Abstract
Human herpesvirus 6 (HHV6) encephalitis and Wernicke’s encephalopathy are treatable yet frequently undiagnosed causes of encephalopathy in pediatric recipients of allogeneic and autologous hematopoietic cell transplantation. Here we review representative cases of both conditions to highlight specific and relevant neurologic features which prompted effective diagnosis and treatment. Two patients with confusion accompanied by seizures, memory changes, or specific visual hallucinations and HHV6 detectable by PCR in cerebrospinal fluid had improvement in viral load with ganciclovir or foscarnet treatment. Two patients had confusion, ataxia, or ocular changes and low serum thiamine levels, which resolved with parenteral thiamine. In all cases, definitive diagnosis and treatment were facilitated by a high index of suspicion and search for specific pathognomonic neurologic deficits accompanying the confusional state. It is critical to clinically differentiate these two conditions from other common neurologic syndromes occurring after transplant, allowing potentially improved patient outcomes by prompt diagnosis, and effective treatment.
Keywords: hematopoietic cell transplant, central nervous system, human herpesvirus 6 encephalitis, Wernicke’s encephalopathy, thiamine
Introduction
Hematopoietic cell transplantation is performed for the treatment of various hematologic malignancies (leukemia, lymphoma), solid tumors, and a variety of non-malignant hematopoietic and metabolic diseases.1 Prior to transplantation, the patient (recipient) is prepared with high doses of chemotherapy and/or radiation to destroy the hematopoietic system and to suppress multiple immune cell subsets in efforts to prevent rejection of the donor graft. Post-transplant central nervous system complications occur in 11—65% of transplant recipients, and 9—17% of cases may be fatal.1–4 Acute confusional states including alterations in mental status, disorientation, memory changes, visual or auditory hallucinations, somnolence, and seizures commonly develop in children with cancer.5 Most transplant recipients experience a transient and profound immunosuppression while awaiting hematopoietic stem cell engraftment and recovery. Additionally, in the case of allogeneic transplants, immunosuppressive medications to prevent graft rejection and graft-versus-host disease render these patients susceptible to specific infectious and non-infectious central nervous system complications. Donor cell immune recovery can be divided into three dominant phases after bone marrow transplant: the pre-engraftment period (phase I, days 0—30 after transplant), post-engraftment period (phase II, days 30—100), and the late phase (phase III, after day 100). The timing and etiologies of central nervous system complications vary according to the specific immune defects of the patient during these post-transplant time intervals.1,6
In Phase I, the recipient’s immune system has notable defects in phagocyte and cytotoxic and helper lymphocyte function, as the hematopoietic system is recovering from preparative treatment conditioning with high-dose chemotherapy and/or radiation. The most common central nervous system complications are infectious, especially fungal, gram-negative, and viral infections. During phase II, profound systemic immunosuppression used to prevent or treat graft-versus-host disease in allogeneic transplant recipients predisposes to further central nervous system complications withHHV6, cytomegalovirus, fungi, toxoplasma, and gram-positive bacteria. During phase III, ongoing immune suppression amplified by the direct immunosuppressive effects of chronic graft-versus-host disease may occur. Common central nervous system complications during phase III include infections caused by fungi, varicella zoster virus, and atypical viral infections including progressive multifocal leukoencephalopathy. Under-recognized non-infectious central nervous system complications such as nutritional or metabolic deficiencies, including Wernicke’s encephalopathy, are more likely during post-transplant phase I and II and have been reported in 5.5% of such patients at autopsy.1,2 Encephalopathic symptoms in Wernicke’s encephalopathy are due to a relatively thiamine-depleted state in bone marrow transplant patients due to increased metabolic needs during marrow and immune reconstitution coupled with chronic thiamine under-replacement, chemotherapy-induced nausea and vomiting, and potentially tumor consumption of thiamine.7
Both HHV6 encephalitis and thiamine deficiency are curable if definitively diagnosed and treated early in their course; however, diagnostic errors and delays often cause less than optimal outcomes for these patients. We present a limited case series of pediatric cancer patients assigned one of these diagnoses following evaluation for acute confusional states post-transplant at St. Jude Children’s Research Hospital. We review the clinical presentation, laboratory data, and neuroimaging findings, and outline specific clinical and imaging features which should assist the astute clinician to rapidly arrive at the correct diagnosis and to initiate definitive therapy.
Methods
Patients
All allogeneic and autologous hematopoietic cell transplant patients at St. Jude Children’s Research Hospital between 2008 and 2013 with chart records indicating acute confusion or encephalopathy were included in the study. Of these cases, patients who presented with similar neurological findings without recorded diagnosis of HHV6 or Wernicke’s encephalopathy were excluded. A total of 4 hematopoietic cell transplant patients met inclusion criteria for encephalopathic state with associated HHV6 infection or Wernicke’s encephalopathy documented in the laboratory data and in neurological consultation.
Data Collection and Analysis
All data were collected retrospectively using the St. Jude Department of Bone Marrow Transplantation and Cellular Therapy database, using de-identified patient data. Data collection and analysis were performed according to the rules and regulations of the Health Information Portability and Accountability Act and St. Jude Institutional Review Board. A written protocol was submitted to and approval obtained from the St. Jude Institutional Review Board prior to data analysis.
Diagnostic and treatment procedures
Testing of peripheral blood samples for HHV6 by quantitative PCR was performed in the St. Jude Children’s Research Hospital Molecular Microbiology Laboratory. Whole blood HHV6 PCR was defined as positive if greater than 2,000 copies/mL. Cerebrospinal testing of cerebrospinal fluid by PCR was performed by Focus Diagnostics, Inc. (Cypress, CA) and defined as positive if greater than 500 copies/mL. Thiamine reference values (whole blood) followed the norms established by the St. Jude Clinical Diagnostics laboratory (normal range, 78—185 nmol/L). Thiamine deficiency was defined at any laboratory value below the lower limit of the reference range.
