Abstract
Aims
The impact of exercise training on the right heart and pulmonary circulation has not yet been invasively assessed in patients with pulmonary hypertension (PH) and right heart failure. This prospective randomized controlled study investigates the effects of exercise training on peak VO2/kg, haemodynamics, and further clinically relevant parameters in PH patients.
Methods and results
Eighty-seven patients with pulmonary arterial hypertension and inoperable chronic thrombo-embolic PH (54% female, 56 ± 15 years, 84% World Health Organization functional class III/IV, 53% combination therapy) on stable disease-targeted medication were randomly assigned to a control and training group. Medication remained unchanged during the study period. Non-invasive assessments and right heart catheterization at rest and during exercise were performed at baseline and after 15 weeks. Primary endpoint was the change in peak VO2/kg. Secondary endpoints included changes in haemodynamics. For missing data, multiple imputation and responder analyses were performed. The study results showed a significant improvement of peak VO2/kg in the training group (difference from baseline to 15 weeks: training +3.1 ± 2.7 mL/min/kg equals +24.3% vs. control −0.2 ± 2.3 mL/min/kg equals +0.9%, P < 0.001). Cardiac index (CI) at rest and during exercise, mean pulmonary arterial pressure, pulmonary vascular resistance, 6 min walking distance, quality of life, and exercise capacity significantly improved by exercise training.
Conclusion
Low-dose exercise training at 4–7 days/week significantly improved peak VO2/kg, haemodynamics, and further clinically relevant parameters. The improvements of CI at rest and during exercise indicate that exercise training may improve the right ventricular function. Further, large multicentre trials are necessary to confirm these results.
Keywords: Pulmonary hypertension, Cardiac rehabilitation, Right heart catheterization, Cardiac output, Cardiac index
See page 45 for the editorial comment on this article (doi:10.1093/eurheartj/ehv440)
Introduction
Previous studies in patients with idiopathic pulmonary arterial hypertension (IPAH),1–5 inoperable chronic thrombo-embolic pulmonary hypertension (CTEPH),6 associated PAH (APAH) in connective tissue disease,7 and APAH in congenital heart disease8 have shown beneficial effects of exercise training as add-on to disease-targeted medical therapy increasing exercise capacity and quality of life (QoL).1,4,5 In four smaller studies, a randomized, controlled trial design has been performed.1,3,5,9 These trials assessed patients with IPAH and inoperable CTEPH and showed an improvement of 6 min walking distance (6MWD), cardiorespiratory function, fatigue, activity, and QoL parameters by exercise training. In further prospective, uncontrolled trials, similar results have been obtained and long-term follow-up revealed excellent 1- and 2-year survival rates of 95–100% in the patients who had participated in the training programme and continued training at home.4,6–8,10 The sample size of all these studies was relatively small (19–183 patients). Despite these limitations, the recommendation for this thoroughly supervised rehabilitation including exercise training has been upgraded to Class I, with a Level of Evidence of A within the last world congress in Nice in 2013.11,12 This recommendation was combined with the request to perform further randomized controlled multicentre trials.12 The mechanisms for the improvement of symptoms, exercise, and functional capacity and the possible effects on prognosis are still not completely understood.
The improvement of muscle function such as increase in capillarization and change of fibre type by exercise training2 has already been shown in PH. There is also evidence that exercise training influences the pulmonary vasculature,13 with a regulating effect on pulmonary vascular remodelling. The effect of exercise training on peak oxygen consumption (VO2/kg) is inconsistent in PH trials. Few uncontrolled trials using exercise training in an outpatient setting did not observe a significant improvement in VO2.2,14,15 Up to now, there is no study investigating the effect of exercise training on peak VO2/kg as primary endpoint, allowing a confirmative conclusion. Furthermore, there is growing evidence that training may improve right ventricular (RV) function. Animal models suggested a beneficial effect of training on the RV, such as a reduction of RV end-diastolic pressure and increase in RV capillary density (+86%, P < 0.05).16,17
The effect of exercise training on cardiopulmonary function and RV haemodynamics is of high interest and needs to be further assessed using cardiopulmonary exercise testing (CPET) and right heart catheterization (RHC) before and after training. Recent publications have shown that peak oxygen uptake (VO2), cardiac index (CI),18 and systolic pulmonary arterial pressure during exercise are important independent predictors for survival.19–21
Therefore, the aim of this study was to perform a large, prospective, randomized trial to investigate the impact of exercise training on peak VO2 as an important prognostic factor in patients with severe PAH or CTEPH and right heart failure. The study was also aimed to provide first insights into the effects of exercise training on invasively measured haemodynamics.
Methods
Study population and design
Patients with PAH and inoperable or persistent CTEPH and chronic right heart failure who were stable on disease-targeted medication for at least 2 months prior to inclusion were randomly assigned to a control and a training group. Medication remained unchanged during the study period. Clinical examinations including RHC were performed at baseline and after 15 weeks. During the study, patients of the control group did not receive any advice on exercise training (Figure 1).
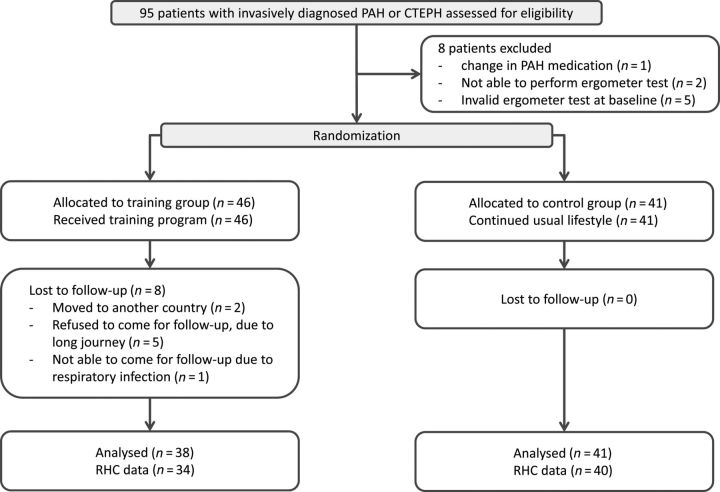
Figure 1.
