Abstract
Neuroimaging studies of mindfulness training (MT) modulate anterior cingulate cortex (ACC) and insula among other brain regions, which are important for attentional control, emotional regulation and interoception. Inspiratory breathing load (IBL) is an experimental approach to examine how an individual responds to an aversive stimulus. Military personnel are at increased risk for cognitive, emotional and physiological compromise as a consequence of prolonged exposure to stressful environments and, therefore, may benefit from MT. This study investigated whether MT modulates neural processing of interoceptive distress in infantry marines scheduled to undergo pre-deployment training and deployment to Afghanistan. Marines were divided into two groups: individuals who received training as usual (control) and individuals who received an additional 20-h mindfulness-based mind fitness training (MMFT). All subjects completed an IBL task during functional magnetic resonance imaging at baseline and post-MMFT training. Marines who underwent MMFT relative to controls demonstrated a significant attenuation of right anterior insula and ACC during the experience of loaded breathing. These results support the hypothesis that MT changes brain activation such that individuals process more effectively an aversive interoceptive stimulus. Thus, MT may serve as a training technique to modulate the brain’s response to negative interoceptive stimuli, which may help to improve resilience.
Keywords: mindfulness, fMRI, interoception, insula, military
INTRODUCTION
To improve the brain’s ability to respond to stress is an important goal for both primary prevention of stress-related sequelae, but also for treatment of individuals with psychiatric disorders secondary to trauma exposure. An important component process to target is the brain’s awareness of the body’s internal physical state. Specifically, one approach is to improve interoception (Craig, 2002), i.e. the processing of the body’s internal state and in particular the perturbation of this state as a result of external demands when an individual has to maintain goal-directed action and homeostatic balance (Paulus et al., 2009). Anatomically, these processes take place in the posterior insula which provides topographic and modality-specific interoceptive signals to the anterior insular cortex for integration (Craig, 2003). The anterior insula has reciprocal connections with subcortical, limbic and executive control brain systems, which allows for the integration of interoceptive signals and hedonic evaluation, such as the anterior cingulate cortex (ACC), amygdala, nucleus accumbens and orbitofrontal cortex (Critchley et al., 2004; Craig, 2009). Thus, modifying interoception can be considered an experimental marker for modifying an individual’s ability to respond to stress.
Resilience can be conceptualized as one’s ability to positively adapt to severe stress, trauma and adversity (Luthar et al., 2000), that may help to prevent the development of psychopathology (Campbell-Sills et al., 2006), particularly in individuals with combat exposure (Pietrzak et al., 2009; Green et al., 2010). Recently, we have conducted a series of studies examining emotion and interoceptive processing in highly resilient individuals (e.g. elite athletes, special operations forces), and demonstrated differential activation of the insula and ACC in response to emotional processing and loaded inspiratory breathing (Paulus et al., 2010; Paulus et al., 2012; Simmons et al., 2012; Thom et al., 2012). Moreover, self-reported resilience during emotion processing task is associated with differential activation of insula, ACC and amygdala (Waugh et al., 2008b; Paulus et al., 2010). These results suggest that the ability to perform well under stress, involves modulation of the neural systems are also important in processing interoceptive information.
Among interoceptive processes, modulation of breathing control and awareness has been a unique target for various treatment approaches. Breathing is an interoceptive process that is modulated in the periphery through several sensory airway receptors (Adriaensen and Timmermans, 2011) providing continuous sensory information, which provides discriminative (i.e. what is sensed) and affective information (i.e. how it feels; Davenport and Vovk, 2009). Affective reactions to breathing changes are experienced as equally or more aversive than negative visual stimuli (Pappens et al., 2010), and can serve as a source of threat, leading to increased anxiety (von Leupoldt et al., 2011). One effective method of inducing experimental breathing change is through loaded inspiratory breathing, which can be operationally defined as the degree to which an individual experiences resistance during inspiration (Lopata et al., 1977; Gottfried et al., 1978). In this investigation, resistance during breathing inspiration occurs when the airflow is re-directed through cintered disks that act as resistors during inspiration but not during expiration. Experientially, loaded inspiratory breathing has a sensory component of increased work to inhale but also an affective component that ranges from mild discomfort to intense fear of breathing in. While inspiratory breathing loads (IBL) result in increased activation in the primary and secondary somatosensory cortices and the cerebellum, IBLs do not modulate task-specific brain activation (Hayen et al., 2012). These characteristics, make breathing an ideal experimental probe for the interoceptive system, in particular the insular cortex, as well as a robust aversive interoceptive stimulus. Understanding the neural network involved in adaptive responses to aversive interoceptive stimuli is an important research question to answer when characterizing optimal performance under stress.
Although interoception has been identified as an important function in responding to, and recovering from, high-magnitude stressors, it remains unclear whether underlying substrates can be modified through training. There is evidence to suggest that mindfulness training (MT), that emphasizes interoceptive awareness and attentional control, can alter brain–behavior relationships involved in response to stress. Mindfulness is ‘the awareness that emerges through paying attention on purpose, in the present moment, and non-judgmentally to the unfolding of experience moment by moment’ (Kabat-Zinn, 2003). Mindful awareness is cultivated by providing guided instruction in mindfulness meditation practices, such as, breath-focused attention and body-scanning of sensory experiences, which require non-judgmental, moment-to-moment awareness of the breath or body part. Mindfulness-based stress reduction (MBSR) developed by Kabat-Zinn (1982, 1990), has been shown to reduce stress-related sequelae (e.g. self-reported stress, medical symptoms, neuroendocrine changes) associated with chronic mental health disorders (e.g. Bowen et al., 2006; Hofmann et al., 2010), medical conditions (e.g. Kabat-Zinn et al., 1985; Carlson et al., 2007) and in nonclinical populations (e.g. Grossman et al., 2004; Chiesa and Serretti, 2009; Wolever et al., 2012). There is converging evidence to suggest anatomical (Kang et al., 2013; Lazar et al., 2005; Holzel et al., 2008; Grant et al., 2010; Kang et al., 2013) and functional (Cahn and Polich, 2006; Brefczynski-Lewis et al., 2007; Farb et al., 2007; Ives-Deliperi et al., 2011; Zeidan et al., 2011; Tang et al., 2012; Dickenson et al., 2013) brain changes are associated with MT, particularly in the insula, ACC and prefrontal cortices. Specifically, several studies have documented posterior cingulate cortex (PCC) and medial prefrontal cortex deactivation in experienced meditators (Brewer et al., 2011; Taylor et al., 2011; Pagnoni, 2012; Brewer et al., 2013), suggesting multiple brain regions are actively involved during meditation practices. Taken together mindfulness mediation has the potential to modulate the effects stress exposure across multiple domains including cognitive, behavioral, physiological, functional and neuroanatomical. Of note, while there are multiple effective interventions for the treatment of stress-related sequelae (e.g. PTSD), such as Cognitive Processing Therapy (Resick and Schnicke, 1992) and Prolonged Exposure (McLean and Foa, 2011), MBSR is an evidence based therapy that can be initiated prior to trauma exposure—and thus may have potential as a preventative method.