Treatment for diagnosed HHV6 infection was foscarnet 90 mg/kg intravenously every 12 hours until documented negative cerebrospinal PCR for human herpesvirus 6. Treatment for thiamine deficiency/Wernicke’s encephalopathy was intravenous thiamine 100 mg intravenously daily until normalization of serum thiamine levels or clinical symptoms. In all cases in which symptom resolution was incomplete at time of normalization of cerebrospinal PCR or thiamine levels, treatment was halted based upon normalization of the laboratory parameters.
Results
Case 1
An 8-year-old male with a history of prior matched sibling and matched unrelated donor allogeneic bone marrow transplants for multiply relapsed acute myelogenous leukemia underwent a third allogeneic hematopoietic cell transplant from his father. He had a cumulative history of 19 months of continuous immunosuppression with the calcineurin inhibitor cyclosporine A following his first and second transplants. The patient had a history of cutaneous graft-versus-host disease following prior transplants and was maintained on tacrolimus and sirolimus for immunosuppression, as well as cyclophosphamide for additional anti-leukemic effect due to persistent minimal residual disease. Thirty-eight days following his haplo-identical transplant, he developed intermittent fever, generalized malaise, cough, fatigue, persistent insomnia, and new onset of focal visual hallucinations in which he recurrently saw the same food item in front of him, or a specific family member who was not present. He also experienced episodes of anterograde amnesia, but was otherwise without delirium or disorientation, and developed progressive hypoxemic respiratory failure with diffuse interstitial infiltrates. Magnetic resonance imaging of the brain on post-transplant day 41 showed new restricted diffusion involving bilateral hippocampi with accompanying T2 prolongation (Figure 1A). Electroencephalogram demonstrated diffuse slow background activity but no focality. Cerebrospinal fluid testing on post-transplant day 41 showed white blood cell count 2/mm3, red blood cell count 15/mm3, lymphocytes 94%, monocytes 5%, glucose 62 mg/dL, protein 31 mg/dL and PCR was positive for HHV6 at 607,000 copies/mL (clinical reportable range: > 500 copies/mL) but negative for West Nile RNA, and parechovirus. Peripheral blood was negative for polyoma viruses, herpes simplex virus types 1 and 2, and adenovirus. HHV6 was subsequently detected in the peripheral blood, initially at 9,772 copies/ml (clinical reportable range: > 2,000 copies/mL). He was empirically treated with foscarnet for presumed HHV6 encephalitis and associated pneumonitis. He remained on foscarnet therapy for 3 weeks until blood HHV6 PCR trended below 2,000 copies/ml. All visual hallucinations resolved within 3 days of initiation of foscarnet treatment, and the patient’s mental status returned to his prior baseline with the exception of mild short-term amnesia persisting until most recent follow-up (4.5 years post-transplant). Most recent neuro-imaging at 4.5 years post treatment continued to demonstrate gliotic changes in hippocampi bilaterally at prior sites of increased T2 signal intensity on magnetic resonance imaging (Figure 1B).
Figure 1.
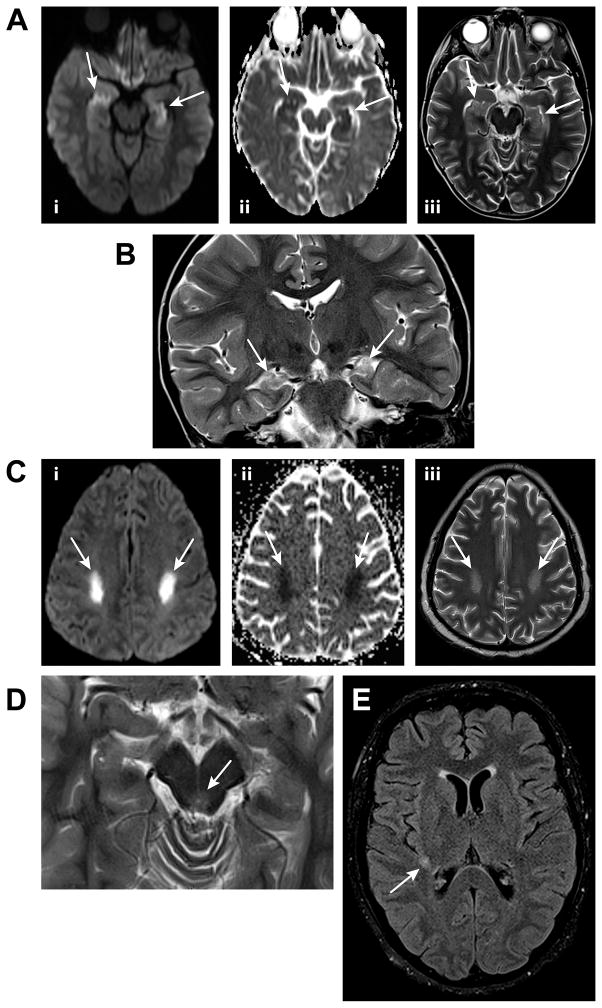
Figure 1A. An axial diffusion-weighted image of the brain for the patient in Case 1 (i) demonstrates high signal in the bilateral hippocampi (arrows) with corresponding low apparent diffusion coefficient values on an apparent diffusion coefficient map (ii), consistent with restricted water diffusion. An axial T2-weighted image (iii) reveals hyperintensity in both hippocampi (arrows). Such findings are associated with human herpesvirus 6 encephalitis.
Figure 1B A coronal T2-weighted image of the brain for the patient in Case 1, obtained approximately 2 years and 3 months after the images in Figure 1A, demonstrates volume loss and hyperintensity in the bilateral hippocampi, consistent with mild atrophy (arrows).
Figure 1C. Axial diffusion-weighted (i) and apparent diffusion coefficient map (ii) images of the brain for the patient in Case 2 demonstrate restricted water diffusion in the posterior portions of the bilateral centrum semiovale (arrows) with corresponding patchy hyperintensity on a T2-weighted image (arrows, iii). These findings are nonspecific but compatible with a toxic myelinopathy.
Figure 1D An axial T2-weighted image of the brain for the patient in Case 3 reveals subtle hyperintensity surrounding the aqueduct of Sylvius. Such hyperintensity may be seen in the setting of Wernicke’s encephalopathy.