Study design. The flowchart shows the number of allocated patients for each treatment group, the number of patients valid for analysis, and the number and reasons for exclusion, respectively (original data).
All patients gave their written informed consent to the study. The study was approved by the Ethics Committee of the University of Heidelberg, Germany. The study was registered at clinicaltrials.gov (NCT01394367).
Outcome measures
Primary endpoint was the change in peak VO2/kg. Secondary endpoints were the change from baseline to 15 weeks in invasively measured haemodynamics at rest and during symptom-limited exercise, 6MWD, workload QoL questionnaire (SF-36), World Health Organization (WHO) functional class, and N-terminal prohormone brain natriuretic peptide (NT-proBNP). QoL questionnaires were analysed in a blinded fashion. Patients in the control group went on with their usual lifestyle during the study. Efficacy parameters were evaluated at baseline and week 15, as described previously.1,10 Assessment of 6MWD, SF-36, and other efficacy parameters were performed by investigators who were blinded to the clinical data. Changes in WHO functional class and Borg dyspnoea scale (with 6 representing no exertion and 20 maximal exertion)22 were also analysed. CPET was carried out as supine cycle exercise, as described previously.1 Systolic and diastolic systemic blood pressures, workload, heart rate, ventilation, and VO2 were measured breath-by-breath. In patients dependent on long-term oxygen therapy, oxygen supplementation was paused throughout and resupplied directly after termination of the test.
RHC was performed at baseline and 15 weeks. Patients were examined on a variable load supine cycle ergometer (model 8420; KHL Corp., Kirkland, WA, USA). The examination at rest and during symptom-limited exercise was performed in a supine position using the transjugular approach with an 8F introducer set (MXI100, MEDEX, Smiths Group Plc, UK). RHC was performed by triple-lumen 7F-Swan-Ganz thermodilution catheters (Edwards Lifesciences, Irvine, CA, USA). Workload started at 25 W and was increased every 2 min for 25 W until symptom-limited exercise capacity was reached. Cardiac output (CO) was measured at least in triplicate at rest and in duplicate at the end of each workload during exercise by thermodilution with a variation of <10% between the measured values. The zero reference point for pressure recordings was set at the level of the right atrium in the mid-axillary line (phlebostatic axis). All examinations and measurements were performed by the same experienced team. There were no complications.
Rehabilitation programme with exercise training
The exercise and respiratory training was performed as described before1,10 and was started in-hospital for 3 weeks in the Rehabilitation Clinic Königstuhl in Heidelberg. We performed a programme especially developed for PH patients with at least 1.5 h/day exercise training (in intervals distributed over the day), consisting of interval cycle ergometer training at low workloads at 7 days a week, walking, dumbbell training of single muscle groups using low weights (500–1000 g), and respiratory training at 5 days/week. The training was continued at home with at least 15 min/day at 5 days a week for the following 12 weeks.
Besides physical training, patients received mental training to improve their perception of their individual physical abilities and limits. Psychological support was offered to all participants. The training programme was closely supervised by physical therapists and physicians specialized in rehabilitation medicine and by PH experts, as described before.1 Adverse events were recorded whenever they occurred. Oxygen saturation and heart rate were monitored continuously throughout the training and were used to adjust the training intensity. When patients' oxygen saturation fell below 90% during exercise, they received oxygen supply (3–10 L/min) throughout the training. Patients who were on oxygen therapy 16–24 h/day before inclusion into this study remained on oxygen throughout the training programme. At discharge from hospital after 3 weeks, patients received an individualized training manual and ordered a cycle ergometer for use at home.
Statistical methods
The analyses were performed by two statisticians (N.E. and C.F.). Data are given as mean values ± standard deviations. For the description of effects, we present changes in absolute values and changes in per cent of the individual baseline value.
Calculation of sample size was based on the primary efficacy endpoint, which was defined as the change in peak VO2/kg between baseline and 15 weeks of exercise training. The null hypothesis to be assessed in the confirmatory analysis states that there is no difference in the changes of the peak VO2/kg value between the intervention and the control group. To detect a placebo-adjusted difference in peak VO2/kg of 2 mL/min/kg, with a standard deviation of 3 mL/min/kg, with a power of 80%, and a two-sided significance level of 5%, it was calculated that 45 patients were required in each treatment arm. Primary efficacy analysis was performed by an analysis of covariance (ANCOVA) model including baseline values as covariate, thereby yielding a power advantage over the standard t-test used for sample size calculation.
The secondary endpoints were compared between groups by an ANCOVA including baseline values as covariate. For analysis of categorical data, the McNemar–Bowden test was used.
To include missing values, we performed two sensitivity analyses: (i) multiple imputation analysis based on multiple regression models with 20 imputed data sets and (ii) a responder analysis regarding missing values and worsening of the parameters as non-responder. For the multiple imputation analysis, the combined results are stated. Threshold between response and non-response was set as 0. The responder analysis with number/percentage of responders vs. non-responders was performed by χ2 test. All tests were two-sided, and P-values less than 0.05 were considered statistically significant. All analyses were carried out with IBM SPSS V20 (IBM Corp. Armonk, NY, USA).
Statement of responsibility
The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Study population and randomization
Ninety-five consecutive patients with invasively diagnosed PAH and inoperable or persistent CTEPH under optimized medication therapy were included (Figure 1). Eight patients were not eligible for the study: one patient had an alteration in his/her medication, two patients were not able to perform an ergometer test, and five patients had an invalid ergometer test at baseline. Thus, the study group consisted of 87 patients who were randomized into a control and a training group (71% PAH, 29% CTEPH, 79% WHO functional class III, 54% female, 56 ± 15 years) (Figure 1 and Table 1).
Table 1.
