson, 1993). Therefore, following prior work (Ayduk & Kross, 2010b; Park, Ayduk, & Kross, 2013), we asked participants to indicate when their experience occurred (memory age: M = 3699.03 days, SD = 4384.83), and controlled for this variable in all analyses.
Physiological Recording and Processing
EEG was recorded with 32 electrodes placed in a nylon cap according to the International 10/20-System with FCz as a common ground reference. The electrooculogram (EOG) was recorded from two additional channels placed beneath the left eye and at Fp1, respectively. EEG and EOG signals were amplified with a band-pass of 0.01 to 30 Hz by BrainCap MR-32 system (Brain Products GmbH, Germany) and sampled with 512 Hz. Impedance for all electrodes was kept below 5kΩ. All data were re-referenced to the averaged M1 and M2 off-line and resampled at 250 Hz. The data were corrected for ocular artifacts (Gratton, Coles, & Donchin, 1983), and visually inspected to remove artifacts. When artifacts occurred in one channel, data from all channels were removed. All artifact-free epochs with 1s in duration were extracted through a Hamming window with 50% overlap to minimize data loss. The data were then subjected to a fast Fourier transform (FFT) algorithm to calculate the power spectra. These power values were averaged across the 1s epochs.
Recent findings indicate that approach-related tendencies (e.g., enhanced positive emotional reactivity) captured via relative left frontal activity is greater for the alpha2 (10–13 Hz) band (e.g., Pizzagalli, Sherwood, Henriques, & Davidson, 2005). Therefore, we obtained total power within the alpha2 band. The power values (YV2/Hz) were log-transformed to normalize the distributions.
In the baseline EEG recording, we obtained two 3-min trials of resting EEG (eyes-open and eyes-closed). The log-transformed power values were averaged across the two trials using the weighted average following Tomarken and colleagues (1992). Specifically, the power value in each trial was multiplied by the number of artifact-free epochs for that trial (eyes-open: M = 343.15, SD = 22.27; eyes-closed: M = 324.86, SD = 90.00). These values were summed and divided by the total number of artifact-free epochs across trials. The epochs that belong to each phase of the reflection task were averaged to yield a mean power density value for each electrode site (Recall: M = 58.80, SD = 137.41; Reflection1: M = 169.31, SD = 15.06; Reflection2: M = 168.55, SD = 16.50).
Finally, asymmetry indices were computed for each phase (Baseline, Recall, Reflection1, Reflection2) by subtracting the log-transformed alpha power on the left site from the log-transformed alpha power on the right site. Since alpha power is inversely related to brain activity (e.g., Davidson, Chapman, Chapman, & Henriques, 1990), higher numbers in these indices indicate greater left (vs. right) cortical activity. On the basis of past research showing stronger effects at F7/F8 (e.g., Harmon-Jones & Sigelman, 2001), our primary analysis focused on this region, with C3/C4 and P3/P4 as comparison sites.
Results
Attrition
Six participants’ EEG data were not analyzable due to an experimenter error (three psychotic BD participants and three HC participants). All other analyses used the total sample.
Analyses Overview
We first examined whether the entire BD group, regardless of their psychosis history, differed from healthy controls (BD vs. HC) on any of the outcome variables we assessed. We found no group differences, Fs < 1.91, ps > .15. Therefore, we subsequently split participants with BD into two subgroups: psychotic BD and non-psychotic BD—and compared both of these subgroups to each other and the healthy control group using a combination of omnibus ANOVAs and follow-up planned comparisons. This analytic strategy allowed us to test our a priori predictions concerning the role that a history of psychosis plays in BD.
Preliminary Analyses
We conducted a series of preliminary analyses to address potential confounding factors. First, the types of positive memories participants recalled included pleasant memories with friends or family (37.7%), experiences in which participants felt loved by a romantic partner (19.7%), achievement of life goals (9.8%), and others (32.8%, e.g., giving a birth, religious experience). This variable did not differ across the groups, χ2(6) = 5.33, p = .50. Content analyses of participants’ essays based on both the coding data and the linguistic analysis index also confirmed that participants recalled memories that did not vary in their degree of positive emotional content, Fs < 1.88, ps > .16.
Next, we analyzed whether baseline affect, task order, or participants’ gender influenced any of outcome variables. Participants did not differ in their baseline affect across the groups, F < 1, ns, and neither this variable nor the order of the baseline EEG trials (eyes-open first vs. eyes-closed first) interacted with group to predict any outcome variable, Fs < 2.21, ps > .12. However, we found a trend toward a gender effect on self-distancing, with females distancing more than males (3.32 vs. 2.59), F(1, 59) = 3.61, p = .06, ηp2 = .06. Therefore, gender was included as an additional covariate in the analysis involving self-distancing. There was no effect of gender on the rest of outcome variables, Fs < 2.15, ps > .14.
