Abstract
Background
Cardiac mast cell (MC) proteases, chymase and tryptase, increase proliferation and collagen synthesis in cultured cardiac fibroblasts. However, the question as to why preventing individually the actions of either protease prevents fibrosis when both are released upon MC activation remains unanswered. Since tryptase has the ability to activate MCs in noncardiac tissues via the protease-activated receptor-2 (PAR-2), there is the possibility that its, in vivo, fibrotic role is due to its ability to induce MC degranulation thereby amplifying the release of chymase.
Methods
This study sought to delineate the interactions between tryptase and chymase in myocardial remodeling secondary to transverse aortic constriction (TAC) for 5 wks in male Sprague Dawley rats untreated or treated with either the tryptase inhibitor, nafamostat mesilate or MC membrane stabilizing drug, nedocromil (n=6/group). In addition, ventricular slices from 6 rat hearts were incubated with tryptase, tryptase plus nafamostat mesilate or chymostatin for 24 h.
Results and Conclusion
The results indicate the presence of PAR-2 on MCs and that tryptase inhibition and nedocromil prevented TAC-induced fibrosis and increases in MC density, activation, and chymase release. Tryptase also significantly increased chymase concentration in ventricular tissue culture media, which was prevented by the tryptase inhibitor. Hydroxyproline concentration in culture media was significantly increased with tryptase incubation as compared to the control group and the tryptase group incubated with nafamostat mesilate or chymostatin. We conclude that tryptase contributes to TAC-induced cardiac fibrosis primarily via activation of MCs and the amplified release of chymase.
Keywords: transverse aortic constriction (TAC), protease-activated receptor 2, hydroxyproline, tryptase inhibitor, chymase inhibitor
1. Introduction
Cardiac mast cells (MCs) have been identified as being causally involved in the development of left ventricular (LV) fibrosis associated with hypertension.[1,2] They produce and release a wide variety of proteases, cytokines, growth factors, vasoactive agents and other biologically active mediators that are capable of mediating tissue remodeling. In particular the proteases, chymase and tryptase, have been shown to increase proliferation and collagen synthesis in cultured cardiac fibroblasts.[3,4] Both are stored in a macromolecular complex in MC cytoplasmic secretory granules and are released upon MC activation. Chymase promotes cardiac fibroblast proliferation and collagen synthesis via the TGF-β1/Smad pathway[3] while tryptase increases cardiac fibroblast proliferation and collagen synthesis via a mechanism involving the activation of protease-activated receptor 2 (PAR-2) and subsequent induction of extracellular signal–regulated kinase signaling.[4,5] Chymase inhibition was also reported to markedly attenuate myocardial fibrosis in the cardiomyopathic hamster,[6] in dogs with tachycardia induced heart failure,[7] in the spontaneously hypertensive rat,[8] and in rats with transverse aortic constriction.[9] Similarly, blockade of tryptase with a PAR-2 antagonist prevented fibrosis in the spontaneously hypertensive rat (SHR) heart.[4] However, these findings raise the question as to how preventing individually the actions of either chymase or tryptase can prevent fibrosis when both are released upon activation of MCs and both have been shown to mediate cardiac fibrosis. Given the fact that tryptase has the ability to activate mast cells in noncardiac tissues via PAR-2,[10] there is the possibility that its primary, in vivo, fibrotic role may be via its ability to induce mast cell degranulation thereby inducing the release of chymase. Thus, this study sought to determine the extent to which tryptase influences MC activation and myocardial chymase levels to delineate the interactions between MC tryptase and chymase in myocardial extracellular matrix remodeling. The results indicate that the elevation in chymase levels with transaortic constriction (TAC) is the result of tryptase-induced MC activation. Accordingly, the resulting fibrosis was directly related to tryptase-induced increases in chymase.
2. METHODS
2.1 Animals
The protocol was approved by the Institution’s Animal Care and Use Committee and was in compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Seven week old male Sprague-Dawley rats, purchased from Harlan Laboratories, were housed under standard environmental conditions and maintained on a rodent diet and tap water ad libitum. Anesthesia for all surgical procedures was achieved by an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) and postoperative analgesia was maintained by the administration of buprenorphine hydrochloride (0.025 mg/kg). At the experimental endpoint and under deep anesthesia, a blood sample was obtained and the heart and lungs were removed and the wet weights of the LV+ septum and lungs were obtained. The basal portion of the LV was fixed in 10% formalin for histological analysis and the apical portion was snap frozen in liquid nitrogen and stored at −80 °C for subsequent analysis. The tibia length (TL) was measured and used to normalize tissue weights.
