Abstract
Background. In August 2012, the Chicago Department of Public Health (CDPH) was notified of acute respiratory illness, including 1 fatality, among a group of meeting attendees who stayed at a Chicago hotel during July 30–August 3, 2012. Suspecting Legionnaires' disease (LD), CDPH advised the hotel to close their swimming pool, spa, and decorative lobby fountain and began an investigation.
Methods. Case finding included notification of individuals potentially exposed during July 16–August 15, 2012. Individuals were interviewed using a standardized questionnaire. An environmental assessment was performed.
Results. One hundred fourteen cases were identified: 11 confirmed LD, 29 suspect LD, and 74 Pontiac fever cases. Illness onsets occurred July 21–August 22, 2012. Median age was 48 years (range, 22–82 years), 64% were male, 59% sought medical care (15 hospitalizations), and 3 died. Relative risks for hotel exposures revealed that persons who spent time near the decorative fountain or bar, both located in the lobby were respectively 2.13 (95%, 1.64–2.77) and 1.25 (95% CI, 1.09–1.44) times more likely to become ill than those who did not. Legionella pneumophila serogroup 1 was isolated from samples collected from the fountain, spa, and women's locker room fixtures. Legionella pneumophila serogroup 1 environmental isolates and a clinical isolate had matching sequence-based types. Hotel maintenance records lacked a record of regular cleaning and disinfection of the fountain.
Conclusions. Environmental testing identified Legionella in the hotel's potable water system. Epidemiologic and laboratory data indicated the decorative fountain as the source. Poor fountain maintenance likely created favorable conditions for Legionella overgrowth.
Keywords: fountain outbreak, legionellosis, Pontiac fever
Legionnaires' disease (LD), an often-severe pneumonia, and Pontiac fever (PF), a milder flu-like illness, are caused by environmental exposure to Legionella bacteria. Cases of LD have increased 3- to 5-fold during the past 10 years [1], and outbreaks with high case counts of LD and PF are atypical [2, 3]. Legionella-related illness can occur when water contaminated with Legionella becomes aerosolized and is inhaled or aspirated [4–6]. Outbreaks have been linked to both community and hospital settings as well as aerosol-producing devices which include the following: whirlpools, decorative fountains, and cooling towers [2–4, 6–18].
On August 14, 2012, the Chicago Department of Public Health (CDPH) was contacted by an occupational health nurse from Company A regarding 30 employees experiencing illness after meetings held at a Chicago hotel (Hotel X) during July 30–August 3, 2012. Symptoms reported by these 30 employees included fever, cough, and diarrhea. A few employees had also been diagnosed with pneumonia, at least 2 were known to be hospitalized, and 1 employee had died on August 13 while hospitalized for an acute respiratory illness. Overall, ∼600 Company A employees from various regional offices in the United States had been at Hotel X for meetings: 427 employees attended a sales meeting held July 30–August 1, 2012; 266 employees attended a leadership meeting held August 2–3, 2012; and ∼80 employees attended both meetings.
Hotel X is a 600-room hotel that opened in November 2010. The hotel occupies the lower portion (basement level through 12th story) of a historic building in Chicago. Before opening, the hotel performed major renovations to their portion of the building, including installation of new plumbing. A restaurant is located on the second floor, and a bar serving food and drinks is located in the lobby. At the time of this outbreak, a decorative fountain was located in the center of the lobby; the bar seating area was immediately adjacent to the fountain. A day spa (which includes a steam room), pool, whirlpool, fitness room, and locker rooms are on the lower level.
After initial notification, the CDPH contacted the hotel to begin the investigation and learned that the hotel had already received a separate report of acute respiratory illness in a hotel guest not affiliated with Company A. The CDPH's investigation focused on identifying the type and source of respiratory illness and the prevention of additional cases.