MT may have some beneficial effects in military populations who are routinely exposed to extreme environments and are challenged by highly stressful events. The MT intervention, referred to as mindfulness-based mental fitness training (MMFT), was created by a former U.S. Army officer with many years of training in MBSR and trauma resilience, and contains many features of the well-established MBSR protocol. MMFT was previously found to improve working memory capacity in active-duty military (Jha et al., 2010). The primary aim of this study was to determine if MMFT affects how individuals process aversive interoceptive stimuli. In other words, is MMFT able to modulate the brains response to interoceptive distress in marines preparing for combat deployment.
METHODS
Participants
The study was conducted at the Marine Corps Base, Camp Pendleton, California, and at the University of California, San Diego (UCSD) Keck Center for Functional MRI. The Naval Health Research Center (NHRC) and UCSD Institutional Review Boards approved this study and all participants signed informed consents. Participants were recruited from a convenience sample of two Marine infantry battalions scheduled to undergo pre-deployment training. Within those battalions, eight platoons were randomly selected for study assignment; four platoons were assigned to the MMFT group (n = 153) and four platoons to the control group (n = 134). Throughout the experiment, participants remained within their organic platoon, which was organized for an upcoming deployment. Approximately five participants from each platoon (n = 42) volunteered for functional MRI (fMRI); additional written consent was obtained from the volunteers prior to the baseline assessment. From the original sub-sample of marines that agreed to complete the fMRI component of the study (n = 42), 19 MMFT and 16 control group marines completed both fMRI scans, and will be included in the analysis (see Study Design for details). The groups did not differ in age (F(1,34) = 2.943, P > 0.05) or years of education (F(1,34) = 3.733, P > 0.05; Table 1).
Table 1.
Demographics and self-report measures of study participants
| MMFT (n = 19) | Control (n = 16) | ||||
|---|---|---|---|---|---|
| Variable | Mean(Std) | ||||
| Age | 22.35(3.30) | 20.81(1.10) | |||
| Years of education | 12.37(0.76) | 12.00(0.00) | |||
| MMFT T1 | Control T1 | MMFT T2 | Control T2 | |
|---|---|---|---|---|
| (n = 14) | (n = 14) | (n = 14) | (n = 14) | |
| Pittsburgh sleep quality index | 9.93(4.62) | 6.57(3.76) | 8.79(4.48) | 6.79(3.80) |
| (n = 12) | (n = 10) | (n = 12) | (n = 10) | |
| Response to stressful experiences scale | 58.92(13.11) | 66.10(13.44) | 59.42(11.25) | 64.20(14.13) |
| (n = 11) | (n = 9) | (n = 11) | (n = 9) | |
| Five facets of mindfulness questionnaire | 129.55(14.50) | 125.55(17.24) | 120.64(7.75) | 128.44(15.57) |
MMFT, mindfulness-based mental fitness training; T1, baseline assessment; T2 post-training assessment.
Study design
Participants underwent two fMRI scans: (i) baseline assessment, which occurred prior to the MMFT course and (ii) post-training, which occurred approximately two weeks following the MMFT course and one week following the Infantry Immersive Trainer. For a more detailed description of the study see Johnson et al., submitted).
Intervention
MMFT is a 20-h course taught over 8 weeks, including eight 2-h class sessions (four in the first 2 weeks), an individual practice interview in week 3 and a 4-h workshop with extended silent practice to refine skills in week 6. Designed for individuals with prior exposure to prolonged stress and trauma, MMFT blends MT with concepts and skills from sensorimotor psychotherapy and Somatic Experiencing. MMFT emphasizes interoceptive awareness by cultivating attentional control and tolerance for challenging experience, both external (e.g. harsh environments) and internal (e.g. physical pain, intense emotions). It focuses on enhancing stress resilience by teaching body-based skills for supporting self-regulation of the stress response, its effects and emotions. Didactics highlight how these skills are applicable to military stress inoculation training, complex decision making and operational missions. Outside of class sessions, Marines were encouraged to practice mindfulness and self-regulation skills, for at least 30 min, in several practice periods daily. Exercises are initially guided with audio CDs, but over time, audio support is not necessary. See Stanley (2014) for details.
Self-report measures
Several personality and symptom assessment questionnaires were administered at baseline and post-training including: five facets of mindfulness questionnaire (FFMQ; Baer et al., 2006), designed to measure the five primary facets of mindfulness (observing, describing, acting with awareness, non-judging of inner experience and non-reactivity to inner experience); response to stressful experiences scale (RSES; Johnson et al., 2011) designed to measure 6-processes that promote resilient responses to high-magnitude stressors (active-coping, spirituality, cognitive flexibility, meaning-making, self-efficacy, restoration); and the Pittsburgh Sleep Scale (Buysse et al., 1989), designed to assess subjective sleep quality and disturbance over the preceding 1-month time interval.