Baseline characteristics
| Baseline characteristics | Control | Training |
|---|---|---|
| Patients, n | 41 | 46 |
| Gender, male/female | 20/21 | 20/26 |
| Age (years) | 57 ± 15 | 55 ± 15 |
| Height (cm) | 171 ± 8 | 170 ± 9 |
| Weight (kg) | 79 ± 18 | 75 ± 18 |
| WHO functional class, no. (%) baseline | ||
| II | 6 (15%) | 8 (18.2%) |
| III | 30 (75%) | 36 (81.8%) |
| IV | 4 (10%) | 0 (0%) |
| Diagnosis | ||
| Pulmonary arterial hypertension | 26 (63.4%) | 35 (76.1%) |
| CTEPH | 15 (36.5%) | 11 (23.9%) |
| NT-proBNP (pg/mL) | ||
| Baseline | 1114 ± 1386 | 1163 ± 2520 |
| Right heart catheterization | ||
| Mean pulmonary arterial pressure (mmHg) | 37.6 ± 11.8 | 41.0 ± 11.7 |
| Pulmonary vascular resistance (dyn × s /cm5) | 512 ± 338 | 540 ± 267 |
| Central venous pressure (mmHg) | 7.1 ± 4.7 | 7.5 ± 3.7 |
| Pulmonary arterial oxygen saturation (%) | 64.3 ± 9.4 | 64.7 ± 9.9 |
| Pulmonary arterial wedge pressure (mmHg) | 9.4 ± 3.8 | 9.4 ± 3.5 |
| Cardiac index (L/min/m2) | 2.69 ± 0.89 | 2.68 ± 0.73 |
| PAH-targeted medication | ||
| Endothelin receptor antagonists | 29 (70.7%) | 33 (71.7%) |
| Phosphodiesterase-5-inhibitors | 30 (73.2%) | 31 (67.4%) |
| Prostanoids inhaled | 6 (14.6%) | 3 (6.5%) |
| Prostanoids per os | 0 (0%) | 1 (2.2%) |
| Prostanoids intravenous | 0 (0%) | 0 (0%) |
| Calcium channel blockers | 3 (7.3%) | 5 (10.9%) |
| Imatinib | 1 (2.4%) | 0 (0%) |
| Soluble guanylate cyclase-stimulator | 3 (7.3%) | 6 (13%) |
| Combination therapy | ||
| Monotherapy | 14 (35%) | 13 (33.3%) |
| Dual therapy | 22 (55%) | 20 (51.3%) |
| Triple therapy | 4 (10%) | 6 (15.4%) |
| Oxygen therapy, yes/no | 20/21 | 17/25 |
Values are mean ± standard deviation.
For the category WHO functional class, values were missing for one patient in the control group and two patients in the training group. For the category NT-proBNP at baseline, values were missing for 12 patients in the control group. For the categories mean pulmonary arterial pressure and pulmonary vascular resistance, values were missing for seven patients in the control group and six patients in the training group. For the category central venous pressure, values were missing for 10 patients in the control group and 8 in the training group. For the category pulmonary artery oxygen saturation, 20 values were missing for the control group and 16 for the training group. For the category pulmonary arterial wedge pressure, eight values were missing for the control group and six for the training group. For the category cardiac index, seven values were missing for both training and control groups.
The control and training groups did not differ in their baseline characteristics, disease severity, or medication (Table 1).
Primary endpoint: change in peak oxygen uptake
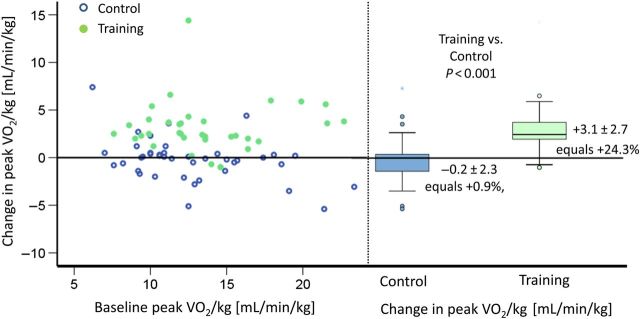
Peak VO2/kg significantly improved in the training group with +3.1 ± 2.7 mL/min/kg (baseline 13.3 ± 3.6 mL/min/kg; mean increase relative to baseline +24.3%) vs. −0.2 ± 2.3 mL/min/kg (baseline 12.7 ± 4.0 mL/min/kg; mean increase relative to baseline +0.9%) in the control group (P < 0.001; Figure 2 and Table 2). In the training group, 91.7% of the patients reached a peak VO2/kg of ≥11.5 mL/min/kg, compared with the control group with 50%.
Figure 2.
Primary endpoint: change in peak oxygen uptake. Left graph: The abscissa shows peak VO2/kg at baseline, and the ordinate shows change of peak VO2/kg from baseline after 15 weeks for each patient. The solid points represent patients of the training group, and the circles represent patients of the control group. This graph shows equal distribution of baseline peak VO2 in both groups as well as changes after 15 weeks of exercise training. Change in peak VO2 did not correlate with baseline peak VO2. Right graph: the boxplots at the right side show median change of VO2 max in the control vs. training group after 15 weeks. A significant increase can be shown in the training group (P < 0.001), whereas the control group shows a small reduction in peak VO2 after 15 weeks. Multiple imputations showed the same P-value.
Table 2.