2.2 Transverse aortic constriction and drug treatment
A transverse aortic constriction (TAC) or sham surgical procedure was performed as described elsewhere.[11] Briefly, a medial skin incision from the neck to the upper chest was made and a cranial portion of the sternum opened. This approach allowed for the direct visualization of the transverse aorta without having to enter the pleural space. The transverse aorta between the right innominate and left carotid artery was constricted to the outside diameter of a 22-gauge needle using 6–0 silk suture. Sham surgery was performed without banding the aorta. Three days prior to creation of TAC, groups of rats either remained untreated or were administered nedocromil (Ne), a mast cell stabilizing drug, or nafamostat mesilate (NM), a potent and selective tryptase inhibitor. Ne was delivered at a rate of 30 mg·kg 1·day 1 via time release pellets (Innovative Research of America, Sarasota, FL) that were implanted subcutaneously.[12] NM was dissolved in sterile water and administrated by a daily intraperitoneal injection (5 mg/kg/day).[13] These treatments were continued until animal sacrifice. The animals were grouped as follows: sham operated (Sham, n=6) and transverse aortic constriction with no treatment (TAC, n=6), with Ne treatment (Ne-TAC, n=6) and with NM treatment (NM-TAC, n=6). Five wks after TAC or sham surgery, the rats were sacrificed and tissue and blood samples were collected as described above.
2.3 Transthoracic echocardiography
On the day of sacrifice, the rats were sedated with isoflurane (~ 1.5%) and LV size and function was evaluated echocardiographically using a Vevo 770 High-Resolution Imaging System with a 37.5-MHz high-frequency linear transducer (VisualSonics Inc. Toronto, ON, Canada). LV internal diameter and posterior wall thickness at end-systole and-diastole were measured from short-axis M-mode images recorded at the papillary muscle level. Fractional shortening and ejection fraction were calculated using VisualSonics’ Measurement Software standard formulae.
2.4 LV tissue slice culture
Six, eight week old male Sprague-Dawley rats were deeply anesthetized and a median sternotomy performed aseptically and the heart removed and washed in cold sterile saline. The LV plus septum was separated from the rest of the heart and filled with 2.5% agarose and then placed into a metal cylinder containing agarose. The LV was sliced perpendicular to the long axis (250 to 300 μm in thickness) using a Brendel/Vitron Tissue Slicer (Vitron Organ Slicing Tech., Tuscon, AZ). To determine the effects of tryptase on cardiac mast cell activation and collagen production, 16 to 20 LV slices from each heart were randomly divided into four groups with four or more slices per well: 1) culture media (Waymonth + 10% FBS) alone (Con); 2) culture media with tryptase (Try, 100 mU/ml, Promega, Madison, WI);[14,15] 3) culture media with tryptase and NM (Try-NM, 10 μg/ml);[16] and 4) culture media with tryptase and chymostatin (Try-Ch, 10 μM, MP Biomedicals, Solon, OH).[17] NM and Ch were added to the culture media 30 min prior to addition of tryptase. After 24 h of incubation (37 °C, 95% O2, and 5% CO2), the slices from each well were weighed, snap frozen and stored at −80 °C. The culture media from each well was aliquoted, snap frozen and stored at −80 °C.
2.5 Histology
Formalin-fixed LV tissue was embedded in paraffin and 5 μm thick cross sections were stained with collagen specific picrosirius red.[18] Twenty microscopic fields (100 X) per section devoid of perivascular collagen were imaged. Interstitial collagen volume fraction (CVF) was determined from these images using ImageProPlus 6.0. (Media Cybernetics, Inc., Bethesda, MD) and was expressed as percent of collagen stained area in the field. LV MCs were stained with toluidine blue.[19] MC density in the section was calculated by dividing the number of MCs per section by the section area measured with a calibrated imaging densitometer (Bio-Rad). If one or more extruded granules were visible adjacent to the MC, the cell was considered to be degranulating.[20] The degranulating MCs were counted and divided by the total MC population in the section to yield the percent degranulation. To localize PAR-2 in cardiac MCs, formalin-fixed LV tissue sections from sham rat hearts were immunostained with PAR-2 antibody and mouse ABC staining system (SCBT Inc., Santa Cruz, CA), which was followed by alcian blue staining, a specific stain for MCs.[17]
2.6 Western blot, ELISA and Hydroxyproline measurement
Total protein from LV tissue was extracted using RIPA buffer and a protease inhibitor cocktail (Pierce, Rockford, IL). Protein samples were fractionated by SDS-PAGE and then transferred to nitrocellulose membranes. The membranes were incubated with corresponding primary antibodies against tryptase (abcam, Cambridge MA), and GAPDH (SCBT Inc., Santa Cruz, CA), subsequently incubated with HRP-conjugated secondary antibodies and detected with the ECL Detection Kit (Thermo Scientific, Rockford, IL). The protein expression was quantified with Image J (NIH) and adjusted to GAPDH. Plasma tryptase levels were measured using a rat MC tryptase ELISA kit (Mybiosource, San Diego, CA) and chymase levels in plasma and culture media were measured using a rat chymase ELISA kit (Uscn Life Science Inc, Wuhan, PRC) according to the manufacturer’s instructions. For the measurement of chymase, culture media was concentrated by using centrifugal filter devices (Millipore, Tullagreen, Carrigtwohill Co Cork IRL). Hydroxyproline levels in culture media were measured using a hydroxyproline colorimetric assay kit (Biovision, Milpitas CA).
2.7 Statistical analysis
Results are presented as mean ± SEM. For comparison between groups, one-way analysis of variance (ANOVA) was performed, followed by the Tukey post-hoc test. Statistical significance was set at a P value < 0.05.