Behavioral interoceptive assessment
Using an IBL task, we have demonstrated that this task reliably generates a stress response and robust brain activation of the interoceptive system (Paulus et al., 2012). Participants wore a nose clip and breathed through a mouthpiece with a non-rebreathing valve (2600 series, Hans Rudolph) that maintained an airtight seal. The resistance loads consisted of sintered bronze disk placed in a series in a Plexiglas tube (loading manifold), with stoppered ports inserted between the disks. Prior to entering the scanner, each participant completed three different levels of breathing restriction: baseline (no-load), 10, 20 and 40 cm H2O/l/s, breathing loads were presented in ascending order, lasted 1-min each with a minute break in between each load. Of note, during the fMRI task only the 40 cm H2O/l/s breathing restriction was employed. The following instructions were given: ‘This task examines how people feel when breathing becomes difficult. You will breathe through a hose, which makes breathing-in more difficult. It is important for you to know that this test is not physically harmful, but you may feel uncomfortable when you breathe through the hose. You can stop at any time if breathing becomes too difficult. You will be asked to breathe through the hose several times. We would like you to complete a one-page rating scale after each trial’. Participants were asked to rate their experience on a 10 cm Visual Analog Scale, which ranged from ‘not at all’ to ‘extremely’ on the following dimensions: pleasant; unpleasant; and intense.
Functional MRI IBL task
The basic experimental approach is analogous to the task described above. Inside the scanner, the mouthpiece was positioned comfortably between the lips and was attached to the scanner head coil to eliminate the need to for the participant to contract mouth muscles. Participants performed a simple continuous performance task during the paradigm; they were instructed to press a button that corresponded to the direction pointed by an arrow on the screen (left arrow = left button, right arrow = right button). Participants’ accuracy and response latency were recorded and analyzed to determine effects of anticipation and stimulus presentation. At the same time, the background color of the arrow served as a cue to the impending presentation of the breathing load (grey = no load, yellow = 25% chance of load). We utilized randomly varied inter-trial intervals between each anticipation phase. There were three conditions: (i) anticipation: the background color of the stimulus signals an impending restricted period; and (ii) breathing load: during the change in background color there was a 25% probability that the participant experienced a 40 s period of restricted breathing; and (iii) post-breathing load: immediately following the 40 s period of restricted breathing. Subjects were requested to maintain a consistent breathing pace during the scan and exhaled CO2 was measured. The 25% probability was introduced in order to maximize the opportunity to measures the effects of anticipating an aversive interoceptive stimulus. This event-related paradigm consisted of 2 scans with 256 repetitions, yielding a total scan duration of 17 min and 4 s. The duration of each condition was ‘jittered’ in time to maximize the resolution of the hemodynamic response function. The primary behavioral variable was performance accuracy and latency during the three conditions, and the primary neuroimaging dependent measure was the activation in functionally constrained regions of interest during the anticipation and stimulus condition relative to the baseline condition (for additional task-related details see Paulus et al., 2012).
Scanning parameters
Imaging data was acquired at the UCSD Center for Functional MRI on a 3T GE shortbore scanner (GE MR750), equipped with an eight-channel high bandwidth receiver. A high-resolution anatomical image was obtained, which consisted of a sagittally acquired spoiled gradient recalled (SPGR) sequence (172 sagittal slices; FOV 25 cm; matrix: 192 x 256 (interpolated to 256 x 256); slices thickness: 1 mm; TR: 8 ms; TE: 3 ms; flip angel: 12°). A standard gradient echo-planar images (EPI) pulse sequence was used to acquire T2*-weighted functional images (40 axial slices, FOV: 230 mm, matrix: 64 x 64; slice thickness: 3 mm; TR: 2000 ms; TE 32 ms; flip angle: 90°). Rapid image T2* acquisition was obtained via GE’s ASSET scanning, a form of sensitivity encoding (SENSE), which uses parallel imaging reconstruction to allow for sub k-space sampling.
Image analysis pathway
Participant-level data were processed with Analysis of Functional Neuroimages (AFNI) software package (Cox, 1996), including temporal and spatial alignment, motion and outlier detection, concatenation, deconvolution and Talairach transformation. Orthogonal regressors were computed for four conditions: (i) anticipation, (ii) breathing load and (iii) post-breathing interval. A task-based reference function corresponding to time interval of the regressor of interest was convolved with a gamma variate function (Boynton et al., 1996) that modeled the prototypical 6- to 8-s delay hemodynamic response function (Friston, 1995) and the temporal dynamics of the hemodynamic response (typically 12–16 sec; Cohen, 1997). In addition, three motion parameters were obtained for each participant and were used to adjust for EPI intensity changes due to motion artifacts. In addition, if the average of any one of these parameters exceeded 2 standard deviations from the mean or if movement exceeded the size of the voxel (4 mm) participants were excluded; however, no participant was excluded based on this criterion. Using the AFNI program, 3dDeconvolve, multivariate regressor analysis was used to relate changes in EPI intensity to differences in task characteristics. The main dependent measure was present signal change, which was spatially smoothed with a 6 mm full-width half-maximum Gaussian filter.
Regions of interest
Analyses were constrained to a priori regions of interest (ROI), which included the ACC and anterior insula. These, a priori, anatomically defined ROIs were constructed using a data-driven approach that combined Talairach stereotactic definition and grey matter probabilities based on high resolution T1 images from a group of 43 healthy adults (Fonzo et al., 2013). Using SPM5 (Statistical Parametric Mapping software; http://www.fil.ion.ucl.ac.uk/spm) implemented in Matlab 7.5.0 (MathWorks, Natick, Massachusetts), grey matter probabilities were determined by applying grey matter segmentation for each subject, which yielded voxel-wise probabilities of assignment to grey matter, across all subjects. The grey matter probability maps were spatially normalized to Talairach stereotactic space, with the boundaries of each region determined based on maximizing sensitivity and specificity for each ROI. The masks were then applied to functional MRI datasets to extract signals from voxels located in selected regions.
Group level analysis
The main dependent measure was percent signal change during the anticipation, stimulation and post-stimulation period, which were entered into a mixed effects model (Littell et al., 2000). Data were analyzed with linear mixed effects models in R (http://cran.r-project.org/), which estimates parameters using Maximum Likelihood Estimation and estimates effects using specific contrast matrices. The fixed factors were defined as the group (MMFT vs control), experimental condition (anticipation, stimulation and post-stimulus intervals), and time (baseline vs post-training), and subject was entered as a random factor. To guard against Type I error, voxel-wise statistics were calculated using the AFNI program Alphasim, which estimates statistical significance based on Monte Carlo stimulations. Based on the Alphasim program, it was determined that, given the spatial smoothing of 6 mm FWHM and a voxel-wise P < 0.01, the volume threshold for clusterwise probability of 0.05 for the anterior insula was 256 µl, for the ACC was 448 µl. Only clusters meeting these criteria were considered for further analysis.