We also examined whether mood symptoms influenced the BD subgroup differences we observed. The two subgroups did not differ on their manic symptoms (ASRM scores; Psychotic BD: 4.40 vs. Non-psychotic BD: 2.93), F(1, 35) = 1.58, p = .22, ns, but BD participants with a history of psychosis were less depressed than those without a history of psychosis (BDI scores; 7.71 vs. 13.47), F(1, 37) = 7.16, p < .05, ηp2 = .16. Neither depressive symptoms nor manic symptoms predicted any of our dependent variables, rs < −.21, ps > .10. In addition, controlling for depressive and/or manic symptoms did not substantively alter any of the results we report.
Finally, the two BD subgroups did not differ on any of their clinical and health characteristics including the number of medications (Psychotic BD: 1.84 vs. Non-psychotic BD: 2.19), F(1, 39) < 1, ns, comorbidity, past or current diagnoses of substance abuse or dependence, or smoking status, χ2s (1) < 1.12, ps > .28, and controlling for these variables did not influence any subgroup differences we report below. Thus, we do not discuss these variables further.
Spontaneous Self-Distancing
We predicted that BD participants would spontaneously self-distance less than healthy controls when they reflected on their positive memories. We further predicted that this group difference would be more pronounced among BD participants with a history of psychosis. To test these predictions, we compared the three groups (Psychotic BD vs. Non-psychotic BD vs. HC) using ANCOVA with group as a between-subjects factor and memory age and gender as covariates.
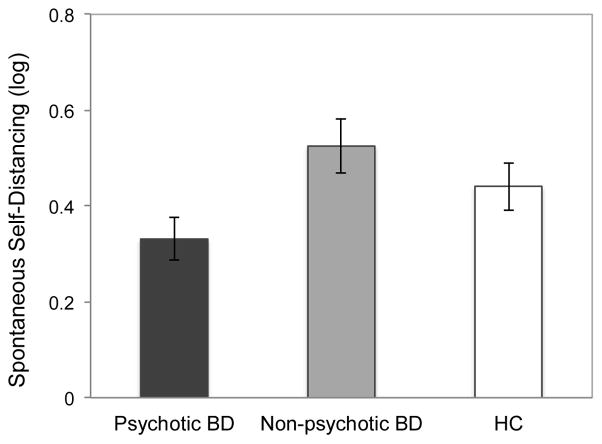
The effect of group was significant, F(2, 56) = 3.86, p < .05, ηp2 = .12. As Fig. 1 illustrates, BD participants with a history of psychosis self-distanced less than both those without a history of psychosis (.33 vs. .53), F(1, 56) = 7.37, p < .01, ηp2 = .12, and healthy controls (.33 vs. .44), F(1, 56) = 2.70, p = .10, ηp2 = .05, though the latter effect fell short of conventional standards of statistical significance. The latter two groups did not differ, F = 1.24, p = .27.
Figure 1.
Group differences in spontaneous self-distancing. Memory age and gender were controlled.
Neural Signals of Emotional Reactivity
Next, we examined whether the pattern of results we observed for spontaneous self-distancing across the three groups was mirrored in the neurophysiological signals of emotional reactivity that we assessed.
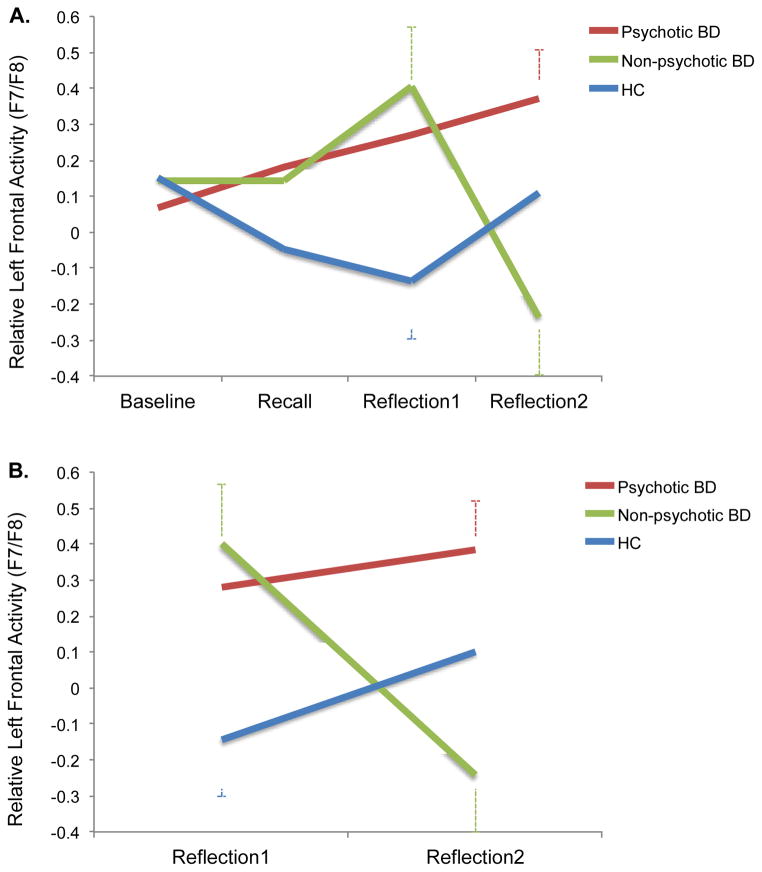
To examine group differences in neurophysiological reactivity, we conducted a 3 (Group) x 4 (Phase: Baseline vs. Recall vs. Reflection1 vs. Reflection2) repeated measures of ANCOVA on the asymmetry index on F7/F8 with group as a between-subjects factor, phase as a within-subjects factor, and memory age as a covariate. There was no main effect of group, F(2, 51) = 1.30, p > .28. But, the Group x Phase interaction was significant, F(6, 153) = 2.49, p < .05, ηp2 = .09. As Fig. 2-1 illustrates, this interaction reflects the fact that the three groups showed distinct profiles of brain responses across the task.3
Figure 2.