3. RESULTS
3.1 Tryptase Inhibition Prevented TAC-Induced LV Hypertrophy and Fibrosis
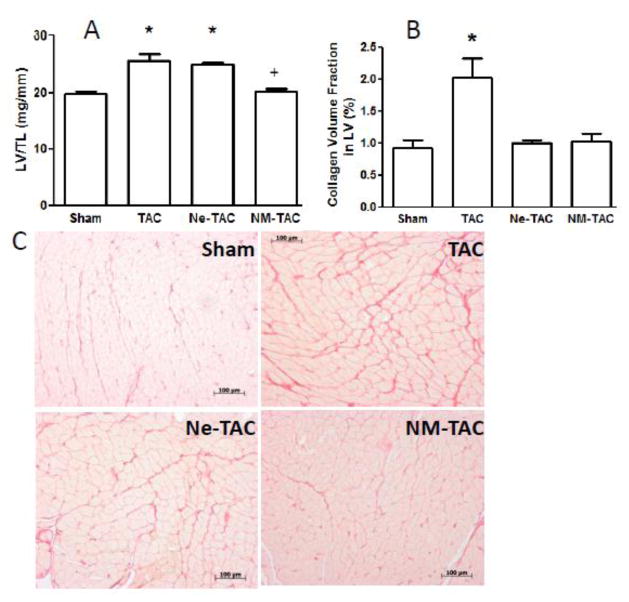
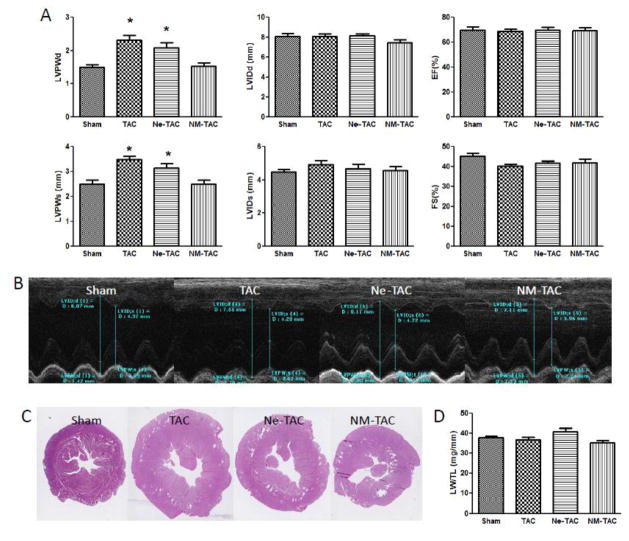
The ability of the tryptase inhibitor, nafamostat mesilate (NM), and MC stabilizing compound nedocromil (Ne) on LV hypertrophy and fibrosis are shown in figures 1 and 2. Included in figure 2 are representative LV echocardiograms taken at the papillary muscle level (Fig 2B) and photomicrographs of LV coronal sections (Fig 2C) 5 weeks post TAC or sham surgery. NM was able to significantly prevent the increase in LV weight normalized to tibial length (Fig 1A) and end-diastolic and end-systolic posterior wall thickness (Fig 2A) that occurred following 5 wks of transverse aortic constriction in the untreated rats. While Ne had no effect on TAC-induced hypertrophy, both NM and Ne were able to prevent the cardiac fibrosis secondary to TAC (Fig 1B). There were no significant group differences in echocardiographically measured LV internal diameter, ejection fraction, and fractional shortening (Fig 2A), and normalized lung weight (Fig 2D) indicating that LV systolic function remained compensated in the untreated and treated TAC groups.
Figure 1.
Transverse aortic constriction (TAC) or sham surgery was performed in 7-wk-old male rats with continuous administration of either nedocromil (Ne) or nafamostat mesilate (NM) initiated 3 days prior to surgery. Group differences in the ratio of left ventricular weight (LVW) to tibia length (TL) and interstitial collagen volume fraction 5 wks after surgery are presented in panels A and B, respectively. Representative microscopic images of myocardium stained with collagen-specific picrosirius red are depicted for the 4 groups in panel C (X100 magnification). All values are mean ± SEM, N=6; *P<0.05 vs. Sham, +P<0.05 vs. TAC and Ne-TAC.
Figure 2.
Transverse aortic constriction (TAC) or sham surgery was performed in 7-week-old male rats with administration of either nedocromil (Ne) or nafamostat mesilate (NM) three days prior to TAC surgery. The changes in LV posterior wall thickness (LVPW) and internal diameter (LVID) at the end-diastole (LVPWd, LVIDd,) and-systole (LVPWs, LVIDs), LV ejection fraction (EF) and fractional shortening(FS), determined by echocardiography, are shown in Panel A. Shown in Panel B and C are the representative LV echocardiograms taken at the papillary muscle level and photomicrographs of left ventricle (LV) coronal section, respectively, 5 weeks post TAC or sham surgery. Panel D shows the ratio of lung weight (LW) to tibia length (TL). All values are mean ± SEM, N=6, *p<0.05 vs. Sham.
