Abstract
Hyponatremia and several other CNS pathologies are associated with substantial astrocytic swelling. To counteract cell swelling, astrocytes lose intracellular osmolytes, including l-glutamate and taurine, through volume regulated anion channel (VRAC). In vitro, when swollen by exposure to hypo-osmotic medium, astrocytes lose endogenous taurine paradoxically faster than l-glutamate or l-aspartate. Here, we explored the mechanisms responsible for differences between the rates of osmolyte release in primary rat astrocyte cultures. In radiotracer assays, hypo-osmotic efflux of preloaded [14C]taurine was indistinguishable from d-[3H]aspartate and only 30–40% faster than l-[3H]glutamate. However, when we used HPLC to measure the endogenous intracellular amino acid content, hypo-osmotic loss of taurine was ~5-fold greater than l-glutamate, and no loss of l-aspartate was detected. The dramatic difference between loss of endogenous taurine and glutamate was eliminated after inhibition of both glutamate reuptake (with 300 µM TBOA) and glutamate synthesis by aminotransferases (with 1 mM aminooxyacetic acid, AOA). Treatment with TBOA+AOA made reductions in the intracellular taurine and l-glutamate levels approximately equal. Taken together, these data suggest that swollen astrocytes actively conserve intracellular glutamate via reuptake and de novo synthesis. Our findings likely also explain why in animal models of acute hyponatremia, extracellular levels of taurine are dramatically elevated with minimal impact on extracellular l-glutamate.
Keywords: Astrocytes, excitatory amino acids, glutamate transport, volume-regulated anion channel, cell swelling, taurine
Hyponatremia is defined as a reduction in blood serum sodium (Na+) levels below 135 mM. It is the most common fluid-electrolyte disorder encountered in clinical practice and is very frequently seen in hospitalized patients and elderly individuals in long-term care (Fraser and Arieff 1997; Adrogue and Madias 2000; Upadhyay et al. 2006). Systemic Na+ concentration is closely monitored in emergency care settings, because acute hyponatremia is potentially life-threatening and even moderate reductions in blood [Na+] strongly increase the risk of death from various co-morbid states (Fraser and Arieff 1997; Upadhyay et al. 2006; Podesta et al. 2015). In the majority of cases, hyponatremia develops due to improper retention of water, and is associated with decreases in systemic osmolarity. Although all tissues are affected, the brain represents the target organ of this disorder (Adrogue and Madias 2000). Acute hyponatremia leads to headaches, nausea, fatigue, confusion and hallucinations. In its most severe form, the disease progresses to seizures and numerous brain stem-related deficits, such as dysregulation of blood pressure, heart rate, thermal and respiratory controls, with severe risk of coma and death (Fraser and Arieff 1997; Adrogue and Madias 2000; Podesta et al. 2015).
The most dangerous neurological changes in acute hyponatremia develop due to brain edema which causes deficits in cerebral circulation and herniation of the brainstem. However, the “milder” neurological deficits are related to osmotic changes in neural cells. A decrease in systemic osmolarity triggers water movement into the CNS and causes cellular swelling. Amongst all brain cell types, swelling is primarily seen in astrocytes, particularly in the astrocytic processes surrounding blood vessels (Wasterlain and Torack 1968; Manley et al. 2000; Risher et al. 2009). Hence, astroglial cells are the focal point of model in vitro studies on functional consequences of cellular edema. The exact reasons for selective astrocytic swelling remain poorly understood. It is thought, however, that increases in astroglial cell volume may be related to high water permeability of the plasmalemma and high propensity of astrocytes to accumulate ions and neurotransmitters (Kimelberg 1995; Sykova 1997; Mongin and Kimelberg 2005a).
As the vast majority of animal cells, astrocytes respond to swelling through the regulatory release of osmotically active molecules. Such release drives efflux of osmotically obligated water and mediates regulatory volume decrease or RVD (Medrano and Gruenstein 1993; O'Connor et al. 1993; Pasantes-Morales et al. 1994). RVD is usually accomplished via concurrent stimulation of volume-sensitive K+ channels and volume-regulated anion channels (VRAC), which cooperatively mediate loss of intracellular K+, Cl−, and bicarbonate (Lang et al. 1998; Mongin and Orlov 2001; Hoffmann et al. 2009). Loss of inorganic ions is the main factor in the CNS adaptation to acute hyponatremia since it counteracts extreme tissue swelling. Yet, along with inorganic osmolytes, swollen cells also lose a variety of small organic molecules, including l-glutamate, l-aspartate, the amino sulfonic acid taurine, myo-inositol, and others. Efflux of organic osmolytes plays a minor role in RVD, but is essential for the long-term adaptation to chronic hypo-osmolarity, in many tissues including the brain (Gullans and Verbalis 1993; Wehner et al. 2003; Hoffmann et al. 2009).
The movement of negatively charged and uncharged organic molecules shares the same pathway with Cl− and HCO3− – the ubiquitously expressed VRAC (Strange et al. 1996; Nilius et al. 1997; Akita and Okada 2014). Although VRAC was functionally characterized in many cell types as early as the 1980s and 1990s, its molecular nature has been uncovered only during the last year (reviewed in Pedersen et al. 2015). Two laboratories independently identified the LRRC8 protein family members as subunits of the hetero-hexameric VRAC (Qiu et al. 2014; Voss et al. 2014). Our group found LRRC8 expression in astroglial cells, and established its critical contribution to the hypo-osmotic release of taurine and the excitatory neurotransmitters, l-glutamate and l-aspartate (Hyzinski-Garcia et al. 2014). Swelling-activated release of l-glutamate determines hyperexcitability and likely mediates many other neurological manifestations in hyponatremia (Gullans and Verbalis 1993; Pasantes-Morales et al. 2002).
In addition to impact on l-glutamate release, cell swelling may also disrupt brain glutamate metabolism. One of the main functions of astrocytes is to control the levels of extrasynaptic glutamate, via activities of the Na+-dependent astrocyte transporters, GLAST and GLT-1 (Danbolt 2001). Inside the astrocyte, glutamate is converted to glutamine by the cytosolic enzyme glutamine synthetase, or metabolized in the TCA cycle after conversion to α-ketoglutarate by mitochondrial transaminases and/or glutamate dehydrogenase. Astrocytes release newly synthesized glutamine to supply neurons with the substrate for synthesis of glutamate (and GABA), thus completing the glutamate-glutamine cycle in the brain (Bak et al. 2006; McKenna 2007). In hyponatremia, this normal chain of events is disrupted, leading to dramatic increases in extracellular l-glutamate and profound reductions in the levels of extracellular l-glutamine (Taylor et al. 1995; Haskew-Layton et al. 2008; Hyzinski-Garcia et al. 2011).
While modeling in astrocyte cultures the effects of cellular swelling on glutamate transport and metabolism, we found that changes in intracellular levels of endogenous l-glutamate and l-aspartate were perplexingly small and inconsistent with the high permeability of VRAC for these excitatory amino acids (Hyzinski-Garcia et al. 2011). This apparent “conservation” of l-glutamate and l-aspartate was particularly striking when compared to the robust loss of intracellular taurine. Therefore, in the present work, we explored potential mechanisms responsible for differences in the release rates of various osmolytes from swollen astrocytes.
Materials and Methods
Materials
β-Alanine, aminooxyacetic acid hydrochloride (AOA), deoxyribonuclease I (DNase I) from bovine pancreas, β-mercaptoethanol, l-methionine sulfoximine (MSO), o-phthalaldehyde, poly-d-lysine, and all salts and buffers were purchased from Sigma-Aldrich (St Louis, MO, U.S.A.). dl-threo-β-benzyloxyaspartic acid (TBOA) was from Bio-Techne/Tocris (Minneapolis, MN, U.S.A.). HPLC-grade methanol was from EMD Millipore (Billerica, MA, U.S.A.). Cell culture media, heat-inactivated horse serum, antibiotics, and the recombinant protease TrypLE were acquired from Life Technologies/Invitrogen (Grand Island, NY, U.S.A.). d-[3H]aspartate, l-[3H]glutamate and [14C]taurine were from PerkinElmer/New England Nuclear (Boston, MA, U.S.A.).
