Oral vancomycin and oral metronidazole have several limitations with regard to their use in the treatment of Clostridium difficile infections (CDIs); however, oral vancomycin has been considered the gold standard in clinical trials. In June 2012, fidaxomicin received Health Canada approval for the treatment of CDIs. Its chemistry, mechanisms of action and pharmacological properties are discussed, along with its potential role in CDI therapy.
Keywords: Clostridium difficile, Fidaxomicin, Infection, Recurrence, Treatment
Abstract
BACKGROUND:
Due to the limitations of existing treatment options for Clostridium difficile infection (CDI), new therapies are needed.
OBJECTIVE:
To review the available data on fidaxomicin regarding chemistry, mechanisms of action and resistance, in vitro activity, pharmacokinetic and pharmacodynamic properties, efficacy and safety in clinical trials, and place in therapy.
METHODS:
A search of PubMed using the terms “fidaxomicin”, “OPT-80”, “PAR-101”, “OP-1118”, “difimicin”, “tiacumicin” and “lipiarmycin” was performed. All English-language articles from January 1983 to November 2014 were reviewed, as well as bibliographies of all articles.
RESULTS:
Fidaxomicin is the first macrocyclic lactone antibiotic with activity versus C difficile. It inhibits RNA polymerase, therefore, preventing transcription. Fidaxomicin (and its active metabolite OP-1118) is bactericidal against C difficile and exhibits a prolonged postantibiotic effect (approximately 10 h). Other than for C difficile, fidaxomicin demonstrated only moderate inhibitory activity against Gram-positive bacteria and was a poor inhibitor of normal colonic flora, including anaerobes and enteric Gram-negative bacilli. After oral administration (200 mg two times per day for 10 days), fidaxomicin achieved low serum concentration levels but high fecal concentration levels (mean approximately 1400 μg/g stool). Phase 3 clinical trials involving adults with CDI demonstrated that 200 mg fidaxomicin twice daily for 10 days was noninferior to 125 mg oral vancomycin four times daily for 10 days in regard to clinical response at the end of therapy. Fidaxomicin was, however, reported to be superior to oral vancomycin in reducing recurrent CDI and achieving a sustained clinical response (assessed at day 28) for patients infected with non-BI/NAP1/027 strains.
CONCLUSION:
Fidaxomicin was noninferior to oral vancomycin with regard to clinical response at the end of CDI therapy. Fidaxomicin has been demonstated to be as safe as oral vancomycin, but superior to vancomycin in achieving a sustained clinical response for CDI in patients infected with non-BI/NAP1/027 strains. Caution should be exercised in using fidaxomicin monotherapy for treatment of severe complicated CDI because limited data are available. Whether fidaxomicin is cost effective (due to its significantly higher acquisition cost versus oral vancomycin) depends on the acceptable willingness to pay threshold per quality-adjusted life year as a measure of assessing cost effectiveness.
Abstract
HISTORIQUE :
Étant donné le peu de traitements de l’infection à Clostridium difficile (ICD), il faut en trouver de nouveaux.
OBJECTIF :
Examiner les données sur les caractéristiques chimiques, les mécanismes d’action, la résistance, l’activité in vitro, les propriétés pharmacocinétiques et pharmacodynamiques, l’efficacité et l’innocuité de la fidaxomicine dans les essais cliniques, ainsi que la place qu’elle occupe dans les traitements.
MÉTHODOLOGIE :
Les chercheurs ont fouillé dans PubMed à l’aide des termes fidaxomicin, OPT-80, PAR-101, OP-1118, difimicin, tiacumicin et lipiarmycin. Ils en ont extrait tous les articles en anglais entre janvier 1983 et novembre 2014, de même que les bibliographies de tous les articles.
RÉSULTATS :
La fidaxomicine est la première lactone macrocyclique à résister au C difficile. Elle inhibe la polymérase de l’ARN et, par conséquent, en empêche la transcription. La fidaxomicine (et son métabolite actif, l’OP-1118) est bactéricide contre le C difficile et possède un effet postantibiotique prolongé (environ dix heures). À par d’autres infections que le C difficile, la fidaxomicine a une activité inhibitrice modérée contre les bactéries Gram positif et est un mauvais inhibiteur de la flore colique normale, y compris les anaérobies et les bacilles entériques Gram négatif. Après son administration par voie orale (200 mg deux fois par jour pendant dix jours), la fidaxomicine était peu concentrée dans le sérum, mais très concentrée dans les selles (moyenne d’environ 1 400 μg/g par selle). Des essais cliniques de phase 3 auprès d’adultes atteints d’une ICD a démontré qu’à la fin du traitement, la réponse clinique à la prise de 200 mg de fidaxomicine pendant dix jours n’était pas inférieure à celle de la prise de 125 mg de vancomycine par voie orale quatre fois par jour pendant dix jours. La fidaxomicine était toutefois supérieure à la vancomycine par voie orale pour réduire les ICD récurrentes et parvenir à une réponse clinique soutenue (évaluée le jour 28) chez les patients infectés par d’autres souches que les BI/NAP1/027.
CONCLUSION :
La réponse clinique de la fidaxomicine n’était pas inférieure à celle de la vancomycine par voie orale à la fin du traitement de l’ICD. Il est démontré que la fidaxomicine est tout aussi sécuritaire que la vancomycine par voie orale, mais qu’elle est supérieure à cet antibiotique pour assurer une réponse clinique soutenue à l’ICD chez les patients infectés par d’autres souches que les BI/NAP1/027. Il faut faire preuve de prudence lorsqu’on utilise la monothérapie à la fidaxomicine pour traiter une IDC très complexe, car il existe peu de données sur le sujet. L’efficience de la fidaxomicine (en raison de son coût d’acquisition beaucoup plus élevé que celui de la vancomycine par voie orale) dépend de la volonté acceptable de payer un seuil par année de vie pondérée par la qualité
Until 2011, oral vancomycin was the only therapy approved by the United States (US) Food and Drug Administration to treat Clostridium difficile infections (CDIs), and is currently considered the gold standard comparator in clinical trials. The most recent US guidelines for treating CDIs, published by the Society for Healthcare and Epidemiology of America and the Infectious Disease Society of America, recommends immediate cessation of antimicrobial treatment followed by therapy with oral metronidazole or oral vancomycin (1). Oral metronidazole at a dose of 500 mg three times daily for 10 to 14 days is preferred for a mild or moderate first episode. Oral vancomycin at 125 mg four times daily for 10 to 14 days is the agent of choice for a severe first episode. In severe, complicated CDI cases (presence of hypotension, ileus, shock or megacolon), oral vancomycin with or without intravenous metronidazole is recommended. First recurrent CDIs are to be treated similarly to an initial episode, while a tapered and/or pulse regimen of oral vancomycin is recommended for second and subsequent episodes.
Several limitations exist with current CDI therapies. While oral metronidazole is effective in treating mild to moderate CDIs (2), it has been demonstrated to be less effective than oral vancomycin for severe CDIs in two of three clinical trials (2–6). Oral vancomycin disrupts normal gut flora (7), and has a four times per day dosing regimen (1,8). Both oral vancomycin and oral metronidazole have been associated with colonization of vancomycin-resistant enterococci (VRE) (9–11). Recurrent infection occurs in approximately 20% to 30% of patients (12–14), with higher CDI recurrence rates observed in patients who have experienced multiple episodes (12) and in subgroups of high-risk patients (oncology, renal impairment, concomitant antibiotics, increased age, previous CDI episode) (15–19).
Fidaxomicin (previously known as OPT-80, PAR-101, tiacumicin B and difimicin) received Health Canada approval (Dificid, Merck and Co, USA) in June 2012 for the treatment of adults with a CDI (Dificid product monograph) (20). Fidaxomicin is marketed as a 200 mg tablet and is recommended to be administered orally twice daily for 10 days. The purpose of the present article was to review the available data on fidaxomicin regarding chemistry, mechanisms of action and resistance, in vitro activity, pharmacokinetic and pharmacodynamic properties, efficacy and safety in clinical trials, and place in therapy.
