Abstract
Substituted allylic amines and their derivatives are key structural motifs of many drug molecules and natural products. A general, mild, and practical palladium-catalyzed γ-arylation of tertiary allylic amines, one of the most challenging Heck arylation substrates, has been developed. The γ-arylation products were obtained in excellent regio- and stereo-selectivity. Moreover, novel and potent adenylyl cyclase inhibitors with the potential for treating neuropathic and inflammatory pain have been identified from the γ-arylation products.
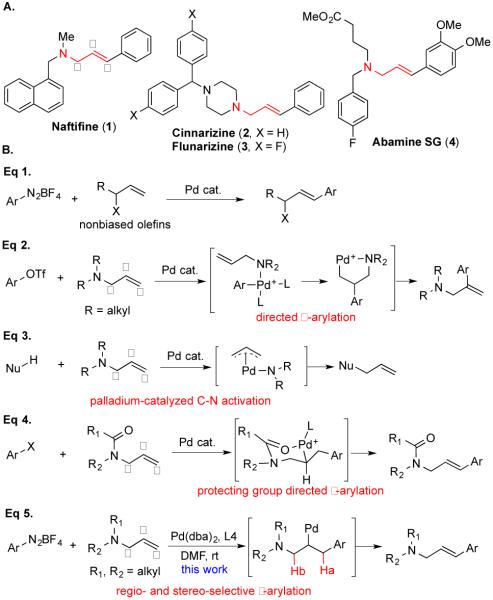
γ-Arylated N,N-dialkylallylamines are ubiquitous structural motifs and have been found widely in biologically active natural products, life-saving drugs, and other molecules of important functions.[1] For example, naftifine (1, Figure 1A) is an allylamine antifungal drug and selectively blocks sterol biosynthesis via inhibition of the squalene 2,3-expoxidase enzyme.[2] Cinnarizine (2) and flunarizine (3) are effective calcium channel blockers and are characterized as antihistamines. They have been widely used for various conditions such as nausea, vertigo, migraine, and vascular diseases.[3] Abamine SG (4) is an abscisic acid biosynthesis inhibitor to target nine-cis-epoxycarotenoid dioxygenase.[4] Various strategies have been developed for the construction of γ-arylated N,N-dialkylallylamine derivatives.[5] One of the most desirable and straightforward ways would be a Heck arylation of N,N-dialkylallylamines at the terminal olefinic carbon (γ-position). In general, Heck arylations work well with activated and biased olefins such as acrylates and styrenes.[6] Recently, significant progresses in the Heck arylation of electronically nonbiased olefins have been made by the groups of Sigman (cf. Eq 1, Figure 1B), Zhou, Jamison, Stahl and others.[7] Because of these elegant studies, regio- and stereo-selective arylation of simple terminal olefins such as allylic alcohols, homoallylic alcohols and other un-activated olefins have become important and powerful synthetic tools in making complex and functional molecules.
Figure 1.
Selected allylic amine drugs, Heck arylation of nonbiased olefins, current status of arylation of allylic amine derivatives and our method.
Despite these significant advancements, stereoselective γ-arylation of N,N-dialkylallylamines persist as a great synthetic challenge due to the following difficulties presented by N,N-dialkylallylamine substrates. (I) The strong basicity and coordinating capability of these tertiary amines make most of the Heck arylation conditions invalid for γ-arylation. For example,Hallberg and Wu have independently reported palladium-catalyzed Heck arylation of N,N-dialkylallylamines and in both cases, β-arylated products were obtained predominantly due to the intrinsic directing ability of the nitrogen atom (Eq 2, Figure 1B).[8] (II) Oxidative addition of palladium (0) catalyst with allylamines to form π-allyl palladium species completes with the Heck arylation, which renders allylamines as allyl group donors (Eq 3, Figure 1B) or an amine source.[8a] Zhang and Tan have independently reported some elegant examples of using allylamines as allyl donors.[9] (III) Selective β-hydride elimination (Ha vs Hb, Eq 5, Figure 1B) to form the desired product remains as an issue as well. In order to get around these intrinsic competing factors and obtain desired γ-arylated products, protecting and directing group strategies have been employed (Eq 4, Figure 1B).[10] The basic nitrogen atoms are masked as amides, carbamates, etc. The protecting groups also serve as directing groups to ensure the arylation takes place at the γ-position. Still, in some of these cases poor regioselectivity or β-arylation were obtained.[11] In addition to the installation of the required protecting and directing groups, extra steps are required to remove them or convert them to the desired alkyl groups. Our recent need of diverse γ-arylated N,N-dialkylallylamines with potential CNS activity[12] (cf. 7aa, Table 1) prompted us to develop a straightforward synthesis of them (Eq 5, Figure 1B). Herein, we report a mild palladium-catalyzed regio- and stereo-selective γ-arylation of N,N-dialkylallylamines and the identification of potent adenylyl cyclase inhibitors with therapeutic potential for treating neuropathic and inflammatory pain from the arylation products.