Preparation of primary astrocyte cultures
Primary astrocytes were prepared from 1–2-day old Sprague-Dawley rats as previously described elsewhere (Mongin et al. 2011). The animal procedures were approved by the Institutional Animal Care and Use Committee of the Albany Medical College. Briefly, neonatal rats were euthanized by rapid decapitation. Cortical tissue was separated from the meninges and hippocampi, collected into ice-cold Opti-MEM, and minced. Cells in the tissue were enzymatically dissociated in a solution of the recombinant protease TrypLE, which was additionally diluted with Opti-MEM (1:1, v/v) and supplemented with DNase I (1 mg/ml). Three TrypLE extractions were carried at 37°C with slow stirring. The first extraction was discarded. The last two extractions were combined, isolated cells were sedimented by brief centrifugation at 1,000 g, and resuspended in minimum essential medium (MEM) containing 10% heat inactivated horse serum (HIHS), 50 U/mL penicillin, and 50 µg/mL streptomycin. Dissociated cells were then plated on poly-d-lysine coated T-75 culture flasks at the low density of 200,000 cells per flask. The primary cultures were grown in MEM/10% HIHS plus antibiotics for 2 to 3 weeks in a humidified atmosphere of 5% CO2/balance air at 37°C. Cell culture medium was changed twice per week. Confluent cultures contained ≥98% of astrocytes as periodically checked by staining with monoclonal antibodies against the astroglial cell marker, glial fibrillary acidic protein (Sigma-Aldrich, G3893).
HPLC analysis of the intracellular amino acid content
To determine the intracellular amino acid levels, confluent primary astrocytes were replated on 60-mm Petri dishes 2–3 days prior to the assay. Before the experiment, cells were washed from culture media with the HEPES-buffered Basal medium containing (in mM) 135 NaCl, 3.8 KCl, 1.2 MgSO4, 1.3 CaCl2, 1.2 KH2PO4, 10 HEPES, and 10 d-glucose (pH 7.4, osmolarity 288–290 mOsm), and pre-incubated in the same medium for 30 min at 37°C. The Basal medium was then changed to either Basal or hypo-osmotic media, some of which additionally contained inhibitors of glutamate transport and metabolism as indicated in the text and figure legends. In the hypo-osmotic medium osmolarity was reduced to ~185 mOsm or ~250 mOsm through decreasing [NaCl] to 77 mM and 115 mM, respectively, while the concentrations of all other constituents remained the same. Cells were incubated in experimental media for 30 min at 37°C, and then lysed in a solution of 5 mM HEPES plus 1 mM EDTA (pH=7.4), scraped using rubber cell scrapers and sonicated for 4 min in a bath with ice-cold water. The resulting lysates were clarified by 4-min centrifugation at 12,000 g and analyzed for amino acid content. The amino acid levels were quantified by HPLC after derivatization with o-phthalaldehyde in the presence of β-mercaptoethanol using an Agilent 1200 HPLC setup with fluorescence detector (Agilent Technologies, Santa Clara, CA, U.S.A.). Fluorescence values were compared to the calibration standards of l-aspartate, l-glutamate, l-glutamine, taurine and l-alanine, which were processed on the same day. The amino acid levels were then normalized to the protein content within each well, which was determined using the bicinchoninic acid protein assay kit (Thermo Fisher Scientific/ Pierce, Rockford, IL, U.S.A.) per the manufacturer’s instructions.
Radiotracer amino acid efflux assays
To determine and compare the release rates for taurine and l-glutamate, we performed radiotracer assays with [14C]taurine and l-[3H]glutamate in primary astrocytes. Cells were replated on 18×18 mm coverslips 1–2 days prior to the experiment. On the day of the experiment, cells were pre-treated with the irreversible glutamine synthetase inhibitor l-methionine sulfoximine (MSO) for one hour at 37°C, in order to reduce cytosolic metabolism of l-[3H]glutamate (Mongin et al. 2011). After the MSO pre-treatment, astrocytes were loaded with radiotracers by placing each coverslip into a 35-mm Petri dish containing l-[3H]glutamate (4 µCi/mL) and [14C]taurine (2 µCi/mL) in MEM/HIHS for 40 minutes at 37°C. In a separate set of experiments cells were loaded in a similar fashion with [14C]taurine (2 µCi/mL) and the non-metabolizable glutamate analogue d-[3H]aspartate (4 µCi/mL). The coverslips were then washed from extracellular radiotracers with HEPES-buffered Basal medium (for composition see above), and transferred to a Lucite perfusion chamber maintained at 37°C throughout experiment. This chamber has a depression on the bottom to accommodate the coverslip, and a Teflon screw lid that leaves a ~200–250 µm space above the cells. Astrocytes were continuously superfused with HEPES buffered Basal medium or hypo-osmotic medium, in which osmolarity was reduced by ~30% to 198–200 mOsm, or by ~14% to 250 mOsm. One-min superfusate fractions were collected at a flow rate of ~1.2 ml/min into scintillation vials. Due to the small volume of the Lucite chamber, the medium inside it is completely exchanged at least five times each minute. [3H] and [14C] contents in each fraction were then determined individually using a TriCarb 2900 scintillation counter (PerkinElmer, Waltham, MA, U.S.A) after adding Ecoscint A scintillation fluid (National Diagnostics, Atlanta, GA, U.S.A.). At the end of each experiment, cells on the coverslip were lysed using a solution of 2% SDS and 8 mM EDTA (pH=7.4). The one-min fractional radiotracer release rates were calculated in relation to the isotope remaining in the cells at each time point, using an Excel-based custom program.
Comparison of astrocytic l-glutamate and taurine uptake rates
To quantitatively compare the uptake rates for taurine and l-glutamate, primary astrocytes were re-seeded in poly-d-lysine covered 24-well multi-well plates. Cells were washed from tissue culture media with HEPES-buffered Basal medium (for composition see above) and additionally equilibrated in the same medium for 40 min. The uptake reaction was initiated by adding either Basal or Hypo-osmotic media (250 mOsm/115 mM NaCl or 185 mOsm/77 mM NaCl, as indicated), which additionally contained 0.5 µCi/mL l-[3H]glutamate and 0.3 µCi/mL [14C]taurine. The concentrations of both amino acids were adjusted to 2 µM by adding unlabeled l-glutamate and taurine. After 20-min incubation at 37°C, uptake was terminated by four washes with warm Basal medium. Astrocytes in each well were lysed by adding 1 mL of solution containing 2% SDS and 8 mM EDTA. The rate of accumulation of [3H]- and [14C]-labeled amino acids was then determined by liquid scintillation counting as described above and normalized to the specific activities of l-[3H]glutamate and [14C]taurine, and the protein content.
Statistical Analysis
Statistical comparisons were performed using t-test or ANOVA with post hoc Fisher's least significant difference (LSD) test for multiple comparisons, where appropriate. In time-course experiments, the rates of amino acid release were compared using two-way ANOVA. In all cases, p values less than 0.05 were considered statistically significant. Origin 8.0 software (OriginLab, Northampton, MA, U.S.A) was used for statistical analysis and graphing purposes.