CHEMISTRY
Fidaxomicin is a first-in-class macrocyclic antibacterial agent for treatment of CDIs (21). It is an unsaturated, 18-membered macrocyclic lactone ring with a 7-carbon sugar constituent at carbon 12 and a 6-deoxy sugar at carbon 21 (Figure 1). Fidaxomicin is produced as a byproduct of fermentation by the actinomycete Dactylosporangium aurantiacum subspecies hamdenesis and has a molecular weight of 1056 g/mol. In vivo, fidaxomicin is primarily hydrolyzed at the fourth position isobutyryl ester by an unknown esterase to produce its main metabolite, OP-1118, which also provides resistance against C difficile.
Figure 1).
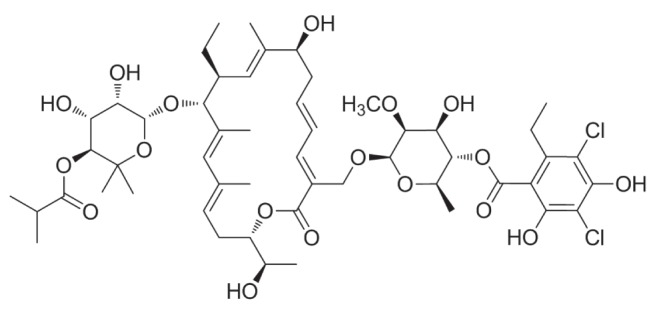
Chemical structure of fidaxomicin
MECHANISM OF ACTION
Fidaxomicin produces its antibacterial effects by inhibiting bacterial RNA polymerase at transcription initiation (22,23). Although fidaxomicin and rifamycins are both inhibitors of bacterial transcription, fidaxomicin acts at an earlier step in the transcription initiation pathway (24). Specifically, fidaxomicin binds to the DNA template-RNA polymerase complex and prevents the initial separation of DNA strands (ie, formation of the open DNA template-RNA polymerase complex), which precedes messenger RNA synthesis by inhibiting the σ subunit (23,25). Fidaxomicin’s unique target site may explain its limited spectrum of antimicrobial activity because σ subunits differ among bacterial species (26). Isolates of C difficile resistant to rifamycins or to other antimicrobial classes (cephalosporins, fluoroquinolones, clindamycin) are not cross-resistant to fidaxomicin (23,24,27).
MECHANISM OF RESISTANCE
A study to determine the frequency of spontaneous (single step) resistance to fidaxomicin at four and eight times the minimum inhibitory concentration (MIC) in C difficile demonstrated low mutation rates (<1.4×10−9) (24). The resistant clones demonstrated stable reduced susceptibility (MICs of 2 μg/mL or 4 μg/mL) and carried mutations in either rpoB (Gln1074Lys or Val1143Phe) or rpoC (Asp237Tyr) genes, which cluster around the fidaxomicin binding site on RNA polymerase and did not demonstrate cross-resistance with other classes of antibiotics, including rifamycins (24). Another study using site-directed mutagenesis revealed that an isolate of C difficile with a Val1143Asp mutation demonstrated impaired fitness and delayed growth (28). Other reported laboratory-generated mutations included β’ Arg89Gly, β Gln1074His, β Val1143Gln and β Val1143Asp (29).
Resistance to fidaxomicin did not develop during treatment in either phase 3 clinical study, although a single isolate from a cured patient (200 mg twice daily of fidaxomicin for 10 days) presented an elevated fidaxomicin MIC of 16 μg/mL at the time of recurrence (30). This isolate contained a single mutation in rpoC (Val1143Gly) (31).
Babakhani et al (32) and Leeds et al (33) generated stable (fidaxomicin MIC 1 μg/mL to 4 μg/mL) mutants by serial passage in the laboratory. Leeds et al (33) found mutations in rpoB and CD22120 (marR homologue), a mechanism outside of the RNA polymerase. Based on the available data, resistance to fidaxomicin is not expected; however, prospective collection of long-term surveillance data is prudent.
MICROBIOLOGY
Fidaxomicin is a narrow-spectrum agent that has been demonstrated to be selectively active against Gram-positive anaerobes (Table 1), including Clostridium (particularly C difficile and Clostridium perfringens) (31,34–41). It is less active against Gram-positive, nonspore-forming bacilli (eg, Propionibacterium and Lactobacilli) and Peptostreptococci (37), and is poorly active against anaerobic Gram-negative bacilli (38). Fidaxomicin MICs for most aerobic and anaerobic Gram-negative bacilli (eg, Enterobacteriaceae, Pseudomonas, Campylobacter, Helicobacter, Haemophilus, Bacteroides, Fusobacterium, Porphyromonas, Prevotella and Veillonella) exceed 32 μg/mL to 64 μg/mL (31). Fidaxomicin is inactive (MIC >64 μg/mL) against Candida species (31).
TABLE 1.
In vitro activity of fidaxomicin against Gram-positive bacteria other than Clostridium difficile
| Bacterium | Fidaxomicin MIC, μg/mL | Isolates tested, n |
|---|---|---|
| Aerococcus | 0.5–16 | 10 |
| Bacillus cereus | 1 | 2 |
| Bifidiobacterium | ≤0.015–0.125 | 22 |
| Bifidiobacterium longum | 0.125 | 1 |
| Clostridium perfringens | ≤0.015–0.125 | 22 |
| Clostridium innocuum | >32 | 20 |
| Clostridium ramosum | >32 | 20 |
| Eggerthella lenta | ≤0.015–0.25 | 20 |
| Enterrococcus faecalis | 0.5–4 | 63 |
| Enterococcus faecium | 1–8 | 64 |
| Eubacterium limosum | 16–>32 | 20 |
| Finegoldia magna | 0.5–2 | 21 |
| Lactobacillus | ≤0.015–>32 | 24 |
| Lactobacillus acidophilus | >32 | 2 |
| Lactobacillus casei | 1–2 | 2 |
| Lactobacillus rhamnosus | 8–16 | 2 |
| Micrococcus luteus | ≤0.125 | 4 |
| Micromonas micros | 0.125 | 1 |
| Parvimonas micra | ≤0.015–2 | 20 |
| Peptostreptococcus anaerobius | ≤0.015–0.03 | 22 |
| Peptostreptococcus (Peptoniphilus) asaccharolyticus | 1 | 2 |
| Peptococcus (Finegoldia) magna | 0.5 | 1 |
| Peptococcus (Micromonus) micros | 0.125 | 1 |
| Propionibacterium acnes | 8 | 2 |
| Staphylococcus aureus | 2–16 | 100 |
| Staphylococcus epidermidis | 1–4 | 3 |
| Staphylococcus intermedius | 4 | 1 |
| Staphylococcus, coagulase-negative | ≤0.05–8 | 60 |
| Streptococcus agalactiae | 16–32 | 2 |
| Streptococcus anginosus | 4–>32 | 21 |
| Streptococcus constellatus/intermedius | 4–>32 | 26 |
| Streptococcus pyogenes | 4–16 | 23 |
| Streptococcus pneumoniae | >32 | 2 |
| Streptococcus sanguinis | 32 | 1 |
Table 2 summarizes the in vitro activities of fidaxomicin, OP-1118, metronidazole and vancomycin against C difficile, tested using the currently published Clinical and Laboratory Standards Institute method (27,30,31,41–45). Fidaxomicin possesses potent activity against C difficile, including MICs required to inhibit growth of 50% of organisms (MIC50s) ranging from 0.06 μg/mL to 0.25 μg/mL and MICs required to inhibit growth of 90% of organisms (MIC90s) ranging from 0.125 μg/mL to 0.5 μg/mL (27,30,31,41–45). Hecht et al (42) and Citron et al (43) did not identify any difference in MIC related to restriction endo-nuclease analysis BI (NAP1/O27) group status. Goldstein et al (30) reported higher MICs for fidaxomicin, vancomycin, metronidazole and rifaximin for BI isolates than for non-BI isolates. Goldstein et al (30) and Louie et al (46) also reported that fidaxomicin susceptibility of baseline isolates did not predict clinical cure, failure or recurrence. In comparison with fidaxomicin, vancomycin and metronidazole, MIC90s for C difficile were 0.5 μg/mL to 2 μg/mL and 1 μg/mL, respectively (Table 2). The antibacterial activity of OP-1118 (MIC90, 8 μg/mL) was approximately eight to 16 times lower than the activity of fidaxomicin (31).