Behavioral data analysis
All behavioral data analyses were carried out with SPSS 20.0 (IBM, Chicago, IL). Repeated measures ANOVA (RM-ANOVA) were run to examine group differences (MMFT vs control) across time (baseline vs post-training), separately, for the FFMQ, RSES and PSQI. In addition, RM-ANOVA were run to examine group differences (MMFT vs control) across time (baseline vs post-training), separately for accuracy and response latency performance during the IBL task (baseline, anticipation, breathing load and post-breathing load), and for VAS ratings of pleasantness, unpleasantness and intensity during behavioral IBL task (breathing load: baseline, 10, 20 and 40 cm H2O/l/s).
RESULTS
Self-report results
The scores of FFMQ, RSES and PSQI did not differ between groups and did not change differentially across time (group-by-time; Table 1). However, it should be noted that due to scheduling conflicts (e.g. attending mandatory training during data collection times), there are several missing data points at baseline and post-training for the self-report measures. Specifically, of the marines from the control group that participated in both fMRI sessions (n = 16), only nine had self-report data at both time points. As such, correlational analyses between self-report measures and brain activation were no conducted
Subjective breathing load
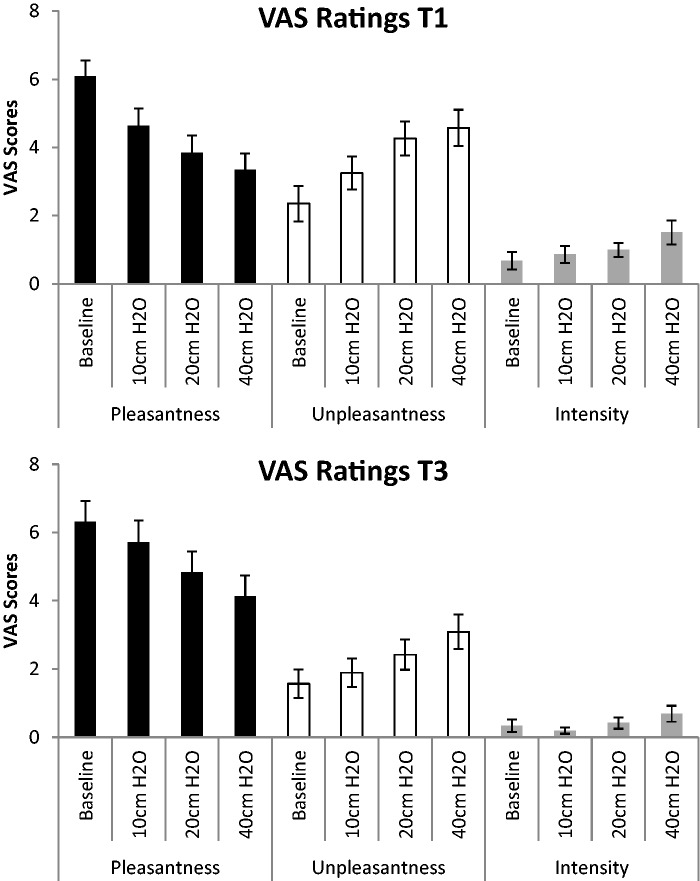
There was an overall effect for breathing load (baseline, 10, 20 and 40 cm H2O/l/s) for VAS pleasantness ratings [F(3,99) = 151.225, P < 0.001], VAS unpleasantness ratings [F(3,99) = 81.938, P < 0.001] and VAS intensity ratings [F(3,99) = 4.625, P < 0.005; Figure 1]. Post hoc analysis revealed that VAS pleasantness ratings significantly decreased as load increased (P < 0.001). For VAS unpleasantness ratings, although there were no significant differences in unpleasantness ratings between baseline and 10 cm H2O/l/s, all other conditions were significantly more unpleasant than baseline. For VAS intensity ratings, 40 cm H2O/l/s was rated as significantly more intense than all of the conditions. There was a main effect of time for VAS unpleasantness ratings [F(1,33) = 7.399, P < 0.01] and VAS intensity ratings [F(1,33) = 10.378, P < 0.003], such that, for both ratings, unpleasantness and intensity significantly decreased from baseline to post-training (Figure 1). Finally, there was a main effect of group for VAS pleasantness [F(1,33) = 5.408, P = 0.027]; overall, across time and breathing condition, the control group rated the breathing restriction task as more pleasant than the MMFT group (Figure 2). Although breathing load resulted in a more aversive experience (decreased pleasantness, increased unpleasantness and intensity), there were no differences in VAS ratings between the MMFT and control groups across time; in other words, there was no effect on the MMFT course in the self-report ratings during the breathing restriction task.
Fig. 1.
There was a main effect load, averaged over time (baseline and post-training) and group (MMFT and control); for pleasantness, both groups’ ratings decreased with increasing load. For unpleasantness, both groups’ ratings increased for 20 and 40 cm H2O. For intensity, both groups’ ratings for 40 cm H2O were significantly greater than the other loads. Additionally, there was a main effect of time averaged over load (10, 20, and 40 cm H2O/l/s) and group (MMFT and control); VAS unpleasantness and intensity ratings decreased from baseline to post-training.
Fig. 2.
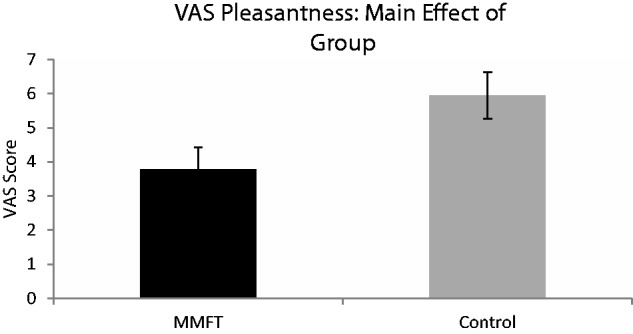
There was a main effect group, averaged over time (baseline and post-training) and load (10, 20, and 40 H2O/l/s) for pleasantness, such that the control group rated the breathing restriction task as more pleasant than the MMFT group.