Results
Effect of hypo-osmotic media on intracellular amino acid levels and transmembrane amino acid fluxes in primary astrocytes
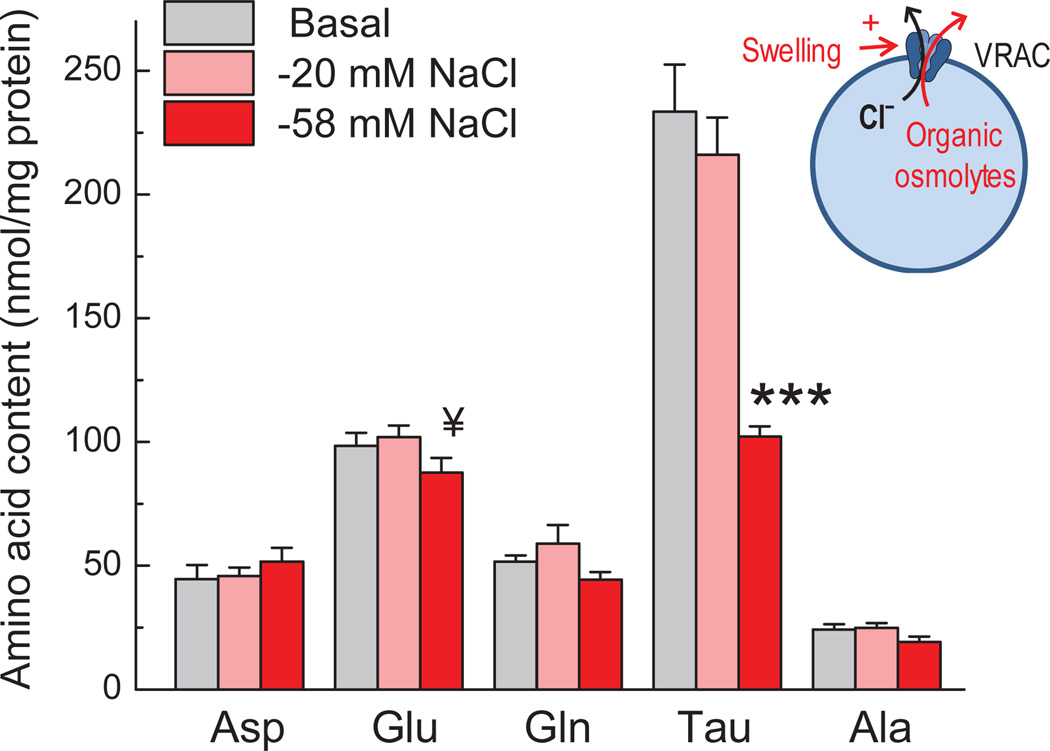
Cultured astrocytes, when exposed to hypo-osmotic media, actively regulate their volume via loss of intracellular osmotically active molecules, including a number of cytosolic amino acids (Pasantes Morales and Schousboe 1988; Kimelberg et al. 1990; Olson 1999; Hyzinski-Garcia et al. 2011). We started this study by comparing the effect of exposure to media with different osmolarities on the intracellular content of five major endogenous amino acids. As shown in Fig. 1, exposing astrocytes for 30 min to strongly hypo-osmotic medium (reduction of NaCl from 135 to 77 mM; 185 mOsm, ~36% decrease in osmolarity) caused a 56.2% reduction in the level of the major intracellular osmolyte taurine, 20.6% drop in the content of l-alanine, 11.1% decrease of l-glutamate, 14.2% loss of l-glutamine, and an apparent 15.6% increase in the intracellular levels of l-aspartate. With the exception of taurine content, none of the observed changes reached the level of statistical significance. However, these changes were further reproduced and statistically validated in the subsequent more detailed analysis (see section “Inhibitors of Na+-dependent glutamate transport and transamination accelerate hypo-osmotic loss of intracellular l-glutamate”). In contrast to strong hypo-osmolarity, a more physiologically relevant drop in the sodium content to 115 mM (osmolarity reduction by ~14%) did not produce measurable changes in the intracellular amino acid content (Fig. 1). In the subsequent experiments, we focused on ~5-fold difference between reductions in l-glutamate and taurine content in 185 mOsm medium.
Fig. 1. Hypo-osmotic cell swelling triggers preferential loss of taurine as compared to four other intracellular amino acids.
Primary rat astrocytes were incubated in either Basal or hypo-osmotic media for 30 min. In hypo-osmotic media NaCl concentration was reduced from 135 to 115 or 77 mM, with corresponding reductions of osmolarity by ~14 and ~36%. Intracellular levels of five amino acids were measured in cell lysates using an HPLC assay, and normalized to total protein content. Data are the mean values ± SEM (n=5–7). ***p<0.001, “−58 mM NaCl” vs. “Basal” and “−20 mM NaCl”; ¥p<0.1, “−58 mM NaCl” vs. “−20 mM NaCl” (one-way ANOVA with Fisher LSD post hoc test). Ala, alanine; Asp, aspartate; Glu, glutamate; Gln, glutamine; Tau, taurine.
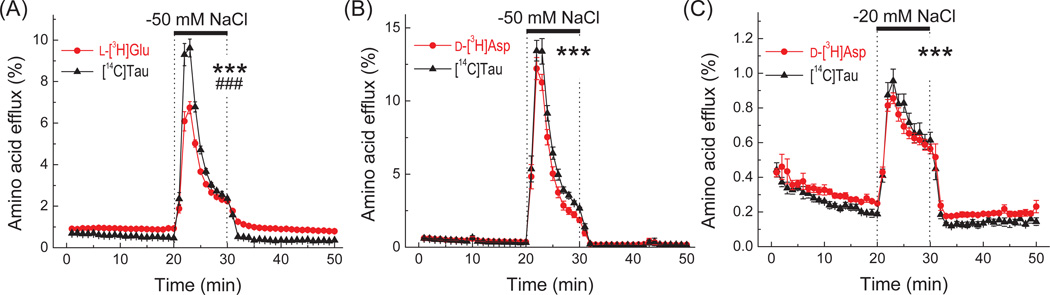
It is generally accepted that during cell volume regulation, amino acids are predominantly released via the swelling-activated anion channel VRAC (see Abdullaev et al. 2006 and Introduction for additional references). Therefore, the simplest explanation for significant differences between hypo-osmotic reductions in the cellular contents of taurine vs. l-glutamate and other amino acids is a higher permeability of VRAC to taurine. To test if this is the case in cultured astrocytes, we simultaneously measured the release of radiolabeled [14C]taurine and l-[3H]glutamate, which were pre-loaded into the cells prior to each experiment. The caveat of such measurements is that astrocytes rapidly metabolize l-glutamate. In mature astrocyte cultures, up to 85% of accumulated glutamate is converted to glutamine by glutamine synthetase, although the highest reported conversion rates may require addition of NH4+ to the assay media (Farinelli and Nicklas 1992; McKenna et al. 1996; Mongin et al. 2011). The balance of accumulated glutamate, which is not processed by glutamine synthetase, enters the TCA cycle via transamination and oxidative deamination, or converted to aspartate and pyruvate/lactate (Farinelli and Nicklas 1992; McKenna et al. 1996). To reduce the intracellular conversions of l-[3H]glutamate, we pre-preincubated astrocytes with the irreversible inhibitor of glutamine synthetase, MSO (Ronzio et al. 1969). Inhibition of glutamate dehydrogenase (GDH) was not practical since GDH contribution to glutamate metabolism is smaller, and, more importantly, because GDH inhibitors interfere with activity of plasmalemmal glutamate transporters (Farinelli and Nicklas 1992; McKenna et al. 1996; Whitelaw and Robinson 2013). When exposed to hypo-osmotic medium, the MSO-treated cells had a 42.6%-higher maximal release rate for [14C]taurine as compared to l-[3H]glutamate (Fig. 2A). The integral 10-min hypo-osmotic [14C]taurine release exceeded that of l-[3H]glutamate by 30.5%. To address the caveat of residual l-[3H]glutamate metabolism and its contribution to the observed release values, we additionally measured the efflux rates for simultaneously pre-loaded [14C]taurine and the non-metabolizable l-glutamate analogue, d-[3H]aspartate. To facilitate the direct comparisons, these latter experiments were performed under identical conditions (including the MSO treatment). As seen in Fig. 2B, [14C]taurine and d-[3H]aspartate release were essentially indistinguishable.
Fig. 2. Effect of hypo-osmotic media on astrocytic l-[3H]glutamate/d-[3H]aspartate and [14C]taurine release measured in the same cells.
In (A) and (B), primary astrocytes were pre-incubated with the glutamine synthetase inhibitor methionine sulfoximine for 1 h, and then pre-loaded with [14C]taurine (Tau) and either l-[3H]glutamate (Glu) or its non-metabolizable analogue d-[3H]aspartate (Asp) for 40 min. In (C), the design was the same, except no methionine sulfoximine was added and change in osmolarity was milder. Cells were superfused with basal (iso-osmotic) or hypo-osmotic media, in which [NaCl] was reduced as indicated. Data are the mean values ±SEM with n=7 (in A and B), or n=4 (in C). ***p<0.001, basal release values vs. hypoosmotic release for l-[3H]Glu, d-[3H]Asp, and [14C]Tau. ###p<0.001, l-[3H]Glu vs. [14C]Tau under hypo-osmotic conditions only (two-way ANOVA).