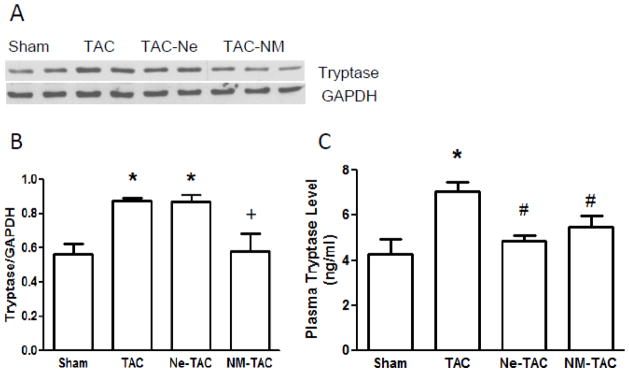
3.2 Tryptase Inhibition Prevented TAC-Increased Myocardial and Plasma Tryptase Protein Content
Myocardial tryptase protein content for the 4 groups, as determined by Western blot (fig 3A), is depicted in Figure 3B. Also, the plasma tryptase levels, which are assumed to include the tryptase released from myocardial MCs, are shown in Figure 3C. As can be seen, TAC significantly increased myocardial tryptase protein content and plasma tryptase level. MC stabilization by Ne prevented TAC-induced elevation of plasma tryptase level but had no effect on tissue tryptase protein content. However, tryptase inhibition by NM prevented both TAC-induced increases in myocardial tryptase protein content and plasma tryptase level, which indicates the presence of a positive feedback loop of mast cell tryptase in the myocardium that was enhanced by TAC.
Figure 3.
Transverse aortic constriction (TAC) or sham surgery was performed in 7-wk-old male rats with continuous administration of either nedocromil (Ne) or nafamostat mesilate (NM) started 3 days prior to surgery. Five wks after surgery, left ventricle tissue tryptase protein content was measured by Western blot (panels A and B). Plasma tryptase levels were measured using an ELISA kit (panel C). All values are mean ± SEM, N=6; *P<0.05 vs. Sham; +P<0.05 vs. TAC and Ne-TAC; #p<0.05 vs. TAC.
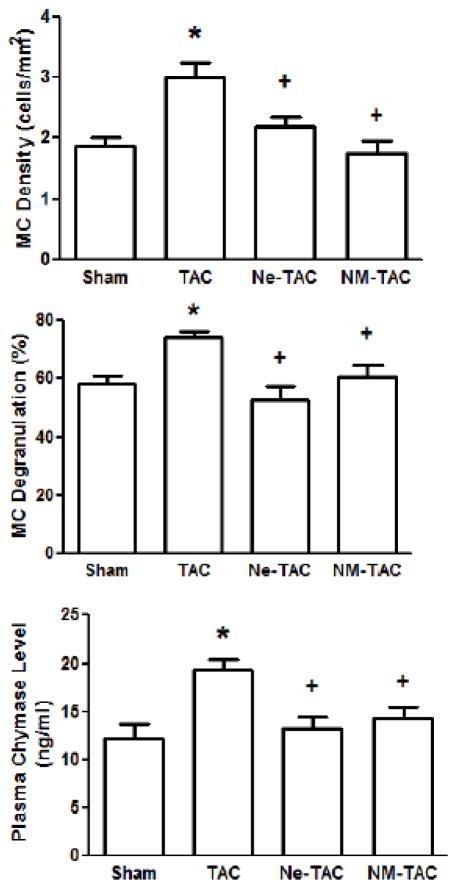
3.3 TAC-Induced Myocardial Mast Cell Activation Was Prevented by Tryptase Inhibition
As shown in Figure 4, LV MC density and degranulation were significantly increased in the TAC group compared with that in the Sham group. Plasma chymase levels, which are assumed to include the chymase released from myocardial MCs, were also significantly increased in the TAC group relative to the Sham group. The TAC-induced increases in LV MC density and degranulation and plasma chymase levels did not occur in the Ne-TAC group. Interestingly, the TAC-induced increases did not occur in NM-TAC group either, indicating tryptase inhibition prevented TAC-induced increases in cardiac MC density and activation and chymase release.
Figure 4.
Transverse aortic constriction (TAC) or sham surgery was performed in 7-wk-old male rats with continuous administration of either nedocromil (Ne) or nafamostat mesilate (NM) started 3 days prior to TAC surgery. Five wks after surgery, LV mast cell (MC) density and degranulation were measured using toluidine blue stained tissue (panels A and B, respectively). Plasma chymase levels were measured using an ELISA kit (panel C). All values are mean ± SEM; N=6; *P<0.05 vs. Sham; +P<0.05 vs. TAC.