Figure 1E An axial T2-weighted fluid attenuated inversion recovery image of the brain for the patient in Case 4 demonstrates a small nonspecific hyperintense focus in the posterior right subinsular region (arrow).
Case 2
A 16-year old male with a history of relapsed acute myelogenous leukemia underwent unrelated double cord blood transplant. On post-transplant day 24, with no signs of engraftment, he was intubated for respiratory distress with pulmonary nodules and had a complicated course including influenza B, disseminated vancomycin resistant enterococcus, and Streptococcus viridans bacteremia. On post-transplant day 26, with normal serum oxygenation and electrolytes and on steady dosage of sedation and paralytics for ongoing intubation, he had a generalized tonic-clonic seizure progressing to non-responsiveness to commands or painful stimuli, and persistent agitation despite maximal sedation. Magnetic resonance imaging of the brain showed symmetric bilateral frontoparietal centrum semiovale/corona radiata white matter signal abnormality with restricted diffusion. Cerebrospinal fluid was tested on post-transplant day 29 and showed white blood cell count 0/mm3, red blood cell count 4,497/mm3, lymphocyte 60%, monocyte 40%, glucose 83 mg/dL, protein 33 mg/dL; and PCR was positive for HHV6 in the cerebrospinal fluid at 16,613 copies/mL (clinical reportable range > 500 copies/mL), negative for cytomegalovirus, Epstein-Barr virus, polyoma viruses, and parechovirus. Electroencephalogram on post-transplant day 29 demonstrated diffuse slowing of the background. Repeat magnetic resonance imaging on post-transplant day 30 showed interval increase in restricted diffusion and signal abnormality bilaterally in the frontoparietal white matter/centrum semiovale (Figure 1C). On post-transplant day 31, peripheral blood PCR detected HHV6 at 2,000 copies/mL; (clinical reportable range > 2,000 copies/mL) and peripheral blood viral testing was negative for cytomegalovirus, adenovirus, Epstein-Barr virus, parvovirus B19, herpes simplex virus 1, herpes simplex virus 2, and varicella zoster virus. The patient was treated for presumed HHV6 encephalitis with foscarnet with normalization of cerebrospinal fluid HHV6 (PCR < 500 copies/mL) after 10 days of therapy. A serum thiamine level was obtained on this patient and was 38 (normal, 78—185 nmol/L) on post-transplant day 30. The patient underwent empiric replacement with thiamine intravenously 100 mg/day for five days. During this time, the patient remained intubated secondary to respiratory failure, presumed due to associated pneumonia and vancomycin resistant enterococcus sepsis. Subsequent re-imaging on post-transplant day 37 showed extension of the prior lesions into the pyramidal tracts and pons. The patient continued without signs of definitive engraftment through day 42 and ultimately developed severe metabolic acidosis precipitated by veno-occlusive disease and cardiorespiratory compromise requiring resuscitation on post-transplant day 43, and was disconnected from life support following consultation with the patient’s family after extensive resuscitative efforts. In light of multiple potential contributing factors to death, autopsy was conducted and was consistent with severe lactic acidosis contributed by impaired tissue perfusion in the setting of veno-occlusive disease of the liver and pulmonary vasculature, with resultant poor cardio-respiratory reserve, combined with potential effects of linolelozid. Focused brain autopsy demonstrated multifocal necrotizing leukoencephalopathy of the brain, which was deemed compatible with viral infectious processes as well as with overlapping potential neuronal toxicities of multiple drugs.
Case 3
A 5-year-old male diagnosed with medulloblastoma arising from the vermis of the cerebellum underwent a subtotal resection followed by a gross total resection with ventriculo-peritoneal shunt placement and craniospinal radiotherapy of 23.4 Gy with focal boost to the vermis at 55.8 Gy. This was followed by high-dose chemotherapy with autologous stem cell transplant over three courses. On post-transplant day 25 of course 3, he presented with a several day history of unsteadiness and acute onset of photophobia, eyelid myoclonus, and horizontal nystagmus without any confusion. His course was complicated by febrile illness, pancreatitis, decreased oral intake, and prolonged parenteral nutrition. Initial magnetic resonance imaging on post-transplant day 26 showed mild nonspecific hyperintensity on T2-weighted images in the periaqueductal region (Figure 1D). Electroencephalogram was not performed. Neurological examination was significant for mild lateral nystagmus, but no confusion or ataxia. Infectious evaluation of fever was significant for Clostridum difficile colitis. Nasopharyngeal wash submitted for viral PCR was negative for adenovirus, human metapneumovirus, influenza A and B, Respiratory Syncytial Virus, and parainfluenza virus type 1, 2 and 3. No abnormalities were found with computed tomography to suggest ventriculo-peritoneal shunt dysfunction. Given history of prolonged parenteral nutrition administration, clinical suspicion resulted in empiric 100 mg intravenous thiamine replacement for possible Wernicke’s encephalopathy. Thiamine level drawn prior to treatment was low at 74 nmol/L (normal, 78—185 nmol/L). The patient’s symptoms completely resolved by the next day. Thiamine was not continued and follow up neuroimaging less than three weeks later showed a slight decrease in prominence of mild nonspecific T2 hyperintensity in the periaqueductal region.