To further, check if l-glutamate and taurine are released with comparable rates under more physiological conditions, we measured [14C]taurine and d-[3H]aspartate efflux in medium, in which NaCl content was reduced by 20 mM (~14% change in medium osmolarity). Again, the release values were also nearly identical (Fig. 2C), suggesting quite similar VRAC permeability rates for taurine and cytosolic excitatory amino acids.
Inhibitors of Na+-dependent glutamate transport and transamination accelerate hypo-osmotic loss of intracellular l-glutamate, but do not affect content of taurine
A small 1.3-fold difference in the integral release values for [14C]taurine and l-[3H]glutamate and identical rates for release of [14C]taurine and d-[3H]aspartate, which are shown in Fig. 2, are clearly at odds with the dramatic (up to 5-fold) difference between the loss of endogenous intracellular taurine and l-glutamate, or the paradoxical lack of changes in the intracellular levels of l-aspartate shown in Fig. 1. We, therefore, hypothesized that l-glutamate and l-aspartate are preserved due to reuptake by the Na+-dependent glutamate transporters and/or synthesized de novo from TCA intermediates via the reactions of transamination.
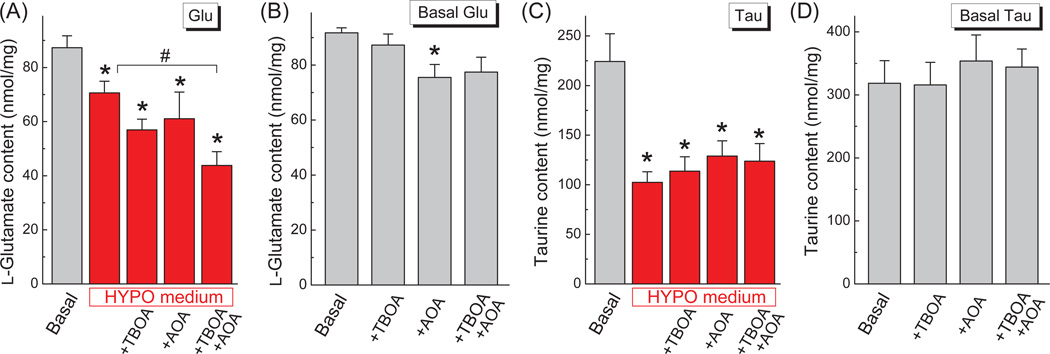
In order to test our hypothesis, we treated astrocytes with the inhibitor of glutamate transport 300 µM TBOA, the broad spectrum aminotransferase inhibitor 1 mM AOA, or the combination of both agents. The effective concentrations for these compounds were selected based on prior studies in cultured astrocytes (see for example Farinelli and Nicklas 1992; Bowens et al. 2013). As shown in Fig. 3A, treatments with either the glutamate transport inhibitor TBOA or the broad spectrum transaminase inhibitor AOA, showed a trend for accelerating l-glutamate loss from swollen cells (34.9 and 30.1%, respectively, vs. 19.2% under hypo-osmotic conditions without inhibitors). Importantly, when cells were treated with a combination of TBOA and AOA, the effect of both compounds was additive, and swollen cells lost approximately 50% of intracellular l-glutamate (Fig 3A). The qualitatively similar effect of TBOA and AOA on intracellular L-glutamate levels was observed in the cells exposed to mild hypo-osmotic medium (250 mOsm/115 mM NaCl, see Supplemental fig. 1).
Fig. 3. Inhibition of glutamate reuptake and metabolism augments loss of intracellular l-glutamate from swollen astrocytes, without impacting loss of taurine.
(A) The effects of the glutamate transport inhibitor TBOA (300 µM) and the transaminase inhibitor AOA (1 mM) on the swelling-induced changes in the intracellular content of glutamate (Glu). Cells were exposed to the specified conditions for 30 min, lysed, and their amino acid content was determined by HPLC. The data are the means±SEM (n=5–8). (B) As a control, the same glutamate content assays were performed under Basal conditions. The data are the means ± SEM (n=4). (C) The effects of TBOA and AOA on the swelling-induced changes in the intracellular content of taurine (Tau) in the same cellular lysates as in A. The data are the means ± SEM (n=5–8). (D) As a control, the same taurine content assays as in C were performed under Basal conditions. The data are the means ± SEM (n=4). *p<0.05, Basal; #p<0.05, HYPO vs. HYPO+TBOA+AOA (one-way ANOVA and Fisher’s LSD post hoc test).
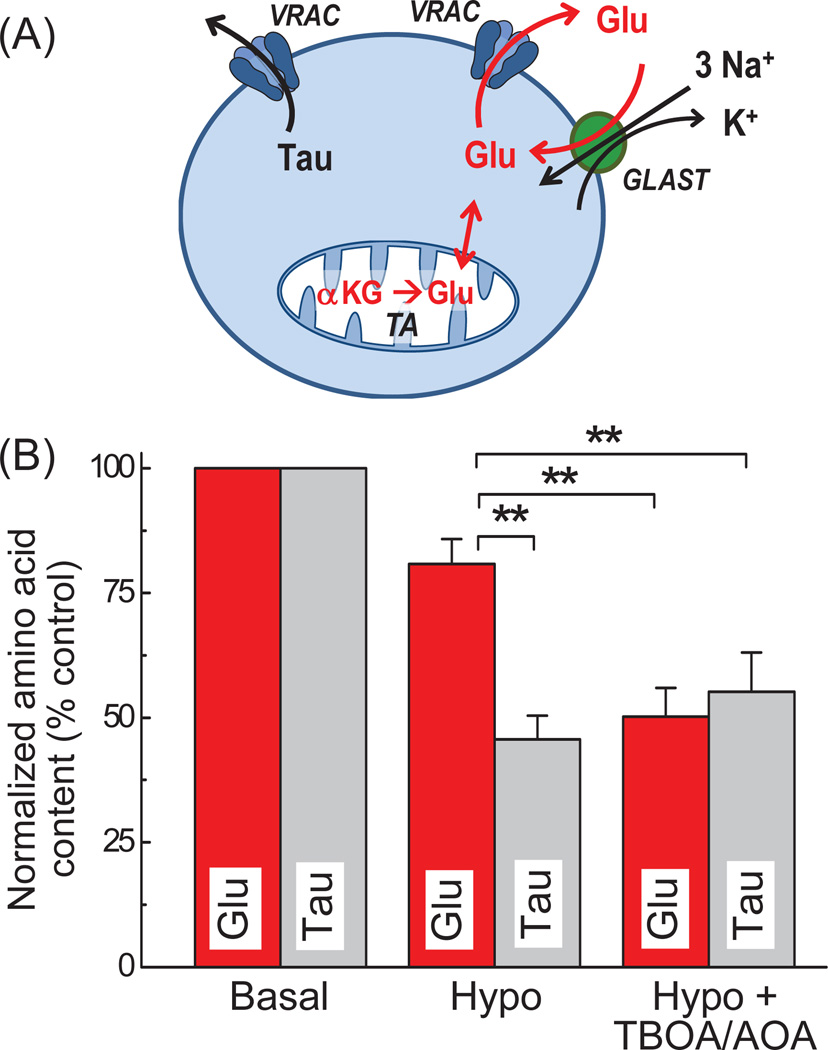
In the same samples, the endogenous taurine was again reduced in response to cells swelling by more than 50%, but neither of the tested inhibitors nor their combination showed any additional effect (Fig. 3C). When we compared the relative release values in cells treated with the combination of TBOA and AOA, the reductions in taurine and l-glutamate levels were not different (see Fig. 3A and 3C, and Discussion).
