Abstract
Aims
Posttranslational modification, such as phosphorylation, plays an essential role in regulating activation of endothelial NO synthase (eNOS). In the present study, we aim to determine whether eNOS could be phosphorylated and regulated by a novel serine/threonine–protein kinase Pim1 in vascular endothelial cells (ECs).
Methods and results
Using immunoprecipitation and protein kinase assays, we demonstrated that Pim1 specifically interacts with eNOS, which leads to a marked phosphorylation of eNOS at Ser-633 and increased production of nitric oxide (NO). Intriguingly, in response to VEGF stimulation, eNOS phosphorylation at Ser-633 exhibits two distinct phases: transient phosphorylation occurring between 0 and 60 min and sustained phosphorylation occurring between 2 and 24 h, which are mediated by the protein kinase A (PKA) and Pim1, respectively. Inhibiting Pim1 by either pharmacological inhibitor SMI-4a or the dominant-negative form of Pim1 markedly attenuates VEGF-induced tube formation, while Pim1 overexpression significantly increases EC tube formation and migration in an NO-dependent manner. Importantly, Pim1 expression and eNOS phosphorylation at Ser-633 were substantially decreased in high glucose-treated ECs and in the aorta of db/db diabetic mice. Increased Pim1 expression ameliorates impaired vascular angiogenesis in diabetic mice, as determined by an ex vivo aortic ring assay.
Conclusion
Our findings demonstrate Pim1 as a novel kinase that is responsible for the phosphorylation of eNOS at Ser-633 and enhances EC sprouting of aortic rings from diabetic mice, suggesting that Pim1 could potentially serve as a novel therapeutic target for revascularization strategies.
Keywords: eNOS, Pim1, Phosphorylation, Angiogenesis, Diabetes
1. Introduction
Endothelium-derived nitric oxide (NO) is an important regulator of vascular function and plays a key role in regulation of leucocyte adhesion to the endothelium, endothelial cell (EC) survival, angiogenesis, and inflammation.1 NO is produced by endothelial NO synthase (eNOS), whose function is tightly controlled and modulated at the transcriptional, post-transcriptional, and phosphorylation levels.2 Phosphorylation plays critical roles in regulating eNOS activation in response to a variety of stimuli, and one such stimuli, hyperglycaemia, has been shown to decrease eNOS activity and NO production by impairing eNOS phosphorylation,3 which eventually leads to the development of cardiovascular complications in diabetes.
Several important phosphorylation sites have been identified, such as Tyr-81, Ser-114, Thr-495, Ser-615, Ser-633, Tyr-657, and Ser-1177 (in human eNOS).4–8 Among them, phosphorylation of eNOS at Ser-615, Tyr-81, Ser-633, and Ser-1177 are associated with eNOS activation and an increased production of NO,4,6,7,9 while phosphorylation at Thr-495 and Tyr-657 has been shown to attenuate eNOS activation.8 Phosphorylation of eNOS at Ser-1177, mainly by Akt, has extensively been studied to significantly augment eNOS activity, under various pathophysiological circumstances.4,5,9 eNOS has also been shown to be phosphorylated at residue Ser-633 and this phosphorylation has been shown to augment eNOS activity under both basal and stimulated conditions, hence playing an important role in the response of ECs to shear stress and statin treatment.10 Although protein kinase A (PKA), extracellular signal-regulated kinase (ERK) 1/2, and AMP-activated protein kinase (AMPK) have been shown to phosphorylate eNOS at Ser-633,10–12 the protein kinase(s) responsible for the eNOS phosphorylation at Ser-633, particularly under pathophysiological states, still remain largely obscured.
Pim kinases, composed of three members, namely Pim1, 2, and 3, are highly conserved between species.13 Pim1 is a serine/threonine–protein kinase with 313-amino acid residues that has been implicated in multiple important cellular functions, such as proliferation, differentiation, apoptosis, and tumorigenesis.14 At the molecular levels, Pim1 is positively regulated by transcription 3(STAT3) and protein kinase B (PKB/Akt), both of which function as essential modulators of insulin signalling in the heart. Indeed, studies of type I and type II diabetes showed decreased expression of Pim1 in the heart, indicating its potential role in diabetic cardiovascular complications.15 As a serine threonine kinase, Pim1 has been shown to preferentially recognize and phosphorylate the serine/threonine residues in the consensus sequence K/R-K/R-R-K/R-X-S/T, where X is a small side-chain amino acid neither basic nor acidic.16 Intriguingly, when we searched for the consensus Pim1 phosphorylation motif, we found that there was one consensus Pim1 phosphorylation site in eNOS located at Ser-633 (RKRKES). This prompted us to postulate that Pim1 might be a critical regulator implicated in eNOS activation. Therefore, in the present study, we investigated whether Pim1 is an upstream kinase that is capable of phosphorylating eNOS at Ser-633. Furthermore, we investigated whether it is functionally involved in regulating endothelial function under diabetic conditions.
2. Methods
2.1 Cell. culture
Human umbilical vein ECs (HUVECs) were purchased from ATCC and cultured in EBM-2 medium (Lonza) supplemented with EGM-2 BulletKit (Lonza). Cos7 cells were purchased from ATCC and cultured in DMEM supplemented with 10% FBS. HUVECs within three passages were used for the physiological studies.
2.2. Animal model of diabetes
Wild-type (WT) and db/db mice on C57BLKS/J background were obtained from Jackson Laboratory. Animals were fed either regular chow diet or high fat diet (HF) from 8 to 16 week olds as described previously.17 Mice were sacrificed by inhalation of CO2 for the collection of aorta and heart. Studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health following protocols that were reviewed and approved by the Institutional Animal Care and Use Committee at Thomas Jefferson University.
2.3. Plasmid constructs
eNOS mutant S633D and Pim1 mutant K67M (dominant-negative Pim1) cloned into pcDNA3 were constructed using QuikChange II Site-Directed Mutagenesis Kit (Agilent Technologies), and PCR primer was as follows: S633D, 5′-GGCGGAAGAGGAAGGAGGACAGTAACACAGACAGTG-3′ and 5′-CACTGTCTGTGTTACTGTCCTCCTTCCTCTTCCGCC-3′; S633A: 5′-GCGGAAGAGGAAGGA GGCCAGTAACACAGACAG-3′ and 5′-CTGTCTGTGTTACTGGCCTCCTTCCTCTTCCGC-3′; S1177A: 5′-CATACGCACCCAGGCCTTTTCCTTGCAGGAGCG-3′ and 5′-CGCTCCTGCAAGGAAAAGGCCTGGGTGCGTATG-3′; S1177D: 5′-CATACGCACCCAGGACTTTTCCTTGCAGGAGCG-3′ and 5′-CGCTCCTGCAAGGAAAAGTCCTGGGTGCGTATG-3′; K67M, 5′-TTGCCGGTGGCCATCATGCACGTGGAGAAGGAC-3′ and 5′-GTCCTTCTCCACGTGCATGATGGCCACCGGCAA-3′. Mutagenesis was confirmed by DNA sequencing.
2.4. Adenovirus construction
Adenoviruses harbouring Ad-dominant-negative Pim1 (Ad-DN Pim1) were made using AdMax (Microbix) as described previously.18 Ad-Pim1 (GFP-Tagged) was a gift from Dr Mark A. Sussman (San Diego Heart Research Institute, San Diego State University).