Behavioral performance during fMRI breathing load
For response latency, there was no group-by-time-condition interaction. However, there was a time-by-condition interaction for response latency [F(4,99) = 4.223, P = 0.007]; for both time points, individuals (MMFT and control) took longer to respond to the anticipation of the breathing load and the post-breathing load, there were no significant differences between baseline and load (Figure 3). There was also a group-by-condition interaction for response latency [F(3,99) = 3.95, P = 0.036]. The control group has significantly longer response latencies than the MMFT group in the post-load condition (Data not shown). There was a main effect of task for response latency [F(3,99) = 185.696, P < 0.0001]; relative to baseline and load, individuals took longer to respond to the anticipation of the breathing load and the post-breathing load; all latencies were significantly different from one another (Data not shown). There were no significant differences in response latency between baseline and breathing load. Overall, the response latency results show longer response latencies for the anticipation and post-breathing load conditions for all individuals at baseline and post-training.
Fig. 3.
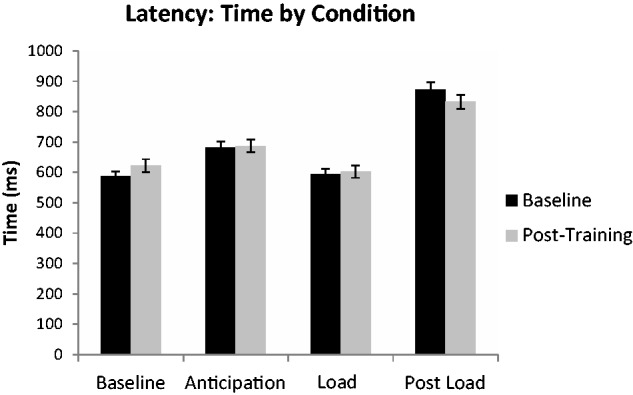
Interaction effect of task by condition for reaction time (latency) during the continuous performance task averaged group (MMFT and control).
There were no significant main effects or interactions for accuracy during the breathing restriction task, indicating no effect of the MMFT course on behavioral performance during the fMRI breathing restriction task.
Neuroimaging results
Group-by-time interaction
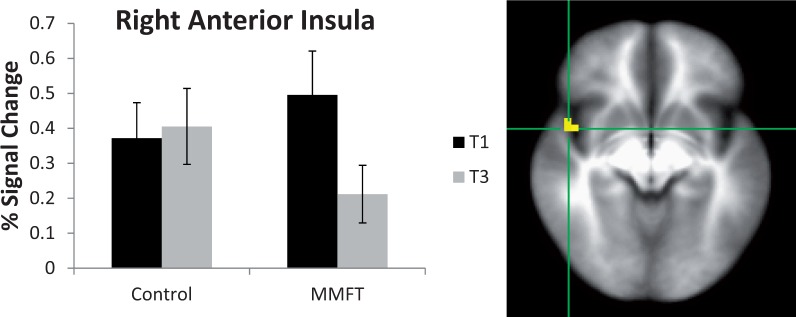
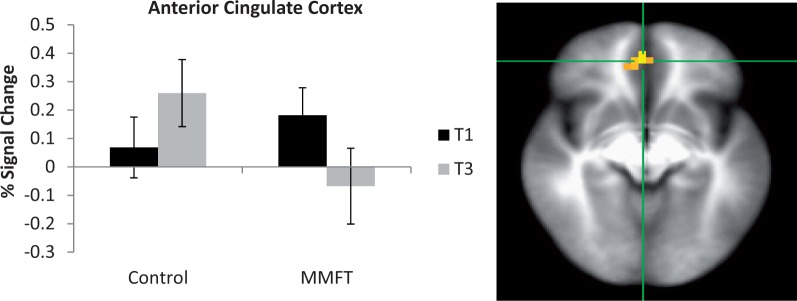
There was a significant group-by-time interaction for breathing restriction in the right anterior insula and ACC (Table 2 and Figures 4 and 5, respectively). Specifically, for the right anterior insula, the MMFT group demonstrated significant attenuated activation following the MMFT course, whereas the control group showed no significant change in activation. Additionally, for the averaged breathing task, the MMFT group demonstrated significant attenuation in ACC, whereas the control group demonstrated a trend for increased brain activation in the post-training period.
Table 2.
Group-by-time interaction of breathing restriction on brain activation
| Volume | x | y | z | Brain area | BA |
|---|---|---|---|---|---|
| 576 | 4 | 44 | −3 | Anterior cingulate | 32 |
| 256 | 45 | 4 | −4 | Right anterior insula |
BA, Brodmann area; Volume (µl), center of mass coordinate, and brain area based on the voxel-wise mixed model group-by-time interaction of breathing restriction.
Fig. 4.
Interaction of group-by-time. Right anterior insula showed attenuated activation in the MMFT group following the MMFT course, whereas the control group showed no change in activation.
Fig. 5.
Interaction of group-by-time. ACC showed attenuated activation in the MMFT group following the MMFT course, whereas the control group showed a trend for increased activation.
DISCUSSION
This investigation aimed to investigate whether individuals who undergo mindfulness-based training will show attenuated behavioral and brain response to aversive interoceptive stimulation. Although we did not find a significant effect of MMFT training on subjective ratings and behavioral measures during aversive interoception, we did find a significant attenuation in neural systems that are important for interoceptive processing. Specifically, marines after MT showed significant attenuation of right anterior insula and ACC, in contrast to those in the training as usual condition who showed a trend for increased brain activation during loaded breathing. These results are consistent with the hypothesis that MT modulates the brain’s response to an aversive interoceptive stimulus such that individuals who have undergone MMFT demonstrate more effective neural modulation when exposed to an aversive interoceptive stimulus.