Table 1.
Condition Optimizationa.
| |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| entry | Pd cat. | x | y | solvent | ligand | base | yield (%)b |
|
| |||||||
| 1 | Pd(dba)2 | 1.5 | 5 | DMF | L1 | - | 54 |
| 2 | Pd(dba)2 | 1.5 | 5 | DMA | L1 | - | 33 |
| 3 | Pd(dba)2 | 1.5 | 5 | DMF | L1 | - | 49c |
| 4 | Pd(dba)2 | 1.5 | 5 | DMF | L1 | Et3N | - |
| 5 | Pd(dba)2 | 1.5 | 5 | DMF | L1 | K2CO3 | - |
| 6 | Pd(dba)2 | 2.0 | 5 | DMF | L1 | - | 73 |
| 7 | Pd(dba)2 | 2.5 | 5 | DMF | L1 | - | 71 |
| 8 | Pd(dba)2 | 2.0 | 8 | DMF | L1 | - | 80 |
| 9 | Pd(dba)2 | 2.0 | 10 | DMF | L1 | - | 85 |
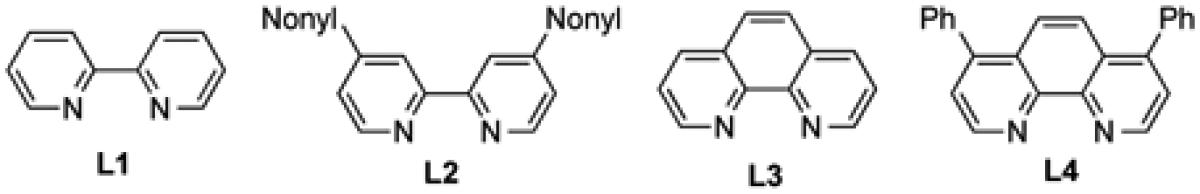
| 10 | Pd(dba)2 | 2.0 | 10 | DMF | L2 | - | 81 |
| 11 | Pd(dba)2 | 2.0 | 10 | DMF | L3 | - | 54 |
| 12 | Pd(dba)2 | 2.0 | 10 | DMF | L4 | - | 90 |
| 13 | Pd(dba)2 | 2.0 | 10 | DMF | - | - | 54 |
| 14 | Pd(OAc)2 | 2.0 | 10 | DMF | L4 | - | 55 |
| 15 | PdCl2 | 2.0 | 10 | DMF | L4 | - | trace |
| 16 | Pd(PPh3)4 | 2.0 | 10 | DMF | L4 | - | trace |
| |||||||
General reaction conditions unless otherwise noted: A mixture of palladium catalyst and ligand in DMF was stirred at rt for 20 min under argon before 5a and 6a were added. The reaction mixture was stirred at rt and monitored by thin-layer chromatography.
Isolated reaction yield.
60°C.