TABLE 2.
Minimum inhibitory concentration (MIC) determinations for fidaxomicin, OP-1118, vancomycin and metronidazole against toxin-positive clinical isolates of Clostridium difficile
| Antimicrobial agent | Isolates tested, n | MIC range, μg/mL | MIC50, μg/mL | MIC90, μg/mL | Reference |
|---|---|---|---|---|---|
| Fidaxomicin | 208 | 0.06–1 | 0.25 | 0.5 | 27 |
| 110 | 0.015–0.25 | 0.125 | 0.125 | 42 | |
| 135 | ≤0.004–8 | 0.125 | 0.25 | 31 | |
| 38 | ≤0.008–0.25 | 0.125 | 0.125 | 43 | |
| 719 | 0.003–1 | 0.125 | 0.25 | 30 | |
| 114 | 0.008–0.125 | 0.06 | 0.125 | 44 | |
| 50 | 0.06–1 | 0.25 | 0.5 | 45 | |
| 50 | 0.03–0.5 | 0.25 | 0.5 | 41 | |
| OP-1118 | 135 | 0.25–>128 | 4 | 8 | 31 |
| Vancomycin | 208 | 0.5–4 | 0.5 | 1 | 27 |
| 719 | 0.25–8 | 1 | 2 | 30 | |
| 114 | 0.125–1 | 0.5 | 0.5 | 44 | |
| Metronidazole | 208 | 0.25–4 | 0.5 | 1 | 27 |
| 719 | 0.02–4 | 0.5 | 1 | 30 | |
| 114 | 0.125–2 | 0.5 | 1 | 44 |
MIC50 MIC required to inhibit the growth of 50% of organisms; MIC90 MIC required to inhibit the growth of 90% of organisms
Fidaxomicin has been reported to have a low ecological impact on the intestinal microbiome (7,47). Babakhani et al (48) speculate, based on in vitro data, that the antibacterial activity of fidaxomicin should not be altered under physiological conditions in the human intestine.
PHARMACOKINETICS
When administered orally, fidaxomicin (similar to oral vancomycin) is minimally absorbed, being excreted almost entirely through the feces (46,49,50). Mean fecal concentration levels (on day 10 of dosing) of fidaxomicin and OP-1118 for patients with CDI who were treated for 10 days with 100 mg, 200 mg or 400 mg per day of fidaxomicin (50 mg, 100 mg and 200 mg twice daily) were 256 μg/g and 393 μg/g, 442 μg/g and 430 μg/g, and 1433 μg/g and 760 μg/g, respectively (46) (Table 3). If the MIC90 for fidaxomicin versus C difficile is 0.5 μg/mL (Table 2) and the mean fecal fidaxomicin concentration is approximately 1400 μg/g, it indicates the mean fecal fidaxomicin concentrations are approximately 2800 times greater than the MIC90 of C difficile (this compares with approximately 1000 times greater than the MIC90 of C difficile for 125 mg oral vancomycin four times per day and approximately one to five times greater than the MIC90 of C difficile for oral metronidazole 500 mg three times per day) (46).
TABLE 3.
Stool and plasma concentrations of fidaxomicin and OP-1118 in patients with Clostridium difficile infection treated for 10 days with fidaxomicin
| Fecal concentrations, μg/g | Fidaxomicin and OP-1118 plasma concentrations, n | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Fidaxomicin, mg/day | n | Fidaxomicin, mean ± SD | OP-1118, mean ± SD | Total, n | Fidaxomicin, ng/mL | OP-1118, ng/mL | |||||||
|
|
|
||||||||||||
| <5 | 5–20 | 20–100 | >100 | <5 | 5–20 | 20–100 | >100 | ||||||
|
|
|
|
|||||||||||
| 100 | 11 | 256±136 | 393±260 | 14 | 12 | 2 | 0 | 0 | 1 | 11 | 2 | 0 | |
| 200 | 9 | 442±238 | 430±263 | 16 | 7 | 8 | 1 | 0 | 2 | 8 | 5 | 1 | |
| 400 | 13 | 1433±975 | 760±373 | 16 | 3 | 11 | 2 | 0 | 0 | 7 | 9 | 1 | |
Adapted from reference 46
In the same open-label dose-ranging trial (46), plasma concentration levels of fidaxomicin and OP-1118 (from patients who received fidaxomicin and had >1 plasma pharmacokinetic sample collected after the first dose) were below the limit of quantification (5 ng/mL) for 22 of 46 patients, and >90% of patients had plasma concentration levels <20 ng/mL (Table 3). In addition, Sears et al (49) demonstrated that fidaxomicin serum concentration levels did not increase compared with controls for patients with mild, moderate or severe renal impairment. Therefore, the majority of fidaxomicin and its active metabolite OP-1118 are not absorbed systemically, rather, they are primarily excreted in the feces following oral administration.
PHARMACODYNAMICS
Fidaxomicin and OP-1118 are bactericidal against C difficile in vitro (at four times the MIC, ≥3 log10 in 48 h), as well as against laboratory generated mutants with reduced susceptibility to fidaxomicin (MIC 1 μg/mL to 4 μg/mL) (32,51). The postantibiotic effect of fidaxomicin extends for approximately 10 h (range 5.5 h to 12.4 h), compared with vancomycin, which has a postantibiotic effect of 0 h to 1.5 h (51).
Fidaxomicin and OP-1118 have been demonstrated to inhibit toxin A and B production in C difficile in vitro (52). The ability of fidaxomicin and OP-1118 to inhibit expression of C difficile toxin A and B, and their gene products (tcdA and tcdB) was examined in vitro for two isolates; one isolate that demonstrated a high level of toxin expression and a second isolate that was a BI (NAP1/O27) strain (52). At ¼× the MIC, fidaxomicin and its metabolite reduced toxin expression by >60% for up to one week. Vancomycin and metronidazole (¼× the MIC) had no effect on toxin expression. At subinhibitory concentrations (¼× the MIC), both fidaxomicin and OP-1118 reduced toxin A-mediated enteritis in a mouse ileum model and cell rounding in human colonic CCD-18Co fibroblasts (53). In clinical trials, during the post-CDI treatment period, there was no difference among fidaxomicin and vancomycin treatment groups in C difficile colony forming units (CFUs) over time; however, toxin expression was reduced by 50% with fidaxomicin therapy (47).
Fidaxomicin, its metabolite OP-1118 and comparator drugs were assessed in vitro for their impact on new spore formation (54). At ¼× the MIC, fidaxomicin and OP-1118 inhibited spore production in both non-BI strains and in a BI strain (NAP1/O27). In contrast, vancomycin, metronidazole and rifaximin at sub-MIC drug concentrations failed to inhibit sporulation. In clinical trials, fecal spore counts (CFU count/g) for patients who had received fidaxomicin were 2.3 log10 lower at 21 to 28 days post-therapy than in patients who had received vancomycin (46). Inhibition of sporulation may provide, in part, a mechanism by which fidaxomicin improves sustained response and lowers the rate of recurrent infection, and may also be useful in decreasing C difficile shedding and transmission. Fidaxomicin and OP-1118 do not interfere with the initiation of spore germination, but rather inhibit outgrowth of vegetative cells from germinated spores (55).