2.5. Immunoprecipitation and western blotting
Immunoprecipitation was performed as described previously.19 Blots were blocked with 5% non-fat milk in phosphate-buffered saline (PBS) with 0.1% Tween 20 (PBST) and then developed with diluted antibodies for Flag (1:1000 dilution; Genescript), eNOS (1:1000 dilution; BD), Pim1 (1:1000 dilution; Santa Cruz Biotechnology), p-eNOS Ser-633 (1:1000 dilution; BD), p-eNOS Ser-1177, Ser-114, Thr-495 (1;1000 dilution; Cell Signaling Technology) at 4°C overnight, followed by incubation with goat anti-rabbit IgG (H + L) (DyLight 680 conjugated, Thermo Scientific) or goat anti-mouse IgG (H + L) (DyLight 800 conjugated, Thermo Scientific) for 1 h. Blots were visualized on an Odyssey Imaging System (LI-COR). The intensity of the bands was quantified by using the Odyssey software.
2.6. Knockdown of Pim1 by small interfering RNA
Small interfering RNA (siRNA) oligonucleotides for human Pim1 (SASI_HS01_0007378: 5′-GAUAUGGUGUGUGGAGAUA-3′, and 5′-UAUCUCCACACACCAUAUC-3′) and a negative control siRNA (MISSION siRNA Universal Negative Control; Sigma-Aldrich) were used for the transfection of HUVEC with Lipofectamine® RNAiMAX transfecting reagent (Invitrogen) in serum-free medium according to the manufacturer's recommendation.
2.7. Real-time quantitative PCR
Real-time quantitative PCR (qRT-PCR) analysis was performed as described previously.19 The primer sequences are described as follows—Pim1: forward, 5′-TCATTAGATGGTGCTTGGCCCTGA-3′ and reverse, 5′-TGTGGAGGTGGATCTCAGCAGTTT-3′; Pim2: forward, 5′-GACACCGCCTCACAGATC-3′ and reverse, 5′-CCTGCACCCACTTTCCATAG-3′; Pim3: forward, 5′-GCTCTCCAAGTTCGGCTC-3′ and reverse, 5′-CTCTCCTTGTCCGCCTTG-3′; Human 18S: forward, 5′-TCAAGAACGAAAGTCGGAGG-3′ and reverse, 5′-GGACATCTAAGGGCATCAC-3′. Human 18S rRNA served as a control for the amount of cDNA present in each sample.
2.8. Determination of NO production in ECs
The NO production by ECs was assessed by quantification of the nitrite content in the supernatant. Nitrite and nitrate levels were determined by the use of a chemiluminescence NO detector (Siever 280i NO Analyser), as described previously.20
2.9. Matrigel angiogenesis assay in vitro
The 96-well culture plates were coated with 50 µL of growth factor-reduced Matrigel per well and then allowed to polymerize for 30 min at 37°C. HUVECs cultured for 24 h in EBM-2 medium were seeded on coated plates at a density of 2 × 104 cells per well in EBM-2 medium supplemented with 0.5% fetal bovine serum and the agents as indicated in the figure legend and then incubated for 18 h at 37°C. Cells were stained with 8 µg/mL Calcein AM. Pictures were taken at ×40 magnification with a digital output camera (Olympus DP11). Total tube length was measured by using the NIH Image program.
2.10. Scratched wound assay
HUVEC monolayer was scraped with a sterile pipette tip to generate a cell-free zone. The cells were then washed with medium and cultured for additional 24 h in the presence and absence of 0.5 mmol/L l-NG-nitroarginine methylester (l-NAME). Photographs were taken by phase-contrast microscopy (Olympus IX 71 microscope).
2.11. Ex vivo aortic ring assay
Mice aortic ring explant cultures were prepared by modification of protocols as described previously.21 96-well plates were covered by 30 µL of Matrigel (Becton Dickenson, Bedford, MA, USA). Aortic rings were prepared from WT and diabetic mice and sliced into 1- to 2-mm-thick rings under a dissecting microscope. Directly infect aortic rings at 8 to 24 rings per well of a 24-well plate by adding Ad-Pim1 or Ad-GFP virus (6.5 × 109 pfus) to each well. Aortic ring was placed on Matrigel in wells and covered with an additional 40 µL of Matrigel. The rings were cultured in 200 µL of endothelial medium (0.5% FBS) for 5–7 days. As microvessels began to branch and develop, some wells with aortic rings were kept as control and other wells were treated with reagents as indicated. Phase-contrast photos of individual explants were taken using an EVOS® FL Cell Imaging System. Analysis of the macrovessel outgrowth was done by a counting number of pixels by NIH Image software program.
2.12. Statistical analysis
Data are expressed as means ± SE. The statistical significance of differences was assessed by Student's t-test or analysis of variance (ANOVA) with Bonferroni's post hoc test, as appropriate; a value of P < 0.05 was considered statistically significant.
3. Results
3.1. Pim1 interacts with eNOS
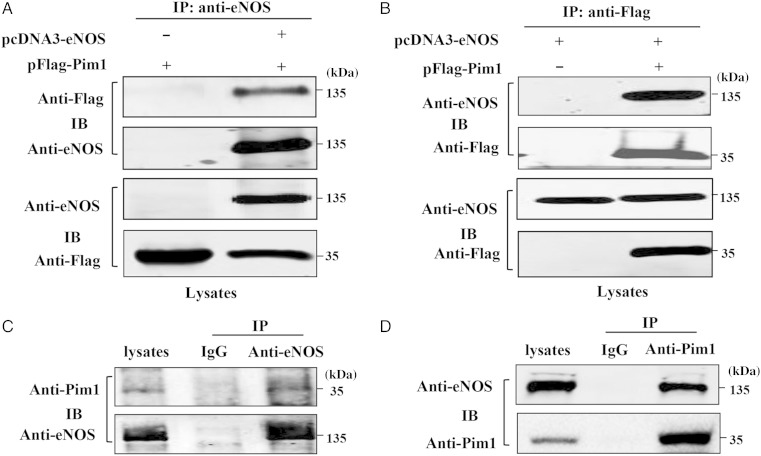
To elucidate the role of Pim1 in regulating eNOS activity, we studied whether eNOS interacts with Pim1 in mammalian cells. To this end, co-immunoprecipitation was performed in Cos7 cells transfected with eNOS and Pim1 expression vectors. Immunoprecipitation of eNOS led to co-immunoprecipitation of Flag-tagged Pim1 when both proteins were co-transfected (Figure 1A). As a control, the anti-eNOS antibody did not immunoprecipitate Flag-tagged Pim1 in the absence of eNOS. Similarly, immunoprecipitation of Flag-tagged Pim1 resulted in co-immunoprecipitation of eNOS, whereas the anti-Flag antibody did not immunoprecipitate eNOS in the absence of Flag-tagged Pim1 (Figure 1B). Together, these findings indicate that eNOS and Flag-tagged Pim1 exist in the same complex in mammalian cells. To further investigate whether Pim1 interacted endogenously with eNOS in ECs, we performed immunoprecipitation with anti-eNOS antibody, anti-Pim1 antibody, and control anti-IgG antibody, respectively, using lysates obtained from HUVECs. We found that endogenous eNOS co-precipitated with endogenous Pim1 in HUVEC cells (Figure 1C and D). Furthermore, we found that VEGF treatment (>2 h) significantly enhances the association of Pim1 and eNOS in HUVECs (see Supplementary material online, Figure S1). Taken together, our results clearly demonstrated that Pim1 interacts with eNOS in ECs.