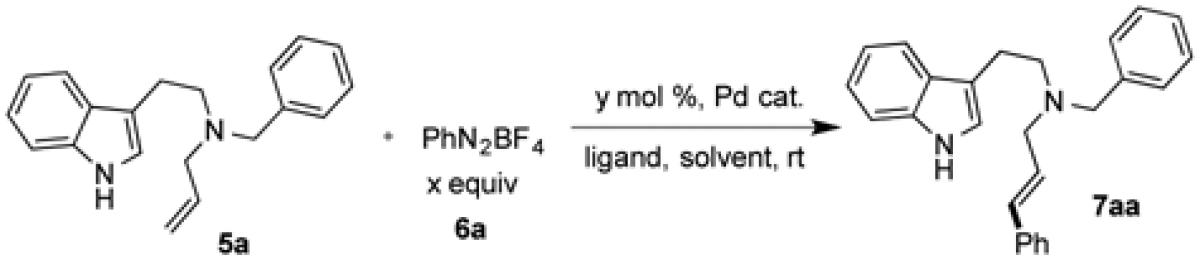
Since aryl diazonium salts have been used frequently in Heck arylation of non-activated olefins,[7d] our investigation started with phenyl diazonium tetrafluoroborate (6a) and a relatively complex and challenging allylic amine substrate, 5a. In order to override the intrinsic coordination of the tertiary nitrogen with palladium center to control the regioselectivity, 2,2’-bipyridine ligand L1 was selected. When 5a (1 equiv.) and 6a (1.5 equiv.) were subjected to the conditions of Pd(dba)2 with L1 (Pd:L = 1:1) at room temperature in DMF, desired product 7aa was obtained in 54% yield. Other solvents such as THF, CH3CN, DMSO, and DMA are inferior to DMF or completely inhibit the reaction (see the Supporting Information). Slightly reduced yield was obtained when the reaction was conducted at an elevated temperature (entry 3). Bases such as triethylamine and K2CO3 shut down the reaction (entry 4-5). Further increase in the amount of 6a or the catalystligand loading has beneficial effect. We also found that among the four nitrogen-containing bidentate ligands tested, L4 gave the best result (entry 12). The reaction yield dropped significantly when several bidentate phosphine ligands were used (see the supporting information). Other palladium catalysts including PdCl2, Pd(PPh3)4 and Pd(OAc)2 were less effective than Pd(dba)2. Overall, under the optimized condition (entry 12), desired product could be produced in 90% yield with excellent regio- and stereo-selectivity.
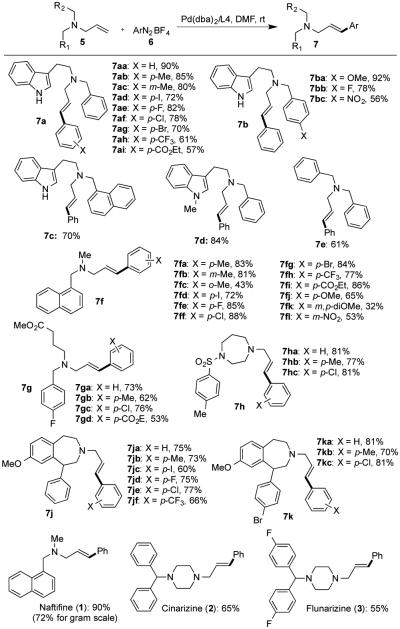
We then evaluated the substrate scope. As summarized in Figure 2, this reaction is very general and compatible with many functional groups including halogens (I, Br, Cl and F), ester groups, amides, nitro group, and indoles. Diazonium salts with both electron withdrawing and electron donating groups are effective coupling partners. A variety of γ-arylated N,N-dialkylallylamines were produced in good to excellent yield, including those derived from azepanes, diazepanes, and piper- azines. Notably, we were able produce naftifine (1) in 90% yield. When reaction was conducted at gram scale, naftifine (1) was obtained at 72% yield. Cinnarizine (2) and flunarizine (3) were synthesized smoothly as well while the corresponding substrates present several challenging factors including two basic nitrogen atoms, potential debenzylation and deallylation reactions.
Figure 2.
Substrate scope.
Additionally, these γ-arylated products could be converted to other useful products as well. For example, amine oxidation followed by [2,3]-sigmatropic rearrangement converted 1 to 11.[13] Dihydroxylation or hydrogenation converted 1 to 12 or 13 respectively in good yields. A one-pot double bond isomerization followed by Picktet-Spengler reaction coverted 7aa to tetrahydro-β-carboline 14.[14] The latter represents the core structure of many bioactive natural products and pharmaceutical molecules.[15]
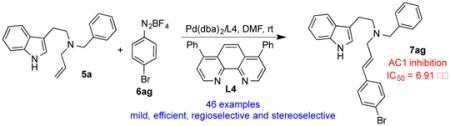
We then evaluated these γ-arylated N,N-dialkylallylamine products for their ability to inhibit adenylyl cyclase activity. Adenylyl cyclases are enzymes that catalyze the production of cAMP from ATP. These enzymes are very important mediators of signaling through G protein-coupled receptors.[16] There are nine different isoforms of membrane-bound adenylyl cyclases, each of which displays unique regulatory properties and expression patterns. Adenylyl cyclase type I (AC1) belongs to a family of adenylyl cyclases that are stimulated by calcium in a calmodulin-dependent manner.[17] Notably, AC1 has been associated with chronic pain responses in several regions of the central nervous system.[18] It has been previously shown that administration of NB001, a small molecule inhibitor of AC1 (IC50 = 10 μM in cell models) leads to analgesic effects in both neuroinflammatory and neuropathic pain rodent models.[19]
To determine whether these γ-arylated products have an inhibitory effect on AC1 activity, HEK (human embryonic kidney) cells stably expressing AC1 (HEK-AC1) were employed and inhibition of Ca2+-stimulated cAMP accumulation was determined. A23187 is a Ca2+ ionophore, which promotes Ca2+ binding to calmodulin in the cells and, subsequent stimulation of AC1. As expected, treatment of HEK-AC1 cells with A23187 led to a robust increase in cAMP accumulation consistent with Ca2+/calmodulin activation of AC1 (data not shown). Several of the γ-arylated N,N-dialkylallylamine products from the 7a-c and 7j series dose-dependently inhibited this cAMP response (Table 2). These compounds displayed IC50 values in the low μM range, with the most potent being 7ag, with an IC50 value of 6.91 (±0.50) μM.