Change echocardiography, CPET, and haemodynamics
| Characteristics | Training group |
Control group |
P-value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Change from baseline to 15 weeks |
Baseline | Change from baseline to 15 weeks |
||||
| Primary analysis set (n = 79: 38 training vs. 41 control) | Absolute | Change in per cent (95% confidence interval) | Absolute | Change in per cent (95% confidence interval) | ANCOVA | ||
| Mean 6 min walking distance (m) | 453 ± 91 | 29 ± 53 | 5.5 (1.7, 9.3) | 413 ± 95 | −12 ± 46 | −1.9 (−6, 2.25) | 0.002 |
| Laboratory parameters | |||||||
| NT-proBNP (pg/mL) | 1163 ± 2520 | −89 ± 1387 | 13.2 (−9, 35) | 1114 ± 1386 | 141 ± 827 | 39.9 (−21, 101) | 0.44 |
| Cardiopulmonary exercise testing | |||||||
| Peak VO2/kg (mL/min/kg) | 13.3 ± 3.6 | 3.1 ± 2.7 | 24.3 (17.1, 31.4) | 12.7 ± 4.0 | −0.2 ± 2.3 | 0.9 (−6.4, 8.3) | <0.001 |
| Peak VO2 (mL/min) | 986 ± 294 | 233 ± 209 | 23.2 (16.6, 30) | 992 ± 275 | −28 ± 150 | −2.4 (−7, 2.2) | <0.001 |
| Oxygen pulse [(mL/min)/min−1] | 8.2 ± 2.4 | 0.6 ± 1.1 | 7.4 (3.5, 11.2) | 8.0 ± 2.1 | 0.1 ± 1.1 | 1.5 (−2.3, 5.3) | 0.07 |
| HR rest (1/min) | 74 ± 11 | 2 ± 10 | 3.8 (−0.8, 8.4) | 80 ± 11 | −4 ± 11 | −4.8 (−8.8, −0.8) | 0.18 |
| HR max (1/min) | 122 ± 21 | 14 ± 15 | 12.8 (8, 17.7) | 125 ± 17 | −4 ± 12 | −2.8 (−5.9, 0.2) | <0.001 |
| RR sys at rest (mmHg) | 114 ± 18 | −5 ± 17 | −3.0 (−7.4, 1.4) | 111 ± 15 | 2 ± 16 | 2.6 (−2, 7.2) | 0.19 |
| RR dia at rest (mmHg) | 72 ± 10 | −2 ± 11 | −1.1 (−5.6, 3.4) | 74 ± 10 | −2 ± 11 | −1.3 (−6.1, 3.6) | 0.78 |
| RR sys max (mmHg) | 140 ± 22 | 13 ± 24 | 10.7 (4.3, 17.1) | 146 ± 25 | −3 ± 23 | −0.2 (−5, 4.6) | 0.008 |
| RR dia max (mmHg) | 82 ± 12 | 3 ± 14 | 6.0 (−0.8, 12.7) | 79 ± 13 | 2 ± 11 | 3.6 (−0.8, 8.0) | 0.34 |
| Workload max (W) | 74 ± 27 | 18 ± 28 | 32.1 (15.8, 48.4) | 72 ± 23 | −1 ± 14 | 1.8 (−6.4, 10.1) | <0.001 |
| Secondary analysis set (n = 59: 31 training vs. 28 control) | |||||||
| RHC at rest | |||||||
| mPAP (mmHg) | 41 ± 12 | −4 ± 10 | −7.3 (−13.2, −1.5) | 38 ± 12 | 5 ± 8 | 16.1 (6.4, 25.8) | 0.007 |
| Cardiac output (L/min) | 5.0 ± 1.4 | 0.7 ± 0.9 | 12.5 (6.3, 18.6) | 5.1 ± 1.8 | −0.5 ± 1.3 | −5.3 (−11.7, 1.1) | <0.001 |
| Cardiac index (L/min/m2) | 2.7 ± 0.7 | 0.2 ± 0.6 | 9.3 (2.4, 16.2) | 2.7 ± 0.9 | −0.3 ± 0.8 | −6.5 (−13, 0.2) | <0.001a |
| PAWP (mmHg) | 9 ± 4 | 2 ± 4 | 24.4 (9.2, 39.7) | 9 ± 4 | −0.3 ± 4.7 | 9.2 (−12, 30) | 0.18 |
| PVR (dyn × s/cm) | 540 ± 267 | −87 ± 151 | −19.3 (−28.4, –10.3) | 512 ± 338 | 87 ± 168 | 34.5 (1, 68) | <0.001a |
| RHC during exercise (exercise n = 49: 28 training vs. 21 control) | |||||||
| mPAP (mmHg) | 66 ± 19 | 2 ± 10 | 5.3 (−0.2, 10.8) | 58 ± 18 | 4 ± 9 | 9.6 (0.7, 18.6) | 0.71 |
| Cardiac output (L/min) | 10.3 ± 3.4 | 2.1 ± 2.7 | 20.4 (11.5, 29.2) | 9.1 ± 2.3 | −0.3 ± 1.2 | −2.0 (−9, 5) | 0.002 |
| Cardiac index (L/min/m2) | 5.4 ± 1.6 | 1.0 ± 1.4 | 19.5 (10.4, 28.5) | 4.7 ± 1.1 | −0.2 ± 0.6 | −4.3 (−12, 3) | 0.002 |
| PAWP (mmHg) | 15 ± 5 | 0.6 ± 6 | 9.7 (−7.5, 26.9) | 15 ± 7 | 0.5 ± 4 | 7.1 (−4, 19) | 0.93 |
| PVR (dyn × s/cm5) | 453 ± 219 | −44 ± 150 | −10.6 (−22.3, 1.1) | 439 ± 272 | 26 ± 175 | 15.1 (−6, 37) | 0.16 |
Values are mean ± standard deviation; two-sided P-values of ANCOVA are given.
NT-proBNP, N-terminal pro-B-type natriuretic peptide; PAP, pulmonary arterial pressure; sys, systolic; dia, diastolic; m, mean; HR, heart rate; RR, blood pressure; TAPSE, tricuspid annular plane systolic excursion; RHC, right heart catheter; PAWP, pulmonary arterial wedge pressure; PVR, pulmonary vascular resistance.
aNo significant difference in multiple imputation analysis. Frequency of missing values within the analysis sets within groups was always below 15%.