Figure 1.
Pim1 interacts with eNOS. (A) and (B) Cos7 cells were co-transfected with Flag-Pim1 and eNOS plasmids. 48 h after co-transfection, immunoprecipitation was performed with either anti-eNOS antibody (A) or anti-Flag antibody (B) to detect the interaction of eNOS with Pim1. (C) and (D) Endogenous eNOS interacts with Pim1 in HUVECs, as determined by immunoprecipitation with anti-eNOS antibody (C) and anti-Pim1 antibody (D), respectively. All experiments were repeated three times.
3.2. Pim1 phosphorylates eNOS at Ser-633
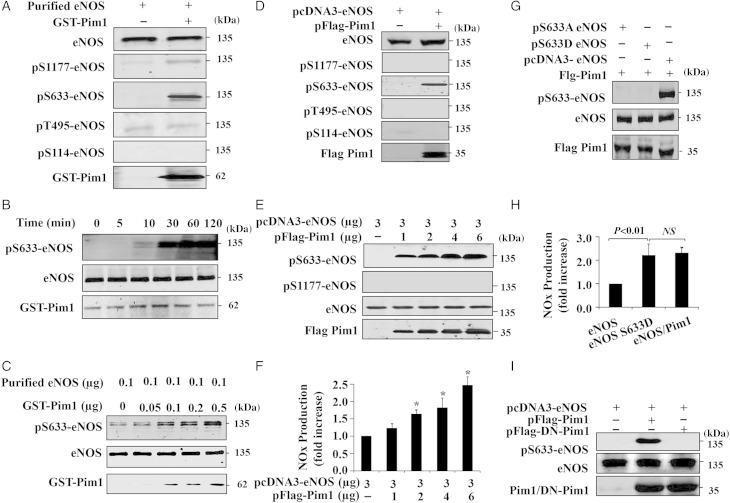
The interaction of Pim1 with eNOS prompted us to hypothesize that Pim1 might be a kinase that mediates eNOS phosphorylation. To test this hypothesis, we performed an in vitro kinase assay using recombinant eNOS and Pim1. We detected the phosphorylation of eNOS by using phospho-specific antibodies against Ser-1177, Ser-633, Thr-495, and Ser-114, respectively. As shown in Figure 2A, incubation of Pim1 with eNOS in the presence of ATP for 30 min led to a robust phosphorylation of eNOS at Ser-633 and a weak phosphorylation at Ser-1177 and Thr-495. In addition, we demonstrated that Pim1 induced eNOS phosphorylation at Ser-633 in a time- and dose-dependent manner, with a maximal phosphorylation observed at 60 min, after the addition of ATP (Figure 2B and C). Taken together, these results indicate that Pim1 efficiently phosphorylates eNOS at Ser-633 in an in vitro kinase assay.
Figure 2.
Pim1 phosphorylates eNOS. (A) In vitro phosphorylation assay indicates eNOS phosphorylation as detected by western blot using the indicated antibodies (repeated three times). (B) Recombinant eNOS and Pim1 were incubated with ATP and the kinase reaction was performed at different time points as indicated (repeated three times). (C) Recombinant eNOS was incubated with increasing amounts of Pim1 and then subjected to in vitro kinase assay (repeated four times). (D) Cos7 cells were co-transfected with eNOS and Pim1 expression vectors. 48 h after transfection, eNOS phosphorylation and expression were determined by western blot (repeated four times). (E) Cos7 cells were co-transfected with 3 µg eNOS expressing plasmid combined with increasing concentration of Pim1 expression vectors. Phosphorylation of eNOS was analysed by western blotting (repeated three times). (F) NO in cell culture medium from (E) was analysed and normalized to pcDNA3-eNOS group (n = 4). *P < 0.05 compared with pcDNA3-eNOS alone (one-way ANOVA). (G) Cos7 cells were co-transfected with either WT eNOS or eNOS mutants (S633A, S633D) plus Pim1 expression vectors. 36 h after transfection, cell lysates were subjected to western blot (repeated four times). (H) NO in cell culture medium (WT eNOS, WT eNOS plus Pim1 and S633D) was determined and normalized to eNOS group (n = 4) (one-way ANOVA). (I) Cos7 cells were co-transfected with WT eNOS or WT eNOS plus WT or dominant-negative Pim1 (DN-Pim1) expression vectors. 36 h after transfection, cell lysates were subjected to western blot analysis (repeated three times).
To determine whether Pim1 kinase phosphorylates eNOS in intact cells, we examined the phosphorylation of eNOS in Cos7 cells co-transfected with eNOS and Pim1 expression plasmids. As shown in Figure 2D, ectopic expression of Pim1 markedly induced eNOS phosphorylation at Ser-633, but it had no effect on the phosphorylation of eNOS at Ser-1177, Thr-495, and Ser-114, as determined by western blot analysis. Furthermore, dose-dependent co-transfection of Pim1 increased eNOS phosphorylation at Ser-633 (Figure 2E), with a concomitant increase in production of NO and cGMP levels in Cos-7 cells (Figure 2F and Supplementary material online, Figure S2A). To confirm the site in eNOS which phosphorylated by Pim1, WT, and eNOS mutant plasmids were co-transfected and western blot was then performed. As shown in Figure 2G, WT–eNOS was phosphorylated by Pim1 at Ser-633, but no phosphorylation was detected in either S633A or S633D eNOS mutant. In addition, co-transfection of Pim1 increased NO production comparable with eNOS phospho-mimetic mutant (S633D) (Figure 2H and Supplementary material online, Figure S2B). This finding was consistent with the previous notion, indicating that eNOS phosphorylation at Ser-633 up-regulates eNOS activity.10,11 Moreover, co-transfection of DN-Pim1 had no effect on eNOS phosphorylation at Ser-633 (Figure 2I), further suggesting that Pim1 kinase activity is indeed responsible for eNOS phosphorylation primarily at Ser-633.