Group differences in relative left (vs. right) cortical activity (F7/F8) in the four task phases (A) and the two reflection phases (B). Memory age was controlled for (A), and memory age and baseline EEG were controlled for (B).
Specifically, BD participants with a history of psychosis showed a steady increase in their relative left frontal activity during the task. When they reached the second reflection period, their activity was significantly greater than their baseline (.36 vs. 08), t(20) = 2.05, p = .05, r = .42. In contrast, BD participants without a history of psychosis showed a similar profile of increasing relative left frontal activity until they peaked at the first reflection period, but they then displayed a significant drop during the second reflection period (.37 vs. −.19), t(14) = 3.49, p < .01, r = .68, which resulted in a significant quadratic effect for the Group x Phase interaction, F(2, 51) = 3.15, p = .05, ηp2 = .11. While healthy controls showed a tendency to decrease their relative left frontal activity from recall to the first reflection period (−.04 vs. −.12), this effect as well as the changes in other phases were not significant, ts(15) < 1.63, ps > .12.
Since the group differences in the relative left frontal activities were evident during the two reflection phases [Reflection1: F(2, 51) = 3.07, p = .05, ηp2 = .11; Reflection2: F(2, 51) = 4.21, p < .05, ηp2 = .14], but not during baseline and recall, Fs < 1, ns, we next conducted a 3 (Group) x 2 (Phase: Reflection1 vs. Reflection2) ANCOVA with baseline EEG and memory age as covariates to better understand the group differences during reflection—the critical phase of the experiment.
This analysis revealed a significant Group x Phase interaction, F(2, 50) = 5.52, p < .01, ηp2 = .18 (see Fig. 2-B). This interaction indicated that BD participants without a history of psychosis showed a significant reduction in their activity from the first reflection period to the second reflection period (.37 vs. −.19), t(13) = 3.38, p < .01, r = .68, whereas those with a history of psychosis maintained elevated activity and displayed no change from the first reflection period to the second reflection period (.28 vs. .36), t(19) < 1, ns. During the reflection period overall, healthy controls remained consistently low in their activity compared to BD participants with a history of psychosis (−.02 vs. .33), F(1, 50) = 4.89, p < .05, ηp2 = .09. BD participants without a history of psychosis fell in between the two groups (.08), but they did not significantly differ from the other two, Fs < 2.40, ps > .12.
Additional analyses confirmed that the group differences were evident in frontal sites (F7/F8), consistent with prior work (e.g., Harmon-Jones & Sigelman, 2001).4 There was no comparable effect of Group x Phase interaction in the comparison regions, C3/C4 and P3/P4: Fs < 1.50, ps > .18, which resulted in a significant Group x Phase x Region 3-way interaction effect, F(12, 306) = 1.91, p < .05, ηp2 = .07.
Subjective Indices of Emotional Reactivity
We then examined the effect of group on three emotion measures. First, we performed a 3 (Group) x 2 (Time: Baseline vs. Post reflection task) repeated measures ANCOVA on happiness with group as a between-subjects factor, time of happiness measurement as a within-subjects factor, and memory age as a covariate. This analysis revealed a significant main effect of time, F(1, 57) = 4.59, p < .05, ηp2 = .07. Participants felt happier after recalling their positive memories compared to their baseline (6.80 vs. 6.32), indicating that our affective manipulation was successful. However, contrastingly sharply with the neural data, there was no significant effect of group or its interaction with time, Fs < 1.39, ps > .25. Similarly, global positive affect and event-specific emotional reactivity also did not differ across the groups, Fs < 1, ns.
Spontaneous Self-Distancing and Emotional Reactivity
Finally, we conducted a series of regression analyses to examine the relationship between self-distancing and our neural and self-report measures of emotional reactivity.5 As predicted, self-distancing correlated negatively with all three self-report emotion measures [happiness: b = −.36, t(56) = −2.14, p < .05; global positive affect: b = −.66, t(56) = −2.80, p < .01; event-specific emotional reactivity: b = −.48, t(55) = −3.58, p < .001]. Self-distancing was also negatively related to the relative left frontal activity during the first reflection period, b = −.53, t(50) = −1.77, p = .08, although this relationship fell just short of conventional levels of statistical significance. These findings suggest that the more participants reported spontaneously self-distancing while reflecting on positive memories, the less emotional reactivity they displayed on both neural and self-report measures. There was no significant relationship between self-distancing and the relative left frontal activity during other task phases, ts < 1, ns.