Unlike oral vancomycin and oral metronidazole, fidaxomicin has minimal effects on the anaerobic colonic flora (7,37,38,46,47,56). Fecal samples from patients with CDI who were treated with oral fidaxomicin (200 mg twice per day) or oral vancomycin (125 mg four times per day) for 10 days showed sparing of major components of the anaerobic microflora (eg, Bacteroides/Prevotella group organisms as well as Clostridium coccoides and Clostridium leptum) with fidaxomicin, but not with vancomycin. In the vancomycin treatment arm, there was a 2 log10 CFU/g to 4 log10 CFU/g reduction in anaerobes (Bacteroides/Prevotella group organisms), which persisted until day 28 of the study (47). The investigators concluded that whereas oral vancomycin and oral fidaxomicin were equally effective in resolving CDI symptoms, preservation of the normal colonic microflora by fidaxomicin was associated with a lower likelihood of CDI recurrence. Nerandzic et al (57) demonstrated that colonization with VRE and Candida were reduced by oral fidaxomicin compared with oral vancomycin in CDI patients who were negative for VRE and Candida before therapy. In patients with stool culture initially negative for VRE, 31% (n=160) of patients acquired VRE when treated with vancomycin versus only 7% (n=115) acquisition of VRE in fidaxomicin-treated patients (P=0.001) (57).
CLINICAL TRIALS
Two large, randomized, multicentre, double-blind phase 3 clinical trials examined the efficacy and safety of oral fidaxomicin versus oral vancomycin in adult patients with CDI (13,14). Study OPT-80-003 comprised 596 patients from the US and Canada (13), and study OPT-80-004 comprised 509 patients from the US, Europe and Canada (14). Patients with confirmed CDI who were ≥16 years of age and had no history or only one previous CDI episode in the past 90 days were eligible for inclusion. The presence of CDI was defined as diarrhea with >3 unformed stools 24 h before randomization and a positive toxin test for toxin A, B or both. Patients who were pregnant or breastfeeding, had previous fidaxomicin exposure, life-threatening or fulminant CDI, toxic megacolon, a history of ulcerative colitis or Crohn disease, or >1 CDI episode in the preceding three months were excluded. Patients were also excluded if they were presently taking other antibiotics for CDI, although they could have received up to four doses of oral vanco-mycin or oral metronidazole in the 24 h before randomization.
Patients were randomized to receive 200 mg of oral fidaxomicin twice daily or 125 mg of oral vancomycin four times daily for 10 days. The primary end point was clinical response (clinical cure). Clinical response was the resolution of diarrhea, defined as ≤3 unformed stools for two consecutive days that was maintained through the duration of therapy with no further CDI treatment required, assessed two days after the treatment period. Secondary end points were recurrence and sustained clinical response (global cure). CDI recurrence was assessed during the 28-day period after completion of therapy and defined as the reappearance of >3 unformed stools in any 24 h period, including a positive toxin test for toxin A, B or both and the need for CDI retreatment. Sustained response was the presence of clinical response and no recurrence or death during the 28-day follow-up period. The modified intent-to-treat (mITT) and per protocol populations were analyzed. The mITT analysis is presented in the present review. A one-sided lower 97.5% CI was used in analysis of the primary end point, with a noninferiority margin of −10% absolute difference. Secondary endpoints of recurrence and sustained clinical response were analyzed using two sided tests of population proportions, with α=0.05.
The treatment outcomes for study OPT-80-003 and OPT-80-004 are summarized in Table 4. In both clinical trials, oral fidaxomicin was noninferior to oral vancomycin for clinical response, with cure rates of approximately 87% in both treatment groups. Fidaxomicin demonstrated superiority to vancomycin for recurrence. The relative reduction in recurrence with fidaxomicin treatment was 39.1% and 52.8% in study OPT-80-003 and OPT-80-004, respectively. Fidaxomicin was also superior for sustained clinical response, demonstrating higher global cure rates compared with vancomycin in the two trials. In study OPT-80-003 (13) (North America only), 38.1% of patients were infected with the BI/NAP1/027 strain. The lower CDI recurrence rate among patients treated with fidaxomicin relative to vancomycin was only observed for those infected with non-BI/NAP1/027 strains (13). Infection rates with the BI/NAP1/027 strain differed according to region in study OPT-80-004, with rates of 45.9% in the US and Canada, and 10.4% in Europe (14). In the OPT-80-004 study, CDI recurrence rates were numerically lower among fidaxomicin-treated patients infected with the BI/NAP1/027 and non-BI/NAP1/027 strains relative to those receiving vancomycin; however, the difference was statistically significant only for the non-BI/NAP1/027 subgroup (14).
TABLE 4.
Summary of treatment outcomes for patients with Clostridium difficile infection treated with fidaxomicin (FDX) or vancomycin (VAN) from two phase 3 randomized double-blind clinical trials (modified intent to treat)
| Study | Clinical response | Patients recurrence | Sustained response | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| FDX | VAN | Difference | P | FDX | VAN | Difference | P | FDX | VAN | Difference | P | |
| OPT-80-003 | 88.2 | 85.8 | 2.4 | NI | 15.4 | 25.3 | −9.9 | 0.005 | 74.6 | 64.1 | 10.5 | 0.006 |
| OPT-80-004 | 87.7 | 86.8 | 0.9 | NI | 12.7 | 26.9 | −14.2 | <0.001 | 76.6 | 63.4 | 13.2 | 0.001 |
Post hoc analysis of the data from the two phase 3 trials were performed for subgroups of patients at high risk for acquiring CDI and/or at increased risk for recurrent disease. These subgroups, described in separate publications, included patients of increased age, patients receiving concomitant antimicrobials, patients with a previous CDI episode, patients with cancer and patients with underlying renal dysfunction (15–19,58). The risk for recurrence among patients treated with oral fidaxomicin relative to oral vancomycin, stratified according to subgroup, is presented in Table 5. The effect of increasing age on the outcome of treatment for CDI with oral fidaxomicin in comparison with oral vancomycin was evaluated by Louie et al (18) using regression modelling. Study participants were stratified into age categories according to 10 year increments, with the lower category including patients from 18 to 40 years of age. The model predicted a 17% increased probability for CDI recurrence for each decade after age 40. Treatment with fidaxomicin was associated with a 60% lower risk for recurrence in multivariate analysis that included adjustment for patient age (18).
TABLE 5.
Recurrence in subpopulations of patients with Clostridium difficile infection treated with fidaxomicin or vancomycin in a post hoc analysis of two phase 3 randomized double-blind clinical trials
| Subpopulation |
Percentage of patients with recurrence
|
|||
|---|---|---|---|---|
| Fidaxomicin | Vancomycin | Difference | P | |
| Concomitant antibiotics* | 16.9 | 29.2 | −12.3 | 0.048 |
| Previous CDI episode* | 19.7 | 35.5 | −15.8 | 0.045 |
| Cancer | 13.5 | 29.6 | −16.1 | 0.018 |
| Renal impairment | ||||
| Stage 2 | 14.3 | 24.3 | −10.0 | 0.040 |
| Stage 3 | 21.4 | 33.0 | −11.6 | 0.080 |
| Stage 4 or higher | 14.7 | 31.6 | −16.9 | 0.092 |
Mullane et al (17) evaluated the efficacy of oral fidaxomicin in comparison with oral vancomycin for the treatment of CDI among patients receiving concomitant antimicrobials. Topical antimicrobials, antimicrobials used for the treatment of CDI, and antifungal and antiviral agents with no antibacterial activity were excluded from the concomitant antimicrobial definition. Results of this analysis have been published only for the per protocol population. Among 999 patients, 275 (27.5%) received concomitant antimicrobials at some time during the study, with 192 (19.2%) receiving concomitant antimicrobials concurrent with the study medication. Clinical cure was significantly lower among patients who received concomitant antimicrobials concurrent with CDI treatment, relative to those that did not (84.4% versus 92.6%; 8.2% absolute difference 95% CI 3.0% to 13.9%). Among patients who received concomitant antimicrobials concurrent with the study medications, clinical cure was 90% for patients treated with oral fidaxomicin versus 79.4% for patients treated with oral vancomycin (P=0.04). Recurrence of CDI among patients who received concomitant antimicrobials at any time during the study was lower for those treated with fidaxomicin than those who received vancomycin (16.9% versus 29.2%; P=0.048) (17).