3.3. VEGF induces sustained eNOS phosphorylation at Ser-633 through Pim1 kinase
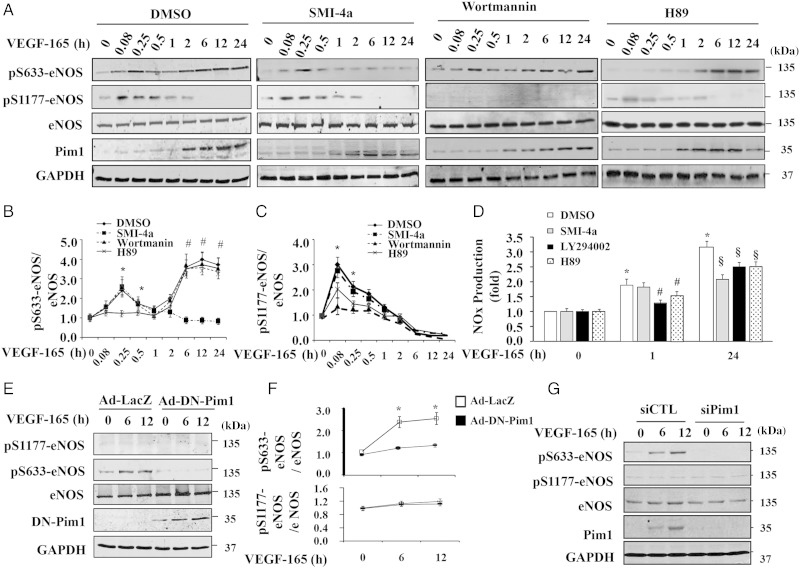
To investigate whether eNOS is a physiological substrate of Pim1, we examined eNOS phosphorylation in HUVECs in response to the stimulation of VEGF, which has been previously shown to induce expression of Pim1 in endothelia cells.22 eNOS phosphorylation in response to VEGF stimulation was determined in the presence and absence of three kinase inhibitors (e.g. SMI-4a, Pim1 inhibitor; Wortmannin, PI3K/Akt inhibitor; H89, PKA inhibitor) at different time points for up to 24 h by western blot analysis. Consistent with previous reports,23 VEGF induced a rapid phosphorylation of eNOS at Ser-1177 beginning at 5 min and lasting for up to 60 min, which was markedly inhibited by pre-treatment of cells with the PI3K inhibitor Wortmannin and slightly inhibited by PKA inhibitor H89 (Figure 3A–C), indicating that VEGF-induced phosphorylation of eNOS at Ser-1177 is mainly PI3K/Akt-dependent. As shown in Figure 3A, VEGF significantly induced Pim1 expression in a time-dependent manner, which began at 1 h and sustained for up to 24 h. Interestingly, VEGF-induced phosphorylation of eNOS at Ser-633 displayed a biphasic course. This biphasic course consisted of transient phosphorylation during the first 1 h of VEGF stimulation and sustained phosphorylation occurring between 1 and 24 h. Furthermore, treatment of HUVECs with the PKA inhibitor H89 significantly inhibited VEGF-induced transient eNOS phosphorylation at Ser-633 during the first 1 h stimulation, but barely affected VEGF-induced sustained phosphorylation of eNOS at Ser-633 (Figure 3B), indicating that VEGF-induced eNOS transient phosphorylation at Ser-633 was mainly mediated by PKA. Moreover, treatment of HUVECs with SMI-4a, a specific Pim1 inhibitor,24 completely inhibited VEGF-induced sustained phosphorylation of eNOS at Ser-633, but barely affected the VEGF-induced Ser-1177 and Ser-633 phosphorylation during the first 1 h stimulation (Figure 3B and C), suggesting that Pim1 is responsible for the VEGF-induced sustained eNOS phosphorylation at Ser-633. Furthermore, inhibition of Pim1 by SMI-4a substantially inhibited VEGF-stimulated NO accumulation, further indicating the physiological importance of Pim1 in VEGF-induced eNOS activation in ECs (Figure 3D). Furthermore, we found that thrombin and histamine induced transient phosphorylation of eNOS at Ser-1177 and Ser-633 beginning at 5 min and lasting for up to 30 min, but barely affected sustained eNOS phosphorylation and Pim1 expression (see Supplementary material online, Figure S3A and B), indicating that VEGF, thrombin, and histamine induce eNOS phosphorylation at Ser-633 probably via a different pathway. To address the specificity of pharmacological inhibition of Pim1 by SMI-4a, we transduced the cells with Ad-DN-Pim1. Likewise, adenovirus-mediated overexpression of DN-Pim1 completely inhibited VEGF-induced Ser-633 phosphorylation at 6 and 12 h after VEGF stimulation (Figure 3E and F). Transfection of HUVECs with Pim1-specific siRNA markedly inhibited both basal and VEGF-induced expression of Pim1 and eNOS phosphorylation at Ser-633 (Figure 3G). Taken together, our results suggested that VEGF stimulation induces a biphasic phosphorylation of eNOS: rapid and transient phosphorylation at Ser-1177 and Ser-633, which are mainly mediated by Akt and PKA pathways; and sustained phosphorylation of eNOS at Ser-633, which is largely dependent on the activity of Pim1 kinase (see Supplementary material online, Figure S4).
Figure 3.
VEGF induces Pim1 expression and sustained phosphorylation of eNOS at Ser-633 in HUVECs. (A) HUVECs were pre-treated with either DMSO or Pim1 inhibitor SMI-4a (10 µmol/L), PI3K inhibitor wortmannin (100 nmol/L), and PKA inhibitor H89 (10 µmol/L) for 2 h and then stimulated with 50 ng/mL VEGF-165 for different time points as indicated. Phosphorylation of eNOS and expression of eNOS and Pim1 were then analysed by western blot. (B) Quantitative analysis phosphorylation of Ser-633-eNOS (n = 4). *P < 0.05 compared with H89 at indicated time points; #P < 0.05 compared with SMI-4a at indicated time points by two-way ANOVA. (C) Quantitative analysis phosphorylation of Ser-1177-eNOS (n = 4). *P < 0.05 compared with LY294002 at indicated time points by two-way ANOVA. (D) NO in cell culture medium at 1 and 24 h after addition of VEGF was analysed and normalized to VEGF 0 h/DMSO group (n = 4). *P < 0.05 compared with DMSO at 0 h; #P < 0.05 vs. DMSO at 1 h after VEGF stimulation; §P < 0.05 vs. DMSO at 24 h after VEGF stimulation (two-way ANOVA). (E) DN-Pim1 attenuates VEGF-induced eNOS phosphorylation at Ser-633. HUVECs were transduced with either Ad-Lacz or Ad-DN-Pim1 virus (50 MOI). 48 h after transduction, cells were then stimulated with 50 ng/mL VEGF-165 for 6 and 12 h. The phosphorylation and expression of eNOS were determined by western blot. (F) Quantitative analysis of p-eNOS/total eNOS ratio (n = 4). *P < 0.05 compared with Ad-DN-Pim1 by two-way ANOVA. (G) 72 h after transfection of either Pim1 siRNA (siPim1) or control siRNA (siCTL), cells were then stimulated with 50 ng/mL VEGF-165 for 6 and 12 h. The eNOS phosphorylation was determined by western blot (n = 4).