Case 4
A 21-year-old female with relapsed Non-Hodgkin lymphoma underwent allogeneic matched sibling marrow transplantation complicated by a history of decreased appetite. She presented on post-transplant day 29 with waxing and waning alertness, confusion, and difficulty expressing herself. This was accompanied by visual hallucinosis (described as visualizing a vehicle in front of her) and tactile hallucinosis (described as feeling objects move within her body). Her neurological exam on post-transplant day 35 was remarkable for ocular abnormalities with dilated pupils, sluggishly reactive to accommodation and to light. She had labile mood, mild expressive and receptive aphasia, and no ataxia. Magnetic resonance imaging of the brain on post-transplant day 35 showed a new nonspecific 7–8 mm in diameter white matter lesion within the right posterior subinsular/peritrigonal area (Figure 1E) with increased diffusivity on diffusion-weighted imaging. Electroencephalogram on post-transplant day 37 showed mild diffuse background slowing. Cerebrospinal fluid testing done on post-transplant day 35 was normal with 0/mm3 white blood cells, 62/mm3 red blood cells, 95% lymphocytes, 5% monocytes, glucose 55 mg/dL, protein 42 mg/dL, and cytology negative for malignant cells. Cerebrospinal fluid was negative for cytomegalovirus, Ebstein-Barr virus, adenovirus, parechovirus, enterovirus, and HHV6. Serum viral testing was also negative for cytomegalovirus, Epstein-Barr virus, adenovirus, HHV6, and herpes simplex virus types 1 and 2. Clinical suspicion given history of poor nutritional intake resulted in empiric treatment on post-transplant day 29 with intravenous thiamine 100 mg daily for Wernicke’s encephalopathy. Serum thiamine level drawn prior to thiamine administration was low at 38 nmol/L (normal, 78—185 nmol/L). Her neuro-cognitive symptoms gradually improved as she was treated on intravenous thiamine replacement 100 mg per day for 10 days, after which thiamine was discontinued and she was discharged without focal neurologic or mental status alterations on post-transplant day 38.
Discussion
When patients undergoing hematopoietic stem cell transplant present with encephalopathic signs or symptoms such as change in mental status, drowsiness, or short-term memory loss, the differential diagnosis should include the following: direct drug effects, electrolyte abnormalities or other metabolic disturbances, intracranial hemorrhage secondary to thrombocytopenia, immunosuppressant-related hyperperfusion encephalopathy, autoimmune paraneoplastic syndromes, a wide range of infectious etiologies including reactivation of latent herpesviruses,8 and central nervous system manifestations of nutritional deficiencies, which should include Wernicke’s encephalopathy.9 Though significant literature exists to guide diagnosis and medical and/or surgical management of the former several conditions after transplant, there is a paucity of data to guide the differential diagnosis of the latter two conditions.
Human Herpesvirus 6 encephalitis
HHV6 was first described as a cause of encephalitis in 1986. It is a ubiquitous neurotropic virus latent in most adults. Primary infection usually occurs in the first two years of life and presents with a characteristic rash (“roseola infantum”) and fever, frequently with a single febrile seizure at time of defervescence.8,10,11 This virus usually remains latent after primary infection. Systemic reactivation typically occurs with acquired immunocompromise or immunosuppression, and can occur in up to 78% of hematopoietic stem cell transplant patients, usually during either Phase I or II of pos-transplant immune recovery. In the post-transplant setting, HHV6 reactivation may be associated with primary activation or recrudescence of graft-versus-host disease. This is presumed to be due to the induction of interleukin 6 and tumor necrosis factor-alpha from immune cells including antigen presenting cells and activated T cells as a result of HHV6 reactivation.1,12–14 Post-transplant immunosuppressive agents used to prevent graft-versus-host disease including calcineurin inhibitors and T-cell depletive antibodies increase the risk of HHV6 reactivation by inhibiting cytolytic T cell and natural killer cell anti-viral activity.15 Systemic human herpesvirus reactivation in immunocompromised patients can cause hepatitis, mononucleosis, chronic fatigue, pneumonitis, and encephalitis, and increases the risk for graft failure following bone marrow transplant.16
The severity of reactivation-associated symptoms and sequelae, including neurologic sequelae, arising from HHV6 reactivation can be reduced only by prompt recognition and treatment with a HHV6-inactivating anti-viral agent such as ganciclovir or foscarnet. There are two distinct variants of HHV6, serotype A and B. Primary infection with variant B typically causes the febrile illness and rash as mentioned in infancy and is typically self-limiting and benign. Reactivation and infection with serotype B is typically found in transplant patients. The significance of serotype A is less known, but has been reported more commonly in oncologic (non-transplant) patients.12 The specific reasons behind this differential prevalence of serotypes in non-transplant versus post-transplant patients is not known, but suggests that some aspect of the heavily or more chronically immunosuppressed state is required for serotype B encephalitis. Foscarnet is active against both serotypes A and B of HHV6, whereas ganciclovir has been reported for prophylaxis and treatment of the B serotype.12,17,18 Treatment should continue until resolution of detectable virus by PCR in cerebrospinal fluid or blood. Typically successful treatment is associated with rapid resolution of or significant improvement in symptomatology, without recurrence unless immunosuppression is re-initiated.8,19–25
Neurological symptoms may be non-specific, but localizing symptoms such as anterograde amnesia and/or focal visual hallucinosis help direct the astute clinician to HHV6 encephalitis.1,26
Detection of HHV6 in the cerebrospinal fluid by PCR is substantive evidence of active HHV6 infection.27 Most cases of HHV6 encephalitis are presumed to be associated with endogenous viral reactivation, rather than deriving from the donor graft as a new primary infection.13 Cerebrospinal fluid PCR retains its sensitivity even after short courses of antiviral therapy, which allows prompt empirical treatment. PCR testing has thus widely supplanted serologic testing, which requires two to four weeks after acute infection for development of a diagnostic rise in antibody titers. The specificity of the cerebrospinal fluid qualitative PCR can be compromised and result in false positive detection of HHV6 when breakdown of the blood-brain barrier or blood contamination of cerebrospinal fluid with blood-derived viral nucleic acid occurs.27 Quantitative real-time PCR provides the viral replication level in the central nervous system to establish a diagnosis. Obtaining serial quantitative PCR while on anti-viral therapy may demonstrate declining viral loads to prove efficacy of treatment.28 In our patients, quantitative PCR in the cerebrospinal fluid was used in determining the viral loads of HHV6 in patients who had an intact blood brain barrier to aid in the establishing diagnosis of encephalitis, and then serial quantitative PCR was used to determine efficacy of specific treatment.