Exposure to extreme environments can result in declines in emotional, cognitive and physiological functioning. Combat deployment has been associated with neurocognitive changes (Vasterling et al., 2006), and there is evidence to suggest that pre-deployment training compromises cognitive function including learning, memory, and visual vigilance (Lieberman et al., 2002). Moreover, a recent meta-analysis indicates that 13.2% of operational infantry units following deployment to Iraqi and Afghanistan meet criteria for post-traumatic stress disorder (Kok et al., 2012). Finding ways to improve resilience, that is—an individual’s ability to successfully cope with (and bounce back from) stress, may help to reduce the short- and long-term sequelae associated with exposure to extreme environments. In this study, MT resulted in significant attenuation of right insular activation during an aversive breathing load task. These findings are consistent with (i) the proposed role of the insula as part of the neural network subserving interoception (Craig, 2002; Critchley et al., 2004), (ii) attenuated right insular activation in elite athletes during aversive interoceptive breathing load (Paulus et al., 2012), (iii) literature documenting the effectiveness of MT in the treatment of stress-related sequelae for a number of clinical disorder and medical disorders (e.g. Carlson et al., 2007; Hofmann et al., 2010) and (iv) modulation of insula activation during interoceptive attention following MT (Farb et al., 2013).
Similar to other sensory modalities (i.e. vision, audition), interoceptive breathing loads are organized along the dimensions of valence and arousal, and represent a homeostatic balance between metabolic activity and affective arousal (Gomez et al., 2008) are experienced as aversive (Pappens et al., 2010), and can result in increased anxiety (von Leupoldt et al., 2011). Self-reported anxiety, particularly concerns regarding the physical features of anxiety, is associated with increased anterior insula activation in response to negatively valenced stimuli (Killgore et al., 2011). Activation in the anterior insula is associated with interoceptive awareness and level of anxiety, such that the level of attention to interoceptive body states was associated with increased levels of anxiety (Terasawa et al., 2013). These findings are consistent with the proposed model suggesting the inability to adequately predict one’s internal body state (i.e. the difference between the value of the anticipated/predicted and value of the current interoceptive state), is associated with insular dysfunction and increased risk for anxiety disorders (Paulus and Stein, 2006). Conversely, resilient individuals generate more efficient body prediction errors, as a way of adapting to extreme environments (Paulus and Stein, 2006). In the support of this proposal, we have previously shown that highly resilient individuals, such as elite adventure racers (Paulus et al., 2012) and U.S. NAVY Sea, Air and Land Forces (SEALs; Simmons et al., 2012), demonstrate improved performance and attenuated insular activation during an aversive interoceptive breathing and emotion detection tasks. These findings suggest that relative to controls, highly resilient individuals are less affected by aversive interoceptive and emotionally demanding tasks. Behaviorally, higher levels of self-reported resilience are associated with a bias towards positive emotions when faced with uncertain or negative emotions (Tugade and Fredrickson, 2004; Arce et al., 2009) and these individuals demonstrate faster recovery following the anticipation of an aversive stimulus (Waugh et al., 2008a), which may contribute to increased coping abilities. Taken together, these results suggest that insular cortex plays an important role in resilience, and MT may modulate the brains response to aversive interoceptive conditions in a manner similar to what has been observed in elite warfighters.
The anterior insula is part of the interoceptive neural network; it integrates information from multiple brain regions, ranging from physiologically driven motivational states to emotional awareness, which serves to maintain interoceptive homeostasis (Craig, 2002; Critchley et al., 2004). This brain area has reciprocal connections with the ACC, which generates an error signal that determines allocation of attentional resources based on interoceptive information (Botvinick et al., 1999; van Veen and Carter, 2002; Botvinick et al., 2004). Anterior insula and the ACC facilitate cognitive control by switching between the central-executive control network and default-mode network (Sridharan et al., 2008; Menon and Uddin, 2010). Mindful meditation is also associated with improved cognitive functioning (Jha et al., 2010; Zeidan et al., 2010). In particular, attentional control is thought to be one of the primary mechanisms of mediation (Baer, 2003; Shapiro et al., 2006; Holzel et al., 2011). As part of MT, individuals are trained to focus their attention and are instructed to return their attention to their focus point (e.g. breath, body sensations, emotions) when they become distracted. Research has shown that individuals who undergo meditation training show improved attention (Wenk-Sormaz, 2005; Jha et al., 2007; Tang et al., 2007; Jensen et al., 2012). In the present study the CPT was designed to be easy so it would not draw attentional resources away from the interoceptive task, as such, we were not anticipating group differences or changes in performance secondary to the MMFT intervention. As expected, the failure to find significant differences in accuracy and reaction time is likely due to the relative ease of the behavioral CPT task and not a reflection of failure to improve attention. Neuroimaging studies of experienced meditators have documented increased ACC activation during tasks of attention (Holzel et al., 2007) and focused meditation (Lazar et al., 2000; Newberg et al., 2001; Brewer et al., 2011), and increased cortical thickness (Lazar et al., 2005; Grant et al., 2010). Given that previous studies have shown that meditation training results in improved attention, in combination with the present results of attenuated ACC and insular activation in response to aversive interoceptive stimulation, we speculate that MT may potentially improve attentional control, such that there is more efficient allocation of attentional resources during stressful experiences.
As part of the fMRI inspiratory breathing restriction task, Marines performed a simple continuous performance task, during which a change in the background color served as a cue for the possibility (25% chance) of an impending breathing load. This task served several purposes: (i) ensured that the Marines’ maintained focus on the task, (ii) provided information regarding response accuracy and latency and (iii) provided the opportunity to measure the effects of anticipating an aversive interoceptive stimulus. There were no significant effects for response accuracy—average response accuracy performance for both groups was at or above the 96th percentile (data not shown), which suggests that the Marines maintained focus on the task. However, for response latency (i.e. reaction time), there was a main effect of task, such that reaction times were longer for the anticipation, post-anticipation and post-load conditions relative to the baseline and load condition. In other words, changes in reaction time indicated that individuals showed significant slowing whenever a change in the interoceptive condition occurred, i.e. when individuals had to prepare for or recover from changes in the interoceptive state. However, there was no group (control vs MMFT) by time (baseline vs post-training) interaction, suggesting that the MMFT training did not modulate behavioral performance during the fMRI loaded breathing task.
This study has several limitations. First, there was no active control group, i.e. marines who did not participate in MMFT training underwent the usual pre-deployment training. Therefore, it is possible that the mere time spent in MMFT training contributed to group differences. Second, we did not identify a clear brain behavior relationship, i.e. the brain activation results were not related to changes in measures of mindfulness or resiliency, which may be due to small sample size. Thus, future studies with larger sample sizes and active comparison groups are needed to elucidate the relationship between modulation of brain activation due to MT and self-report measures of mindfulness and resiliency is important for understanding the functional implications of MT. Nevertheless, this investigation contributes to the generalizability of potentially resilience-building effects of MT. Specifically, the subjects were not treatment seeking, did not suffer from a mental health condition and had not been previously exposed to concepts of mindfulness and yet showed significant brain changes as a consequence of training.