METHODS
Case Definitions
We defined 3 case categories for this investigation. A confirmed case of LD was defined as a person who stayed at or visited the hotel with onset of illness between 2 and 14 days of exposure to Hotel X, and with radiographically or autopsy-confirmed pneumonia, and with laboratory evidence of Legionella infection. Laboratory evidence included at least 1 of the following: isolation through culture of any Legionella organism from respiratory secretions, lung tissue, pleural fluid, or other normally sterile fluid; detection of Legionella pneumophila serogroup 1 (Lp1) antigen in urine; seroconversion, specifically a 4-fold greater rise in specific antibody titer to Lp1 between acute and convalescent titers; or detection of Lp1 by molecular testing (polymerase chain reaction [PCR]) in respiratory secretions. A suspect case of LD was defined as illness in person who stayed at or visited Hotel X with onset between 2 and 14 days of exposure to the hotel who had pneumonia confirmed by radiographic report or by clinical diagnosis but without laboratory confirmation of Legionella infection. A case of PF was defined as fever, either subjective or documented, in a person who stayed at or visited Hotel X with onset of illness within 3 days of exposure to hotel and at least 1 of the following symptoms: headache, cough, shortness of breath, myalgias, vomiting, or diarrhea, and who did not meet definition for confirmed or suspect LD.
Case Finding
Heightened surveillance for additional cases was performed. On August 14, 2012, we attempted to contact organizers of all other events held during mid-July through mid-August 2012 at Hotel X (n = 77). On August 15, 2012, notifications about the outbreak were made to local, statewide, and national public health agencies. To define the exposure period, the CDPH chose the earliest potential exposure date to accommodate all hotel guests and visitors known to date with respiratory illness; the last possible exposure date corresponded to the date when possible water sources were removed from public access. On August 19, 2012, the hotel was instructed to contact all guests with stays between July 16 and August 15, 2012 notifying them of their potential exposure to LD. Hotel management attempted to reach all hotel guests by phone and/or mail. The CDPH issued press releases to augment the hotel's direct notification of guests. The press releases instructed hotel guests experiencing acute respiratory illness to contact the CDPH. To accommodate these reports, the CDPH operated a telephone hotline from August 21 through September 7, 2012.
Cohort Study
To determine an etiology and risk factors for disease, we performed a cohort study involving hotel guests who stayed at or visited Hotel X between July 16 and August 15, 2012. A standardized questionnaire was developed to identify potential sources of exposure and to obtain information about the clinical characteristics of illness using information from initial case interviews and elements of the Centers for Disease Control and Prevention (CDC)'s unknown respiratory disease outbreak questionnaire [19]. Two versions of the questionnaire were used during the investigation: (1) a self-administered electronic version for Company A meeting attendees and (2) a paper version for CDPH staff to administer to individuals reporting illness via the hotline. Case-patients were compared with controls, defined as a person with absence of any illness.
For individuals who indicated by survey that they submitted clinical specimens or had chest x-rays performed by their private physicians (n = 90), the CDPH attempted to obtain clinical records to confirm the diagnosis rather than relying solely on the patient self-report.
Environmental Investigation
Water samples and biofilm swab specimens were collected according to Illinois Department of Public Health (IDPH) guidelines [20] on August 15, 17, and 18, 2012 from multiple sites, including the decorative fountain, whirlpool spa, pool, locker rooms, and hotel guest rooms. Legionella isolation and confirmations were carried out by EMSL Analytical, Inc. (Cinnaminson, NJ) using direct fluorescent antibody stains for Legionella species: Legionella anisa, Legionella bozemanii, Legionella dumoffii, Legionella gormanii, Legionella jordanis, Legionella longbeachae, Legionella maceachernii, Legionella micadei, Legionella sainthelensi, and each L pneumophila serogroup (1–14).
Laboratory Methods
The CDC performed monoclonal antibody (MAb) and sequence-based typing (SBT) on environmental and clinical isolates. All isolates were MAb typed by an immunodot method using MAb1 [21], MAb75 [22], and MAb2 [21] as previously described [23]. A mix of MAb1 and MAb75 was used to confirm that the L pneumophila isolates tested were serogroup 1; MAb2 was used to identify MAb2-positive strains.
Sequence-based typing was performed using the European Society of Clinical Microbiology and Infectious Diseases Study Group for Legionella Infections SBT protocol for epidemiological typing of L pneumophila (version 5.0) with M13-tagged primers [24]. For each isolate, the combination of 7 alleles was defined as a 7-digit allelic profile by using the predetermined order flaA, pilE, asd, mip, mompS, proA, and neuA (eg, 1-4-3-1-1-1-1) and a sequence type (ST) represented by a number (eg, ST1) [25, 26].