3.4 Tryptase Simulated Chymase Release and Increased Collagen Production in Cultured LV Tissue Slices
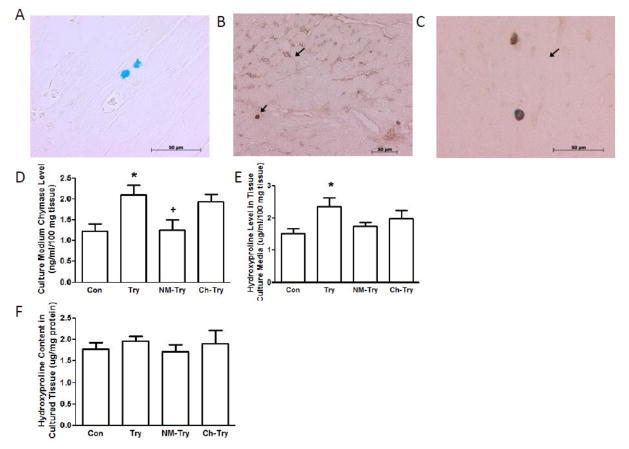
The results presented in figures 3 and 4 indicate that TAC-induced increases in myocardial tryptase are responsible for increases in MC density, MC activation, and chymase release. To further investigate the ability of tryptase to activate myocardial MCs, the presence of the tryptase receptor, PAR-2 on myocardial MCs was examined in sham rat hearts (figs 5A, 5B and 5C) and in LV tissue slices incubated with tryptase plus NM or Ch (figs 5D, 5E and 5F). PAR-2 was found to be localized to interstitial cells (fig 5B) and myocardial MCs by double staining for PAR-2 and MCs (fig 5C); PAR-2 density was greater in myocardial MCs than in the other interstitial cells. Tryptase significantly increased chymase concentration in LV tissue culture media, which was prevented by the tryptase inhibitor, NM, but not by chymase inhibitor, Ch, (fig 5D), verifying the ability of tryptase to activate myocardial MCs to release chymase. The effects of tryptase on collagen production were also examined in LV tissue culture slices and media by the measurement of hydroxyproline, a nonproteinogenic amino acid found only in collagen and elastin in mammals. There were no group differences in hydroxyproline concentration in the slices (fig 5F). However, it was significantly increased in the culture media of the group incubated with tryptase as compared to the control group and the Try groups incubated with NM and Ch (fig 5E).
Figure 5.
Panel A shows mast cells stained with mast cell specific alcian blue. Panel B illustrates single immunostaining of PAR-2 (arrow heads) localization of PAR-2 in LV interstitial cells. Panel C demonstrates the presence of the tryptase receptor, PAR-2, on myocardial mast cells that were double stained with immunostaining for protease-activated receptor 2 (PAR-2) and alcian blue for mast cell (arrow heads in panel C). LV tissue slices from 8-wk-old male rats were cultured in Waymonth media for 24 hr untreated (Con) or treated with tryptase (Try), Try plus nafamostat mesilate (NM-Try) or Try plus chymostatin (Ch-Try). Chymase concentrations in culture media were measured using an ELISA kit (panel D). Hydroxyproline concentrations in culture media and the tissue slices are shown in panels E and F, respectively. All values are mean ± SEM; N=6; *P<0.05 vs. Con; +P<0.05 vs. Try.
4. DISCUSSION
Tryptase, which is almost exclusively found in MCs,[21] is associated with the pathogenesis of fibrosis such as that occurring in the lung,[22] kidney,[23] and skin.[24] Recently, it has been reported that tryptase causes fibrosis in the hypertensive heart as well as a PAR-2-mediated increase in collagen production in cultured cardiac fibroblasts.[4,5] Alternatively the other major MC protease, chymase, also causes increased collagen synthesis by cultured cardiac fibroblasts.[3] However, the fact that the in vivo inhibition of either tryptase or chymase results in the prevention of fibrosis, raises the question as to whether the fibrotic actions of these two MC proteases are part of a common pathway. Given the fact that tryptase has been shown to activate MCs in noncardiac tissue via the PAR-2 receptor,[10] its primary, in vivo, fibrotic role may be via its ability to induce MC degranulation thereby increasing the synthesis/release of chymase. Indeed, this is the major finding of this study.
TAC for five wks induced LV hypertrophy and fibrosis along with increases in cardiac MC density, degranulation and release of tryptase and chymase. Treatment of TAC animals with the MC membrane stabilizing agent, Ne, prevented cardiac fibrosis, but not LV hypertrophy, corroborating our previous report using the SHR model[5] which represents a distinctly different model of pressure overload. Further, tryptase inhibition by NM, an extremely potent and selective tryptase inhibitor,[25] prevented the TAC-induced LV fibrosis, indicating that MC tryptase played a crucial role in the adverse TAC-induced LV myocardial remodeling. This fits with our previous report that blockade of PAR-2 prevented cardiac fibrosis in the SHR.[4] Tryptase inhibition also attenuated MC tryptase synthesis and release. Furthermore, inhibition of tryptase prevented the TAC-induced increase in MC density and degranulation, resulting in reduced MC chymase release. This provides evidence that the effects of tryptase on cardiac fibrosis may be via its regulation of chymase. Tryptase-stimulated histamine release has been reported to occur in the tonsils, lungs and skin,[26–28] which in the case of skin was PAR-2 mediated.[28] The fact that we have shown herein the presence of PAR-2 on cardiac MCs strongly suggest that, in vivo, tryptase activates cardiac MCs via PAR-2 to enhance MC density and chymase release in an autocrine fashion.