3.4. Inhibition of Pim1 attenuates VEGF-induced tube formation
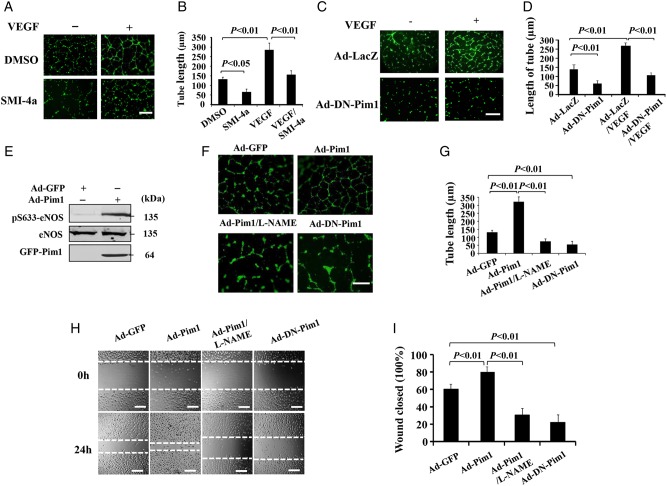
Pim1 phosphorylates eNOS at Ser-633 and promotes NO production, which prompted us to investigate whether Pim1 affects NO-mediated downstream events, such as angiogenesis. To this end, we performed Matrigel tube formation assay using HUVECs. As shown in Figure 4, VEGF strongly induces the tube formation, which was markedly inhibited by both Pim1 inhibitor SMI-4a (Figure 4A and B) and DN-Pim1 (Figure 4C and D). Furthermore, we investigated the potential role of Pim1 in ECs by adenovirus-mediated overexpression of Pim1. As expected, overexpression of Pim1 in HUVECs induced a robust phosphorylation of eNOS at Ser-633 (Figure 4E). Accordingly, endothelial tube formation was significantly induced by overexpression of Pim1, but not by DN-Pim1. This effect appeared to be NO-dependent, because it was inhibited by co-treatment with the NOS inhibitor l-NAME (Figure 4F and G). Likewise, overexpression of Pim1, but not DN-Pim1, significantly increased EC migration, which was also attenuated by the eNOS inhibitor l-NAME (Figure 4H and I). Taken together, these data demonstrated that Pim1 enhances angiogenic effects, at least in part, through the Pim1/NO pathway.
Figure 4.
Pim1 mediates VEGF-induced EC angiogenesis. (A) Phase-contrast photomicrograph demonstrates the endothelial in vitro tube formation on Matrigel. Scale bars = 400 μm. (B) Quantitative analysis of tube length from (A) (n = 20 field, repeated five times) (one-way ANOVA). (C) VEGF-induced the formation of capillary-like structures were inhibited by DN-Pim1. Scale bars = 400 μm. (D) Quantitative analysis of tube length from (C) (n = 20 field, repeated five times) (one-way ANOVA). (E) HUVECs were transduced with either Ad-Pim1 (GFP tagged) or Ad-GFP viruses. 48 h after transduction, eNOS phosphorylation and expression were analysed by western blotting using the indicated antibodies (n = 5). (F) HUVECs were infected with Ad-GFP, Ad-Pim1, or Ad-DN-Pim1 viruses. 36 h after transduction, the tube formation on the Matrigel was determined in the presence and absence of l-NAME. Scale bars = 400 μm. (G) Quantitative analysis of tube length was performed by using Image J as shown in (F) (n = 20 field, repeated five times) (one-way ANOVA). (H) HUVECs were transfected with either Ad-Pim1 or Ad-DN-pim1 virus for 36 h and then starved for 12 h. The HUVECs monolayer was scraped and cultured for additional 24 h in the presence and absence of 0.5 mmol/L l-NAME. The scratched areas were photographed. Scale bars = 200 μm. (I) Quantitative analysis of cell migration (n = 5) (one-way ANOVA).
3.5. High glucose impairs expression of Pim1 and phosphorylation of eNOS at Ser-633 in ECs
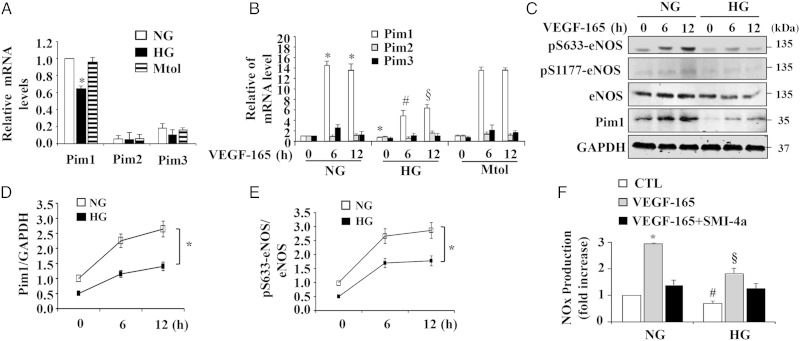
The Pim1, Pim2, and Pim3 serine/threonine kinases are highly conserved between species.13 As shown in Figure 5A, Pim1 is the predominant isoform of Pim kinase expressed in ECs, as determined by qRT-PCR. High glucose treatment substantially inhibited both basal and VEGF-stimulated Pim1 expression in HUVECs, but had no effect on expression of Pim2 and Pim3 (Figure 5A and B), indicating the specificity of high glucose on Pim1 expression in ECs. Consistent with mRNA levels, Pim1 protein was significantly increased after VEGF stimulation for 6 and 12 h. However, high glucose markedly attenuated the Pim1 protein expression under both basal and VEGF-stimulated conditions (Figure 5C and D). Likewise, phosphorylation of eNOS at Ser-633 at 6 and 12 h after VEGF stimulation was markedly inhibited under hyperglycaemic conditions (Figure 5C and E). Finally, both basal and VEGF-induced NO production were significantly impaired by high glucose treatment (Figure 5F and Supplementary material online, Figure S5). Taken together, these results suggested that Pim1 may play a critical role in endothelial dysfunction under hyperglycaemic conditions.
Figure 5.
High glucose attenuates Pim1 expression and eNOS phosphorylation in HUVECs. (A) and (B) HUVECs were treated with 5 mmol/L d-glucose (normal glucose, NG), 25 mmol/L d-glucose (high glucose, HG) or 5 mmol/L d-glucose plus 20 mmol/L d-mannitol (Mtol) for 48 h. The expression of Pim1, Pim2, and Pim3 were measured by quantitative PCR in the absence (A) or presence of 50 ng/mL VEGF-165 (B) stimulation for 6 h and 12 h (n = 3). *P < 0.05 compared with NG or Mtol group. #P < 0.05 compared with NG at 6 h after VEGF-165; §P < 0.05 compared with NG at 12 h after VEGF-165 (one-way ANOVA). (C) HUVECs were treated with 25 mmol/L d-glucose (high glucose, HG) or normal glucose (NG) for 48 h, and then stimulated with 50 ng/mL VEGF-165 for 6 and 12 h. Expression of Pim1, eNOS, and eNOS phosphorylation were measured by western blot. (D) and (E) Quantitative analysis of pim1 expression and eNOS phosphorylation at Ser-633 (n = 3). *P < 0.05 NG compare with HG (two-way ANOVA). (F) Normal glucose or high glucose-treated HUVECs were pre-treated with 10 µmol/L SMI-4a for 2 h, and then stimulated with VEGF-165 for 12 h, NO production was determined (n = 4). *P < 0.05 compared with NG/CTL or NG/SMI-4a; #P < 0.05 compared with NG/CTL; §P < 0.05 compared with NG/VEGF-165 (two-way ANOVA).