Cornely et al (19) compared treatment with oral fidaxomicin and oral vancomycin in a subset of patients with a first CDI recurrence. The published results for this subgroup analysis included 128 patients in the per protocol population who experienced an episode of CDI in the three months before randomization. Clinical cure for patients among this subpopulation was >90% for both fidaxomicin and vancomycin. However, a second recurrence was less frequent among patients who received fidaxomicin (19.7% versus 35.5% for vancomycin; P=0.045) (19).
Oral fidaxomicin compared with oral vancomycin for the treatment of CDI among patients with cancer was assessed by Cornely et al (15). Patients with solid tumours and/or hematological malignancies were identified according to system organ class and preferred term from active medical history entries of case report forms after coding by MedDRA version 10.0 (MedDRA, USA) according to indications for concomitant medication entries and treatment-emergent adverse events. In total, 183 patients in the mITT population with active cancer were identified. The likelihood of CDI recurrence following treatment for patients with and without cancer was similar. Among patients with cancer, recurrence occurred in 13.5% of those treated with fidaxomicin versus 29.6% treated with vancomycin (P=0.018) (15). Recently, Esmaily-Fard et al (59) treated 22 cancer patients (lymphomas, leukemias and solid tumours) with CDI using fidaxomicin. Fidaxomicin was used in these patients because of recurrent CDI (16 of 22 [72%] patients) or failure of both oral metronidazole and oral vancomycin (six of 22 [28%] patients). Despite 19 of 22 (86%) patients receiving concomitant antimicrobials during CDI treatment, clinical response occurred in 91% of patients and overall sustained clinical response was observed in 82%. The researchers concluded that in cancer patients, fidaxomicin is an effective treatment for a first episode of CDI after failure of standard therapies and for treatment of recurrent CDI (59). However, in view of the small number of patients evaluated and the study design (retrospective case series) further data are required to support these conclusions.
Treatment of CDI with fidaxomicin in comparison with vancomycin among the subgroup of patients with renal impairment was evaluated by Mullane et al (16). Creatinine clearance (CrCl) was estimated using the Cockcroft-Gault equation with serum creatinine from a blood sample obtained on day 1 before the first dose of study medication. Patients were stratified according to renal function using criteria from the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (60) as follows: normal (CrCl >90 mL/min/1.73 m2), stage 2 (CrCl 60 mL/min/1.73 m2 to 89 mL/min/1.73 m2), stage 3 (CrCl 30 mL/min/1.73 m2 to 59 mL/min/1.73 m2), stage 4 (CrCl 15 mL/min/1.73 m2 to 30 mL/min/1.73 m2), stage 5 (CrCl <15 mL/min/1.73 m2). At baseline, 57.9% of patients in the mITT population with a CrCl estimate available had abnormal renal function. In a multivariate analysis, patients with stage 2 or higher chronic kidney disease were less likely to be cured of CDI (OR 0.53; P=0.03), and patients with stage 3 or greater chronic kidney disease were more likely to have a recurrence (OR 1.8; P=0.024). Oral fidaxomicin was associated with a lower risk for recurrent CDI relative to oral vancomycin, independent of renal function (16). The reader is cautioned about placing extensive weighting on data obtained from the subgroup analysis because the data obtained from these post hoc analyses were not as rigorous as those obtained from analyses of primary end point data.
REAL-WORLD EXPERIENCE
Several observational studies describing the real-world use of fidaxomicin have been published (61–65). Vargo et al (61) evaluated the clinical outcome of 61 patients treated for CDI with fidaxomicin at a single centre. Fifty-five (90.2%) patients received treatment for CDI in the preceding 30 days, concomitant antimicrobials were used by 60.7%, and severe infection was present in 31.1%. Slightly more than one-half of the patients received fidaxomicin in combination with another recognized treatment for CDI. Clinical cure was achieved in 72.1% of patients. Among patients who achieved clinical cure, recurrence was documented in 13.6%. Eiland et al (62) also assessed the clinical efficacy of fidaxomcin in a single centre, retrospective cohort study. Sixty patients were included in the analysis. Severe or severe-complicated disease was present in 45% of patients, concomitant antimicrobials were being administered to 55%, and 43.3% were being treated for a second or greater CDI episode. Overall, 96.7% of patients achieved clinical success, with recurrence documented in 10.3%. The difference in clinical efficacy reported between these two real-world evaluations and the phase 3 fidaxomicin clinical trials likely relates to differences among the patient populations studied (13,14,61,62).
Clutter et al (63) evaluated fidaxomicin for the treatment of CDI in recipients of a solid organ or hematopoietic stem cell transplant in comparison with conventional therapy (oral vancomycin and/or oral metronidazole). Fifty-nine transplant recipients were included in the analysis. Clinical cure was documented among 67% of patients (10 of 15) that received fidaxomicin versus 89% of patients (41 of 44) who received conventional therapy (P=0.06, not significant). Recurrence occurred in 7% of patients in both study groups. The non-randomized design of the study and small number of patients included make it difficult to draw significant conclusions concerning the efficacy of fidaxomicin relative to conventional therapy. Penziner et al (64) assessed the efficacy of fidaxomicin for the treatment of CDI among patients admitted to a critical care unit in comparison with patients treated on a general medicine ward. In total, 50 patients were included in the analysis, of whom 20 were receiving care in a critical care unit. Forty percent of patients treated in a critical care unit setting had severe-complicated disease as opposed to 10% of patients on a general medicine ward (P=0.031). Clinical cure was reported in 60% of patients treated in a critical care unit versus 67% of those treated on a general medicine ward (P=0.9). The response rate for patients having severe or severe-complicated CDI, irrespective of study location (critical care unit or medicine ward), was only 46%, in comparison with a response rate of 81% for patients with mild to moderate disease (P=0.02). Life-threatening or fulminant CDI was an exclusion criterion for the two large phase 3 fidaxomicin clinical trials (13,14). The results from Penziner et al (64) support caution with using fidaxomicin monotherapy to treat severe-complicated CDI until further data are available.
Novel fidaxomicin treatment regimens have also been assessed among patients with multiple CDI recurrences. Soriano et al (65) evaluated the efficacy of fidaxomicin administered as a 10-day chaser following a treatment course of oral vancomycin (n=8 patients). The study patients had between three and 10 CDI episodes. Five of the eight patients (62%) did not experience a further CDI recurrence following the fidaxomicin chaser. The same investigators also assessed a tapering regimen of fidaxomicin over 14 to 21 days following a 10-day fidaxomicin treatment course. The tapering regimen was evaluated in 11 patients who had between three and 11 CDI episodes. Nine patients (82%) did not experience a further recurrence of CDI. The reader is cautioned about placing extensive weighting on these real-world experience data because frequently these data are noncomparative and the studies include only a small number of patients treated.
ADVERSE EFFECTS
Fidaxomicin has been well tolerated in clinical trials. In the two phase 3 clinical studies (OPT-80-003 and OPT-80-004), adverse events were not significantly different among the fidaxomicin and vancomycin treatment groups (13,14,66). Adverse events possibly or definitely related to study treatment were primarily gastrointestinal in nature, and included nausea, vomiting, diarrhea, abdominal pain and constipation (13,14). Gastrointestinal adverse events requiring study discontinuation occurred in 2.3% of patients treated with fidaxomicin versus 1.4% of patients receiving vancomycin (P=0.24) (66). At present, there are a lack of data regarding fidaxomicin use during pregnancy (66).