Table 2.
AC1 inhibition.
| Compound | 7ag | 7aa | 7c | 7je |
|---|---|---|---|---|
| IC50 (μM) | 6.91 (±0.50) | 9.92 (±2.28) | 8.95 (±1.55) | 9.10 (±0.82) |
In summary, we have developed a general and practical palladium-catalyzed γ-arylation of tertiary allylic amines. The arylated products were obtained in high regio- and stereoselectivity. We have applied it to prepare several drug molecules including naftifine, cinarizine, flunarizine and their analogs. Moreover, we have identified potent adenylyl cyclase type I inhibitors from the γ-arylation products. These novel AC1 inhibitors are of great potential for treating neuropathic and inflammatory pain. The new synthetic capability developed here will also facilitate the optimization of these lead compounds in terms of potency, isoform selectivity and pharmacological properties.
Supplementary Material
Scheme 1.
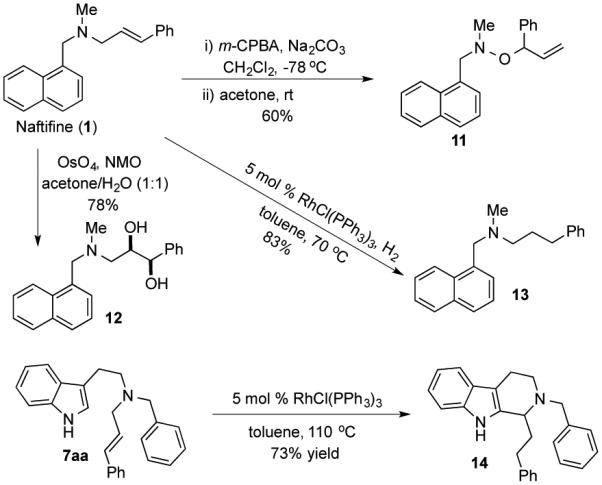
Diversification of the γ-arylation products.
ACKNOWLEDGMENT
We thank Professor Yu Xia and her group at Purdue University for mass spectrometric assistance. M. D. thanks startup support from the Purdue Chemistry Department and Center for Cancer Research. We thank the NIH P30CA023168 for supporting shared NMR resources to Purdue Center for Cancer Research.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Experimental procedures and characterization for new compounds are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interests.