3.6. Overexpression of Pim1 augments EC sprouting of aortic rings from diabetic mice
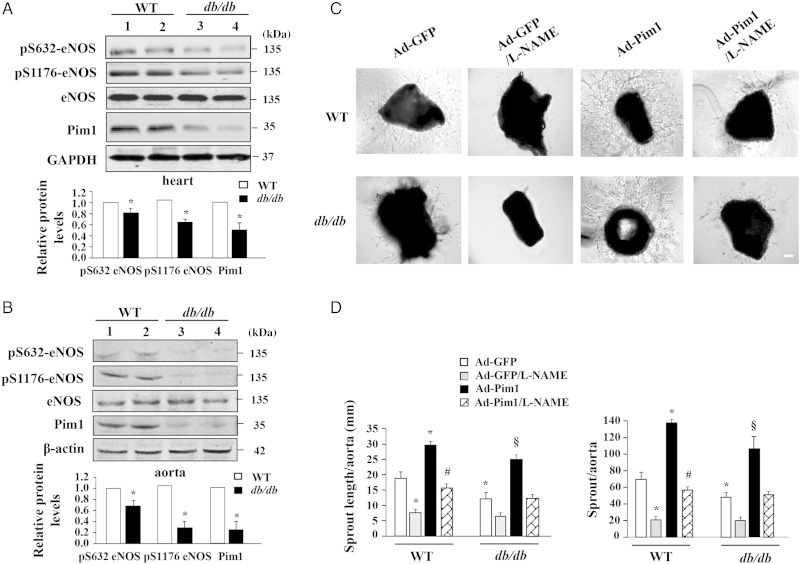
To further substantiate the functional significance of the Pim1/eNOS pathway under diabetic conditions, we investigated the expression of Pim1 and phosphorylation of eNOS in diabetic db/db mice fed with an HF, which exhibit some similarities to type 2 diabetic patients with dyslipidaemia, as reported previously.17 As shown in Figure 6A and B, Pim1 expression and eNOS phosphorylation at Ser-1176 and Ser-632 (mouse eNOS sequence) were significantly decreased in both diabetic aortas and hearts. Furthermore, we performed an ex vivo microvessel sprouting assay to define the functional significance of Pim1/eNOS in vascular angiogenesis by using aortic rings obtained from normal and diabetic mice. As shown in Figure 6C and D, EC sprouting was significantly attenuated in diabetic mice, when compared with normal control mice. Adenovirus-mediated overexpression of Pim1 markedly enhanced EC sprouting in aortic rings obtained from both normal and diabetic mice. Furthermore, the endothelial origin of the sprouts induced by Pim1 overexpression was confirmed by immunostaining with anti-CD31 antibody (see Supplementary material online, Figure S6). Taken together, our results demonstrated that the Pim1/eNOS pathway is impaired in diabetic mice, and overexpression of Pim1 could efficiently ameliorate the impairment of angiogenesis under diabetic conditions.
Figure 6.
Overexpression of Pim1 ameliorates impairment of angiogenesis in ex vivo. (A) and (B) Hearts and aortas were harvested from control C57BLKS/J mice (WT) and db/db mice. eNOS phosphorylation and expression of eNOS and Pim1 were analysed by western blot. *P < 0.01 compared with WT mice (Student's t-test), n = 6. (C) Aortic rings were incubated with Ad-Pim1 and Ad-GFP in opti-MEM medium for 24 h and then embedded in Matrigel. As microvessels began to branch and develop, some wells were pre-treated with 0.5 mmol/L l-NAME (n = 4 per group). Phase-contrast photos of individual explants were taken using EVOS® FL Cell Imaging System. (D) Analysis of the microvessel growth (sprout length and sprout number) was performed by image J program. *P < 0.05 compared with WT/Ad-GFP group; #P < 0.05 compared with WT/Ad-Pim1 group; §P < 0.01 compared with either db/db/Ad-GFP group or db/db/Ad-Pim1/L-NAME group (two-way ANOVA). n = 4. Scale bars = 2000 μm.
4. Discussion
In the present study, we, for the first time, identify Pim1 as a novel kinase that mediates eNOS phosphorylation at Ser-633 and induces NO production under both in vitro and ex vivo conditions. We also demonstrate the interaction between Pim1 and eNOS by co-immunoprecipitation of Pim1 and eNOS in native ECs and Cos7 cells co-transfected with eNOS and Pim1 cDNAs. We confirm phosphorylation of eNOS at Ser-633 by an in vitro kinase assay using recombinant eNOS and Pim1, as well as in intact cells by western blot analysis. Moreover, phosphorylation of eNOS by Pim1 substantially increased eNOS activity and NO production. In vascular ECs, VEGF substantially increased expression of Pim1, accompanied by a sustained phosphorylation of eNOS at Ser-633. Interestingly, our findings show that VEGF stimulation induces a biphasic phosphorylation of eNOS, with a rapid and transient phosphorylation at Ser-1177 and Ser-633, which are mainly mediated by Akt and PKA, respectively. In addition, VEGF-induced sustained phosphorylation of eNOS at Ser-633 mainly depends on the activity of Pim1 kinase. Ectopic expression of Pim1 significantly increases EC migration and tube formation. Importantly, reduced Pim1 expression is essentially involved in hyperglycaemia-induced inhibition of Ser-633 phosphorylation in both ECs and aortas from diabetic mice. Finally, we demonstrate that overexpression of Pim1 significantly ameliorates hyperglycaemia-induced impairment of microvessel sprouting. This serves as further indication that Pim1 may represent a novel therapeutic target for the treatment of diabetic vascular complications.
eNOS plays a critical role in maintaining cardiovascular homeostasis by controlling NO bioavailability, and its activity is tightly regulated at multiple levels.25 Phosphorylation is believed to be one of the major mechanisms regulating eNOS activation.23,26 To date, seven specific phosphorylation sites have been identified in human eNOS. These include Tyr81, Ser114, Thr495, Ser615, Ser633, Tyr657, and Ser1177.4–6,8,26 The relative contribution of these phosphorylation sites to eNOS activation, however, remains largely elusive. The eNOS phosphorylation of Ser-1177 is rapid and calcium dependent, which has been proposed to be critical for eNOS activation in response to several stimuli, including VEGF, shear stress, and 3-hydroxy-3-methylglutaryl-coenzyme A inhibitors (statins).4,11 In contrast to the Ser-1177 phosphorylation, eNOS phosphorylation at Ser-633 is relatively slower and calcium independent. So, phosphorylation at Ser-633 has been suggested to maintain persistent eNOS activity without changing the intracellular calcium level, after an initial activation induced by Ser-1177 phosphorylation.27,28 Indeed, several kinases, including PKA, PKG, and AMP-activated protein kinase (AMPK), have recently been shown to phosphorylate eNOS at Ser-633 and to mediate eNOS activation in response to various stimulations, including shear stress, VEGF, and statins.10,27,28 In contrast to most previous studies that normally examined eNOS phosphorylation within 2 h, we decided to examine eNOS phosphorylation for 24 h after VEGF stimulation in the present study. Strikingly, we found that, in addition to inducing a fast and transient phosphorylation of eNOS at Ser-1177 and Ser-633, VEGF robustly induced Pim1-dependent sustained eNOS phosphorylation at Ser-633, and this phosphorylation lasted for at least 24 h. In this regard, our findings, for the first time, provided compelling evidence, demonstrating that eNOS is a physiological substrate of Pim1. Additionally, Pim1-mediated eNOS phosphorylation may serve as an efficient mechanism for sustained eNOS activation under certain pathophysiological conditions.