As a control, we exposed astrocytes to the same inhibitors under iso-osmotic conditions. In non-swollen cells, the glutamate transport inhibitor TBOA did not change intracellular l-glutamate levels (Fig. 3B). The transaminase inhibitor AOA significantly reduced the intracellular l-glutamate content by 17.7%; however, this effect was substantially smaller than when AOA was added to hypo-osmotic medium (compare Fig. 3A to 3B). Consistent with hypo-osmotic data, the same two inhibitors or their combination did not change the intracellular levels of taurine under iso-osmotic conditions (Fig. 3D). Thus, the effects of TBOA and AOA appear to be specific, and related to changes in glutamate transport and metabolism.
For the sake of simplicity, we present data for other amino acids (l-aspartate, l-glutamine and l-alanine) in the supplement only (Supplemental fig. 2). Endogenous l-aspartate levels were not significantly affected by hypo-osmotic medium or any of the tested treatments (Supplemental fig. 2). We speculate that this is due to the fact that the bulk of intracellular l-aspartate is located within the mitochondria and that the mitochondrial pool is slowly exchanged with the cytosol. The pattern of changes in l-glutamine levels was very similar to that of l-glutamate, albeit with higher variability (Fig. 1 and Supplemental fig. 2). The l-alanine content was significantly reduced by cell swelling but not additionally affected by the inhibitors of glutamate transport and transamination (Supplemental fig. 2).
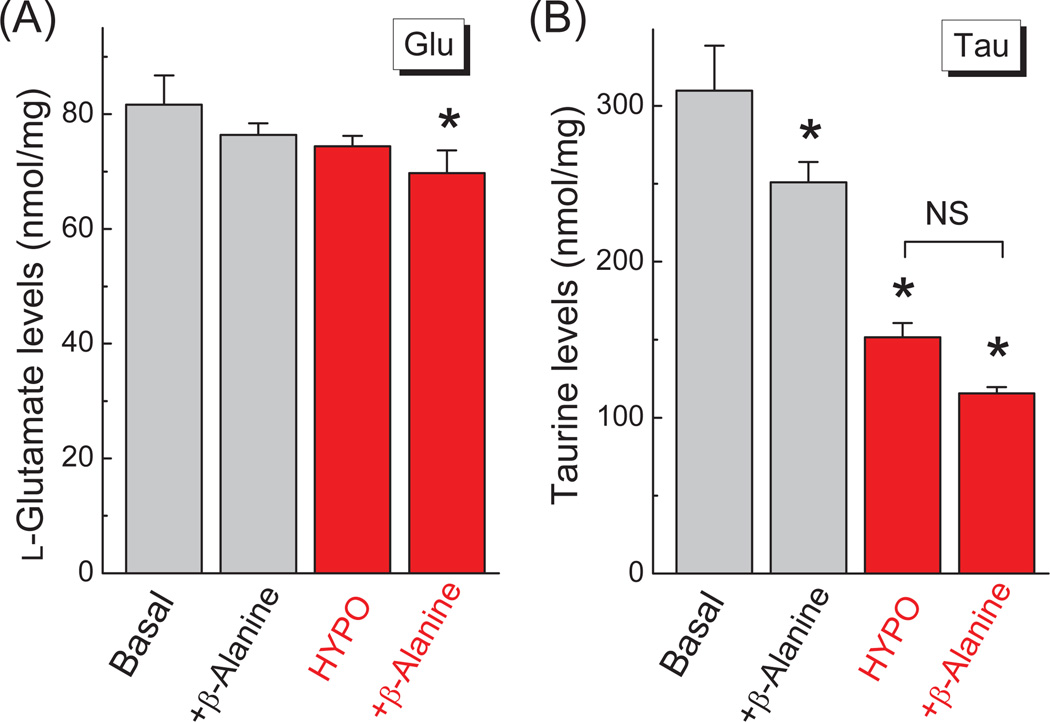
To evaluate if inhibition of taurine reuptake accelerates hypoosmotic loss of intracellular taurine, much like the effect of the glutamate transport blocker TBOA on intracellular glutamate, we used the competitive TauT transporter inhibitor β-alanine (Larsson et al. 1986; Nishimura et al. 2010). Sub-mM levels of β-alanine, which are sufficient to selectively suppress taurine uptake, caused a ~25% drop in the intracellular taurine levels under both basal and hypo-osmotic conditions (Fig. 4A). This effect is most likely determined by the previously reported heteroexchange of β-alanineout for taurinein (Larsson et al. 1986). Unfortunately, non-transportable selective inhibitors of TauT are not available. Nevertheless, since β-alanine did not cause “selective” acceleration of taurine loss from swollen cells, it is unlikely that TauT can preserve intracellular levels of taurine. As a control, the effects of β-alanine on intracellular levels of glutamate was observed in the same cells and did not produce any significant changes under basal or hypoosmotic conditions (Fig 4B).
Fig. 4. The competitive inhibitor of the taurine transporter TauT, β-alanine, alters intracellular amino acid levels in a fashion, which is not dependent on cell swelling.
(A) Effect of 800 µM β-alanine on the intracellular content of glutamate (Glu) under iso-osmotic and hypo-osmotic (−58 mM NaCl) conditions. Amino acid levels were determined in cell lysates after a 30-min exposure to the specified conditions. (B) Effect of β-alanine on the intracellular content of taurine (Tau) in the same cellular lysates as in A. The data are the means ± SEM (n=3). *p<0.05 vs. Basal (one-way ANOVA with Fisher’s LSD post hoc test).
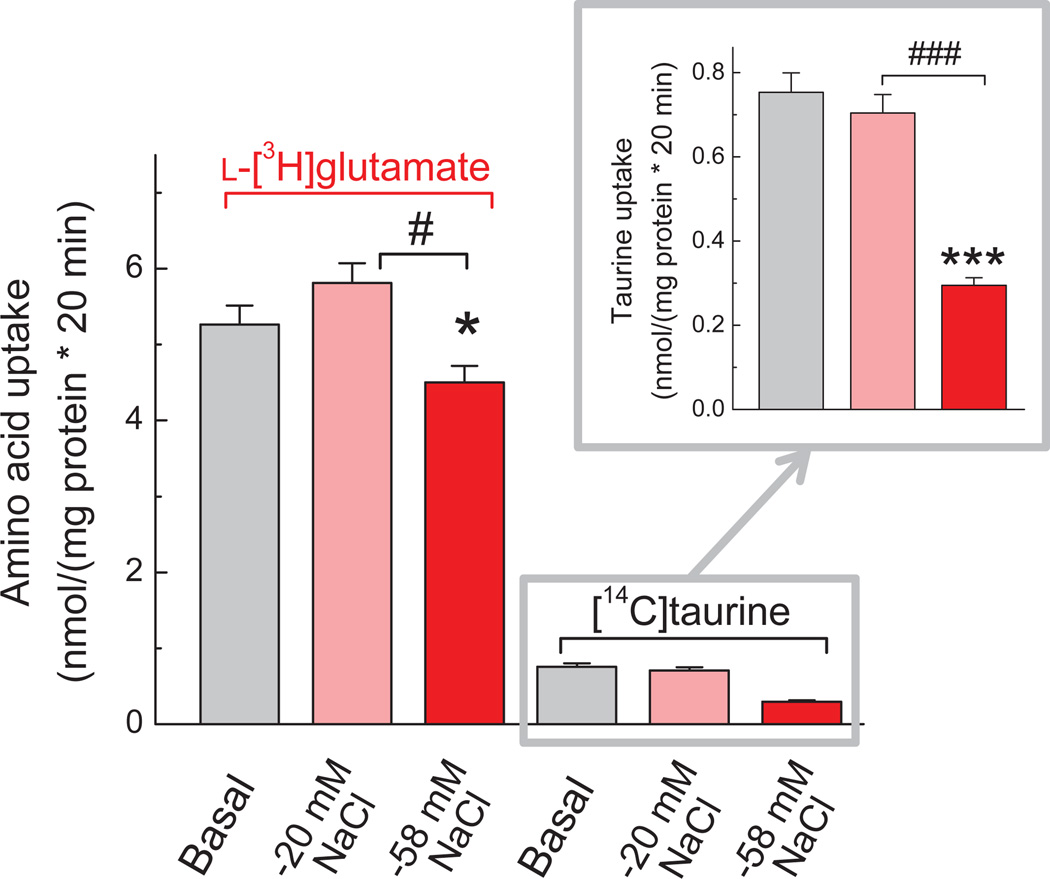
To further explore the relevance of glutamate and taurine transport, we measured the effects of medium osmolarity on astrocytic uptake of l-[3H]glutamate and [14C]taurine. The uptake rates were measured in the same cells to negate any potential differences between cell culture preparations. Under the basal conditions, calculated uptake rates for l-glutamate were >7-fold higher than for taurine, reflecting differences in densities and/or activities of the two amino acid transporters in astrocytes (Fig. 5). In mildly hypoosmotic medium (−20 mM NaCl, 250 mOsm) there was no significant change in uptake of either l-[3H]glutamate or [14C]taurine as compared to the basal conditions. In contrast, strongly hypoosmotic medium (−58 mM NaCl, 185 mOsm), inhibited both l-[3H]glutamate and [14C]taurine accumulation. Interestingly, as seen in Fig. 5, the effects on [14C]taurine uptake were much more dramatic (60% inhibition, p<0.001), as compared to l-[3H]glutamate (14.4% inhibition, p<0.05). The variance between degrees of inhibition is likely related to much higher reliance of TauT on the transmembrane Na+ gradient, as compared to the glutamate transporters GLAST and GLT-1 (see for example Haskew-Layton et al. 2008).