Statistical Analysis
Data from the online questionnaire were electronically transferred to a Microsoft Excel spreadsheet, and analyses were done with SAS software (version 9.3; SAS Institute). Relative risks and 95% confidence intervals (CIs) were calculated to assess categorical exposure variables. Fisher's exact test was used to calculate P values when an expected cell was <5. In all data analyses, P < .05 was considered to be significant.
RESULTS
Case Finding
We reached 48 of 77 organizers of other events held at Hotel X during mid-July through mid-August 2012. Of these, 5 event organizers noted that they had heard about complaints of illness. Dates for 4 of 5 of these events coincided with the exposure period. One event was held on July 15, 2012, but the one person who reported illness did not have symptoms consistent with either LD or PF. As a result of our notifications, several event organizers contacted participants and queried them for symptoms of illness. Some of these participants in turn reported their illness via the CDPH hotline.
One hundred forty-five people who contacted the CDPH by telephone completed a questionnaire; 328 people associated with Company A completed an electronic questionnaire using the Internet. Of this combined total, 114 cases were identified: 11 confirmed LD, 29 suspect LD, and 74 PF cases.
Cohort Study
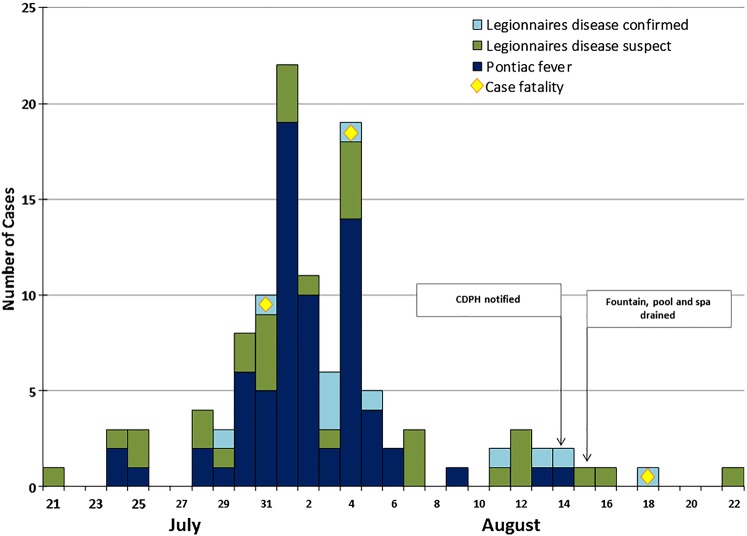
Illness onsets for all cases occurred July 21–August 22, 2012 (Figure 1). Median age was highest for confirmed LD cases (65 years [range, 49–82 years]) and lowest for PF cases (44 years [range, 22–65 years]). In all 3 case categories, there was a higher percentage of males. Fifty-nine percent of all cases sought medical care. Eighty-two percent of confirmed LD cases and 12% of suspect LD cases were hospitalized compared with 3% of PF cases. Three (27%) of the 11 confirmed LD cases died (Table 1). One hundred fourteen case-patients reported the following symptoms: fever (99%), cough (69%), shortness of breath (65%), and vomiting or diarrhea (57%). Respiratory symptoms were more common in LD cases, whereas gastrointestinal symptoms were similar for both PF and LD cases (Table 1). Eleven PF case-patients had chest radiography, and none of these had pneumonia. Detailed histories were obtained for the confirmed LD cases and revealed that 1 case-patient was obese, 1 was human immunodeficiency virus positive, 1 suffered from chronic obstructive pulmonary disease, and 5 reported hypertension or cardiovascular disease. Two case-patients were current smokers and 2 were former smokers.
Figure 1.
Epidemic curve of case by date and symptom onset (number of cases = 114). The light blue color represents confirmed cases of legionella, the green color represents suspect cases of legionella, and the dark blue color represents cases of Pontiac fever. The yellow diamond represents cases that expired.