If patients are unable to undergo lumbar puncture, the diagnosis of HHV6 encephalitis may be made by satisfying 2 of the 3 criteria: (1) typical clinical presentation with neurologic symptoms listed above, (2) detection of human HHV6 DNA in peripheral blood, or (3) limbic encephalopathy with selective involvement of medial temporal lobe on magnetic resonance imaging.26,29 As one might expect, these criteria require a high index of clinical suspicion for HHV6 encephalitis with neurologic findings in the post-transplant patient. HHV6 reactivation has a predilection for the medial temporal lobes, classically with symmetric and bilateral involvement.6,8,12,30,31 The localization of abnormal signal intensity in the mesio-temporal lobes correlates well with the neurologic deficit of anterograde amnesia with or without visual hallucinosis.8,32,33
In HHV6 encephalitis, magnetic resonance imaging features often evolve with chronicity of the lesions after the onset of neurological symptoms. During the early phase, at 0 to 2 days after onset of neurological symptoms, the medial temporal lobes classically demonstrate high signal intensity on T2-weighted fluid attenuated inversion recovery images, diffusion-weighted imaging, and T2-weighted images. This abnormal signal intensity typically persists on T2-weighted images. T2-weighted fluid attenuated inversion recovery images for 30 days after onset of neurological symptoms and gradually resolves with anti-viral treatment.31 Long-range follow-up magnetic resonance imaging of the brain in Case 1 demonstrated mild volume loss in the mesial temporal lobes (Figure 1B). This suggests the potential for long-term hippocampal parenchymal injury, which could well explain this patient’s persistent short-term memory loss.8,19 In vitro, astrocytes infected with HHV6 can demonstrate a marked decrease in glutamate transporter (excitatory amino acid transporter 2) expression, which has been proposed as one explanation for viral-induced long-term hippocampal injury following human herpes virus 6 reactivation in the cerebrum.34
Though localizing lesions to the mesio-temporal lobes serves as a diagnostic clue for HHV6 encephalitis, extra-temporal areas of demyelination and necrotizing inflammatory lesions during and following HHV6 encephalitis are atypical findings. Atypical regions of presentation include the extrahippocampal regions such as basal ganglia, diencephalon, brainstem, and spinal cord.19,35,36
Case 1 describes a patient with symptoms of anterograde amnesia, confusion, and visual hallucinosis, neuroimaging consistent with hippocampal involvement, and detection of HHV6 in peripheral blood and cerebrospinal fluid. This patient was treated with foscarnet and had a favorable clinical outcome despite significant and rapid progression of his neurologic deficits prior to diagnosis and initiation of foscarnet therapy. The patient in case 2 developed a seizure with post-ictal depressed consciousness persisting greater than 24 hours, as well as classic detection of HHV6 by PCR in cerebrospinal fluid, yet with nonspecific neuroimaging showing progressive extrahippocampal involvement. Although the patient died due to multi-systemic issues, foscarnet treatment allowed normalization of HHV6 levels in cerebrospinal fluid. The outcome of treatment of human herpesvirus 6 encephalitis can vary from near complete recovery to death, based upon current reports.8, 20,37–39 A theme common to all published reports is that prompt treatment with intravenous foscarnet or ganciclovir optimizes the chances for neurologic recovery and minimizes sequelae, even in highly immunocompromised chemotherapy and post-transplant patients.1,8,12,40
Wernicke’s Encephalopathy
Thiamine (vitamin B1) deficiency encephalopathy was first described by Carl Wernicke as an acute superior hemorrhagic encephalitis (initially confused with polioencephalitis).41 The clinical syndrome was first associated with thiamine deficiency in the 1940’s by Campbell and Russell.42 Though commonly associated with chronic alcoholism, Wernicke’s encephalopathy can also occur due to inadequately supplemented, prolonged total parenteral nutrition or protracted vomiting that may occur after hematopoietic stem cell transplant during the cytopenic phase while awaiting engraftment and resolution of gastrointestinal mucositis.9,43 Though Wernicke’s encephalopathy is characterized by the classical triad of altered mental status, ataxia, and opthalmoplegia,2,44 only one-third of patients manifest the complete triad.7 Mental status changes are the most frequent symptoms and signs, occurring in 82% of these patients.2 Ocular signs other than opthalmoplegia include conjugate gaze palsies and horizontal and vertical nystagmus.9 Other systemic clues to this syndrome may include the appearance of a “raspberry tongue”, metabolic acidosis, or other neurologic findings including confusion, papilledema, nystagmus, and blindness.44 Memory may also be severely affected as evidenced through functional connectivity maps, mammillothalamic connectivity impairment affecting verbal and nonverbal memory.45 Systemic tendency for thiamine deficiency may also be augmented in hematopoietic stem cell transplant recipients due to the high metabolic demand for thiamine during synthesis of bone marrow and immune progenitors associated with bone marrow engraftment and early immune reconstitution, as well as with tissue healing. Whereas thiamine deficiency is well-documented to have very serious systemic and neurologic sequelae if untreated, prompt recognition and treatment results in rapid resolution of symptoms and optimizes long-range neurologic outcome.46 If untreated, Wernicke’s encephalopathy can progress to permanent cortical blindness and/or coma, severe lactic acidosis, and death. Undertreated or inadequately treated patients may develop Korsakoff’s psychosis or a chronic syndrome of combined retrograde and anterograde amnesia and neuroimaging may show excessive mammillary body, cerebellar, and cerebral shrinkage.7,47