Secondary endpoints
Haemodynamics and right heart function
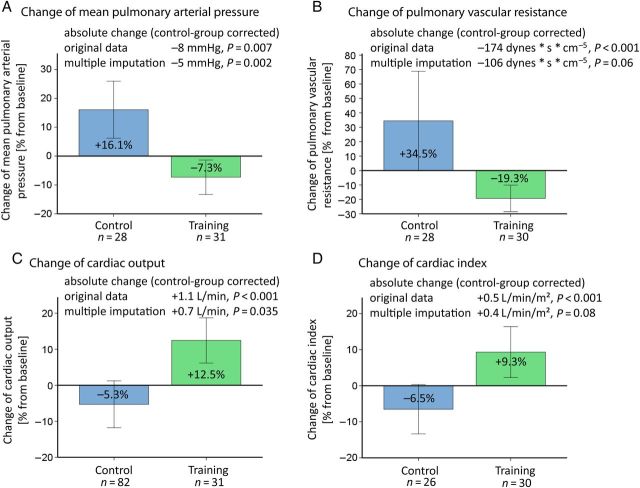
RHC was performed at baseline and after 17 ± 7.5 weeks in 74 of 87 (81.6%) patients: 34 patients of the training group and 40 patients of the control group agreed to invasive assessment of haemodynamics by RHC. Haemodynamics at rest and during exercise significantly improved in the training group, compared with the control group (Figure 3). The measurements at rest (Figure 3) revealed a significant improvement of CI (training +0.2 ± 0.6 L/min/m2; +9.3% vs. control −0.3 ± 0.9 L/min/m2; −6.5%, P < 0.001), CO (training +0.7 ± 0.9 L/min/m2; +12.5% vs. control −0.5 ± 1.3 L/min/m2; −5.3%, P < 0.001), mean pulmonary arterial pressure (mPAP) at rest (training −4 ± 10 mmHg; −7.3% vs. control +4 ± 8 mmHg; +16.1%, P = 0.007), and pulmonary vascular resistance (training −87 ± 151 mmHg; −19.3% vs. control +87 ± 168 mmHg; +34.5%, P < 0.001) in the training group, compared with the control group and baseline. P-values for multiple imputations are shown in Figure 3.
Figure 3.
Secondary endpoints: haemodynamic function. Results from RHC for mean pulmonary arterial pressure (A), pulmonary vascular resistance (B), cardiac output (C) and cardiac index (D) at rest. The graphs depict the change of each parameter in per cent from baseline to 15 weeks after exercise training/no training. The mean changes of the training group, compared with baseline and control, as absolute values are given at the top of each graph with corresponding P-values of the ANCOVA for original data and multiple imputations. The bars are representing two times standard error.
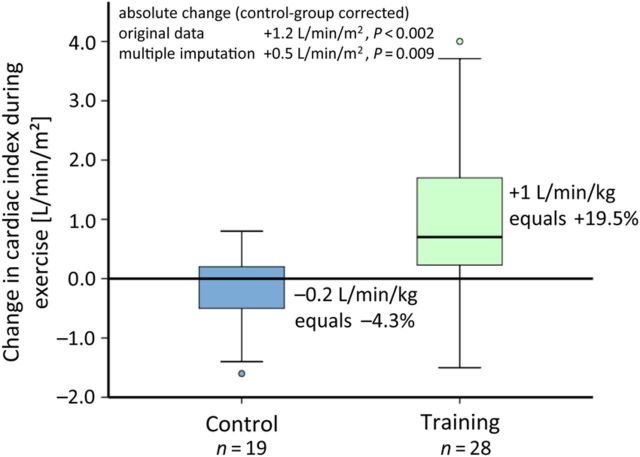
Measurements during exercise baseline vs. 15 weeks revealed a significant improvement of CI during exercise (training +1.0 ± 1.4 L/min/m2; +19.5% vs. control −0.2 ± 0.6 L/min/m2; −4.3%, P = 0.002; Figure 4) and further haemodynamic parameters in the training group, compared with the control group (Figure 3 and Table 2). Both time points were also analysed for the same workload, which corresponds either to the maximum of baseline or follow-up examination. The analysis at the same workload showed the same results with significant improvement of both CI and CO during exercise (difference from baseline: CO training +1.41 ± 2.13 L/min vs. control −0.38 ± 0.29 L/min, CI training +0.66 ± 1.06 L/min/m2 vs. control −0.23 ± 0.66 L/min/m2, both P = 0.001 for original data; for multiple imputation: both P = 0.03 compared with baseline and control).
Figure 4.
Haemodynamics during exercise. The distribution of absolute changes in CI during exercise (P < 0.001) is shown by a boxplot for each group. The training group improved CI by 19.5%, whereas the control group had a decrease of 4.3%. P-values are given for the ANCOVA for original data and multiple imputations.
Further clinical parameters
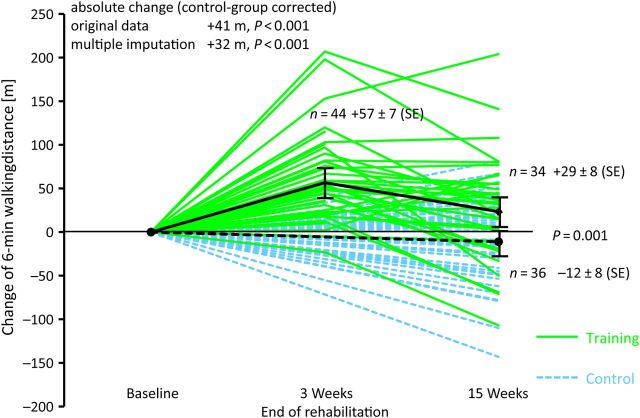
After 15 weeks of treatment, the 6MWD and change in the workload during CPET significantly improved in the training compared with the control group (control group corrected change: 6MWD +41 m, P = 0.001 and CPET +19 W, P < 0.001; Figure 5 and Table 2).
Figure 5.
6MWD. The figure shows the absolute changes in 6MWD for each patient: patients of the training group as solid lines and patients of the control group as dashed lines. Mean changes are given with darker lines, and error bars indicate ±1 standard error of the mean. Exercise training significantly improved 6MWD compared with baseline (P = 0.002). P-values are given for the ANCOVA for original data and multiple imputations.
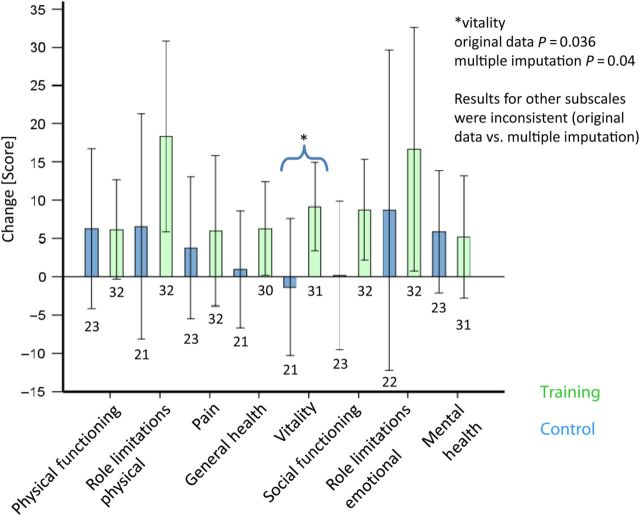
At baseline, all patients had significantly compromised QoL scores compared with the general population. Only for the subscale vitality, P-values were significant for both original data and multiple imputation (for original data P = 0.036 and for multiple imputation P = 0.042) (Figure 6).