Table 1.
Demographic, Symptoms, and Clinical Characteristics of Patients With Pontiac Fever and Legionnaires' Disease
| Characteristic | Patients With Pontiac Fever (n = 70) | Patients With Suspect Legionnaires’ Disease (n = 33) | Patients With Confirmed Legionnaires’ Disease (n = 11) |
|---|---|---|---|
| Age, median (range) | 44 (22–65) | 52 (35–78) | 65 (49–82) |
| Male sex | 41 (59) | 21 (64) | 10 (91) |
| Fever | 70 (100) | 32 (97) | 10 (91) |
| Cough | 38 (58)a | 29 (88) | 9 (90)b |
| Shortness of breath | 33 (50)a | 24 (77)b | 8 (80)b |
| Vomiting | 9 (14)a | 7 (23)c | 4 (40)c |
| Diarrhea | 31 (48)a | 15 (50)c | 4 (40)b |
| Hospitalized | 2 (3) | 4 (12) | 9 (82) |
| Deaths | 0 (0) | 0 (0) | 3 (27) |
| Smoke | 6 (9) | 4 (12) | 2 (18) |
| Asthma | 5 (7) | 3 (9) | 0 (0) |
a Denominator differs from total noted in respective disease category, but missing data does not exceed n = 5.
b Denominator differs from total noted in respective disease category, but missing data does not exceed n = 3.
c Denominator differs from total noted in respective disease category; n = 10.
Relative risks for hotel exposures revealed that persons who spent time near the decorative fountain or bar were respectively 2.13 (95%, 1.64–2.77) and 1.25 (95% CI, 1.09–1.44) times more likely to become ill than those who did not. All other associations were not significant (Table 2).
Table 2.
Epidemiologic Data (Relative Risk) for Exposures Assessed
| Exposure | Total No. Exposed | No. Ill Exposed | Total No. Not Exposed | No. Ill Not Exposed | Attack Rate % | Relative Risk | 95% Confidence Interval | P Value |
|---|---|---|---|---|---|---|---|---|
| Decorative lobby fountain | 123 | 65 | 173 | 37 | 52.85 | 2.13 | 1.64–2.77 | <.0001 |
| Lobby bar | 211 | 86 | 88 | 20 | 40.76 | 1.25 | 1.09–1.44 | .0030 |
| Pool | 4 | 3 | 252 | 96 | 75.00 | 4.76 | .50–45.10 | .3018 |
| Whirlpool spa | 5 | 3 | 251 | 96 | 60.00 | 2.38 | .40–13.99 | .3779 |
| Took a shower | 236 | 91 | 20 | 8 | 38.56 | 1.00 | .92–1.07 | .8991 |
| Took a bath | 50 | 18 | 206 | 81 | 36.00 | 0.89 | .53–1.50 | .6660 |
| Steam room | 4 | 2 | 252 | 97 | 50.00 | 1.59 | .23–11.08 | .6416 |
| Sauna | 3 | 1 | 253 | 98 | 33.33 | 0.79 | .07–8.63 | 1.0000 |
Environmental Investigation Results
Legionella pneumophila serogroup 1 was isolated from samples collected from the decorative fountain, whirlpool spa, and women's locker room fixtures (Table 3). Legionella pneumophila serogroup 1 environmental isolates and a clinical isolate reacted positively with MAb2, which is often called a “virulent marker” [27, 28]. The isolates belonged to the same sequence type ST36 (3-4-1-1-14-9-1). Hotel engineer records showed a lack of regular fountain cleaning and no record of disinfection levels within the fountain. In addition, the fountain had been constructed with narrow caliber piping, which might have limited water flow, and also contained submerged lighting. The pool, decorative fountain, and spa were drained on August 15, 2012, and the spa and locker rooms were closed to the public on August 23, 2012. Hotel X removed the fountain August 30–31, 2012. Per IDPH regulations, 2 consecutive negative test results were required for these facilities (spa, pool, and locker rooms) to reopen. These negative tests were obtained from sampling that occurred on September 20 and October 18, 2012.
Table 3.