To verify the in vivo observation that tryptase stimulated MC chymase release in TAC rats and to determine the extent to which chymase mediates the effects of tryptase on myocardial fibrosis, cultured LV tissue slice experiments were performed. In this preparation, myocardial cells retain their 3-D structural integrity, intercellular interactions and extracellular attachments and the myocardial response to perturbations is solely intrinsic in that it is void of factors, such as circulating inflammatory cells, cytokines, variations in the neuro-hormonal background, and variations in preload, afterload and contractility. Also, multiple perturbations on tissue from an individual heart are possible thereby minimizing biological variability. Incubating LV tissue slices with exogenous tryptase resulted in MC chymase release into the culture media, confirming that tryptase induces MC release of chymase in the myocardial tissue. This was prevented by the tryptase inhibitor but not by a chymase inhibitor, indicating that chymase does not have autocrine actions on MCs, at least in regard to chymase release. Most importantly, incubation of LV slices with tryptase increased the synthesis of hydroxyproline (a surrogate marker of collagen) which was prevented by tryptase inhibition and, importantly, by chymase inhibition. These findings represent direct evidence that the pro-fibrotic actions of tryptase in the LV are mediated by chymase.
In an earlier study, we reported the following: myocardial tryptase content was increased in SHR; blockade of PAR-2 prevented myocardial fibrosis in SHR; and tryptase increased cultured cardiac fibroblast conversion to the myofibroblast phenotype and collagen production via PAR-2.[5] Collectively, these observations led us to conclude that tryptase contributed to myocardial fibrosis via activation of PAR-2 on cardiac fibroblasts.[4] However, given the findings reported herein that tryptase increases MC density and chymase release, it seems likely that the antifibrotic effects of PAR-2 blockade in SHR may primarily have been attributed to the blockade of PAR-2 on MCs. While the contribution of PAR-2 on fibroblasts remains a possibility, our previous cultured fibroblast studies did not detect collagen synthesis in response to tryptase (100 mU/mL) until after 72 hrs of treatment,[5] as opposed to the 24 hrs of tryptase (100 mU/mL) incubation of LV tissue slices studied herein. We have reported collagen synthesis by isolated cardiac fibroblasts at 24 hrs,[4] however, that required 1000 mU/mL (~1000 ng/mL) of tryptase, 10 times higher than that used in the LV slice experiments and ~140 times higher than the plasma level of tryptase (~7 ng/ml) determined in TAC hearts in the present study. Although, it is important to note that tryptase concentration may be substantially higher at the local level where MCs are degranulating. Interestingly though, isolated cardiac fibroblasts do proliferate in response to tryptase (100 mU/mL) after 24 hrs. Thus, tryptase activation of PAR-2 on fibroblasts may be important in initiating their proliferation, whereas PAR-2 initiation of chymase release by MCs may be the stimulus for collagen production by fibroblasts. Chymase can contribute to fibrosis by the formation of angiotensin II and activation of transforming growth factor (TGF)-β1.
To the best of our knowledge, there are no specific tryptase inhibitors available. Although NM has been found to be an extremely potent and selective tryptase inhibitor,[25] it is also capable of inhibiting a variety of other serine proteases at higher concentrations. Thus a possible shortcoming to the study lies in the fact that we do not know whether trypsin and other serine proteases associated with fibrosis were inhibited by NM at the dose used in the present and previous studies.[13] However, there has been no specific report to indicate that NM is capable of inhibiting chymase. Therefore, it is highly unlikely that the antifibrotic effects of NM in the present study were related to its inhibition of chymase.
5. PERSPECTIVES
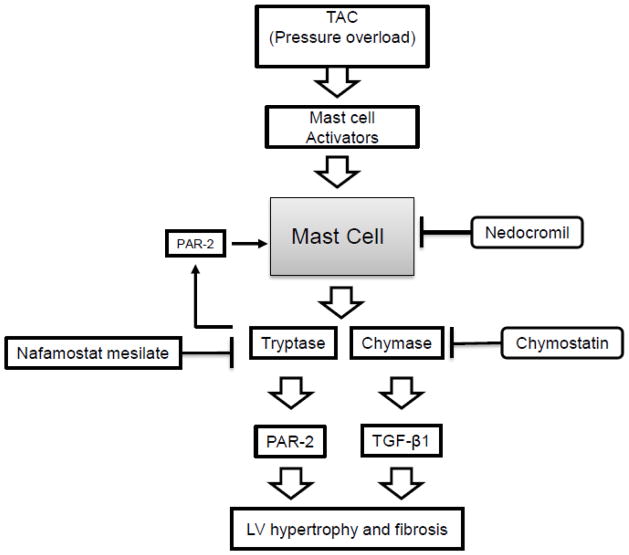
As summarized in Figure 6, the results herein delineate the interactions between MC tryptase and chymase involved in hypertension-related cardiac fibrosis. TAC-increased myocardial tryptase and chymase release from MCs. The released tryptase via PAR-2 further activated MCs and chymase release thus amplifying the effects of MCs on TAC-induced myocardial fibrosis. Tryptase inhibition prevented TAC-induced myocardial fibrosis primarily by preventing activation of MCs and the subsequent increase in released chymase. The fact that chymostatin completely prevented TAC-induced fibrosis indicates that the myocardial levels of tryptase are insufficiently elevated to directly stimulate fibroblast collagen production. These findings resolve the issue as to why in vivo inhibition of either tryptase or chymase results in the prevention of fibrosis when both are released from activated MCs. Moreover, the involved pathway is such that treatment with a tryptase or chymase inhibitor or a MC membrane stabilizing drug will prevent pressure overload-induced fibrosis.