Figure 6.
QoL. Mean changes of QoL ± 2 standard errors of the mean and sample sizes below each bar. The bars of the control group are shown on the left side of each category. Exercise and respiratory training significantly improved the subscale score for vitality (P = 0.036), compared with the control group, in which this parameter slightly worsened. The subscales role limitations due to physical restrictions (P = 0.099), role limitations due to emotional restrictions (P = 0.09), and general health perception (0.091) were significant in trend in the original data, but showed inconsistent findings in multiple imputation.
For evaluation of WHO functional class, the prepared sheets were not filled in by the investigators for >50% of the patients, so this parameter was not analysed. NT-proBNP values varied largely within the population at baseline also due to comorbidities such as renal insufficiency and did not show a statistically significant difference between groups (training 979 ± 2811 vs. control 1074 ± 1424; P = 0.44). Echocardiography data showed no statistically significant change in right heart areas and systolic pulmonary arterial pressure between groups. Tricuspid annular plane systolic excursion significantly decreased in the training group compared with the control group.
Sensitivity analysis: responder vs. non-responder
A sensitivity analysis was performed for primary and secondary endpoints treating missing values as non-responders. Change in workload (training 54% vs. control 21% responders, P = 0.002), peak VO2 (training 74% vs. control 44%, P = 0.006), mPAP (training 64% vs. control 28%, P = 0.001), CO (training 59% vs. control 23%, P = 0.001), CI (training 51% vs. control 21%, P = 0.005), PVR (training 64% vs. control 26%, P = 0.001), CO during exercise (training 59% vs. control 21%, P = 0.001), and CI during exercise (training 56% vs. control 23%, P = 0.003) remained statistically significant.
Discussion
This large prospective, randomized, controlled trial evaluates the effect of exercise training on peak VO2/kg as primary endpoint and invasively measured haemodynamics in patients with severe PAH and inoperable or persistent CTEPH. To the best of our knowledge, there is only one previous publication performing RHC after training in 20 patients with left heart failure.23 The results of the study showed an improvement of peak VO2/kg, CI, and CO during exercise, suggesting that this treatment might improve RV function. Other prognostic parameters such as CI at rest, pulmonary vascular resistance, mPAP, 6MWD, QoL parameters, and exercise capacity significantly improved in the training group. Bearing in mind that these effects were reached as add-on in patients with optimized medical treatment, low-dose exercise training was very effective.
Effect on peak oxygen uptake and RV function
The results of this study confirm previous findings that exercise training improves peak VO2/kg in patients with severe PH by approximately 15–25%.1,4,7,8,10 Study results that did not show positive effects of exercise training on peak VO2/kg2,14,15 may be evoked by less intensive training due to an outpatient setting and by a lower training frequency of three times a week or less. Peak VO2 is linearly associated with RV function.24,25 Both were severely reduced in our patients at rest and during exercise. RV dysfunction is a crucial factor contributing to functional impairment and mortality. Improvement of peak VO2/kg is possibly due to improvement of capillary density of the skeletal muscle, which has been identified before in patients with IPAH.2
CI and CO significantly improved at rest, during maximal exercise, and when comparing the baseline and follow-up assessments at the same workload. The improvements in haemodynamics at rest and during exercise point out that training may also be beneficial for the RV function, which is a strong prognostic predictor in these patients.19,20,26,27 CI during exercise has recently been detected to be the most important independent haemodynamic predictor of survival in PH in the investigated cohort,18,20 correlated well with the clinical data, and was even more informative than haemodynamics at rest.
Mechanism of exercise training in athletes and patients with left heart failure
Previous studies in healthy athletes have shown that endurance exercise training causes a significant increase in skeletal muscle capillarization, characterized by an elevated capillary density and capillary-to-fibre ratio.28–30 The physiological adaptation contributes to enhanced aerobic capacity by increased transport, conductance, and extraction of oxygen in skeletal muscles. In patients with left heart failure, exercise training has shown to improve ventilatory efficiency,31 the reversal of skeletal muscle atrophy,32 the attenuation of endothelial dysfunction,33 and inflammatory mediators.34–36 Besides these ‘peripheral effects’ on skeletal muscle function, it is not known yet whether there is a direct effect of exercise training on the heart muscles. Erbs et al.37 showed improvement of left ventricular ejection fraction, stroke volume, and CO at peak exercise after 12 weeks of exercise training in patients with advanced chronic left heart failure.24 Wisloff et al.38 reported that after 12 weeks of aerobic-moderate continuous training and aerobic-intense interval training, left ventricular ejection fraction of cardiac patients increased. This study confirmed the effects of exercise training by invasive measurements of pulmonary circulation in patients with PAH/CTEPH and right heart failure.
Limitations
RHC was an optional assessment in this study. Thus, a referral bias may have occurred as only 83% of the patients received RHC. The haemodynamic effects of this study may therefore serve as first results to plan prospective, randomized studies on the effects of exercise training on haemodynamics at rest and during exercise. The training group showed a significant increase in heart rate during exercise when compared with the control group. This may be a hint for a higher volitional effort. However, haemodynamics also showed a significant improvement when examinations were compared for the same workload.
In a randomized controlled trial, a responder analysis does not reflect a worst-case scenario and is therefore not appropriate as a mere sensitivity analysis. The findings of both responder analysis and multiple imputations, however, showed almost the same significant parameters as the original data analysis.
NT-proBNP values, which are associated with disease progression, showed no statistical difference between changes of control and training groups. This may also be influenced by the large variation of values, as in six patients NT-proBNP was highly increased due to renal insufficiency.
It is a general issue of exercise training studies that they cannot be performed in a blinded fashion and that a referral bias towards highly motivated patients with a better outcome may occur.