The diagnosis of Wernicke’s encephalopathy also relies on a high index of clinical suspicion, as there are no specific diagnostic abnormalities in cerebrospinal fluid, brain imaging, or electroencephalogram. Magnetic resonance imaging is currently the most valuable tool to support diagnosis,46 with a high (93%) specificity. However, due to poor sensitivity (53%), a normal magnetic resonance image of the brain cannot be reliably used to exclude the disease.47 Typical radiologic findings on magnetic resonance imaging include symmetric, hyper-intense T2 signal in the medial thalami, surrounding the aqueduct of Sylvius and in the tectal plate, as well as enhancement and/or shrinkage of the mammillary bodies.47–49 In thiamine deficiency, imaging changes may also be seen in locations such as the cerebellum, cranial nerve nuclei, floor of the fourth ventricle and cerebral cortex as well as in other locations. Neuropathological findings may include extensive periventricular hemorrhagic foci affecting the thalamus, midbrain, pons, and medulla oblongata.2 These radiographic changes result from the central role of thiamine as a co-factor in carbohydrate and lipid metabolism and in neurotransmitter production in these regions of the central nervous system. Additionally, thiamine deficiency contributes to blood brain barrier breakdown, allowing susceptible areas including the medial thalamus and periaqueductal gray regions to undergo neuronal necrosis.7 Electroencephalographic findings are non-specific and non-diagnostic, ranging from normal to severe background slowing without epileptiform discharges.7
Thiamine deficiency may occur within as few as 18—20 days without nutrition in patients receiving strict thiamine-free diets. An often overlooked clinical pearl is that patients receiving long-term glucose-containing intravenous solutions including total parenteral nutrition require larger thiamine stores (and thus potential thiamine replacement) to adequately metabolize their high carbohydrate intake. Wernicke’s encephalopathy is seen in post-transplant patients with prolonged use of transparental nutrition who were not given thiamine supplementation, suggesting the addition of thiamine is necessary.9 Delay in empiric treatment with thiamine while awaiting evaluations of neurologic symptoms can result in progressive and permanent neurological damage and death. Early parenteral replacement with thiamine can rapidly reverse symptoms and prevent this permanent neurological injury.7 Post-mortem studies confirm that Wernicke’s encephalopathy is commonly underdiagnosed in the pediatric population. One study showed that only 21.4% of patients displayed the clinical triad of ocular abnormalities, mental status changes, and ataxia at onset. Eventually 32.1% demonstrated the triad. 71.4% had at least two components of the triad. In 41.9%, however, diagnosis was made only at postmortem examination. With prompt treatment, 44.4% fully recovered, however 55.5% of those treated were left with cognitive difficulties, nystagmus, or ataxia.50 Thus, it is important for the transplant clinician to seriously consider Wernicke’s encephalopathy as a possible cause of neurological symptoms after transplant.9,43
Cases 3 and 4 had a clinical history of persistent nausea and vomiting, prolonged parenteral nutrition use, or prolonged decreased appetite, accompanied by some or all of the Wernicke’s encephalopathy triad of visual changes or nystagmus, mental status deterioration, and ataxia. Case 3 had lateral nystagmus, unsteady gait, and magnetic resonance imaging evidence of very subtle periaqueductal hyperintensity on T2-weighted images. Case 4 had confusion with visual and tactile hallucinosis, ocular changes, expressive dysphasia, and labile mood. Neuroimaging showed a non-specific white matter lesion in the right posterior subinsular/peritrigonal area. A high index of clinical suspicion prompted neuroimaging, documentation of thiamine deficiency by pre-treatment assessment of serum thiamine level, and empiric therapy with parenteral thiamine without delay while awaiting the results of the diagnostic evaluation. This resulted in resolution of the symptom complex for both of these patients. Both patients experienced a good clinical outcome. Case 2 emphasizes the importance of maintaining an inclusive differential diagnosis, in which both HHV6 encephalitis and thiamine deficiency were detected and both were treated in a critically ill patient while awaiting definitive diagnostic evaluation.
In conclusion, HHV6 encephalitis and Wernicke’s encephalopathy are two often underdiagnosed yet readily treatable causes of encephalopathy after hematopoietic cell transplantation, particularly in the first year after transplant. A better appreciation and understanding of the subtle neurologic features and localizing findings of these syndromes in children undergoing transplantation should aid prompt evaluation, detection, and treatment, thus reducing long-term neurologic sequelae and potentially improving survival in these patients.
Acknowledgments
The authors thank the St. Jude Department of Bone Marrow Transplantation and Cellular Therapy data management office for data collection and review, and Crystal Melloh (Department of Pathology) for data retrieval.
Funding Support
This research was supported by K08 #HL-088260-06 (NHLBI) (AP),the V Scholar Award of the V Foundation for Cancer Research (AP) and the American Lebanese Syrian Association of Charities (ALSAC), the funding arm of St. Jude Children’s Research Hospital (ZS and AP).
Footnotes
Authorship Contribution
The first author of this manuscript, Zsila Sadighi, performed chart analysis and drafted and edited the final manuscript. The senior author of this manuscript, Asha Pillai, provided direct patient care, developed the concept, reviewed data, drafted the original manuscript, and edited the final manuscript. Other co-authors provided critical patient-specific data, radiographic images and reports, and assisted in writing.
Declaration of Conflicting Interests
The authors have no conflict of interest to disclose.
Ethical Approval
This study was approved by the St. Jude Children’s Research Hospital Institutional Review Board as a retrospective chart review under the classification of exempt research. No animal subjects were used.