It should be noted that hypersensitivity reactions to fidaxomicin have been reported postmarketing. Iarikov et al (67) summarized data for 12 patients presenting with a hypersensitivity reaction in association with fidaxomicin use. Onset of symptoms occurred between 1 h and 7 days after starting fidaxomicin. The clinical presentation included facial, tongue or throat swelling, a burning sensation in the throat and rash. In two patients, symptoms recurred with re-exposure to fidaxomicin (67). The US Food and Drug Administration has added a warning about the possibility of hypersensitivity reactions to the fidaxomicin prescribing information (Dificid monograph). Hypersensitivity to fidaxomicin is listed as a contraindication to the use of this antimicrobial (Dificid monograph).
PHARMACOECONOMICS
Oral fidaxomicin has demonstrated superiority to oral vancomycin in achieving a sustained clinical response for CDI (in patients infected with non-BI/NAP1/027 strains), and this superiority is maintained in both severe and non-severe CDI, as well as in patients with a high risk for recurrent CDI (68). Whole-genome sequencing has recently demonstrated that this is due to fidaxomicin significantly reducing both the risk for relapse and reinfection (69). The increase in efficacy of oral fidaxomicin in preventing recurrent CDI relative to oral vancomycin should, however, be balanced with the increased cost of this antimicrobial. The acquisition cost of a 10-day course of fidaxomicin is significantly higher (five to >20 times) than the cost of a 10-day course of oral vancomycin (depending on the vancomycin formulation used). Wagner et al (70) developed a decision-tree model to determine the incremental cost per recurrence avoided by treating patients having severe CDI with oral fidaxomicin in comparison with oral vancomycin. This model considered patients treated in the Canadian health care system, and costs were presented in Canadian dollars. For a cohort of 1000 patients, the model predicted that treatment of severe CDI with fidaxomicin would result in an incremental cost of $13,202 per recurrence avoided. Furthermore, among 1000 patients with recurrent CDI, treatment with fidaxomicin would result in an incremental cost of $18,190 per second recurrence avoided. Overall, use of fidaxomicin for the treatment of patients with severe CDI was associated with a cost increase for the Canadian health care system (70).
Stranges et al (71) performed a cost utility analysis comparing oral fidaxomicin versus oral vancomycin for CDI treatment, using a decision analytic model from a third-party payer perspective (United States). These investigators reported an incremental cost-effectiveness ratio of USD$67,576/quality adjusted life-year (QALY) with fidaxomicin. Their analysis suggested that fidaxomicin may be cost effective in the US health care system based on a willingness to pay threshold of USD$100,000/QALY (71). Nathwani et al (72) used a one year time horizon Markov model with seven health states to analyze the cost-effectiveness of oral fidaxomicin versus oral vancomycin for the treatment of CDI from the perspective of Scottish public health care providers. This analysis was limited to patients with severe CDI or a first CDI recurrence. The main outcome was the incremental cost-effectiveness ratio expressed as a cost per QALY in British pounds, interpreted using a willingness to pay threshold of UK£20,000/QALY and UK£30,000/QALY. Fidaxomicin was found to be cost effective for severe CDI (incremental cost-effectiveness ratio = UK£16,529/QALY) and dominant (more effective and less costly) in patients who were treated for a first recurrence (72). A pharmacoeconomic analysis may be warranted for hospitals that are considering adding fidaxomicin to the formulary.
GUIDELINES/PLACE IN THERAPY
The European Society of Clinical Microbiology and Infectious Diseases recently published updated guidelines regarding the treatment of CDI (73). For treatment of an initial episode of nonsevere CDI, oral metronidazole is recommended, while oral fidaxomicin is considered a possible alternative therapy. For treatment of severe CDIs, oral vancomycin is recommended, with fidaxomicin again considered to be a potential alternative therapy. The guidelines further caution that there are no data currently available to support the use of fidaxomicin in life-threatening CDIs related to exclusion criteria in the two large phase 3 trials. Fidaxomicin is a recommended antimicrobial for treating patients with a first recurrence of CDI and for patients experiencing multiple recurrences (73). Public Health England also published guidelines in 2013 regarding therapy for CDI (74). These guidelines suggest consideration of fidaxomicin for patients with severe CDIs who are believed to be at high risk for recurrence, including elderly patients with multiple comorbidities and patients who are receiving concomitant antimicrobials. Fidaxomicin is further recommended by Public Health England as the preferred option for patients with recurrent CDI, regardless of disease severity (74). North American CDI treatment guidelines from the Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America have not been updated since fidaxomicin received US Food and Drug Administration approval for CDI treatment (1).
SUMMARY
Fidaxomicin is noninferior to oral vancomycin in terms of clinical response at the end of CDI therapy. Fidaxomicin has been demonstrated to be as safe as oral vancomycin, but superior to vancomycin in achieving a sustained clinical response of CDI in patients infected with non-BI/NAP1/027 strains. Fidaxomicin superiority in patients infected with non-BI/NAP1/027 strains is maintained in both severe and nonsevere CDI, as well as in patients with a high risk for recurrent CDI. Caution should be exercised in using fidaxomicin monotherapy for treatment of severe complicated CDI because limited data are available. Whether fidaxomicin is cost effective (due to its significantly higher acquisition cost versus oral vancomycin) depends on the willingness to pay threshold per QALY as a measure of assessing cost effectiveness.
Footnotes
DISCLOSURES AND SOURCES OF FUNDING: GGZ has received research grants supported by Cubist US and Merck US. The remainder of the authors have no financial relationships or conflicts of interest to declare.
REFERENCES
- 1.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:413–55. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 2.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–7. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 3.Louie TJ, Gerson M, Grimard D, et al. Results of a phase III trial clinical trial comparing tolevar, vancomycin and metronidazole in patient with Clostridium difficile-associated diarrhea (CDAD). 47th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); Chicago. September 17–20, 2007; (Abst K-425a). [Google Scholar]
- 4.Bouza E, Dryden M, Mohammed R, et al. Results of a phase III trial comparing tolevamer, vancomycin and metronidazole in patients with Clostridium difficile-associated diarrhoea. 18th European Congress of Clincal Microbiology and Infectious Diseases (ECCMID); Barcelona. April 19–22, 2008; (Abst O464). [Google Scholar]
- 5.Johnson S, Louie TJ, Gerding D, et al. Vancomycin metronidazole or tolevamer for Clostridium difficile infection: Results from two multinational randomized controlled trials. Clin Infect Dis. 2014;59:345–54. doi: 10.1093/cid/ciu313. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox MH. The trials and tribulations of treating Clostridium difficile infection – one step backward one step forward but still progress. Clin Infect Dis. 2014;59:355–7. doi: 10.1093/cid/ciu316. [DOI] [PubMed] [Google Scholar]
- 7.Tannock GW, Munro K, Taylor C, et al. A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiol. 2010;156:3354–9. doi: 10.1099/mic.0.042010-0. [DOI] [PubMed] [Google Scholar]
- 8.Venugopal AA, Johnson S. Fidaxomicin: A novel macrocyclic antibiotic approved for treatment of Clostridium difficile infection. Clin Infect Dis. 2012;54:568–74. doi: 10.1093/cid/cir830. [DOI] [PubMed] [Google Scholar]