Conclusion
This prospective, randomized, controlled trial confirms that low-dose exercise training at 4–7 days/week as add-on to PAH-targeted medication significantly improves peak VO2. The study indicates that exercise training improves cardiopulmonary parameters such as pulmonary vascular resistance and CI at rest and during exercise, leading to an increase in exercise capacity and QoL. The results of the study suggest that this therapy can improve RV function and other important prognostic parameters. The precise mechanism through which exercise-based interventions benefit PH patients still remains unclear. Further, larger multicentre trials using CI during exercise and time to clinical worsening as primary endpoints are necessary to confirm these results and to assess the impact of this therapy on patient outcomes.
Acknowledgements
We would like to thank all patients who participated, the PH e.V. for their support, and all physicians, therapists, and physiotherapists of the Rehabilitation-Clinic Koenigstuhl, Heidelberg, who took part in the rehabilitation programme.
Funding
Funding to pay the Open Access publication charges for this article was provided by centre for pulmonary hypertension, Thoraxclinic at the University of Heidelberg.
Conflict of interest: none declared.
References
- 1. Mereles D, Ehlken N, Kreuscher S, Ghofrani S, Hoeper MM, Halank M, Meyer FJ, Karger G, Buss J, Juenger J, Holzapfel N, Opitz C, Winkler J, Herth FF, Wilkens H, Katus HA, Olschewski H, Grunig E. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation 2006;114:1482–1489. [DOI] [PubMed] [Google Scholar]
- 2. de Man FS, Handoko ML, Groepenhoff H, van 't Hul AJ, Abbink J, Koppers RJ, Grotjohan HP, Twisk JW, Bogaard HJ, Boonstra A, Postmus PE, Westerhof N, van der Laarse WJ, Vonk-Noordegraaf A. Effects of exercise training in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2009;34:669–675. [DOI] [PubMed] [Google Scholar]
- 3. Fox BD, Kassirer M, Weiss I, Raviv Y, Peled N, Shitrit D, Kramer MR. Ambulatory rehabilitation improves exercise capacity in patients with pulmonary hypertension. J Card Fail 2011;17:196–200. [DOI] [PubMed] [Google Scholar]
- 4. Grünig E, Lichtblau M, Ehlken N, Ghofrani HA, Reichenberger F, Staehler G, Halank M, Fischer C, Seyfarth HJ, Klose H, Meyer A, Sorichter S, Wilkens H, Rosenkranz S, Opitz C, Leuchte H, Karger G, Speich R, Nagel C. Safety and efficacy of exercise training in various forms of pulmonary hypertension. Eur Respir J 2012;40:84–92. [DOI] [PubMed] [Google Scholar]
- 5. Chan L, Chin LM, Kennedy M, Woolstenhulme JG, Nathan SD, Weinstein AA, Connors G, Weir NA, Drinkard B, Lamberti J, Keyser RE. Benefits of intensive treadmill exercise training on cardiorespiratory function and quality of life in patients with pulmonary hypertension. Chest 2013;143:333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagel C, Prange F, Guth S, Herb J, Ehlken N, Fischer C, Reichenberger F, Rosenkranz S, Seyfarth HJ, Mayer E, Halank M, Grünig E. Exercise training improves exercise capacity and quality of life in patients with inoperable or residual chronic thromboembolic pulmonary hypertension. PLoS ONE 2012;7:e41603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grunig E, Maier F, Ehlken N, Fischer C, Lichtblau M, Blank N, Fiehn C, Stockl F, Prange F, Staehler G, Reichenberger F, Tiede H, Halank M, Seyfarth HJ, Wagner S, Nagel C. Exercise training in pulmonary arterial hypertension associated with connective tissue diseases. Arthritis Res Ther 2012;14:R148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becker-Grunig T, Klose H, Ehlken N, Lichtblau M, Nagel C, Fischer C, Gorenflo M, Tiede H, Schranz D, Hager A, Kaemmerer H, Miera O, Ulrich S, Speich R, Uiker S, Grunig E. Efficacy of exercise training in pulmonary arterial hypertension associated with congenital heart disease. Int J Cardiol 2013;168:375–381. [DOI] [PubMed] [Google Scholar]
- 9. Weinstein AA, Chin LM, Keyser RE, Kennedy M, Nathan SD, Woolstenhulme JG, Connors G, Chan L. Effect of aerobic exercise training on fatigue and physical activity in patients with pulmonary arterial hypertension. Respir Med 2013;107:778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grünig E, Ehlken N, Ghofrani A, Staehler G, Meyer FJ, Juenger J, Opitz CF, Klose H, Wilkens H, Rosenkranz S, Olschewski H, Halank M. Effect of exercise and respiratory training on clinical progression and survival in patients with severe chronic pulmonary hypertension. Respiration 2011;81:394–401. [DOI] [PubMed] [Google Scholar]
- 11. Galie N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, McGoon MD, McLaughlin VV, Preston IR, Rubin LJ, Sandoval J, Seeger W, Keogh A. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol 2013;62(Suppl.):D60–D72. [DOI] [PubMed] [Google Scholar]
- 12. Galie N, Simonneau G, Barst RJ, Badesch D, Rubin L. Clinical worsening in trials of pulmonary arterial hypertension: results and implications. Curr Opin Pulm Med 2010;16(Suppl. 1):S11–S19. [DOI] [PubMed] [Google Scholar]
- 13. Weissmann N, Peters DM, Klopping C, Kruger K, Pilat C, Katta S, Seimetz M, Ghofrani HA, Schermuly RT, Witzenrath M, Seeger W, Grimminger F, Mooren FC. Structural and functional prevention of hypoxia-induced pulmonary hypertension by individualized exercise training in mice. Am J Physiol Lung Cell Mol Physiol 2014;306:L986–L995. [DOI] [PubMed] [Google Scholar]
- 14. Martinez-Quintana E, Miranda-Calderin G, Ugarte-Lopetegui A, Rodriguez-Gonzalez F. Rehabilitation program in adult congenital heart disease patients with pulmonary hypertension. Congenit Heart Dis 2010;5:44–50. [DOI] [PubMed] [Google Scholar]