The biological role of Pim1 has previously been reported for ECs and vascular smooth muscle cells (VSMCs).29,30 For example, the loss of Pim1 has been shown to increase the EC adhesiveness and inhibit VSMC proliferation.29,30 Furthermore, accumulating evidence indicates that Pim1 is expressed in vivo in ECs during angiogenesis and induced by VEGF in HUVECs.22,31 Inhibition of Pim1 has been shown to inhibit VEGF-induced proliferation and migration and to attenuate capillary formation and EC sprouting in ECs, although the molecular mechanism mediating this process is completely unknown.22,30 Since eNOS-derived NO has been shown to be critically involved in EC proliferation, migration, and in vivo angiogenesis,23,32 the identification of eNOS as a substrate of Pim1 in ECs may not only provide novel mechanistic insights into the Pim1-mediated angiogenesis. It may also unveil a potential therapeutic target for the treatment of angiogenesis-related human diseases, such as cancer metastasis. Indeed, the pathological roles of Pim1 in cancer progression and metastasis have recently received considerable attention.33 Furthermore, inhibition of Pim1 kinase activity has been shown to diminish cancer cell migration and invasion.14 In this regard, it would be very interesting to determine whether Pim1-mediated angiogenesis is involved in cancer progression and metastasis, and whether Pim1 is a valid target of anti-angiogenic cancer therapies.
NO plays an important role in modulating vascular homeostasis.34 Endothelial dysfunction, as characterized by reduced NO bioavailability, is a hallmark of diabetic cardiovascular complications in both human and animal models.6,8 Although the reduced phosphorylation of eNOS is not seen in all papers using db/db models, our results are consistent with previous reports indicating that eNOS phosphorylation at S1176 was diminished in vasculature of db/db mice.35,36 Several recent studies have indicated that Pim1 might be involved in diabetic cardiomyopathy,37 and its roles in endothelial dysfunction, however, remain largely unknown. Indeed, researchers have shown that expression of Pim1 is significantly decreased in the hearts of diabetic mice.15 Increased Pim1 expression by AAV9-mediated gene delivery significantly improves diastolic function and prevents left ventricular dilation and failure in diabetic mice.15 In the present study, we found that Pim1 expression is markedly reduced in both hearts and aortas of db/db diabetic mice. Accordingly, phosphorylation of eNOS at Ser-633 and aortic sprouting were substantially attenuated in diabetic mice, further implicating the functional significance of the Pim1/eNOS pathway in endothelial dysfunction and angiogenesis impairment in diabetes. Importantly, Pim1 was recently shown to play critical roles in preserving mitochondrial integrity in cardiac cells under various pathological conditions.38–40 Since hyperglycaemia-induced mitochondrial dysfunction, increased ROS production, and apoptosis in vascular tissues have been implicated in endothelial dysfunction and impaired angiogenesis in diabetes,41,42 it would be interesting to investigate whether Pim1 protects endothelial function through its anti-oxidative and anti-apoptotic effects under diabetic states. Furthermore, since laminar shear stress has been shown to increase Pim1 expression in mesenchymal stem cells,43 it would be interesting to investigate whether shear stress increases Pim1 expression in ECs and whether Pim1-induced eNOS Ser-633 phosphorylation mediates eNOS activation in response to shear stress. Ongoing studies are currently testing these hypotheses.
In summary, we have shown that Pim1 interacts with eNOS in ECs. This interaction is specific, as it induces eNOS phosphorylation at Ser-633; it is functional, as it leads to increase eNOS activity; and it is patho-physiologically important, as it mediates angiogenic effects under both basal and diabetic conditions. These findings suggest that Pim1 may be an important therapeutic target for the treatment of vascular complications under diabetic states.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This research was supported by the U.S. National Institutes of Health (HL103869) and the Chinese Natural Science Foundation (no. 81170114 and no. 81370418) to J.S.
Acknowledgements
We thank Jennifer Wilson for editing the manuscript and Dr Mark Sussman from SDSU Heart Institute for kindly providing Pim1 adenovirus.
Conflict of interest: none declared.
References
- 1.Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch 2010;459:793–806. [DOI] [PubMed] [Google Scholar]
- 2.Qian J, Fulton D. Post-translational regulation of endothelial nitric oxide synthase in vascular endothelium. Front Physiol 2013;4:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musicki B, Burnett AL. Endothelial dysfunction in diabetic erectile dysfunction. Int J Impot Res 2007;19:129–138. [DOI] [PubMed] [Google Scholar]
- 4.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 1999;399:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 2001;88:E68–E75. [DOI] [PubMed] [Google Scholar]
- 6.Fulton D, Ruan L, Sood SG, Li C, Zhang Q, Venema RC. Agonist-stimulated endothelial nitric oxide synthase activation and vascular relaxation. Role of eNOS phosphorylation at Tyr83. Circ Res 2008;102:497–504. [DOI] [PubMed] [Google Scholar]
- 7.Michell BJ, Harris MB, Chen ZP, Ju H, Venema VJ, Blackstone MA, Huang W, Venema RC, Kemp BE. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J Biol Chem 2002;277:42344–42351. [DOI] [PubMed] [Google Scholar]
- 8.Loot AE, Schreiber JG, Fisslthaler B, Fleming I. Angiotensin II impairs endothelial function via tyrosine phosphorylation of the endothelial nitric oxide synthase. J Exp Med 2009;206:2889–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo Z, Fujio Y, Kureishi Y, Rudic RD, Daumerie G, Fulton D, Sessa WC, Walsh K. Acute modulation of endothelial Akt/PKB activity alters nitric oxide-dependent vasomotor activity in vivo. J Clin Invest 2000;106:493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y, Zhu Y, DeFea K, Pan S, Tsai MD, Shyy JY. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res 2009;104:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, Jo H. Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol 2002;283:H1819–H1828. [DOI] [PubMed] [Google Scholar]
- 12.Xiao Z, Wang T, Qin H, Huang C, Feng Y, Xia Y. Endoplasmic reticulum Ca2+ release modulates endothelial nitric-oxide synthase via extracellular signal-regulated kinase (ERK) 1/2-mediated serine 635 phosphorylation. J Biol Chem 2011;286:20100–20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narlik-Grassow M, Blanco-Aparicio C, Carnero A. The PIM family of serine/threonine kinases in cancer. Med Res Rev 2014;34:136–159. [DOI] [PubMed] [Google Scholar]
- 14.Nawijn MC, Alendar A, Berns A. For better or for worse: the role of Pim oncogenes in tumorigenesis. Nat Rev Cancer 2011;11:23–34. [DOI] [PubMed] [Google Scholar]