References
- 1.Yoshida S, Hayakawa K, Yamamoto A, et al. The central nervous system complications of bone marrow transplantation in children. Eur Radiol. 2008;18(10):2048–2059. doi: 10.1007/s00330-008-1000-3. [DOI] [PubMed] [Google Scholar]
- 2.Bleggi-Torres LF, de Medeiros BC, Werner B, et al. Neuropathological findings after bone marrow transplantation: an autopsy study of 180 cases. Bone marrow Transplant. 2000;25(3):301–307. doi: 10.1038/sj.bmt.1702140. [DOI] [PubMed] [Google Scholar]
- 3.Wiznitzer M, Packer RJ, August CS, Burkey ED. Neurological complications of bone marrow transplantation in childhood. Ann Neurol. 1984;16(5):569–576. doi: 10.1002/ana.410160507. [DOI] [PubMed] [Google Scholar]
- 4.Patchell RA, White CL, 3rd, Clark AW, et al. Neurologic complications of bone marrow transplantation. Neurology. 1985;35(3):300–306. doi: 10.1212/wnl.35.3.300. [DOI] [PubMed] [Google Scholar]
- 5.DiMario FJ, Jr, Packer RJ. Acute mental status changes in children with systemic cancer. Pediatrics. 1990;85(3):353–360. [PubMed] [Google Scholar]
- 6.Coley SC, Jager HR, Szydlo RM, Goldman JM. CT and MRI manifestations of central nervous system infection following allogeneic bone marrow transplantation. Clin Radiol. 1999;54(6):390–397. doi: 10.1053/crad.1999.0200. [DOI] [PubMed] [Google Scholar]
- 7.Kuo SH, Debnam JM, Fuller GN, de Groot J. Wernicke’s encephalopathy: an underrecognized and reversible cause of confusional state in cancer patients. Oncology. 2009;76(1):10–18. doi: 10.1159/000174951. [DOI] [PubMed] [Google Scholar]
- 8.Gorniak RJ, Young GS, Wiese DE, et al. MR imaging of human herpesvirus-6-associated encephalitis in 4 patients with anterograde amnesia after allogeneic hematopoietic stem-cell transplantation. AJNR Am J Neuroradiol. 2006;27(4):887–891. [PMC free article] [PubMed] [Google Scholar]
- 9.Baek JH, Sohn SK, Kim DH, et al. Wernicke’s encephalopathy after allogeneic stem cell transplantation. Bone Marrow Transplant. 2005;35(8):829–830. doi: 10.1038/sj.bmt.1704893. [DOI] [PubMed] [Google Scholar]
- 10.De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18(1):217–245. doi: 10.1128/CMR.18.1.217-245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zerr DM, Fann JR, Breiger D, et al. HHV-6 reactivation and its effect on delirium and cognitive functioning in hematopoietic cell transplantation recipients. Blood. 2011;117(19):5243–5249. doi: 10.1182/blood-2010-10-316083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshikawa T. Human herpesvirus-6 and -7 infections in transplantation. Pediatr Transplant. 2003;7(1):11–17. doi: 10.1034/j.1399-3046.2003.02094.x. [DOI] [PubMed] [Google Scholar]
- 13.Yoshikawa T, Kojima S, Asano Y. Human herpesvirus-6 infection and bone marrow transplantation. Leuk Lymphoma. 1992;8(1–2):65–73. doi: 10.3109/10428199209049819. [DOI] [PubMed] [Google Scholar]
- 14.Dockrell DH, Paya CV. Human herpesvirus-6 and -7 in transplantation. Rev Med Virol. 2001;11(1):23–36. doi: 10.1002/rmv.299. [DOI] [PubMed] [Google Scholar]
- 15.Vu T, Carrum G, Hutton G, et al. Human herpesvirus-6 encephalitis following allogeneic hematopoietic stem cell transplantation. Bone marrow Transplant. 2007;39(11):705–709. doi: 10.1038/sj.bmt.1705666. [DOI] [PubMed] [Google Scholar]
- 16.Nakayama T, Okada F, Ando Y, et al. A case of pneumonitis and encephalitis associated with human herpesvirus 6 (HHV-6) infection after bone marrow transplantation. Br J Radiol. 2010;83(996):e255–258. doi: 10.1259/bjr/19375793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seeley WW, Marty FM, Holmes TM, et al. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology. 2007;69(2):156–165. doi: 10.1212/01.wnl.0000265591.10200.d7. [DOI] [PubMed] [Google Scholar]
- 18.Tokimasa S, Hara J, Osugi Y, et al. Ganciclovir is effective for prophylaxis and treatment of human herpesvirus-6 in allogeneic stem cell transplantation. Bone Marrow Transplant. 2002;29(7):595–598. doi: 10.1038/sj.bmt.1703423. [DOI] [PubMed] [Google Scholar]
- 19.Wainwright MS, Martin PL, Morse RP, et al. Human herpesvirus 6 limbic encephalitis after stem cell transplantation. Ann Neurol. 2001;50(5):612–619. doi: 10.1002/ana.1251. [DOI] [PubMed] [Google Scholar]
- 20.Bethge W, Beck R, Jahn G, et al. Successful treatment of human herpesvirus-6 encephalitis after bone marrow transplantation. Bone marrow Transplant. 1999;24(11):1245–1248. doi: 10.1038/sj.bmt.1702065. [DOI] [PubMed] [Google Scholar]
- 21.Singh N, Paterson DL. Encephalitis caused by human herpesvirus-6 in transplant recipients: relevance of a novel neurotropic virus. Transplantation. 2000;69(12):2474–2479. doi: 10.1097/00007890-200006270-00002. [DOI] [PubMed] [Google Scholar]
- 22.Kawano Y, Miyazaki T, Watanabe T, et al. HLA-mismatched CD34-selected stem cell transplant complicated by HHV-6 reactivation in the central nervous system. Bone Marrow Transplant. 2000;25(7):787–790. doi: 10.1038/sj.bmt.1702220. [DOI] [PubMed] [Google Scholar]
- 23.Yoshikawa T. Significance of human herpesviruses to transplant recipients. Curr Opinion Infect Dis. 2003;16(6):601–606. doi: 10.1097/00001432-200312000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Yoshikawa T, Asano Y, Ihira M, et al. Human herpesvirus 6 viremia in bone marrow transplant recipients: clinical features and risk factors. J Infect Dis. 2002;185(7):847–853. doi: 10.1086/339411. [DOI] [PubMed] [Google Scholar]
- 25.Yoshikawa T, Suzuki K, Ihira M, et al. Prediction of human herpesvirus 6 infection after allogeneic bone marrow transplantation. Blood. 1998;92(7):2597–2599. [PubMed] [Google Scholar]