Environmental Results (August 15–18, 2012)
| Location, Sample, or Specimen | Sample Type | Isolate | CFU/Specimen | CFU/mL |
|---|---|---|---|---|
| Decorative fountain | ||||
| General area (1) | Swab | Legionella pneumophila group 1 | 9100 | |
| Legionella anisa group 1 | 100 | |||
| General area (2) | Swab | N.D. | N.D. | |
| Top (pooled water) | Swab | L pneumophila group 1 | 25 000 | |
| L anisa group 1 | 114 000 | |||
| Inlet, northside | Swab | L anisa group 1 | 100 | |
| Ornamental feature | Swab | N.D. | N.D. | |
| Water line under ornamental feature | Swab | N.D. | N.D. | |
| Whirlpool spa | ||||
| Spa | Water | N.D. | N.D. | |
| Spa backwash | Water | N.D. | N.D. | |
| Spa backwash | Swab | N.D. | N.D. | |
| Skimmers | Swab | L pneumophila group 1 | 50 | |
| Lint strainers (2) | Swab | N.D. | N.D. | |
| Filters (2) | Swab | N.D. | N.D. | |
| Spa surfaces (4) | Swab | N.D. | N.D. | |
| Pool | ||||
| Pool | Water | L pneumophila group 1 | <1 CFU/mL | |
| Pool backwash | Water | N.D. | N.D. | |
| Pool backwash | Swab | N.D. | N.D. | |
| Skimmers | Swab | N.D. | N.D. | |
| Lint strainer | Swab | N.D. | N.D. | |
| Filters (2) | Swab | N.D. | N.D. | |
| Pool surfaces (3) | Swab | N.D. | N.D. | |
| Drinking fountain | Swab | N.D. | N.D. | |
| Locker rooms | ||||
| Men's shower | Swab | L anisa group 1 | 800 | |
| Men's sink | Swab | N.D. | ||
| Women's shower | Swab | L pneumophila group 1 | 4200 | |
| Women's sink | Swab | Legionella pneumophila group 1 | 4800 | |
| L anisa group 1 | 11 700 | |||
| Hotel guest rooms | ||||
| Room A, shower head and sink faucets (3) | Swab | N.D. | N.D. | |
| Room B, shower head and sink faucet (2) | Swab | N.D. | N.D. | |
Abbreviations: CFU, colony-forming unit; N.D., not detected.
Laboratory Investigation
Of the 11 confirmed LD cases, 10 individuals had Lp1 antigen detected in their urine, 1 individual had a sputum culture positive for Lp1, and 2 individuals had Lp1 detected by PCR of bronchoalveolar lavage fluid and sputum, respectively. Testing at the CDC showed that the sole clinical isolate from a confirmed LD case and Lp1 environmental isolates had matching sequence-based types (Table 4).
Table 4.
Sequence-Based Typing of Legionella pneumophila Serogroup 1 on Clinical and Environmental Isolatesa
| Isolate | Patient or Environmental Location | MAb (No.) | flaA | pilE | asd | mip | mompS | proA | neuA | ST |
|---|---|---|---|---|---|---|---|---|---|---|
| D7089 | LD confirmed case 1 | 1,2 | 3 | 4 | 1 | 1 | 14 | 9 | 1 | 36 |
| D7108 | Women's locker room sinks | 1,2 | 3 | 4 | 1 | 1 | 14 | 9 | 1 | 36 |
| D7104 | Spa skimmer | 1,2 | 3 | 4 | 1 | 1 | 14 | 9 | 1 | 36 |
| D7091 | water/fountain | 1,2 | 3 | 4 | 1 | 1 | 14 | 9 | 1 | 36 |
Abbreviations: LD, Legionnaires’ disease; MAb, monoclonal antibody; ST, sequence type.
a All 7 loci were identical for both clinical and environmental isolates.