- 9.Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC) MMWR Recomm Rep. 1995;44:1–13. [PubMed] [Google Scholar]
- 10.ASHP therapeutic position statement on the preferential use of metronidazole for the treatment of Clostridium difficile-associated disease. Am J Health Syst Pharm. 1998;55:1407–11. doi: 10.1093/ajhp/55.13.1407. [DOI] [PubMed] [Google Scholar]
- 11.Al-Nassir WN, Sethi AK, Li Y, et al. Both oral metronidazole and oral vancomycin promote overgrowth of vancomycin-resistant enterococci during treatment of Clostridium difficile-associated disease. Antimicrob Agents Chemother. 2008;52:2403–6. doi: 10.1128/AAC.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: Old therapies and new strategies. Lancet Infect Dis. 2005;5:549–57. doi: 10.1016/S1473-3099(05)70215-2. [DOI] [PubMed] [Google Scholar]
- 13.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–31. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 14.Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: A double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281–9. doi: 10.1016/S1473-3099(11)70374-7. [DOI] [PubMed] [Google Scholar]
- 15.Cornely OA, Miller MA, Fantin B, et al. Resolution of Clostridium difficile-associated diarrhea in patients with cancer treated with fidaxomicin or vancomycin. J Clin Oncol. 2013;31:2493–9. doi: 10.1200/JCO.2012.45.5899. [DOI] [PubMed] [Google Scholar]
- 16.Mullane KM, Cornely OA, Crook DW, et al. Renal impairment and clinical outcomes of Clostridium difficile infection in two randomized trials. Am J Nephrol. 2013;38:1–11. doi: 10.1159/000351757. [DOI] [PubMed] [Google Scholar]
- 17.Mullane KM, Miller MA, Weiss K, et al. Efficacy of fidaxomicin versus vancomycin as therapy for Clostridium difficile infection in individuals taking concomitant antibiotics for other concurrent infections. Clin Infect Dis. 2011;53:440–7. doi: 10.1093/cid/cir404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louie TJ, Miller MA, Crook DW, et al. Effect of age on treatment outcomes in Clostridium difficile infection. J Am Geriatr Soc. 2013;61:222–30. doi: 10.1111/jgs.12090. [DOI] [PubMed] [Google Scholar]
- 19.Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: Fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(S2):S154–61. doi: 10.1093/cid/cis462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DIFICID® Product Monograph. Optimer Pharmaceuticals, Inc; 2013. [Google Scholar]
- 21.Johnson AP. Drug evaluation: OPT-80, a narrow-spectrum macrocyclic antibiotic. Curr Opin Investig Drugs. 2007;8:168–73. [PubMed] [Google Scholar]
- 22.Osburne MS, Sonenshein AL. Inhibition by lipiarmycin of bacteriophage growth in Bacillus subtilis. J Virol. 1980;33:945–53. doi: 10.1128/jvi.33.3.945-953.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Artsimovitch I, Seddon J, Sears P. Fidaxomicin is an inhibitor of the initiation of bacterial RNA synthesis. Clin Infect Dis. 2012;55(Suppl 2):S127–31. doi: 10.1093/cid/cis358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babakhani F, Seddon J, Sears P. Comparative microbiological studies of transcription inhibitors fidaxomicin and the rifamycins in Clostridium difficile. Antimicrob Agents Chemother. 2014;58:2934–7. doi: 10.1128/AAC.02572-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gualtieri M, Villain-Guillot P, Latouche J, et al. Mutation in the Bacillus subtilis RNA polymerase β’ subunit confers resistance to lipiarmycin. Antimicrob Agents Chemother. 2006;50:401–2. doi: 10.1128/AAC.50.1.401-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wostem MM. Eubacterial sigma-factors. FEMS Microbiol Rev. 1998;22:127–50. doi: 10.1111/j.1574-6976.1998.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 27.Karlowsky JA, Laing NM, Zhanel GG. In vitro activity of OPT-80 tested against clinical isolates of toxin-producing Clostridium difficile. Antimicrob Agents Chemother. 2008;52:4163–5. doi: 10.1128/AAC.00476-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuehne SA, Dempster AW, Heeg D, et al. Use of allelic exchange to characterize the impact of rpoB/C mutations on fitness of Clostridium difficile and sensitivity to fidaxomicin. 23rd European Congress of Clincal Microbiology and Infectious Diseases (ECCMID); Berlin. April 27–30, 2013. [Google Scholar]
- 29.Seddon J, Babakhani F, Sears P. Mutant prevention concentration of fidaxomicin for Clostridium difficile. 52nd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); San Francisco. September 9–12, 2012; (Abstr A-1274). [Google Scholar]
- 30.Goldstein EJC, Citron DM, Sears P, et al. Comparative susceptibilities to fidaxomicin (OPT-80) of isolates collected at baseline, recurrence, and failure from patients in two phase III trials of fidaxomicin against Clostridium difficile infection. Antimicrob Agents Chemother. 2011;55:5194–9. doi: 10.1128/AAC.00625-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein EJC, Babakhani F, Citron DM. Antimicrobial activities of fidaxomicin. Clin Infect Dis. 2012;55(Suppl 2):S143–8. doi: 10.1093/cid/cis339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babakhani F, Gomez A, Robert N, Sears P. Postantibiotic effect of fidaxomicin and its major metabolite, OP-1118, against Clostridium difficile. Antimicrob Agents Chemother. 2011a;55:4427–9. doi: 10.1128/AAC.00104-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leeds JA, Sachdeva M, Mullin S, et al. in vitro selection via serial passage of Clostridium difficile mutants with reduced susceptibility to fidaximicin or vancomycin. J Antimicrob Chemother. 2014;69:41–4. doi: 10.1093/jac/dkt302. [DOI] [PubMed] [Google Scholar]
- 34.Gerber M, Ackermann G. OPT-80 a macrocyclic antimicrobial agent for the treatment of Clostridium difficile infection: A review. Exp Opin Investig Drugs. 2008;17:51–3. doi: 10.1517/13543784.17.4.547. [DOI] [PubMed] [Google Scholar]
- 35.Swanson RN, Hardy DJ, Shipkowitz NL, et al. In vitro and in vivo evaluation of tiacumicius B and C against Clostridium difficile. Antimicrob Agents Chemother. 1991;35:1106–11. doi: 10.1128/aac.35.6.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theriault RJ, Karwowski TP, Jackson M, et al. Tiacumicins a novel complex of 18-membered macrolide antibiotics. I. Taxonomy, fermentation and antibacterial activity. J Antibiotic (Tokyo) 1987;40:567–74. doi: 10.7164/antibiotics.40.567. [DOI] [PubMed] [Google Scholar]
- 37.Credito KL, Appelbaum PC. Activity of OPT-80, a novel macrocyclic, compared with those of eight other agents against selected anaerobic species. Antimicrob Agents Chemother. 2004;48:4430–4. doi: 10.1128/AAC.48.11.4430-4434.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finegold SM, Molitoris D, Vaisanen ML, et al. In vitro activities of OPT-80 and comparator drugs against anaerobic bacteria. Antimicrob Agents Chemother. 2004;48:4898–902. doi: 10.1128/AAC.48.12.4898-4902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beidenbach DI, Ross JE, Putnam SD, Jones RN. In vitro activity of fidaxomicin (OPT80) tested against contemporary isolates of Staphylococcus spp. and Enterococcus spp. Antimicrobial Agents Chemother. 2010;54:2273–5. doi: 10.1128/AAC.00090-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babakhani F, Seddon J, Robert N, et al. Narrow spectrum activity and low fecal binding of OPT-80 and its major hydrolysis metabolite (OP-1118). 47th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); Chicago. September 17 to 20, 2007. [Google Scholar]