- 15. Boutet K, Garcia G, Degano B, Gonzalves-Tavares M, Tcherakian C, Jais X, Humbert M, Escourrou P, Simonneau G, Sitbon O. Results of a 12-week outpatient cardiovascular rehabilitation in patients with idiopathic pulmonary arterial hypertension (PAH). Eur Respir J 2008;32(Suppl 52):240s–41s. [Google Scholar]
- 16. Colombo R, Siqueira R, Becker CU, Fernandes TG, Pires KM, Valenca SS, Souza-Rabbo MP, Araujo AS, Bello-Klein A. Effects of exercise on monocrotaline-induced changes in right heart function and pulmonary artery remodeling in rats. Can J Physiol Pharmacol 2013;91:38–44. [DOI] [PubMed] [Google Scholar]
- 17. Handoko ML, de Man FS, Happe CM, Schalij I, Musters RJ, Westerhof N, Postmus PE, Paulus WJ, van der Laarse WJ, Vonk-Noordegraaf A. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation 2009;120:42–49. [DOI] [PubMed] [Google Scholar]
- 18. Chaouat A, Sitbon O, Mercy M, Poncot-Mongars R, Provencher S, Guillaumot A, Gomez E, Selton-Suty C, Malvestio P, Regent D, Paris C, Herve P, Chabot F. Prognostic value of exercise pulmonary haemodynamics in pulmonary arterial hypertension. Eur Respir J 2014;44:704–713. [DOI] [PubMed] [Google Scholar]
- 19. Grunig E, Tiede H, Enyimayew EO, Ehlken N, Seyfarth HJ, Bossone E, D'Andrea A, Naeije R, Olschewski H, Ulrich S, Nagel C, Halank M, Fischer C. Assessment and prognostic relevance of right ventricular contractile reserve in patients with severe pulmonary hypertension. Circulation 2013;128:2005–2015. [DOI] [PubMed] [Google Scholar]
- 20. Blumberg FC, Arzt M, Lange T, Schroll S, Pfeifer M, Wensel R. Impact of right ventricular reserve on exercise capacity and survival in patients with pulmonary hypertension. Eur J Heart Fail 2013;15:771–775. [DOI] [PubMed] [Google Scholar]
- 21. Bossone E, D'Andrea A, D'Alto M, Citro R, Argiento P, Ferrara F, Cittadini A, Rubenfire M, Naeije R. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J Am Soc Echocardiogr 2013;26:1–14. [DOI] [PubMed] [Google Scholar]
- 22. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–381. [PubMed] [Google Scholar]
- 23. Erbs S, Linke A, Gielen S, Fiehn E, Walther C, Yu J, Adams V, Schuler G, Hambrecht R. Exercise training in patients with severe chronic heart failure: impact on left ventricular performance and cardiac size. A retrospective analysis of the Leipzig Heart Failure Training Trial. Eur J Cardiovasc Prev Rehabil 2003;10:336–344. [DOI] [PubMed] [Google Scholar]
- 24. Lewis GD, Bossone E, Naeije R, Grunig E, Saggar R, Lancellotti P, Ghio S, Varga J, Rajagopalan S, Oudiz R, Rubenfire M. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 2013;128:1470–1479. [DOI] [PubMed] [Google Scholar]
- 25. Argiento P, Vanderpool RR, Mule M, Russo MG, D'Alto M, Bossone E, Chesler NC, Naeije R. Exercise stress echocardiography of the pulmonary circulation: limits of normal and sex differences. Chest 2012;142:1158–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gorcsan J, III, Murali S, Counihan PJ, Mandarino WA, Kormos RL. Right ventricular performance and contractile reserve in patients with severe heart failure. Assessment by pressure–area relations and association with outcome. Circulation 1996;94:3190–3197. [DOI] [PubMed] [Google Scholar]
- 27. McLaughlin VV, Presberg KW, Doyle RL, Abman SH, McCrory DC, Fortin T, Ahearn G. Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest 2004;126(Suppl.):78s–92s. [DOI] [PubMed] [Google Scholar]
- 28. Andersen P. Capillary density in skeletal muscle of man. Acta Physiol Scand 1975;95:203–205. [DOI] [PubMed] [Google Scholar]
- 29. Brodal P, Ingjer F, Hermansen L. Capillary supply of skeletal muscle fibers in untrained and endurance-trained men. Am J Physiol 1977;232:H705–H712. [DOI] [PubMed] [Google Scholar]
- 30. Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev 1992;72:369–417. [DOI] [PubMed] [Google Scholar]
- 31. Coats AJ, Adamopoulos S, Radaelli A, McCance A, Meyer TE, Bernardi L, Solda PL, Davey P, Ormerod O, Forfar C, Conway J, Sleight P. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation 1992;85:2119–2131. [DOI] [PubMed] [Google Scholar]
- 32. Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation 1992;85:1751–1759. [DOI] [PubMed] [Google Scholar]
- 33. Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, Yu J, Adams V, Niebauer J, Schuler G. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation 1998;98:2709–2715. [DOI] [PubMed] [Google Scholar]
- 34. Gielen S, Adams V, Mobius-Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol 2003;42:861–868. [DOI] [PubMed] [Google Scholar]
- 35. Conraads VM, Beckers P, Bosmans J, De Clerck LS, Stevens WJ, Vrints CJ, Brutsaert DL. Combined endurance/resistance training reduces plasma TNF-alpha receptor levels in patients with chronic heart failure and coronary artery disease. Eur Heart J 2002;23:1854–1860. [DOI] [PubMed] [Google Scholar]
- 36. Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol 2005;98:1154–1162. [DOI] [PubMed] [Google Scholar]
- 37. Erbs S, Hollriegel R, Linke A, Beck EB, Adams V, Gielen S, Mobius-Winkler S, Sandri M, Krankel N, Hambrecht R, Schuler G. Exercise training in patients with advanced chronic heart failure (NYHA IIIb) promotes restoration of peripheral vasomotor function, induction of endogenous regeneration, and improvement of left ventricular function. Circ Heart Fail 2010;3:486–494. [DOI] [PubMed] [Google Scholar]
- 38. Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 2007;115:3086–3094. [DOI] [PubMed] [Google Scholar]