DISCUSSION
This investigation describes a combined outbreak of LD and PF among 114 hotel guests and visitors who were exposed to a decorative fountain in a hotel lobby. Decorative fountains in hospitals, restaurants, and hotels have been identified as sources of either LD or PF outbreaks [4, 6, 17, 29, 30]. The large number of both LD and PF cases in this outbreak is unusual for an outbreak associated with a fountain. Ambrose et al [31] noted that previous reports with mixed outbreaks were associated with either cooling towers [7, 11, 12, 14, 15], air-conditioning units (through aerosolization of stagnant sump pump water) [32], or heated whirlpool spas or hot tubs [2, 3, 8, 10, 13, 16, 18]. Rowbotham [33] hypothesized that the etiology of PF might be caused by a hypersensitivity to a cellular component of Legionella or to a protozoan host of the bacteria. Another theory is that PF is associated with exposure to endotoxins produced from viable or nonviable Legionella species [34]. Review of hotel maintenance records indicated that the fountain lacked routine disinfection and had features that may have contributed not only to overgrowth of Legionella, but perhaps bioaccumulation of nonviable Legionella species.
Although the environmental investigation identified Lp1 in the whirlpool spa and the women's locker room fixtures, epidemiologic evidence did not support these water sources as the likely source of infection. The spa was under maintenance during the majority of the exposure period, and <5 cases mentioned using the spa during their hotel stay.
The American Society of Heating Refrigerating and Air-Conditioning Engineers provides guidance on requirements for design, construction, installation, operation, and maintenance of building water systems [35]. The IDPH inspection of the fountain indicated that there were features in the design of the fountain that might have also led to favorable conditions for Legionella growth. As of April 2015, Standard 188P is under revision; the new version will reportedly specifically address issues regarding fountain maintenance and siting. Neither the CDPH nor IDPH currently have regulatory authority over fountain construction and maintenance. In addition, the City of Chicago does not have any ordinances banning fountains from hospitals, hotels, or other buildings.
This investigation is subject to limitations. The first survey was sent to Company A 12 days after the last possible exposure. Because the incubation period for LD can be as long as 14 days, there is a possibility that individuals who were recorded as “well” might have become sick after they responded to the survey. This would have resulted in a weaker association between illness and exposures. Misclassification could have also occurred among PF cases; the case definition did not include diagnostic confirmation, and reported illnesses might have been due to another etiology; however, illness occurred during a period that is generally low for other viral etiologies, such as influenza. We tried to limit misclassification bias in another instance by choosing asymptomatic individuals for controls instead of a combination of asymptomatic individuals and ill individuals who did not meet case definition. This was done due to incomplete information for some of the individuals who responded to the questionnaire. Another limitation occurred when the paper questionnaire design was converted to an electronic version for e-mail distribution; 6 of the exposure questions were changed to “check all that apply” versus “yes/no” as in the original paper version. It was difficult to determine whether the question was skipped or was truly representative of what occurred. In an effort to account for this, we excluded individuals who left all 6 responses for this question blank. For 4 of the 6 variables, only 3–5 individuals out of a total cohort of 256 stated they had been exposed. For “took a shower” and “took a bath” (Table 2), the relative risks were 1.00 and 0.89, respectively, with narrow CIs. Therefore, we are confident that we would have detected an association for these exposures had one existed. Lastly, we are unable to assess for a dose-response relationship for amount of time spent near the fountain. We considered including such a question on the survey, but given the urgency to identify the etiology, we opted to assess only “any time spent” near the implicated exposures.
CONCLUSIONS
This was a large point-source Lp1 outbreak with substantial morbidity and mortality. The epidemiologic and molecular typing data confirmed that the fountain was the likely source of this Legionella outbreak. To the best of our knowledge, this is the first mixed outbreak with large numbers of PF and LD cases that were attributed to exposure to a fountain. Although Legionella spp were identified in other water features at the hotel, none of these were supported by epidemiologic data as the source. Poor fountain maintenance likely created favorable conditions for Legionella overgrowth, resulting in an outbreak of LD and PF [9, 36]. Building water systems require ongoing maintenance and monitoring to reduce conditions that favor Legionella growth and transmission. We suspect that mixed outbreaks are more common than what has been documented in the literature. We recommend that local health officials include both PF and LD in their case finding when investigating legionellosis outbreaks and consider laboratory diagnostics to confirm outbreak-associated cases of PF [8].