Discussion
The current research examined the role that spontaneous self-distancing plays in healthy individuals and two potentially different types of BD groups—those who have a history of psychosis and those who do not—to shed light on the cognitive and neural processes that underlie positive emotion regulation in BD. It generated two key findings.
First, we found that a history of psychosis modulates the way people with BD process idiosyncratic positive information at both the cognitive and neural levels. Specifically, BD participants with a history of psychosis displayed heightened neurophysiological signs of emotional engagement (i.e., relative left frontal activity) as they reflected on their positive memories compared to both healthy controls and individuals with BD who were not characterized by a history of psychosis. Our analyses of the essays participants wrote to describe their thoughts and feelings regarding their positive memories confirmed that the three groups recalled positive memories that did not vary in their degree of positive emotional content, thereby suggesting that BD participants with a history of psychosis magnified their emotional response to the same type of emotional stimuli compared to the other two groups of participants. These findings are also consistent with previous evidence indicating that people with BD show increased approach motivation towards positive stimuli such as reward, indexed by enhanced relative left frontal activity, compared to healthy individuals (Harmon-Jones et al., 2008). Our work extends this study by suggesting that such a tendency is primarily shown among those with a history of psychosis when they respond to highly idiosyncratic positive emotional memories.
It is important to note that the differences between the two subgroups of BD were not explained by any clinical characteristics or mood symptoms. These findings suggest that a history of psychosis may modulate the way people with BD process not only cognitive information, which has been the focus of prior work, but also emotional information. More generally, they suggest that the psychotic and non-psychotic forms of BD may represent distinct clinical phenotypes that are distinguishable on the basis of neurobiological and genetic factors (Pearlson et al., 1995; Potash et al., 2003; Strasser et al., 2005). Future research is needed to further examine this issue, and is important for refining our understanding of BD.
Second, we found that BD participants without a history of psychosis resembled healthy controls on their spontaneous self-distancing scores, but differed from both healthy controls and people with BD who have a history of psychosis in terms of their neurophysiological reactivity. Specifically, BD participants without a history of psychosis showed a steady increase in their relative left frontal activity during the first phase of the reflection period, reflecting their high levels of emotional engagement (similar to participants with a history of psychosis). However, whereas participants with a history of psychosis continued to show high emotional engagement during the second reflection phase of the task, those without a history of psychosis displayed a sharp reduction in their relative left frontal activity during this phase of the task. It is not clear why those without a history of psychosis showed such a disengagement tendency.6 One interpretation of this finding is that this disengagement tendency reflects the attempt of participants in this group to regulate their positive emotions. This interpretation is consistent with recent evidence suggesting that people with BD who do not have a history of psychosis are characterized by less severe illness course, including fewer lifetime frequency of manic episodes (Tohen et al., 2003) and faster illness recovery (Brockington et al., 1983), compared to those who have a history of psychosis. If true, this would explain why the self-distancing scores of BD participants without a history of psychosis—which were assessed immediately following the second reflection phase—resembled that of healthy controls. This is, of course, a post-hoc speculation—future research is needed to test this idea by assessing self-distancing repeatedly throughout the different phases of the reflection task.
It is important to emphasize that had we not used a methodology that allowed us to examine the temporal dynamics of neural responses, we would not have observed many of the differences we observed between the three groups we examined. This underscores the importance of utilizing continuous measures of psychological and biological activities in BD research as well as clinical psychopathology research more generally.
Given prior research documenting that healthy individuals show increased relative left frontal activity in response to positive stimuli, it would seem puzzling that we did not observe any such responses among our healthy control participants. We speculate that the discrepancy between previous studies and our finding is likely due to the type of positive mood induction we used. We used emotional memories to induce positive emotions because they facilitate mental imagery of emotional scenes (Arntz, de Groot, & Kindt, 2005), which in turn is likely to amplify emotional responses in people with BD (Holmes et al., 2008; Holmes & Mathews, 2010). Conversely, previous studies that have revealed increased relative left frontal activity in healthy individuals typically used emotionally evocative stimuli such as films (Davidson, Ekman, Saron, Senulis, & Friesen, 1990; Ekman, Davidson, & Friesen, 1990; Jones & Fox, 1992; Wheeler, Davidson, & Tomarken, 1993) or images (Davidson, Schaffer, & Saron, 1985; Hagemann, Naumann, Becker, Maier, & Bartussek, 1998) to induce positive emotions, which may have had a stronger effect compared to asking participants to reflect on past emotional memories. Future research should explore this issue further by comparing emotional reactivity in response to different positive mood induction procedures among people with BD (both with and without a history of psychosis) and healthy controls.
Several limitations of the current work should be noted. First, although we observed a consistent pattern of results on the neurophysiological and self-distancing measures we administered, we did not observe a similar set of significant results for self-report emotional reactivity. Though unexpected, the asymmetry we observed between participants’ responses on our physiological and self-report emotional reactivity measures is consistent with research indicating that self-report measures of emotion often do not cohere with physiological responses (e.g., Lang, Bradley, & Cuthbert, 1998; Lang, Greenwald, Bradley, & Hamm, 1993). Further research is needed to more fully understand the dissociation between different types of emotional reactivity measures.