Fig. 5. Comparative effects of hypo-osmotic media on uptake of l-[3H]glutamate and [14C] taurine measured in the same cells.
The uptake of two labeled amino acid was measured simultaneously, in media with different osmolarities and NaCl content, for 20 min. The main figure shows values of the l-[3H]glutamate and [14C]taurine uptake on the same scale, after normalization to the protein content. The levels of both amino acids were adjusted to 2 µM by adding unlabeled l-glutamate or taurine. In the inset, the effect of hypo-osmotic media on [14C]taurine uptake is shown on an expanded scale for clarity. Data are the mean values ± SEM (n=8). *p<0.05, ***p<0.001, “−58 mM NaCl” vs. “Basal”. #p<0.05, ###p<0.001, “−20 mM NaCl” vs. “−58 mM NaCl” (one-way ANOVA with Fisher’s LSD post hoc test).
Discussion
The major finding of this study is that astrocytes can conserve intracellular l-glutamate upon exposure to hypo-osmotic media via at least two complementary mechanisms: the Na+-dependent reuptake and mitochondrial synthesis via transamination. Our work mechanistically explains paradoxical preservation of intracellular glutamate and aspartate in swollen astrocytes in vitro (see discussion below). Moreover, our new findings can help in understanding a mismatch between extracellular levels of taurine, glutamate, and other organic osmolytes seen in animal models of hyponatremia.
While studying the effects of hypo-osmotic swelling on astrocytic amino acid content, we found that endogenous taurine is lost at much higher levels as compared to l-glutamate and l-aspartate, despite the fact that these osmolytes share the same release pathway, VRAC (Hyzinski-Garcia et al. 2014) and Figs. 1, 3 in the present work). The preferential loss of taurine has also been observed in several prior in vitro studies in cultured astrocytes, other cell types, and brain slices (Kimelberg et al. 1990; Roy and Malo 1992; Rasmusson et al. 1993; Manolopoulos et al. 1997; Franco et al. 2000). Observations on the preferential release of taurine from glia, and findings that in taurine-depleted cells cell volume regulation is impaired, lead to the suggestion that this atypical amino acid serves as a major regulatory osmolyte, and that its release is regulated via a distinct mechanism (Moran et al. 1994).
There are two potential explanations for the unique osmotic properties of taurine: its specific permeation properties via VRAC, or the existence of multiple taurine release pathways. The first idea originates from the electrophysiological studies, which found that VRAC permeability for taurine exceeded the permeability for l-glutamate and l-aspartate by 3-fold or higher (Banderali and Roy 1992; Manolopoulos et al. 1997), although smaller 1.5–2-fold differences were also reported (Jackson et al. 1994). An important caveat of the electrophysiological experiments measuring taurine currents is that they all have been conducted at pH=8.1–9.0, to increase the charge on the taurine zwitterion. In contrast, l-glutamate and l-aspartate permeabilities have been determined at physiological pH values of 7.3–7.4.
To directly address the first hypothesis that VRAC is more permeable to taurine as compared to l-glutamate, we simultaneously measured the hypo-osmotic induced release of radiolabeled [14C]taurine and l-[3H]glutamate. Although we found that taurine was indeed released at higher rates than l-[3H]glutamate, the difference was quite modest (30–40%) and clearly insufficient to explain the disproportional changes in intracellular amino acid content. Furthermore, when compared under identical conditions, the efflux rates of [14C]taurine and the non-metabolizable analogue of l-glutamate, d-[3H]aspartate, were essentially the same. Supporting the present findings, we found comparable release rates for d-[3H]aspartate and [14C]taurine using a different paradigm of osmotic stress in the previous work (Mongin and Kimelberg 2005b).
The second distinct possibility is that taurine has a separate release pathway, or more than one release mechanism, and for this reason it is lost at higher rates. This second concept is supported by published data on variances between properties of Cl−/I− and taurine efflux pathways in several cell lines (Lambert and Hoffmann 1994; Shennan et al. 1994; Stutzin et al. 1999; Tomassen et al. 2004). Furthermore, several prior publications provided indirect evidence for more than one route for the swelling-activated release of different organic osmolytes (Shennan et al. 1994; Mongin et al. 1999; Shennan and Thomson 2000). The hypothesis of multiple release pathways in astrocytes was largely ruled out by our recent work involving siRNA knockdown of the VRAC component LRRC8A (Hyzinski-Garcia et al. 2014). In HEK293 cells and astrocytes, downregulation of a single LRRC8A protein blocked RVD, eliminated VRAC currents, and prevented the hypo-osmotic loss of endogenous taurine, l-glutamate, and other organic osmolytes (Voss et al. 2014; Hyzinski-Garcia et al. 2014).
Our present work provides a simple alternative explanation for discrepancies between reductions in taurine and l-glutamate in swollen astrocytes. The pharmacological data strongly support the idea that intracellular l-glutamate levels are preserved by rapid reuptake and de novo synthesis. Combination of the glutamate transport blocker TBOA and the transaminase inhibitor AOA, strongly accelerated the loss of l-glutamate, without impacting the intracellular taurine content. In fact, in the presence of TBOA+AOA we found no statistical difference between the losses of two organic osmolytes (see summary diagram in Fig. 6). In order to explore if the taurine transporter TauT also buffers hypo-osmotic loss of intracellular taurine and how this effect may be relevant to differences between behavior of taurine and other amino acids, we used the competitive inhibitor of TauT, β-alanine. β-Alanine caused some reductions in the intracellular taurine content, however, this effect was not cell volume dependent and is likely related to the previously reported heteroexchange of extracellular β-alanine for intracellular taurine (Larsson et al. 1986). In additional assays evaluating TauT activity, we found that the taurine uptake rate was more than 7-fold lower than that of l-glutamate under basal conditions, and this difference was further accentuated in hypoosmotic media. Therefore, the most plausible explanation for ineffective taurine buffering is the low density of TauT in astrocytes and strong inhibition of TauT by decreased extracellular Na+ levels.
Fig. 6. Mechanisms mediating the preservation of intracellular glutamate levels in swollen astroglial cells.
(A) The hypothetical diagram summarizing the role of VRAC, glutamate transporter GLAST, and mitochondrial transamination (TA) in the release and preservation of astrocytic glutamate during cell swelling (see text for additional details). (B) Side-by-side comparison of the intracellular contents of l-glutamate and taurine in primary astrocytes subjected to hypo-osmotic swelling. Addition of TBOA plus AOA completely eliminated the sparing of intracellular glutamate levels and the difference between l-glutamate and taurine behavior in swollen cells. **p<0.01 (n=5–8, one-way ANOVA with Fisher’s LSD post hoc test).