Acknowledgments
Financial support. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Centers for Disease Control and Prevention (CDC). Legionellosis—United States, 2000–2009. MMWR Morb Mortal Wkly Rep 2011; 60:1083–6. [PubMed] [Google Scholar]
- 2.Benin AL, Benson RF, Arnold KE et al. An outbreak of travel-associated Legionnaires’ disease and Pontiac fever: the need for enhanced surveillance of travel-associated legionellosis in the United States. J Infect Dis 2002; 185:237–43. [DOI] [PubMed] [Google Scholar]
- 3.Euser SM, Pelgrim M, Den Boer JW. Legionnaires’ disease and Pontiac fever after using a private outdoor whirlpool spa. Scand J Infect Dis 2010; 42:910–6. [DOI] [PubMed] [Google Scholar]
- 4.Haupt TE, Heffernan RT, Kazmierczak JJ et al. An outbreak of Legionnaires disease associated with a decorative water wall fountain in a hospital. Infect Control Hosp Epidemiol 2013; 33:185–91. [DOI] [PubMed] [Google Scholar]
- 5.Ng V, Tang P, Fisman DN. Our evolving understanding of legionellosis epidemiology: learning to count. Clin Infect Dis 2008; 47:600–2. [DOI] [PubMed] [Google Scholar]
- 6.Palmore TN, Stock F, White M et al. A cluster of cases of nosocomial Legionnaires disease linked to a contaminated decorative water fountain. Infect Control Hosp Epidemiol 2009; 30:764–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell JC, Jorm LR, Williamson M et al. Legionellosis linked with a hotel car park – how many were infected? Epidemiol Infect 1996; 116:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnsed LJ, Hicks LA, Smithee LMK et al. A large travel-associated outbreak of legionellosis among hotel guests: utility of the urine antigen assay in confirming Pontiac fever. Clin Infect Dis 2007; 44:222–8. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC). Legionnaires disease associated with potable water in a hotel—Ocean City, Maryland, October 2003–February 2004. MMWR Morb Mortal Wkly Rep 2005; 54:165–8. [PubMed] [Google Scholar]
- 10.Foster K, Gorton R, Waller J.. Outbreak of legionellosis associated with a spa pool, United Kingdom. Euro Surveill 2006; 11:pii=3053. [DOI] [PubMed] [Google Scholar]
- 11.Fraser DW, Tsai TR, Orenstein W et al. Legionnaires'disease: description of an epidemic of pneumonia. N Engl J Med 1977; 297:1189–97. [DOI] [PubMed] [Google Scholar]
- 12.Girod JC, Reichman RC, Winn WC Jr et al. Pneumonic and nonpneumonic forms of legionellosis: the result of a common-source exposure to Legionella pneumophila. Arch Intern Med 1982; 142:545–7. [PubMed] [Google Scholar]
- 13.Goldberg DJ, Wrench JG, Collier PW et al. Lochgoilhead fever: outbreak of non-pneumonic legionellosis due to Legionella micdadei. Lancet 1989; 333:316–8. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell E, O'Mahony M, Watson JM et al. Two outbreaks of legionnaires’ disease in Bolton Health District. Epidemiol Infect 1990; 104:159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolay N, Boland M, Ward M et al. Investigation of Pontiac-like illness in office workers during an outbreak of Legionnaires’ disease, 2008. Epidemiol Infect 2010; 138:1667–73. [DOI] [PubMed] [Google Scholar]
- 16.Okada M, Kawano K, Kura F et al. [The largest outbreak of legionellosis associated with spa baths: epidemic curve and environmental investigation]. Kasenshogaku Zasshi 2005; 79:365–74. [DOI] [PubMed] [Google Scholar]
- 17.O'Loughlin RE, Kightlinger L, Werpy MC et al. Restaurant outbreak of Legionnaires’ disease associated with a decorative fountain: an environmental and case-control study. BMC Infect Dis 2007; 7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas DL, Mundy LM, Tucker PC. Hot tub legionellosis: Legionnaires’ disease and Pontiac fever after a point-source exposure to Legionella pneuomophila. Arch Intern Med 1993; 153:2597–9. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Unexplained respiratory disease outbreaks: data collection forms. Available at: http://www.cdc.gov/urdo/sampleforms.html. Accessed 15 August 2012.