Another issue that was left unaddressed is the cognitive mechanism that differentiates the two BD subgroups, especially during the second reflection phase when they displayed distinct neurophysiological signals. Future research should directly assess self-distancing as well as other potential emotion regulatory processes (e.g., reappraisal or suppression; Gruber, Hay, & Gross, 2013; Werner & Gross, 2010) that BD participants without a history of psychosis may have engaged in during this later stage of emotional processing.
Finally, the current work focused on positive emotion regulation in BD. Thus, it remains unclear whether the failure to spontaneously self-distance when reflecting on negative emotional experiences also plays a role in BD (both with and without a history of psychosis). Although prior research indicates that self-distancing allows people to reflect adaptively over both positive and negative emotional experiences in a variety of samples, including healthy children (Kross et al., 2011), adult (Kross, 2009; Kross & Ayduk, 2011; Verduyn et al., 2012), sub-clinical (Kross & Ayduk, 2009; Wisco & Nolen-Hoeksema, 2011), and clinical (Gruber et al., 2009; Kross et al., 2012) populations, future research is needed to directly test whether people with BD, particularly those who have a history of psychosis, exhibit similar difficulties in engaging in this process while reflecting on negative experiences.
Concluding Remarks
The current findings suggest that individuals with BD who have a history of psychosis may represent a distinct clinical phenotype that is characterized by dysfunctional positive emotion regulation—in particular, the inability to spontaneously self-distance while reflecting on positive emotional memories. Although future research is needed to more fully characterize the cognitive and neural mechanisms that distinguish such individuals from people with BD who do not have a history of psychosis, the current findings suggest that taking the next steps to address this issue is important from both a basic science and clinical perspective.
Acknowledgments
This study was supported by the Heinz C. Prechter Bipolar Research Fund (MGM), the National Center for Research Resources Grant UL1RR024986 (now at the National Center for Advancing Translational Sciences Grant 2UL1TR000433) (MK), the American Foundation for Suicide Prevention young investigator grant YIG-xxxx-00176-1209 (MK), and the Rachel Upjohn Clinical Scholars Award at the University of Michigan.
Footnotes
Two BD participants were not from the longitudinal study. Their diagnoses were confirmed using the Structured Clinical Interview for the DSM-IV, Patient Edition (SCID-I/P; First, Spitzer, Gibbon, & Williams, 1995).
Excluding these three BD participants did not substantially alter the results. Neither the group differences on self-distancing and relative left frontal activity nor the relationships between self-distancing and emotional reactivity indices were influenced by this exclusion.
Drawing from the Gruber et al. (2009), we expected that the way that self-distancing influences positive emotional reactivity should be identical for all three groups. To demonstrate this point, we tested whether self-distancing interacted with group in influencing the neurophysiological signals of positive emotional reactivity. As predicted, self-distancing did not influence the critical Group x Phase 2-way interaction effect [i.e., non-significant Self-distancing x Group x Phase 3-way interaction effect, F(6, 144) < 1, ns].
Additional analyses confirmed that our findings were not limited to the asymmetry index on F7/F8 in the frontal region. The analyses on two additional pairs of frontal site electrodes (FC5/FC6, F3/F4) showed weak but consistent patterns of Group x Phase interaction effects [FC5/FC6: F(6, 153) = 2.34, p < .05, ηp2 = .08; F3/F4: F(6, 153) = 1.51, p = .18, ηp2 = .06]. When we conducted a 3 (Group) x 4 (Phase) x 3 (Region: F7/F8, FC5/FC6, F3/F4) repeated measures ANCOVA, the critical Group x Phase 2-way interaction remained significant, F(6, 153) = 2.99, p < .01, ηp2 = .11, while the Group x Phase x Region 3-way interaction was not significant, F(12, 306) < 1, ns, indicating that the similar patterns of Group x Phase 2-way interaction were observed across the three pairs of frontal site electrodes.
Influence diagnostics based on Cook’s D indicated that there was one influential data point for all analyses except for the model on happiness. Thus, this participant’s data were excluded from the corresponding analyses.
One might argue that people in the non-psychotic BD group displayed the disengagement tendency because they reverted to negative mood processing at the later stage of reflection. To address this issue, we examined whether the two BD subgroups differed on their self-reported negative affect ratings based on the negative affect (NA) subscale of the PANAS (Watson et al., 1988) after they completed the reflection task. If the non-psychotic BD group decreased their relative left frontal activity because they reverted to more negative mood processing during the final reflection stage, then one might expect to see higher negative affect scores in this group compared to the psychotic BD group. Our analysis showed that this was not the case. The two BD subgroups did not significantly differ from each other on their self-reported negative affect (Psychotic BD: 1.30 vs. Non-psychotic BD: 1.52), F(1, 39) = 1.44, p = .24.
J.P. E.K. and O.A. developed the study concept and designed the study. Testing and data collection were performed by L.O. J.C. and M.K. J.P. performed the data analysis and interpretation under the supervision of E.K. J.P. and E.K. drafted the paper, and O.A. J.G. M.M. and P.D. provided critical revisions. All authors approved the final version of the paper for submission.