CONCLUSIONS
In summary, this study found that an 8-week MT program in active duty infantry marines modulates the brain response to an aversive interoceptive stimulus. Relative to controls, marines who underwent MT showed attenuated right anterior insula and ACC activation during an aversive interoceptive condition. The present findings provide evidence that MT may serve as a training technique to modulate the brain’s response to negative interoceptive stimuli, which may contribute to increased coping abilities.
Conflict of Interest
None declared.
Acknowledgments
We extend our appreciation to Christopher Demuro, Karl Van Orden, Sante Kotturi, Leila El-kara, John Schaldach, Robert Skidmore, Matt Fennell, Rose McDermott, Tom Liu, and Anet Petrosyan. This research was supported by funding from the Office of Naval Research (ONR) Code 30, and Navy Bureau of Medicine and Surgery (BUMED) and by the National Institute of Mental Health (T32-MH018399-26). This material is the result of work supported with resources and the use of facilities at the 1st Marine Expeditionary Force (IMEF), Camp Pendleton, CA, the University of California, San Diego, La Jolla, California, and the VA Center of Excellence Stress and Mental Health (CESAMH).
Footnotes
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Navy, Department of Defense, or the U.S. Government. This research has been conducted in compliance with all applicable federal regulations governing the protection of human subjects in research.
REFERENCES
- Adriaensen D, Timmermans JP. Breath-taking complexity of vagal C-fibre nociceptors: implications for inflammatory pulmonary disease, dyspnoea and cough. Journal of Physiology. 2011;589:3–4. doi: 10.1113/jphysiol.2010.201434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce E, Simmons AN, Stein MB, Winkielman P, Hitchcock C, Paulus MP. Association between individual differences in self-reported emotional resilience and the affective perception of neutral faces. Journal of Affective Disorders. 2009;114:286–93. doi: 10.1016/j.jad.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clinical Psychology: Science and Practice. 2003;10:125–43. [Google Scholar]
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–81. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bowen S, Witkiewitz K, Dillworth, et al. Mindfulness meditation and substance use in an incarcerated population. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors. 2006;20:343–7. doi: 10.1037/0893-164X.20.3.343. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. Journal of Neuroscience. 1996;16:4207–21. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proceedings of the National Academy of the Sciences United States of America. 2007;104:11483–8. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Garrison KA, Whitfield-Gabrieli S. What about the “Self” is Processed in the Posterior Cingulate Cortex? Frontiers in Human Neuroscience. 2013;7:647. doi: 10.3389/fnhum.2013.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of the Sciences United States of America. 2011;108:20254–9. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychological Bulletin. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Cohan SL, Stein MB. Relationship of resilience to personality, coping, and psychiatric symptoms in young adults. Behaviour Research and Therapy. 2006;44:585–99. doi: 10.1016/j.brat.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain, Behavior, and Immunity. 2007;21:1038–49. doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A. Mindfulness-based stress reduction for stress management in healthy people: a review and meta-analysis. Journal of Alternative and Complementary Medicine (New York, N.Y.) 2009;15:593–600. doi: 10.1089/acm.2008.0495. [DOI] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature reviews. Neuroscience. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature reviews. Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Vovk A. Cortical and subcortical central neural pathways in respiratory sensations. Respiratory Physiology & Neurobiology. 2009;167:72–86. doi: 10.1016/j.resp.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Dickenson J, Berkman ET, Arch J, Lieberman MD. Neural correlates of focused attention during a brief mindfulness induction. Social Cognitive and Affective Neuroscience. 2013;8:40–7. doi: 10.1093/scan/nss030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Anderson AK. Mindfulness meditation training alters cortical representations of interoceptive attention. Social Cognitive and Affective Neuroscience. 2013;8:15–26. doi: 10.1093/scan/nss066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2:313–22. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Flagan TM, Sullivan S, et al. Neural functional and structural correlates of childhood maltreatment in women with intimate-partner violence-related posttraumatic stress disorder. Psychiatry Research. 2013;211:93–103. doi: 10.1016/j.pscychresns.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Commentary and opinion: II. Statistical parametric mapping: ontology and current issues. Journal of Cerebral Blood Flow and Metabolism. 1995;15:361–70. doi: 10.1038/jcbfm.1995.45. [DOI] [PubMed] [Google Scholar]
- Gomez P, Shafy S, Danuser B. Respiration, metabolic balance, and attention in affective picture processing. Biological Psychology. 2008;78:138–49. doi: 10.1016/j.biopsycho.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Gottfried SB, Altose MD, Kelsen SG, Fogarty CM, Cherniack NS. The perception of changes in airflow resistance in normal subjects and patients with chronic airways obstruction. Chest. 1978;73:286–8. doi: 10.1378/chest.73.2_supplement.286. [DOI] [PubMed] [Google Scholar]
- Grant JA, Courtemanche J, Duerden EG, Duncan GH, Rainville P. Cortical thickness and pain sensitivity in zen meditators. Emotion (Washington, D.C.) 2010;10:43–53. doi: 10.1037/a0018334. [DOI] [PubMed] [Google Scholar]
- Green KT, Calhoun PS, Dennis MF, Beckham JC. Exploration of the resilience construct in posttraumatic stress disorder severity and functional correlates in military combat veterans who have served since September 11, 2001. The Journal of Clinical Psychiatry. 2010;71:823–30. doi: 10.4088/JCP.09m05780blu. [DOI] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. Journal of Psychosomatic Research. 2004;57:35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Hayen A, Herigstad M, Kelly M, et al. The effects of altered intrathoracic pressure on resting cerebral blood flow and its response to visual stimulation. Neuroimage. 2012;66C:479–88. doi: 10.1016/j.neuroimage.2012.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78:169–83. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Vangel M, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Research. 2011;191:36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Gard T, et al. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Social Cognitive and Affective Neuroscience. 2008;3:55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Hempel H, et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neuroscience Letters. 2007;421:16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Ives-Deliperi VL, Solms M, Meintjes EM. The neural substrates of mindfulness: an fMRI investigation. Social Neuroscience. 2011;6:231–42. doi: 10.1080/17470919.2010.513495. [DOI] [PubMed] [Google Scholar]