Figure 6.
A schematic summary of the results. Mast cell activators are released from myocardial cells in response to TAC. Upon activation, cardiac mast cells release tryptase and chymase into the interstitium. Released tryptase acts in an autocrine fashion to activate mast cells and thus further increase tryptase and chymase release. Nedocromil prevents mast cell tryptase and chymase release while nafamonstat mesilate prevents tryptase-induced mast cell activation. The fact that chymostatin completely prevents TAC-induced fibrosis indicates that the myocardial levels of tryptase are insufficiently elevated to directly stimulate fibroblast collagen production.
9. NOVELTY AND SIGNIFICANCE.
In this study the interactions between MC tryptase and chymase involved in hypertension-related cardiac fibrosis are delineated. The results resolve the issue as to why in vivo inhibition of either tryptase or chymase results in the prevention of fibrosis when both are released from activated mast cells. That is tryptase contributes to hypertension-induced cardiac fibrosis primarily via PAR-2 activation of cardiac mast cells causing the amplified release of chymase, which in turn stimulates fibroblast collagen production.
Acknowledgments
Grant Support: This study funded in part by the Heart, Lung and Blood Institute of the National Institutes of Health (grant #s R21-HL-59981 to JSJ and R00-HL-093215 to SPL)
6. Source of funding
This work was supported in part by the Heart, Lung and Blood Institute of the National Institutes of Health (grant #s R21-HL-59981 to JSJ and R00-HL-093215 to SPL).
Footnotes
7. Conflict(s) of Interest/Disclosure(s)
No conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Janicki JS, Brower GL, Levick SP. The emerging prominence of the cardiac mast cell as a potent mediator of adverse myocardial remodeling. Methods Mol Biol. 2015;1220:121–139. doi: 10.1007/978-1-4939-1568-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levick SP, Meléndez GC, Plante E, McLarty JL, Brower GL, Janicki JS. Cardiac mast cells: the centrepiece in adverse myocardial remodelling. Cardiovasc Res. 2011;89:12–19. doi: 10.1093/cvr/cvq272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao XY, Zhao LY, Zheng QS, Su JL, Guan H, Shang FJ, Niu XL, He YP, Lu XL. Chymase induces profibrotic response via transforming growth factor-beta 1/Smad activation in rat cardiac fibroblasts. Mol Cell Biochem. 2008;310:159–166. doi: 10.1007/s11010-007-9676-2. [DOI] [PubMed] [Google Scholar]
- 4.McLarty JL, Meléndez GC, Brower GL, Janicki JS, Levick SP. Tryptase/Protease-activated receptor 2 interactions induce selective mitogen-activated protein kinase signaling and collagen synthesis by cardiac fibroblasts. Hypertension. 2011;58:264–270. doi: 10.1161/HYPERTENSIONAHA.111.169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levick SP, McLarty JL, Murray DB, Freeman RM, Carver WE, Brower GL. Cardiac mast cells mediate left ventricular fibrosis in the hypertensive rat heart. Hypertension. 2009;53:1041–1047. doi: 10.1161/HYPERTENSIONAHA.108.123158. [DOI] [PubMed] [Google Scholar]
- 6.Takai S, Jin D, Sakaguchi M, Katayama S, Muramatsu M, Sakaguchi M, Matsumura E, Kim S, Miyazaki M. A novel chymase inhibitor, 4-[1-([bis-(4-methyl-phenyl)-methyl]-carbamoyl)3-(2-ethoxy-benzyl)-4-oxo-azetidine-2-yloxy]-benzoic acid (BCEAB), suppressed cardiac fibrosis in cardiomyopathic hamsters. J Pharmacol Exp Ther. 2003;305:17–23. doi: 10.1124/jpet.102.045179. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto T, Wada A, Tsutamoto T, Ohnishi M, Isono T, Kinoshita M. Chymase inhibition prevents cardiac fibrosis and improves diastolic dysfunction in the progression of heart failure. Circulation. 2003;107:2555–2558. doi: 10.1161/01.CIR.0000074041.81728.79. [DOI] [PubMed] [Google Scholar]
- 8.Liu W, Chen J, Xu T, Tian W, Li Y, Zhang Z, Li W. Qiliqiangxin improves cardiac function in spontaneously hypertensive rats through the inhibition of cardiac chymase. Am J Hypertens. 2012;25:250–260. doi: 10.1038/ajh.2011.219. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Jubair S, Janicki JS. Estrogen inhibits mast cell chymase release to prevent pressure overload-induced adverse cardiac remodeling. Hypertension. 2015;65:328–334. doi: 10.1161/HYPERTENSIONAHA.114.04238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’andrea MR, Rogahn CJ, Andrade-Gordon P. Localization of protease-activated receptors -1 and -2 in human mast cells: indications for an amplified mast cell degranulation cascade. Biotech Histochem. 2000;75:85–90. doi: 10.3109/10520290009064152. [DOI] [PubMed] [Google Scholar]