References
- Albus M, Hubman W, Wahlheim C, Sobizack N, Franz U, Mohr F. Contrasts in neuropsychological test profile between patients with firstepisode schizophrenia and first-episode affective disorders. Acta Psychiatrica Scandinavica. 1996;94:87–93. doi: 10.1111/j.1600-0447.1996.tb09830.x. [DOI] [PubMed] [Google Scholar]
- Altman EG, Davis JM, Hedekar DR, Peterson JL. The Altman Self Rating Mania Scale. Biological Psychiatry. 1997;42:948–955. doi: 10.1016/S0006-3223(96)00548-3. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Aminoff SR, Hellvin T, Lagerberg TV, Berg AO, Andreassen OA, Melle I. Neurocognitive features in subgroups of bipolar disorder. Bipolar Disorders. 2013;15:272–283. doi: 10.1111/bdi.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. Journal of Affective Disorders. 2002;68:167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, Glahn DC. Global prefrontal and fronto-amygdala dysconnectivity in Bipolar I Disorder with psychosis history. Biological Psychiatry. 2013;73:565–573. doi: 10.1016/j.biopsych.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arntz A, de Groot C, Kindt M. Emotional memory is perceptual. Journal of Behavior Therapy and Experimental Psychiatry. 2005;36(1):19–34. doi: 10.1016/j.jbtep.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Ayduk Ö, Kross E. Analyzing negative experiences without ruminating: The role of self-distancing in enabling adaptive self-reflection. Social and Personality Psychology Compass. 2010a;4(10):841–854. [Google Scholar]
- Ayduk Ö, Kross E. From a distance: Implications of spontaneous self-distancing for adaptive self-reflection. Journal of Personality and Social Psychology. 2010b;98(5):809–829. doi: 10.1037/a0019205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bellivier F, Golmard JL, Henry C, Leboyer M, Schurhoff F. Admixture analysis of age at onset in bipolar affective disorder. Archives of General Psychiatry. 2001;58:510–512. doi: 10.1001/archpsyc.58.5.510. [DOI] [PubMed] [Google Scholar]
- Bora E, Yücel M, Pantelis C. Neurocognitive markers of psychosis in bipolar disorder: A meta-analytic study. Journal of Affective Disorders. 2010;127(1–3):1–9. doi: 10.1016/j.jad.2010.02.117. [DOI] [PubMed] [Google Scholar]
- Bradley M, Lang P. Measuring emotion: The Self- Assessment Manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1995;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Brockington IF, Hiller VF, Francis AF, Helzer JE, Wainwright S. Definitions of mania: concordance and prediction of outcome. American Journal of Psychiatry. 1983;140:435–439. doi: 10.1176/ajp.140.4.435. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB. EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Chapman JP, Chapman LJ, Henriques JB. Asymmetrical brain electrical activity discriminates between psychometrically-matched verbal and spatial cognitive tasks. Psychophysiology. 1990;27:528–543. doi: 10.1111/j.1469-8986.1990.tb01970.x. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Ekman P, Saron CD, Senulis JA, Friesen WV. Approach-withdrawal and cerebral asymmetry: emotional expression and brain physiology I. Journal of Personality and Social Psychology. 1990;58:330–341. [PubMed] [Google Scholar]
- Davidson RJ, Schaffer CE, Saron C. Effects of lateralized presentations of faces on self-reports of emotion and EEG asymmetry in depressed and non-depressed subjects. Psychophysiology. 1985;22:353–364. doi: 10.1111/j.1469-8986.1985.tb01615.x. [DOI] [PubMed] [Google Scholar]
- Delgado VB, Chaves ML. Mood congruence phenomenon in acutely symptomatic mania bipolar I disorder patients with and without psychotic symptoms. Cognitive Neuropsychiatry. 2013;18(6):477–490. doi: 10.1080/13546805.2012.744303. [DOI] [PubMed] [Google Scholar]
- Di Nardo PA, Moras K, Barlow DH, Rapee RM, Brown TA. Reliability of the DSM-III-R anxiety disorder categories: using the Anxiety Disorders Schedule – Revised (ADIS-R) Archives of General Psychiatry. 1993;50:251–256. doi: 10.1001/archpsyc.1993.01820160009001. [DOI] [PubMed] [Google Scholar]
- Ekman P, Davidson RJ, Friesen WV. The Duchenne smile: emotional expression and brain physiology II. Journal of Personality and Social Psychology. 1990;58:342–353. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for the DSM-IV Axis I Disorders - Patient Edition (SCID I-P, Version 2.0) Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, Velligan DI. The neurocognitive signature of psychotic bipolar disorder. Biological Psychiatry. 2007;62:910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press; 1990. [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography & Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Grossmann I, Kross E. The impact of culture on adaptive vs. maladaptive self-reflection. Psychological Science. 2010;21(8):1150–1157. doi: 10.1177/0956797610376655. [DOI] [PubMed] [Google Scholar]