- 41.Citron DM, Tyrrell KL, Merriam V, Goldstein EJC. Comparative in vitro activities of LFF571 against Clostridium difficile and 630 other intestinal strains of aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 2012;56:2493–503. doi: 10.1128/AAC.06305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hecht DW, Galang MA, Sambol SP, et al. In vitro activities of 15 antimicrobial agents against 110 toxigenic Clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob Agents Chemother. 2007;51:2716–9. doi: 10.1128/AAC.01623-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Citron DM, Babakhani F, Goldstein EJ, et al. Typing and susceptibility of bacterial isolates from the fidaxomicin (OPT-80) phase II study for C. difficile infection. Anaerobe. 2009;15:234–6. doi: 10.1016/j.anaerobe.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Rashid MU, Dalhoff A, Weintraub A, Nord CE. In vitro activity of MCB3681 against Clostridium difficile strains. Anaerobe. 2014;28:216–9. doi: 10.1016/j.anaerobe.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Goldstein EJC, Citron DM, Tyrrell KL, Merriam CV. Comparative in vitro activities of SMT19969, a new antimicrobial agent, against Clostridium difficile and 350 Gram-positive and Gram-negative aerobic and anaerobic intestinal flora isolates. Antimicrob Agents Chemother. 2013;57:4872–6. doi: 10.1128/AAC.01136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Louie T, Miller M, Donskey C, et al. Clinical outcomes, safety, and pharmacokinetics of OPT-80 in a phase 2 trial with patients with Clostridium difficile infection. Antimicrob Agents Chemother. 2009;53:223–8. doi: 10.1128/AAC.01442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Louie TJ, Cannon K, Byrne B, et al. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis. 2012;55(Suppl 2):S132–42. doi: 10.1093/cid/cis338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babakhani F, Seddon J, Robert N, et al. Effects of inoculum, pH, and cations on the in vitro activity of fidaxomicin (OPT-80, PAR-101) against Clostridium difficile. Antimicrob Agents Chemother. 2010;54:2674–6. doi: 10.1128/AAC.01842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sears P, Crook DW, Louie TJ, Miller MA, Weiss K. Fidaxomicin attains high fecal concentrations with minimal plasma concentrations following oral administration in patients with Clostridium difficile infection. Clin Infect Dis. 2012;55(Suppl 2):S116–20. doi: 10.1093/cid/cis337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seddon J, Babakhani F, Sears P. Mutant prevention concentration of fidaxomicin for Clostridium difficile. 52nd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); San Francisco. September 9–12, 2012; (Abstr. A-1274). [Google Scholar]
- 51.Babakhani F, Gomez A, Robert N, Sears P. Killing kinetics of fidaxomicin and its major metabolite, OP-1118, against Clostridium difficile. J Med Microbiol. 2011;60:1213–7. doi: 10.1099/jmm.0.029470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Babakhani F, Bouillaut L, Sears P, et al. Fidaxomicin inhibits toxin production in Clostridium difficile. J Antimicrob Chemother. 2013;68:515–22. doi: 10.1093/jac/dks450. [DOI] [PubMed] [Google Scholar]
- 53.Koon HW, Ho S, Hing TC, et al. Fidaxomicin inhibits Clostridium difficile toxin A-mediated enteritis in the mouse ileum. Antimicrob Agents Chemother. 2014;58:4642–50. doi: 10.1128/AAC.02783-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Babakhani F, Bouillaut L, Gomez A, et al. Fidaxomicin inhibits spore production in Clostridium difficile. Clin Infect Dis. 2012;55(Suppl 2):S162–9. doi: 10.1093/cid/cis453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allen CA, Babakhani F, Sears P, et al. Both fidaxomicin and vancomycin inhibit outgrowth of Clostridium difficile spores. Antimicrob Agents Chemother. 2013;57:664–7. doi: 10.1128/AAC.01611-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chilton CH, Crowther GS, Freeman J, et al. Successful treatment of simulated Clostridium difficile infection in a human gut model by fidaxomicin first line and after vancomycin or metronidazole failure. J Antimicrob Chemother. 2014;69:451–62. doi: 10.1093/jac/dkt347. [DOI] [PubMed] [Google Scholar]
- 57.Nerandzic MM, Mullane K, Miller MA, et al. Reduced acquisition and overgrowth of vancomycin-resistant enterococci and Candida species in patients treated with fidaxomicin versus vancomycin for Clostridium difficile infection. Clin Infect Dis. 2012;55(Suppl 2):S121–6. doi: 10.1093/cid/cis440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mullane KM. Fidaxomicin in Clostridium difficile infection: Latest evidence and clinical guidance. Ther Adv Chronic Dis. 2014;5:69–84. doi: 10.1177/2040622313511285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Esmaily-Fard A, Tverdek FP, Crowther, et al. The use of fidaxomicin for treatment of relapsed Clostridium difficile infections in patients with cancer. Pharmacother. 2014;34:1220–5. doi: 10.1002/phar.1479. [DOI] [PubMed] [Google Scholar]
- 60.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–47. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 61.Vargo CA, Bauer KA, Mangino JE, Johnston JEW, Goff DA. An antimicrobial stewardship program’s real-world experience with fidaxomicin for treatment of Clostridium difficile infection: A case series. Pharmacotherapy. 2014;34:901–9. doi: 10.1002/phar.1451. [DOI] [PubMed] [Google Scholar]
- 62.Eiland EH, Sawyer AJ, Massie NL. Fidaxomicin use and clinical outcomes for Clostridium difficile-associated diarrhea. Infect Dis Clin Pract. 2015;23:32–5. doi: 10.1097/IPC.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clutter DS, Dubrovskaya Y, Merl MY, Teperman L, Press R, Safdar A. Fidaxomicin versus conventional antimicrobial therapy in 59 recipients of solid organ and hematopoietic stem cell transplantation with Clostridium difficile-associated diarrhea. Antimicrob Agents Chemother. 2013;57:4501–5. doi: 10.1128/AAC.01120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Penziner S, Dubrovskaya Y, Press R, Safdar A. Fidaxomicin therapy in critically ill patients with Clostridium difficile infection. Antimicrob Agents Chemother. 2015;59:1776–81. doi: 10.1128/AAC.04268-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soriano MM, Danziger LH, Gerding DN, Johnson S. Novel fidaxomicin treatment regimens for patients with multiple Clostridium difficile infection recurrences that are refractory to standard therapies. Open Forum Infect Dis. 2014 Aug 25;1:ofu069. doi: 10.1093/ofid/ofu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiss K, Allgren RL, Sellers S. Safety analysis of fidaxomicin in comparison with oral vancomycin for Clostridium difficile infection. Clin Infect Dis. 2012;55(Suppl 2):S110–5. doi: 10.1093/cid/cis390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iarikov DE, Alexander J, Nambiar S. Hypersensitivity reactions associated with fidaxomicin use. Clin Infect Dis. 2014;58:537–9. doi: 10.1093/cid/cit719. [DOI] [PubMed] [Google Scholar]
- 68.Cornely OA, Nathwani D, Ivanescu C, et al. Clinical efficacy of fidaxomicin compared with vancomycin and metronidazole in Clostridium difficile infections: A meta-analysis and indirect treatment comparison. J Antimicrob Chemother. 2014;69:2892–900. doi: 10.1093/jac/dku261. [DOI] [PubMed] [Google Scholar]
- 69.Eyre DW, Babakhani F, Griffiths D, et al. Whole-genome sequencing demonstrates that fidaxomicin is superior to vancomycin for peventing reinfection and relapse of infection with Clostridium difficile. J Infect Dis. 2014;209:1446–51. doi: 10.1093/infdis/jit598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wagner M, Lavoie L, Goetghebeur M. Clinical and economic consequences of vancomycin and fidaxomicin for the treatment of Clostridium difficile infection in Canada. Can J Infect Dis Med Microbiol. 2014;25:87–94. doi: 10.1155/2014/793532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stranges PM, Hutton DW, Collins CD. Cost-effectiveness analysis evaluating fidaxomicin versus oral vancomycin for the treatment of Clostridium difficile infection in the United States. Value Health. 2013;16:297–304. doi: 10.1016/j.jval.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 72.Nathwani D, Cornely OA, Van Engen AK, Odufowora-Sita O, Retsa P, Odeyemi IAO. Cost-effectiveness analysis of fidaxomicin versus vancomycin in Clostridium difficile infection. J Antimicrob Chemother. 2014;69:2901–12. doi: 10.1093/jac/dku257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Debast SB, Bauer MP, Kuijper EJ, on behalf of the Committee European Society of Clinical Microbiology and Infectious Diseases: Update on the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(Suppl 2):1–26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 74.Wilcox MH. Updated guidance on the management and treatment of Clostridium difficile infection. < www.his.org.uk> (Accessed September 17, 2014).