- 20.Illinois Department of Public Health (IDPH). Legionnaires’ Disease: Guideline for the Surveillance, Investigation, and Control of Legionnaires’ Disease in Illinois, 2014. Available at http://www.dph.illinois.gov/forms-publications. Accessed 27 July 2015.
- 21.Joly JR, McKinney RM, Tobin JO et al. Development of a standardized subgrouping scheme for Legionella pneumophila serogroup 1 using monoclonal antibodies. J Clin Microbiol 1986; 23:768–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Para MF, Plouffe JF. Production of monoclonal antibodies to Legionella pneumophila serogroups 1 and 6. J Clin Microbiol 1983; 18:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanden GN, Cassiday PK, Barbaree JM. Rapid immunodot technique for identifying Bordetella pertussis. J Clin Microbiol 1993; 31:170–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mentasti M, Fry NK.. Sequence-Based Typing Protocol for Epidemiological Typing of Legionella pneumophila, p. 1–11, 8 October 2012 ed ESCMID Study Group for Legionella Infections; Available at: http://bioinformatics.phe.org.uk/legionella/legionella_sbt/php/protocols/ESGLI%20SBT%20GUIDELINE%20v5.0.pdf. Accessed 27 July 2015. [Google Scholar]
- 25.Gaia V, Fry NK, Afshar B et al. Consensus sequence-based scheme for epidemiological typing of clinical and environmental isolates of Legionella pneumophila. J Clin Microbiol; 43:2047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratzow S, Gaia V, Helbig JH et al. Addition of neuA, the gene encoding N-acylneuraminate cytidylyl transferase, increases the discriminatory ability of the consensus sequence-based scheme for typing Legionella pneumophila serogroup 1 strains. J Clin Microbiol 2007; 45:1965–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helbig JH, Bernander S, Castellani Pastoris M et al. Pan-European study on culture-proven Legionnaires’ disease: distribution of Legionella pneumophila serogroups and monoclonal subgroups. Eur J Clin Microbiol Infect Dis 2002; 21:710–6. [DOI] [PubMed] [Google Scholar]
- 28.Kozak NA, Benson RF, Brown E et al. Distribution of lag-1 alleles and sequence-based types among Legionella pneumophila serogroup 1 clinical and environmental isolates in the United States. J Clin Microbiol 2009; 47:2525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hlady WG, Mullen RC, Mintz CS et al. Outbreak of Legionnaires’ disease linked to a decorative fountain by molecular epidemiology. Am J Epidemiol 1993; 138:555–62. [DOI] [PubMed] [Google Scholar]
- 30.Jones TF, Benson RF, Brown EW et al. Epidemiologic investigation of a restaurant-associated outbreak of Pontiac fever. Clin Infect Dis 2003; 37:1292–7. [DOI] [PubMed] [Google Scholar]
- 31.Ambrose J, Hampton LM, Fleming-Dutra KE et al. Large outbreak of Legionnaires’ disease and Pontiac fever at a military base. Epidemiol Infect 2014; 142:2336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Mahony MC, Stanwell-Smith RE, Tillett HE et al. The Stafford outbreak of Legionnaires’ disease. Epidemiol Infect 1990; 104:361–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rowbotham TJ. Pontiac fever explained? Lancet 1980; 2:969. [DOI] [PubMed] [Google Scholar]
- 34.Fields BS, Haupt T, Davis JP et al. Pontiac fever due to Legionella micdadei from a whirlpool spa: possible role of bacterial endotoxin. J Infect Dis 2001; 184:1289–92. [DOI] [PubMed] [Google Scholar]
- 35.ASHRAE, Standard 188-2015 Legionellosis: Risk Management for Building Water Systems, 1–18. Available at http://www.ashrae.org/resources--publications/bookstore/ansi-ashrae-standard-188-2015-legionellosis-risk-management-for-building-water-systems. Accessed 27 Jul 2015.
- 36.McEvoy M, Batchelor N, Hamilton G et al. A cluster of cases of legionnaires’ disease associated with exposure to a spa pool on display. Commun Dis Public Health 2000; 3:43–5. [PubMed] [Google Scholar]