- Gruber J, Eidelman P, Johnson SL, Smith B, Harvey AG. Hooked on a feeling: Rumination about positive and negative emotion in inter-episode bipolar disorder. Journal of Abnormal Psychology. 2011;120(4):956–961. doi: 10.1037/a0023667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Harvey AG, Johnson SL. Reflective and ruminative processing of positive emotional memories in bipolar disorder and healthy controls. Behaviour Research And Therapy. 2009;47(8):697–704. doi: 10.1016/j.brat.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Hay AC, Gross JJ. Rethinking emotion: Cognitive reappraisal is an effective positive and negative emotion regulation strategy in Bipolar Disorder. Emotion. 2013 doi: 10.1037/a0035249. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Goodkind MS, Kramer JH, Miller BL, Levenson RW. Executive functions and the down-regulation and up-regulation of emotion. Cognition and Emotion. 2012;26(1):103–118. doi: 10.1080/02699931.2011.557291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A, Goodkind MS, Madan A, Kramer JH, Miller BL, Levenson RW. Do tests of executive functioning predict ability to downregulate emotions spontaneously and when instructed to suppress? Cognitive, Affective & Behavioral Neuroscience. 2009;9(2):144–152. doi: 10.3758/CABN.9.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Becker G, Maier S, Bartussek D. Frontal brain asymmetry and affective style: A conceptual replication. Psychophysiology. 1998;35:372–388. [PubMed] [Google Scholar]
- Harmon-Jones E, Abramson LY, Nusslock R, Sigelman JD, Urosevic S, Turonie L, Fearn M. Effect of bipolar disorder on left frontal cortical responses to goals differing in valence and task difficulty. Biological Psychiatry. 2008;63:693–698. doi: 10.1016/j.biopsych.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Sigelman J. State anger and prefrontal brain activity: Evidence that insult-related relative left prefrontal activation is associated with experienced anger and aggression. Journal of Personality and Social Psychology. 2001;80:797–803. [PubMed] [Google Scholar]
- Holmes EA, Geddes JR, Colom F, Goodwin GM. Mental imagery as an emotional amplifier: application to bipolar disorder. Behaviour Research and Therapy. 2008;46(12):1251–1258. doi: 10.1016/j.brat.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Holmes EA, Mathews A. Mental imagery in emotion and emotional disorders. Clinical Psychology Review. 2010;30(3):349–362. doi: 10.1016/j.cpr.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Johnson SL. Life events in bipolar disorder: Towards more specific models. Clinical Psychology Review. 2005;25:1008–1027. doi: 10.1016/j.cpr.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NA, Fox NA. Electroencephalogram asymmetry during emotionally evocative films and its relation to positive and negative affectivity. Brain and Cognition. 1992;20:280–299. doi: 10.1016/0278-2626(92)90021-d. [DOI] [PubMed] [Google Scholar]
- Keck PE, McElroy SL, Havens JR, Altshuler LL, Noeln WA, Frye MA, Post RM. Psychosis in bipolar disorder: Phenomenology and impact on morbidity and course of illness. Comprehensive Psychiatry. 2003;44(4):263–269. doi: 10.1016/S0010-440X(03)00089-0. [DOI] [PubMed] [Google Scholar]
- Kross E. When self becomes other: Towards an integrative understanding of the processes distinguishing adaptive self-reflection from rumination. Annals of the New York Academy of Sciences. 2009;1167:35–40. doi: 10.1111/j.1749-6632.2009.04545.x. [DOI] [PubMed] [Google Scholar]
- Kross E, Ayduk Ö. Boundary conditions and buffering effects: Does depressive symptomology moderate the effectiveness of distanced-analysis for facilitating adaptive self-reflection? Journal of Research in Personality. 2009;43(5):923–927. doi: 10.1016/j.jrp.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Ayduk Ö. Making meaning out of negative experiences by self-distancing. Current Directions in Psychological Science. 2011;20(3):187–191. [Google Scholar]
- Kross E, Ayduk Ö, Mischel W. When asking “why” does not hurt. Distinguishing rumination from reflective processing of negative emotions. Psychological Science. 2005;16(9):709–715. doi: 10.1111/j.1467-9280.2005.01600.x. [DOI] [PubMed] [Google Scholar]
- Kross E, Bruehlman-Senecal E, Park J, Burson A, Dougherty A, Shablack H, Ayduk O. Self-talk as a regulatory mechanism: How you do it matters. Journal of Personality and Social Psychology. doi: 10.1037/a0035173. in press. [DOI] [PubMed] [Google Scholar]
- Kross E, Duckworth AL, Ayduk Ö, Tsukayama E, Mischel W. The effect of self-distancing on adaptive versus maladaptive self-reflection in children. Emotion. 2011;11(5):1032–1039. doi: 10.1037/a0021787. [DOI] [PubMed] [Google Scholar]
- Kross E, Gard D, Deldin P, Clifton J, Ayduk Ö. “Asking why” from a distance: Its cognitive and emotional consequences for people with Major Depressive Disorder. Journal of Abnormal Psychology. 2012;121(3):559–569. doi: 10.1037/a0028808. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biological Psychiatry. 1998;44:1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Torrent C, Tabares-Seisdedos R, Salamero M, Daban C, Balanza-Martinez V, Vieta E. Neurocognitive impairment in bipolar patients with and without history of psychosis. Journal of Clinical Psychiatry. 2008;69:233–239. doi: 10.4088/jcp.v69n0209. [DOI] [PubMed] [Google Scholar]