- 15.Katare R, Caporali A, Zentilin L, Avolio E, Sala-Newby G, Oikawa A, Cesselli D, Beltrami AP, Giacca M, Emanueli C, Madeddu P. Intravenous gene therapy with PIM-1 via a cardiotropic viral vector halts the progression of diabetic cardiomyopathy through promotion of prosurvival signaling. Circ Res 2011;108:1238–1251. [DOI] [PubMed] [Google Scholar]
- 16.Trinh TB, Xiao Q, Pei D. Profiling the substrate specificity of protein kinases by on-bead screening of peptide libraries. Biochemistry 2013;52:5645–5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi K, Forte TM, Taniguchi S, Ishida BY, Oka K, Chan L. The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of Western diet feeding. Metabolism 2000;49:22–31. [DOI] [PubMed] [Google Scholar]
- 18.You B, Jiang YY, Chen S, Yan G, Sun J. The orphan nuclear receptor Nur77 suppresses endothelial cell activation through induction of IkappaBalpha expression. Circ Res 2009;104:742–749. [DOI] [PubMed] [Google Scholar]
- 19.Chen M, Yi B, Sun J. Inhibition of cardiomyocyte hypertrophy by protein arginine methyltransferase 5. J Biol Chem 2014;289:24325–24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Q, Yuan Y, Yi W, Lau WB, Wang Y, Wang X, Sun Y, Lopez BL, Christopher TA, Peterson JM, Wong GW, Yu S, Yi D, Ma XL. C1q/TNF-related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler Thromb Vasc Biol 2011;31:2616–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker M, Robinson SD, Lechertier T, Barber PR, Tavora B, D'Amico G, Jones DT, Vojnovic B, Hodivala-Dilke K. Use of the mouse aortic ring assay to study angiogenesis. Nat Protoc 2012;7:89–104. [DOI] [PubMed] [Google Scholar]
- 22.Zippo A, De Robertis A, Serafini R, Oliviero S. PIM1-dependent phosphorylation of histone H3 at serine 10 is required for MYC-dependent transcriptional activation and oncogenic transformation. Nat Cell Biol 2007;9:932–944. [DOI] [PubMed] [Google Scholar]
- 23.Sun J, Liao JK. Induction of angiogenesis by heat shock protein 90 mediated by protein kinase Akt and endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol 2004;24:2238–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin YW, Beharry ZM, Hill EG, Song JH, Wang W, Xia Z, Zhang Z, Aplan PD, Aster JC, Smith CD, Kraft AS. A small molecule inhibitor of Pim protein kinases blocks the growth of precursor T-cell lymphoblastic leukemia/lymphoma. Blood 2010;115:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun J, Liao JK. Functional interaction of endothelial nitric oxide synthase with a voltage-dependent anion channel. Proc Natl Acad Sci USA 2002;99:13108–13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 2007;42:271–279. [DOI] [PubMed] [Google Scholar]
- 27.Butt E, Bernhardt M, Smolenski A, Kotsonis P, Frohlich LG, Sickmann A, Meyer HE, Lohmann SM, Schmidt HH. Endothelial nitric-oxide synthase (type III) is activated and becomes calcium independent upon phosphorylation by cyclic nucleotide-dependent protein kinases. J Biol Chem 2000;275:5179–5187. [DOI] [PubMed] [Google Scholar]
- 28.Boo YC, Sorescu GP, Bauer PM, Fulton D, Kemp BE, Harrison DG, Sessa WC, Jo H. Endothelial NO synthase phosphorylated at SER635 produces NO without requiring intracellular calcium increase. Free Radic Biol Med 2003;35:729–741. [DOI] [PubMed] [Google Scholar]
- 29.Willert M, Augstein A, Poitz DM, Schmeisser A, Strasser RH, Braun-Dullaeus RC. Transcriptional regulation of Pim-1 kinase in vascular smooth muscle cells and its role for proliferation. Basic Res Cardiol 2010;105:267–277. [DOI] [PubMed] [Google Scholar]
- 30.Walpen T, Peier M, Haas E, Kalus I, Schwaller J, Battegay E, Humar R. Loss of pim1 imposes a hyperadhesive phenotype on endothelial cells. Cell Physiol Biochem 2012;30:1083–1096. [DOI] [PubMed] [Google Scholar]
- 31.Zippo A, De Robertis A, Bardelli M, Galvagni F, Oliviero S. Identification of Flk-1 target genes in vasculogenesis: Pim-1 is required for endothelial and mural cell differentiation in vitro. Blood 2004;103:4536–4544. [DOI] [PubMed] [Google Scholar]
- 32.Kawasaki K, Smith RS Jr., Hsieh CM, Sun J, Chao J, Liao JK. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol Cell Biol 2003;23:5726–5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cen B, Xiong Y, Song JH, Mahajan S, DuPont R, McEachern K, DeAngelo DJ, Cortes JE, Minden MD, Ebens A, Mims A, LaRue AC, Kraft AS. The Pim-1 protein kinase is an important regulator of MET receptor tyrosine kinase levels and signaling. Mol Cell Biol 2014;34:2517–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Triggle CR, Ding H. A review of endothelial dysfunction in diabetes: a focus on the contribution of a dysfunctional eNOS. J Am Soc Hypertens 2010;4:102–115. [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Atochin D, Kashiwagi S, Earle J, Wang A, Mandeville E, Hayakawa K, d'Uscio LV, Lo EH, Katusic Z, Sessa W, Huang PL. Deficient eNOS phosphorylation is a mechanism for diabetic vascular dysfunction contributing to increased stroke size. Stroke 2013;44:3183–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong JC, Yu XY, Huang Y, Yung LM, Lau CW, Lin SG. Apelin modulates aortic vascular tone via endothelial nitric oxide synthase phosphorylation pathway in diabetic mice. Cardiovasc Res 2007;74:388–395. [DOI] [PubMed] [Google Scholar]
- 37.Moore A, Shindikar A, Fomison-Nurse I, Riu F, Munasinghe PE, Ram TP, Saxena P, Coffey S, Bunton RW, Galvin IF, Williams MJ, Emanueli C, Madeddu P, Katare R. Rapid onset of cardiomyopathy in STZ-induced female diabetic mice involves the downregulation of pro-survival Pim-1. Cardiovasc Diabetol 2014;13:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borillo GA, Mason M, Quijada P, Volkers M, Cottage C, McGregor M, Din S, Fischer K, Gude N, Avitabile D, Barlow S, Alvarez R, Truffa S, Whittaker R, Glassy MS, Gustafsson AB, Miyamoto S, Glembotski CC, Gottlieb RA, Brown JH, Sussman MA. Pim-1 kinase protects mitochondrial integrity in cardiomyocytes. Circ Res 2010;106:1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Din S, Mason M, Volkers M, Johnson B, Cottage CT, Wang Z, Joyo AY, Quijada P, Erhardt P, Magnuson NS, Konstandin MH, Sussman MA. Pim-1 preserves mitochondrial morphology by inhibiting dynamin-related protein 1 translocation. Proc Natl Acad Sci USA 2013;110:5969–5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Din S, Konstandin MH, Johnson B, Emathinger J, Volkers M, Toko H, Collins B, Ormachea L, Samse K, Kubli DA, De La Torre A, Kraft AS, Gustafsson AB, Kelly DP, Sussman MA. Metabolic dysfunction consistent with premature aging results from deletion of Pim kinases. Circ Res 2014;115:376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davidson SM, Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res 2007;100:1128–1141. [DOI] [PubMed] [Google Scholar]
- 42.Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med 2012;2012:918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tu ML, Wang HQ, Sun XD, Chen LJ, Peng XC, Yuan YH, Li RM, Ruan XZ, Li DS, Xu YJ, Ke ZJ. Pim-1 is up-regulated by shear stress and is involved in shear stress-induced proliferation of rat mesenchymal stem cells. Life Sci 2011;88:233–238. [DOI] [PubMed] [Google Scholar]