- 11.Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol. 2009;131:471–481. doi: 10.1007/s00418-008-0541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu H, Meléndez GC, Levick SP, Janicki JS. Prevention of adverse cardiac remodeling to volume overload in female rats is the result of an estrogen-altered mast cell phenotype. Am J Physiol Heart Circ Physiol. 2012;302:H811–7. doi: 10.1152/ajpheart.00980.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sendo T, Itoh Y, Goromaru T, Sumimura T, Saito M, Aki K, Yano T, Oishi R. A potent tryptase inhibitor nafamostat mesilate dramatically suppressed pulmonary dysfunction induced in rats by a radiographic contrast medium. Br J Pharmacol. 2003;138:959–67. doi: 10.1038/sj.bjp.0705121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Compton SJ, Cairns JA, Holgate ST, Walls AF. The Role of mast cell tryptase in regulating endothelial cell proliferation, cytokine release, and adhesion molecule expression: tryptase induces expression of mRNA for IL-1b and IL-8 and stimulates the selective release of IL-8 from human umbilical vein endothelial cells. J Immunol. 1998;161:1939–1946. [PubMed] [Google Scholar]
- 15.He S, Gaça MD, Walls AF. A role for tryptase in the activation of human mast cells: modulation of histamine release by tryptase and inhibitors of tryptase. J Pharmacol Exp Ther. 1998;286:289–297. [PubMed] [Google Scholar]
- 16.Sand E, Themner-Persson A, Ekblad E. Mast cells reduce survival of myenteric neurons in culture. Neuropharmacology. 2009;56:522–530. doi: 10.1016/j.neuropharm.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Lu H, Plante E, Meléndez GC, Levick SP, Janicki JS. Stem cell factor is responsible for the rapid response in mature mast cell density in the acutely stressed heart. J Mol Cell Cardiol. 2012;53:469–474. doi: 10.1016/j.yjmcc.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meléndez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension. 2010;56:225–231. doi: 10.1161/HYPERTENSIONAHA.109.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brower GL, Chancey AL, Thanigaraj S, Matsubara BB, Janicki JS. Cause and effect relationship between myocardial mast cell number and matrix metalloproteinase activity. Am J Physiol Heart Circ Physiol. 2002;283:H518–H525. doi: 10.1152/ajpheart.00218.2000. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Guo T, Liang D, Sun Y, Kingery WS, David Clark JD. Substance P Signaling Controls Mast Cell Activation, Degranulation, and Nociceptive Sensitization in a Rat Fracture Model of Complex Regional Pain Syndrome. Anesthesiology. 2012;116:882–895. doi: 10.1097/ALN.0b013e31824bb303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castells M, Irani AA, Schwartz LB. Evaluation of human peripheral blood leukocytes for mast cell tryptase. J Immunol. 1987;138:2184–9. [PubMed] [Google Scholar]
- 22.Akers IA, Parson M, Hill MR, Hollenburg MD, Sanjar S, Laurent GJ, McAnulty RJ. Mast cell tryptase stimulates human lung fibroblast proliferation via protease activate receptor-2. Am J Physiol-Lung Cell Mol Physiol. 2000;278:L193–L201. doi: 10.1152/ajplung.2000.278.1.L193. [DOI] [PubMed] [Google Scholar]
- 23.Sirvent AE, Gonzalez C, Enriquez R, Fernandez J, Millan I, Barber X, Amoros F. Serum tryptase level and markers of renal dysfunction in a population with chronic kidney disease. J Nephrol. 2010;23:282–290. [PubMed] [Google Scholar]
- 24.Frungieri MB, Weidinger S, Meineke V, Kohn FM, Mayerhofer A. Proliferative action of mast-cell tryptase is mediated by PAR2, COX2, prostaglandins, and PPAR_: possible relevance to human fibrotic disorders. Proc Natl Acad Sci US A. 2002;99:15072–15077. doi: 10.1073/pnas.232422999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori S, Itoh Y, Shinohata R, Sendo T, Oishi R, Nishibori M. Nafamostat mesilate is an extremely potent inhibitor of human tryptase. J Pharmacol Sci. 2003;92:420–3. doi: 10.1254/jphs.92.420. [DOI] [PubMed] [Google Scholar]
- 26.He S, Gaca MDA, Walls AF. A role for tryptase in the activation of human mast cells: modulation of histamine release by tryptase and inhibitors of tryptase. J Pharmacol Exp Ther. 1998;286:289–97. [PubMed] [Google Scholar]
- 27.He S, Aslam A, Gaça MD, He Y, Buckley MG, Hollenberg MD, Walls AF. Inhibitors of tryptase as mast cell-stabilizing agents in the human airways: effects of tryptase and other agonists of proteinase-activated receptor 2 on histamine release. J Pharmacol Exp Ther. 2004;309:119–26. doi: 10.1124/jpet.103.061291. [DOI] [PubMed] [Google Scholar]
- 28.Moormann C, Artuc M, Pohl E, Varga G, Buddenkotte J, Vergnolle N, Brehler R, Henz BM, Schneider SW, Luger TA, Steinhoff M. Functional characterization and expression analysis of the proteinase-activated receptor-2 in human cutaneous mast cells. J Invest Dermatol. 2006;126:746–55. doi: 10.1038/sj.jid.5700169. [DOI] [PubMed] [Google Scholar]