The broader significance of this study is that it helps to understand a number of in vivo phenomena. In the acute phase of hyponatremia, microdialysis studies revealed paradoxically high extracellular levels of taurine as compared to other organic osmolytes. For example, several groups found dramatic increases in the microdialysate concentrations of taurine with minimal or no changes in microdialysate levels of l-glutamate, l-aspartate, N-acetylaspartate, or other major cytosolic osmolytes (Wade et al. 1988; Lehmann 1989; Taylor et al. 1995; Estevez et al. 1999). Our present model experiments suggest that glial glutamate transporters can at least partially buffer changes in the extracellular excitatory amino acids levels during acute hyponatremia. Consistent with this idea, we previously found that the microdialysate delivery of the glutamate transport blocker TBOA, more than doubles hyponatremia-induced release of l-glutamate and l-aspartate in the rat cortex (Haskew-Layton et al. 2008). High degrees of hypotonicity are sufficient to override glutamate buffering, leading to progressively higher l-glutamate and l-aspartate release rates, presumably via VRAC, and trigger neurological manifestations of hyponatremia (Wade et al. 1988; Lehmann 1989; Taylor et al. 1995).
Overall, reduction of glutamate release to the extracellular space via the proposed by us buffering of l-glutamate release in the hyponatremic brain is expected to be beneficial. This buffering likely limits hyperexcitability and allows for sustained activity of the glutamate-glutamine cycle and other synthetic processes, which heavily rely on high homeostatic levels of glutamate in the cytosol and other intracellular compartments.
Supplementary Material
Acknowledgements
We thank Maria C. Hyzinski-Garcia for methodological help with HPLC experiments and preparation of primary cell cultures. This work was supported by grant R01 NS061953 from the National Institutes of Health (to A.A.M.).
Abbreviations used
- AOA
aminooxyacetic acid
- HIHS
heat-inactivated horse serum
- LRRC8
leucine rich-repeat containing protein family 8
- MEM
minimal essential medium
- MSO
l-methionine sulfoximine
- RVD
regulatory volume decrease
- TBOA
dl-threo-β-benzyloxyaspartic acid
- TCA
tricarboxylic acid cycle
- VRAC
volume-regulated anion channel
Footnotes
conflict of interest statement
The authors declare that they have no conflict of interest.
References
- Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, Mongin AA. Pharmacological comparison of swelling-activated excitatory amino acid release and Cl− currents in rat cultured astrocytes. J. Physiol. 2006;572:677–689. doi: 10.1113/jphysiol.2005.103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrogue HJ, Madias NE. Hyponatremia. N. Engl. J. Med. 2000;342:1581–1589. doi: 10.1056/NEJM200005253422107. [DOI] [PubMed] [Google Scholar]
- Akita T, Okada Y. Characteristics and roles of the volume-sensitive outwardly rectifying (VSOR) anion channel in the central nervous system. Neuroscience. 2014;275:211–231. doi: 10.1016/j.neuroscience.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- Banderali U, Roy G. Anion channels for amino-acids in Mdck cells. Am. J. Physiol. 1992;263:C1200–C1207. doi: 10.1152/ajpcell.1992.263.6.C1200. [DOI] [PubMed] [Google Scholar]
- Bowens NH, Dohare P, Kuo YH, Mongin AA. DCPIB, the proposed selective blocker of volume-regulated anion channels, inhibits several glutamate transport pathways in glial cells. Mol. Pharmacol. 2013;83:22–32. doi: 10.1124/mol.112.080457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Estevez AY, O'Regan MH, Song D, Phillis JW. Effects of anion channel blockers on hyposmotically induced amino acid release from the in vivo rat cerebral cortex. Neurochem. Res. 1999;24:447–452. doi: 10.1023/a:1020902104056. [DOI] [PubMed] [Google Scholar]
- Farinelli SE, Nicklas WJ. Glutamate metabolism in rat cortical astrocyte cultures. J. Neurochem. 1992;58:1905–1915. doi: 10.1111/j.1471-4159.1992.tb10068.x. [DOI] [PubMed] [Google Scholar]
- Franco R, Quesada O, Pasantes-Morales H. Efflux of osmolyte amino acids during isovolumic regulation in hippocampal slices. J. Neurosci. Res. 2000;61:701–711. doi: 10.1002/1097-4547(20000915)61:6<701::AID-JNR14>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Fraser CL, Arieff AI. Epidemiology, pathophysiology, and management of hyponatremic encephalopathy. Am. J. Med. 1997;102:67–77. doi: 10.1016/s0002-9343(96)00274-4. [DOI] [PubMed] [Google Scholar]
- Gullans SR, Verbalis JG. Control of brain volume during hyperosmolar and hypoosmolar conditions. Annu. Rev. Med. 1993;44:289–301. doi: 10.1146/annurev.me.44.020193.001445. [DOI] [PubMed] [Google Scholar]
- Haskew-Layton RE, Rudkouskaya A, Jin Y, Feustel PJ, Kimelberg HK, Mongin AA. Two distinct modes of hypoosmotic medium-induced release of excitatory amino acids and taurine in the rat brain in vivo. PLoS ONE. 2008;3:e3543. doi: 10.1371/journal.pone.0003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol. Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- Hyzinski-Garcia MC, Rudkouskaya A, Mongin AA. LRRC8A protein is indispensable for swelling-activated and ATP-induced release of excitatory amino acids in rat astrocytes. J. Physiol. 2014;592:4855–4862. doi: 10.1113/jphysiol.2014.278887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyzinski-Garcia MC, Vincent MY, Haskew-Layton RE, Dohare P, Keller RW, Jr, Mongin AA. Hypoosmotic swelling modifies glutamate-glutamine cycle in the cerebral cortex and in astrocyte cultures. J. Neurochem. 2011;118:140–152. doi: 10.1111/j.1471-4159.2011.07289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PS, Morrison R, Strange K. The volume-sensitive organic osmolyte-anion channel VSOAC is regulated by nonhydrolytic ATP binding. Am. J. Physiol. 1994;267:C1203–C1209. doi: 10.1152/ajpcell.1994.267.5.C1203. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Current concepts of brain edema. Review of laboratory investigations. J. Neurosurg. 1995;83:1051–1059. doi: 10.3171/jns.1995.83.6.1051. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J. Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert IH, Hoffmann EK. Cell swelling activates separate taurine and chloride channels in Ehrlich mouse ascites tumor cells. J. Membr. Biol. 1994;142:289–298. doi: 10.1007/BF00233436. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Larsson OM, Griffiths R, Allen IC, Schousboe A. Mutual inhibition kinetic analysis of gamma-aminobutyric acid, taurine, and beta-alanine high-affinity transport into neurons and astrocytes: evidence for similarity between the taurine and beta-alanine carriers in both cell types. J. Neurochem. 1986;47:426–432. doi: 10.1111/j.1471-4159.1986.tb04519.x. [DOI] [PubMed] [Google Scholar]
- Lehmann A. Effects of microdialysis-perfusion with anisoosmotic media on extracellular amino acids in the rat hippocampus and skeletal muscle. J. Neurochem. 1989;53:525–535. doi: 10.1111/j.1471-4159.1989.tb07365.x. [DOI] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Manolopoulos VG, Voets T, Declercq PE, Droogmans G, Nilius B. Swelling-activated efflux of taurine and other organic osmolytes in endothelial cells. Am. J. Physiol. 1997;273:C214–C222. doi: 10.1152/ajpcell.1997.273.1.C214. [DOI] [PubMed] [Google Scholar]
- McKenna MC. The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J. Neurosci. Res. 2007;85:3347–3358. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Sonnewald U, Huang X, Stevenson J, Zielke HR. Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J. Neurochem. 1996;66:386–393. doi: 10.1046/j.1471-4159.1996.66010386.x. [DOI] [PubMed] [Google Scholar]