- Mazzarini L, Colom F, Pacchiarotti I, Nivoli AMA, Murru A, Bonnin CM, Vieta E. Psychotic versus non-psychotic bipolar II disorder. Journal of Affective Disorders. 2010;126:55–60. doi: 10.1016/j.jad.2010.03.028. [DOI] [PubMed] [Google Scholar]
- Mischowski D, Kross E, Bushman B. Flies on the wall are less aggressive: The effect of self-distancing on aggressive affect, cognition, and behavior. Journal of Experimental Social Psychology. 2012;48:1187–1191. [Google Scholar]
- Nigro G, Neisser U. Point of view in personal memories. Cognitive Psychology. 1983;15(4):467–482. [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, HarkavyFriedman J, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of General Psychiatry research. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Park J, Ayduk O, Kross E. Stepping back to move forward: Expressive writing promotes self-distancing. University of Michigan; 2013. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Wong DF, Tune LE, Ross CA, Chase GA, Links JM, Wagner HN. In vivo D2 dopamine receptor density in psychotic and nonpsychotic patients with bipolar disorder. Archives of General Psychiatry. 1995;52:471–477. doi: 10.1001/archpsyc.1995.03950180057008. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW, Booth RJ, Francis ME. Linguistic Inquiry and Word Count (LIWC2007): A computer-based text analysis program [Computer software] Austin, TX: LIWC.net; 2007. [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13(829):833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness: A Source localization study. Psychological Science. 2005;16:805–813. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Potash JB, Zandi PP, Willour VL, Lan TH, Avramopoulos D, Shugart YY, McInnis M. Suggestive linkage to chromosomal regions 13q31 and 22q12 in families with psychotic Bipolar Disorder. American Journal of Psychiatry. 2003;160:680–686. doi: 10.1176/appi.ajp.160.4.680. [DOI] [PubMed] [Google Scholar]
- Robinson JA, Swanson KK. Field and observer modes of remembering. Memory. 1993;1:169–184. doi: 10.1080/09658219308258230. [DOI] [PubMed] [Google Scholar]
- Ryu V, Song D, Hab R, Ha K, Cho H. Prodromes and coping types in bipolar patients with nonpsychotic or psychotic mania. Comprehensive Psychiatry. 2012;53:732–739. doi: 10.1016/j.comppsych.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Savitz J, van der Merwe L, Stein DJ, Solms M, Ramesar R. Neuropsychological status of bipolar I disorder: impact of psychosis. British Journal of Psychiatry. 2009;194:243–251. doi: 10.1192/bjp.bp.108.052001. [DOI] [PubMed] [Google Scholar]
- Simonsen C, Sundet K, Vaskinn A, Birkenaes AB, Engh JA, Faerden A, Andreassen OA. Neurocognitive dysfunction in bipolar and schizophrenia spectrum disorders depends on history of psychosis rather than diagnostic group. Schizophrenia Bulletin. 2011;37(1):78–83. doi: 10.1093/schbul/sbp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser HC, Lilyestrom J, Ashby ER, Honeycutt NA, Schretlen DJ, Pulver AE, Pearlson GD. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biological Psychiatry. 2005;57:633–639. doi: 10.1016/j.biopsych.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Tohen M, Zarate CAJ, Hennen J, Khalsa HM, Strakowski SM, Gebre-Medhin P, Baldessarini RJ. The McLean-Harvard First-Episode Mania Study: Prediction of recovery and first recurrence. American Journal of Psychiatry. 2003;160:2099–2107. doi: 10.1176/appi.ajp.160.12.2099. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Kinney L. Psychometric properties of resting anterior EEG asymmetry: Temporal stability and internal consistency. Psychophysiology. 1992;29:576–592. doi: 10.1111/j.1469-8986.1992.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Verduyn P, Van Mechelen I, Kross E, Chezzi C, Van Bever F. The relationship between self-distancing and the duration of negative and positive emotional experiences in daily life. Emotion. 2012;12(6):1248–1263. doi: 10.1037/a0028289. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Werner K, Gross JJ. Emotion regulation and psychopathology: a conceptual framework. In: Kring A, Sloan D, editors. Emotion regulation and psychopathology. New York: Guilford Press; 2010. [Google Scholar]
- Wheeler RE, Davidson RJ, Tomarken AJ. Frontal brain asymmetry and emotional reactivity: a biological substrate of affective style. Psychophysiology. 1993;30:82–89. doi: 10.1111/j.1469-8986.1993.tb03207.x. [DOI] [PubMed] [Google Scholar]
- Wisco BE, Nolen-Hoeksema S. Effect of visual perspective on memory and interpretation in dysphoria. Behaviour Research and Therapy. 2011;49(6–7):406–412. doi: 10.1016/j.brat.2011.03.012. [DOI] [PubMed] [Google Scholar]