REFERENCES
- (1).Johannsen M, Jørgensen KA. Chem. Rev. 1998;98:1689–1708. doi: 10.1021/cr970343o. For a review. [DOI] [PubMed] [Google Scholar]
- (2).Stütz A, Georgopoulos A, Granitzer W, Petranyi G, Berney D. J. Med. Chem. 1986;29:112–125. doi: 10.1021/jm00151a019. [DOI] [PubMed] [Google Scholar]
- (3).St-Onge M, Dubé P-A, Gosselin S, Guimont C, Godwin J, Archambault PM, Chauny J-M, Frenette AJ, Darveau M, Le Sage N, Poitras J, Provencher J, Juurlink DN, Blais R. Clin. Toxicol. 2014;52:926–944. doi: 10.3109/15563650.2014.965827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Kitahata N, Han S-Y, Noji N, Saito T, Kobayashi M, Nakano T, Kuchitsu K, Shinozaki K, Yoshida S, Matsumoto S, Tsujimoto M, Asami T. Bioorg. Med. Chem. 2006;14:5555–5561. doi: 10.1016/j.bmc.2006.04.025. [DOI] [PubMed] [Google Scholar]
- (5).(a) Xie Y, Hu J, Wang Y, Xia C, Huang H. J. Am. Chem. Soc. 2012;134:20613–20616. doi: 10.1021/ja310848x. For leading references, [DOI] [PubMed] [Google Scholar]; (b) Banerjee D, Junge K, Beller M. Angew. Chem. Int. Ed. 2014;53:1630–1635. doi: 10.1002/anie.201308874. [DOI] [PubMed] [Google Scholar]; (c) Overman LE, Carpenter NE. Org. React. 2005;66:1. For selected reviews. [Google Scholar]; (d) Hartwig JF, Stanley LM. Acc. Chem. Res. 2010;43:1461–1475. doi: 10.1021/ar100047x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ramirez TA, Zhao B, Shi Y. Chem. Soc. Rev. 2012;41:931–942. doi: 10.1039/c1cs15104e. [DOI] [PubMed] [Google Scholar]
- (6).Beletskaya IP, Cheprakov AV. Chem. Rev. 2000;100:3009–3066. doi: 10.1021/cr9903048. For a review. [DOI] [PubMed] [Google Scholar]
- (7).(a) Oestreich M. Angew. Chem. Int. Ed. 2014;53:2282–2285. doi: 10.1002/anie.201310585. For a reveiw. [DOI] [PubMed] [Google Scholar]; (b) Olofsson K, Larhed M, Hallberg A. J. Org. Chem. 1998;63:5076–5079. For examples. [Google Scholar]; (c) Werner E, Mei T-S, Burckle AJ, Sigman MS. Science. 2012;338:1455–1458. doi: 10.1126/science.1229208. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Werner E, Sigman MS. J. Am. Chem. Soc. 2011;133:9692–9695. doi: 10.1021/ja203164p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Werner EW, Sigman MS. J. Am. Chem. Soc. 2010;132:13981–13983. doi: 10.1021/ja1060998. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Hu J, Hirao H, Li Y, Zhou J. Angew. Chem. Int. Ed. 2013;52:8676–8680. doi: 10.1002/anie.201303753. [DOI] [PubMed] [Google Scholar]; (g) Wu C, Zhou J. J. Am. Chem. Soc. 2014;136:650–652. doi: 10.1021/ja412277z. [DOI] [PubMed] [Google Scholar]; (h) Qin L, Ren X, Lu Y, Li Y, Zhou J. Angew. Chem. Int. Ed. 2012;51:5915–5919. doi: 10.1002/anie.201201806. [DOI] [PubMed] [Google Scholar]; (i) Zheng C, Wang D, Stahl SS. J. Am. Chem. Soc. 2012;134:16496–16499. doi: 10.1021/ja307371w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Tasker SZ, Gutierrez AC, Jamison TF. Angew. Chem. Int. Ed. 2014;53:1858–1861. doi: 10.1002/anie.201308391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(a) Olofsson K, Larhed M, Hallberg A. J. Org. Chem. 2000;65:7235–7239. doi: 10.1021/jo000824z. [DOI] [PubMed] [Google Scholar]; (b) Wu J, Marcoux J-F, Davies IW, Reider PJ. Tetrahedron Lett. 2001;42:159–162. [Google Scholar]