- Medrano S, Gruenstein E. Mechanisms of regulatory volume decrease in UC-11MG human astrocytoma cells. Am. J. Physiol. 1993;264:C1201–C1209. doi: 10.1152/ajpcell.1993.264.5.C1201. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Hyzinski-Garcia MC, Vincent MY, Keller RW., Jr A simple method for measuring intracellular activities of glutamine synthetase and glutaminase in glial cells. Am. J. Physiol. Cell Physiol. 2011;301:C814–C822. doi: 10.1152/ajpcell.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongin AA, Kimelberg HK. Astrocytic swelling in neuropathology. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford/New York: Oxford University Press; 2005a. pp. 550–562. [Google Scholar]
- Mongin AA, Kimelberg HK. ATP regulates anion channel-mediated organic osmolyte release from cultured rat astrocytes via multiple Ca2+-sensitive mechanisms. Am. J. Physiol. Cell Physiol. 2005b;288:C204–C213. doi: 10.1152/ajpcell.00330.2004. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Orlov SN. Mechanisms of cell volume regulation and possible nature of the cell volume sensor. Pathophysiology. 2001;8:77–88. doi: 10.1016/s0928-4680(01)00074-8. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Reddi JM, Charniga C, Kimelberg HK. [3H]Taurine and D-[3H]aspartate release from astrocyte cultures are differently regulated by tyrosine kinases. Am. J. Physiol. Cell Physiol. 1999;276:C1226–C1230. doi: 10.1152/ajpcell.1999.276.5.C1226. [DOI] [PubMed] [Google Scholar]
- Moran J, Maar T, Pasantes-Morales H. Cell volume regulation in taurine deficient cultured astrocytes. Adv. Exp. Med. Biol. 1994;359:361–367. doi: 10.1007/978-1-4899-1471-2_37. [DOI] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Prog. Biophys. Mol. Biol. 1997;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Sai Y, Fujii J, Muta M, Iizasa H, Tomi M, Deureh M, Kose N, Nakashima E. Roles of TauT and system A in cytoprotection of rat syncytiotrophoblast cell line exposed to hypertonic stress. Placenta. 2010;31:1003–1009. doi: 10.1016/j.placenta.2010.08.003. [DOI] [PubMed] [Google Scholar]
- O'Connor ER, Kimelberg HK, Keese CR, Giaever I. Electrical resistance method for measuring volume changes in monolayer cultures applied to primary astrocyte cultures. Am. J. Physiol. 1993;264:C471–C478. doi: 10.1152/ajpcell.1993.264.2.C471. [DOI] [PubMed] [Google Scholar]
- Olson JE. Osmolyte contents of cultured astrocytes grown in hypoosmotic medium. Biochim. Biophys. Acta. 1999;1453:175–179. doi: 10.1016/s0925-4439(98)00090-8. [DOI] [PubMed] [Google Scholar]
- Pasantes Morales H, Schousboe A. Volume regulation in astrocytes: a role for taurine as an osmoeffector. J. Neurosci. Res. 1988;20:503–509. doi: 10.1002/jnr.490200415. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Franco R, Ordaz B, Ochoa LD. Mechanisms counteracting swelling in brain cells during hyponatremia. Arch. Med. Res. 2002;33:237–244. doi: 10.1016/s0188-4409(02)00353-3. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Murray RA, Lilja L, Moran J. Regulatory volume decrease in cultured astrocytes. I. Potassium- and chloride-activated permeability. Am. J. Physiol. 1994;266:C165–C171. doi: 10.1152/ajpcell.1994.266.1.C165. [DOI] [PubMed] [Google Scholar]
- Pedersen SF, Klausen TK, Nilius B. The identification of a volume-regulated anion channel: an amazing Odyssey. Acta Physiol (Oxf) 2015;213:868–881. doi: 10.1111/apha.12450. [DOI] [PubMed] [Google Scholar]
- Podesta MA, Faravelli I, Cucchiari D, Reggiani F, Oldani S, Fedeli C, Graziani G. Neurological counterparts of hyponatremia: pathological mechanisms and clinical manifestations. Curr. Neurol. Neurosci. Rep. 2015;15:536. doi: 10.1007/s11910-015-0536-2. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell. 2014;157:447–458. doi: 10.1016/j.cell.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson RL, Davis DG, Lieberman M. Amino acid loss during volume regulatory decrease in cultured chick heart cells. Am. J. Physiol. 1993;264:C136–C145. doi: 10.1152/ajpcell.1993.264.1.C136. [DOI] [PubMed] [Google Scholar]
- Risher WC, Andrew RD, Kirov SA. Real-time passive volume responses of astrocytes to acute osmotic and ischemic stress in cortical slices and in vivo revealed by two-photon microscopy. Glia. 2009;57:207–221. doi: 10.1002/glia.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzio RA, Rowe WB, Meister A. Studies on the mechanism of inhibition of glutamine synthetase by methionine sulfoximine. Biochemistry. 1969;8:1066–1075. doi: 10.1021/bi00831a038. [DOI] [PubMed] [Google Scholar]
- Roy G, Malo C. Activation of amino acid diffusion by a volume increase in cultured kidney (MDCK) cells. J. Membr. Biol. 1992;130:83–90. doi: 10.1007/BF00233740. [DOI] [PubMed] [Google Scholar]
- Shennan DB, McNeillie SA, Curran DE. The effect of a hyposmotic shock on amino acid efflux from lactating rat mammary tissue: stimulation of taurine and glycine efflux via a pathway distinct from anion exchange and volume-activated anion channels. Exp. Physiol. 1994;79:797–808. doi: 10.1113/expphysiol.1994.sp003808. [DOI] [PubMed] [Google Scholar]
- Shennan DB, Thomson J. Further evidence for the existence of a volume-activated taurine efflux pathway in rat mammary tissue independent from volume-sensitive Cl- channels. Acta Physiol Scand. 2000;168:295–299. doi: 10.1046/j.1365-201x.2000.00658.x. [DOI] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am. J. Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Stutzin A, Torres R, Oporto M, Pacheco P, Eguiguren AL, Cid LP, Sepulveda FV. Separate taurine and chloride efflux pathways activated during regulatory volume decrease. Am. J. Physiol. Cell Physiol. 1999;277:C392–C402. doi: 10.1152/ajpcell.1999.277.3.C392. [DOI] [PubMed] [Google Scholar]
- Sykova E. The extracellular space in the CNS: Its regulation, volume and geometry in normal and pathological neuronal function. Neuroscientist. 1997;3:28–41. [Google Scholar]
- Taylor DL, Davies SE, Obrenovitch TP, Doheny MH, Patsalos PN, Clark JB, Symon L. Investigation into the role of N-acetylaspartate in cerebral osmoregulation. J. Neurochem. 1995;65:275–281. doi: 10.1046/j.1471-4159.1995.65010275.x. [DOI] [PubMed] [Google Scholar]
- Tomassen SF, Fekkes D, De Jonge HR, Tilly BC. Osmotic swelling-provoked release of organic osmolytes in human intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2004;286:C1417–C1422. doi: 10.1152/ajpcell.00468.2003. [DOI] [PubMed] [Google Scholar]
- Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am. J. Med. 2006;119:S30–S35. doi: 10.1016/j.amjmed.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science. 2014;344:634–638. doi: 10.1126/science.1252826. [DOI] [PubMed] [Google Scholar]
- Wade JV, Olson JP, Samson FE, Nelson SR, Pazdernik TL. A possible role for taurine in osmoregulation within the brain. J. Neurochem. 1988;51:740–745. doi: 10.1111/j.1471-4159.1988.tb01807.x. [DOI] [PubMed] [Google Scholar]
- Wasterlain CG, Torack RM. Cerebral edema in water intoxication. II. An ultrastructural study. Arch. Neurol. 1968;19:79–87. doi: 10.1001/archneur.1968.00480010097008. [DOI] [PubMed] [Google Scholar]
- Wehner F, Olsen H, Tinel H, Kinne-Saffran E, Kinne RK. Cell volume regulation: osmolytes, osmolyte transport, and signal transduction. Rev. Physiol Biochem. Pharmacol. 2003;148:1–80. doi: 10.1007/s10254-003-0009-x. [DOI] [PubMed] [Google Scholar]
- Whitelaw BS, Robinson MB. Inhibitors of glutamate dehydrogenase block sodium-dependent glutamate uptake in rat brain membranes. Front Endocrinol. (Lausanne) 2013;4:123. doi: 10.3389/fendo.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.