- 26.Mori Y, Miyamoto T, Nagafuji K, et al. High incidence of human herpes virus 6-associated encephalitis/myelitis following a second unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2010;16(11):1596–1602. doi: 10.1016/j.bbmt.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Debiasi RL, Tyler KL. Molecular methods for diagnosis of viral encephalitis. Clin Microbiol Rev. 2004;17(4):903–925. doi: 10.1128/CMR.17.4.903-925.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gautheret-Dejean A, Manichanh C, Thien-Ah-Koon F, et al. Development of a real-time polymerase chain reaction assay for the diagnosis of human herpesvirus-6 infection and application to bone marrow transplant patients. J Virol Methods. 2002;100(1–2):27–35. doi: 10.1016/s0166-0934(01)00390-1. [DOI] [PubMed] [Google Scholar]
- 29.Ogata M, Kikuchi H, Satou T, et al. Human herpesvirus 6 DNA in plasma after allogeneic stem cell transplantation: incidence and clinical significance. J Infect Dis. 2006;193(1):68–79. doi: 10.1086/498531. [DOI] [PubMed] [Google Scholar]
- 30.Niehusmann P, Mittelstaedt T, Bien CG, et al. Presence of human herpes virus 6 DNA exclusively in temporal lobe epilepsy brain tissue of patients with history of encephalitis. Epilepsia. 2010;51(12):2478–2483. doi: 10.1111/j.1528-1167.2010.02741.x. [DOI] [PubMed] [Google Scholar]
- 31.Noguchi T, Yoshiura T, Hiwatashi A, et al. CT and MRI findings of human herpesvirus 6-associated encephalopathy: comparison with findings of herpes simplex virus encephalitis. AJR. American journal of roentgenology. 2010 Mar;194(3):754–760. doi: 10.2214/AJR.09.2548. [DOI] [PubMed] [Google Scholar]
- 32.Cipolotti L, Bird CM. Amnesia and the hippocampus. CCurr Opin Neurol. 2006;19(6):593–598. doi: 10.1097/01.wco.0000247608.42320.f9. [DOI] [PubMed] [Google Scholar]
- 33.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 34.Fotheringham J, Donati D, Akhyani N, et al. Association of human herpesvirus–6B with mesial temporal lobe epilepsy. PLoS Med. 2007;4(5):e180. doi: 10.1371/journal.pmed.0040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Provenzale JM, vanLandingham KE, Lewis DV, et al. Extrahippocampal involvement in human herpesvirus 6 encephalitis depicted at MR imaging. Radiology. 2008;249(3):955–963. doi: 10.1148/radiol.2492071917. [DOI] [PubMed] [Google Scholar]
- 36.Shintaku M, Kaneda D, Tada K, et al. Human herpes virus 6 encephalomyelitis after bone marrow transplantation: report of an autopsy case. Neuropathology. 2010;30(1):50–55. doi: 10.1111/j.1440-1789.2009.01020.x. [DOI] [PubMed] [Google Scholar]
- 37.Drobyski WR, Knox KK, Majewski D, Carrigan DR. Brief report: fatal encephalitis due to variant B human herpesvirus-6 infection in a bone marrow-transplant recipient. N Engl J Med. 1994;330(19):1356–1360. doi: 10.1056/NEJM199405123301905. [DOI] [PubMed] [Google Scholar]
- 38.Denes E, Magy L, Pradeau K, et al. Successful treatment of human herpesvirus 6 encephalomyelitis in immunocompetent patient. Emerg Infect Dis. 2004;10(4):729–731. doi: 10.3201/eid1004.030587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakai R, Kanamori H, Motohashi K, et al. Long-term outcome of human herpesvirus-6 encephalitis after allogeneic stem cell transplantation. BiolBiol Blood Marrow Transplant. 2011;17(9):1389–1394. doi: 10.1016/j.bbmt.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Chamberlain MC, Chowdhary S. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology. 2008;70(6):491–493. doi: 10.1212/01.wnl.0000304028.19061.46. [DOI] [PubMed] [Google Scholar]
- 41.Wernicke C. Die akute hamorrhagische pilioencehpalitis superior. In: Verlag F, editor. Lehrbuch der Gehirnkrankheiten fur Arzte und Studierende. II. Kassel: 1881. pp. 229–242. [Google Scholar]
- 42.Campbell A, Russell WR. Wernicke’s encephalopathy: the clinical features and their probable relationship to vitamin B deficiency. QJM. 1941;10(1):41–64. [Google Scholar]
- 43.Lim YJ, Kim HJ, Lee YJ, et al. Clinical features of encephalopathy in children with cancer requiring cranial magnetic resonance imaging. Pediatric Neurol. 2011;44(6):433–438. doi: 10.1016/j.pediatrneurol.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Bleggi-Torres LF, de Medeiros BC, Ogasawara VS, et al. Iatrogenic Wernicke’s encephalopathy in allogeneic bone marrow transplantation: a study of eight cases. Bone Marrow Transplant. 1997;20(5):391–395. doi: 10.1038/sj.bmt.1700892. [DOI] [PubMed] [Google Scholar]
- 45.Kim E, Ku J, Namkoong K, et al. Mammillothalamic functional connectivity and memory function in Wernicke’s encephalopathy. Brain. 2009;132(Pt 2):369–376. doi: 10.1093/brain/awn311. [DOI] [PubMed] [Google Scholar]
- 46.Sechi G, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6(5):442–455. doi: 10.1016/S1474-4422(07)70104-7. [DOI] [PubMed] [Google Scholar]
- 47.Antunez E, Estruch R, Cardenal C, et al. Usefulness of CT and MR imaging in the diagnosis of acute Wernicke’s encephalopathy. AJR Am J Roentgenol. 1998;171(4):1131–1137. doi: 10.2214/ajr.171.4.9763009. [DOI] [PubMed] [Google Scholar]
- 48.Weidauer S, Nichtweiss M, Lanfermann H, Zanella FE. Wernicke encephalopathy: MR findings and clinical presentation. Eur Radiol. 2003;13(5):1001–1009. doi: 10.1007/s00330-002-1624-7. [DOI] [PubMed] [Google Scholar]
- 49.Zuccoli G, Siddiqui N, Bailey A, Bartoletti SC. Neuroimaging findings in pediatric Wernicke encephalopathy: a review. Neuroradiology. 2010;52(6):523–529. doi: 10.1007/s00234-009-0604-x. [DOI] [PubMed] [Google Scholar]
- 50.Vasconcelos MM, Silva KP, Vidal G, et al. Early diagnosis of pediatric Wernicke’s encephalopathy. Pediatric Neurol. 1999;20(4):289–294. doi: 10.1016/s0887-8994(98)00153-2. [DOI] [PubMed] [Google Scholar]