- (9).(a) Zhao X, Liu D, Guo H, Liu Y, Zhang W. J. Am. Chem. Soc. 2011;133:19354–19357. doi: 10.1021/ja209373k. [DOI] [PubMed] [Google Scholar]; (b) Li M-B, Wang Y, Tan S-K. Angew. Chem. Int. Ed. 2012;51:2968–2971. doi: 10.1002/anie.201109171. [DOI] [PubMed] [Google Scholar]; (c) Wu X-S, Chen Y, Li M-B, Zhou M-G, Tian S-K. J. Am. Chem. Soc. 2012;134:14694–14697. doi: 10.1021/ja306407x. [DOI] [PubMed] [Google Scholar]
- (10).(a) Prediger P, Barbosa LF, Génisson Y, Correia CRD. J. Org. Chem. 2011;76:7737–7749. doi: 10.1021/jo201105z. For examples: [DOI] [PubMed] [Google Scholar]; (b) Ripin DHB, Bourassa DE, Brandt T, Castaldi MJ, Frost HN, Hawkins J, Johnson PJ, Massett SS, Neumann K, Phillips J, Raggon JW, Rose PR, Rutherford JL, Sitter B, Stewart AM, III, Vetelino MG, Wei L. Org. Process Res. Dev. 2005;9:440–450. [Google Scholar]; (c) Alvisi D, Blart E, Bonini BF, Mazzanti G, Ricci A, Zanti P. J. Org. Chem. 1996;61:7139–7146. doi: 10.1021/jo960301k. [DOI] [PubMed] [Google Scholar]; (d) Dong Y, Busacca CA. J. Org. Chem. 1997;62:6464–6465. [Google Scholar]; (e) Reddington MV, Cunninghan-Bryant D. Tetrahedron Lett. 2011;52:181–183. [Google Scholar]; (f) Cacchi S, Fabrizi G, Goggiamani A, Sferrazza A. Org. Biomol. Chem. 2011;9:1727–1730. doi: 10.1039/c0ob01052a. [DOI] [PubMed] [Google Scholar]; (g) Millet A, Baudoin O. Org. Lett. 2014;16:3998–4000. doi: 10.1021/ol5018257. [DOI] [PubMed] [Google Scholar]
- (11).Olofsson K, Sahlin H, Larhed M, Hallberg A. J. Org. Chem. 2001;66:544–549. doi: 10.1021/jo001416y. [DOI] [PubMed] [Google Scholar]
- (12).Ajay, Bemis GW, Murcko MA. J. Med. Chem. 1999;42:4942–4951. doi: 10.1021/jm990017w. [DOI] [PubMed] [Google Scholar]
- (13).(a) Bao H, Qi X, Tambar UK. J. Am. Chem. Soc. 2011;133:1206–1208. doi: 10.1021/ja110500m. [DOI] [PubMed] [Google Scholar]; (b) Bao H, Qi X, Tambar UK. Synlett. 2011:1789–1792. [Google Scholar]
- (14).(a) Ascic E, Jensen JF, Nielsen TE. Angew. Chem. Int. Ed. 2011;50:5188–5191. doi: 10.1002/anie.201100417. [DOI] [PubMed] [Google Scholar]; (b) Ascic E, Hansen CL, Le Quement ST, Nielsen TE. Chem. Commun. 2012;48:3345–3347. doi: 10.1039/c2cc17704h. [DOI] [PubMed] [Google Scholar]; (c) Hansen CL, Clausen JW, Ohm RG, Ascic E, Le Quement ST, Tanner D, Nielsen TE. J. Org. Chem. 2013;78:12545–12565. doi: 10.1021/jo402192s. [DOI] [PubMed] [Google Scholar]; (d) Cai Q, Liang X-W, Wang S-G, Zhang J-W, Zhang X, You S-L. Org. Lett. 2012;14:5022–5025. doi: 10.1021/ol302215u. [DOI] [PubMed] [Google Scholar]; (e) Cai Q, Liang X-W, Wang S-G, You S-L. Org. Biomol. Chem. 2013;11:1602–1605. doi: 10.1039/c3ob00072a. [DOI] [PubMed] [Google Scholar]
- (15).(a) Stöckigt J, Antonchick AP, Wu F, Waldmann H. Angew. Chem. Int. Ed. 2011;50:8538–8564. doi: 10.1002/anie.201008071. [DOI] [PubMed] [Google Scholar]; (b) Cao R, Peng W, Wang Z, Xu A. Curr. Med. Chem. 2007;14:479–500. doi: 10.2174/092986707779940998. [DOI] [PubMed] [Google Scholar]
- (16).Cooper DM, Crossthwait AJ. Trends Pharmacol. Sci. 2006;27:426–431. doi: 10.1016/j.tips.2006.06.002. [DOI] [PubMed] [Google Scholar]
- (17).Sadana R, Dessauer CW. Neurosignals. 2009;17:5–22. doi: 10.1159/000166277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Zhou M. Drug Discovery Today. 2012;17:573–582. doi: 10.1016/j.drudis.2012.01.009. [DOI] [PubMed] [Google Scholar]
- (19).Wang H, Xu H, Wu LJ, Kim SS, Chen T, Koga K, Descalzi G, Gong B, Vadakkan KI, Zhang X, Kaang BK, Zhuo M. Sci. Transl. Med. 2011;3:65ra3. doi: 10.1126/scitranslmed.3001269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.