- Jensen CG, Vangkilde S, Frokjaer V, Hasselbalch SG. Mindfulness training affects attention–or is it attentional effort? Journal of Experimental Psychology. General. 2012;141:106–23. doi: 10.1037/a0024931. [DOI] [PubMed] [Google Scholar]
- Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cognitive, Affective & Behavioral Neuroscience. 2007;7:109–19. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- Jha AP, Stanley EA, Kiyonaga A, Wong L, Gelfand L. Examining the protective effects of mindfulness training on working memory capacity and affective experience. Emotion (Washington, D.C.) 2010;10:54–64. doi: 10.1037/a0018438. [DOI] [PubMed] [Google Scholar]
- Johnson DC, Polusny MA, Erbes CR, et al. Development and initial validation of the Response to Stressful Experiences Scale. Military Medicine. 2011;176:161–9. doi: 10.7205/milmed-d-10-00258. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. General Hospital Psychiatry. 1982;4:33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Mind and Body to Face Stress, Pain, and Illness. New York: Delacorte; 1990. [Google Scholar]
- Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. Journal of Behavioral Medicine. 1985;8:163–90. doi: 10.1007/BF00845519. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Mindfulness-based interventions in context: past, present, and future. Clinical Psychology: Science and Practice. 2003;10:144–56. [Google Scholar]
- Kang DH, Jo HJ, Jung WH, et al. The effect of meditation on brain structure: cortical thickness mapping and diffusion tensor imaging. Social Cognitive and Affective Neuroscience. 2013;8:27–33. doi: 10.1093/scan/nss056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Britton JC, Price LM, Gold AL, Deckersbach T, Rauch SL. Neural correlates of anxiety sensitivity during masked presentation of affective faces. Depression and Anxiety. 2011;28:243–9. doi: 10.1002/da.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok BC, Herrell RK, Thomas JL, Hoge CW. Posttraumatic stress disorder associated with combat service in Iraq or Afghanistan: reconciling prevalence differences between studies. The Journal of Nervous and Mental Disease. 2012;200:444–50. doi: 10.1097/NMD.0b013e3182532312. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Bush G, Gollub RL, Fricchione GL, Khalsa G, Benson H. Functional brain mapping of the relaxation response and meditation. Neuroreport. 2000;11:1581–5. [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16:1893–7. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman HR, Tharion WJ, Shukitt-Hale B, Speckman KL, Tulley R. Effects of caffeine, sleep loss, and stress on cognitive performance and mood during U.S. Navy SEAL training. Sea-Air-Land. Psychopharmacology (Berl) 2002;164:250–61. doi: 10.1007/s00213-002-1217-9. [DOI] [PubMed] [Google Scholar]
- Littell RC, Pendergast J, Natarajan R. Modelling covariance structure in the analysis of repeated measures data. Statistics in Medicine. 2000;19:1793–819. doi: 10.1002/1097-0258(20000715)19:13<1793::aid-sim482>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Lopata M, La Fata J, Evanich MJ, Lourenco RV. Effects of flow-resistive loading on mouth occlusion pressure during CO2 rebreathing. The American Review of Respiratory Disease. 1977;115:73–81. doi: 10.1164/arrd.1977.115.1.73. [DOI] [PubMed] [Google Scholar]
- Luthar SS, Cicchetti D, Becker B. The construct of resilience: a critical evaluation and guidelines for future work. Child Development. 2000;71:543–62. doi: 10.1111/1467-8624.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CP, Foa EB. Prolonged exposure therapy for post-traumatic stress disorder: a review of evidence and dissemination. Expert Review of Neurotherapeutics. 2011;11:1151–63. doi: 10.1586/ern.11.94. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure & Function. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberg A, Alavi A, Baime M, Pourdehnad M, Santanna J, d'Aquili E. The measurement of regional cerebral blood flow during the complex cognitive task of meditation: a preliminary SPECT study. Psychiatry Research. 2001;106:113–22. doi: 10.1016/s0925-4927(01)00074-9. [DOI] [PubMed] [Google Scholar]
- Pagnoni G. Dynamical properties of BOLD activity from the ventral posteromedial cortex associated with meditation and attentional skills. Journal of Neuroscience. 2012;32:5242–9. doi: 10.1523/JNEUROSCI.4135-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappens M, Van den Bergh O, De Peuter S, Bresseleers J, Vansteenwegen D, Van Diest I. Defense reactions to interoceptive threats: a comparison between loaded breathing and aversive picture viewing. Biological Psychology. 2010;84:98–103. doi: 10.1016/j.biopsycho.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Flagan T, Simmons AN, et al. Subjecting elite athletes to inspiratory breathing load reveals behavioral and neural signatures of optimal performers in extreme environments. PloS one. 2012;7:e29394. doi: 10.1371/journal.pone.0029394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Potterat EG, Taylor MK, et al. A neuroscience approach to optimizing brain resources for human performance in extreme environments. Neuroscience and Biobehavioral Reviews. 2009;33:1080–8. doi: 10.1016/j.neubiorev.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Simmons AN, Fitzpatrick SN, et al. Differential brain activation to angry faces by elite warfighters: neural processing evidence for enhanced threat detection. PloS one. 2010;5:e10096. doi: 10.1371/journal.pone.0010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60:383–7. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Johnson DC, Goldstein MB, Malley JC, Southwick SM. Psychological resilience and postdeployment social support project against traumatic stress and depressive symptoms in soldiers returning from Operations Enduring Freedom and Iraqi Freedom. Journal of Special Operations Medicine: A Peer Reviewed Journal for SOF Medical Professionals. 2009;9:67–73. doi: 10.55460/LANU-HRWC. [DOI] [PubMed] [Google Scholar]
- Resick PA, Schnicke MK. Cognitive processing therapy for sexual assault victims. Journal of Consulting and Clinical Psychology. 1992;60:748–56. doi: 10.1037//0022-006x.60.5.748. [DOI] [PubMed] [Google Scholar]
- Shapiro SL, Carlson LE, Astin JA, Freedman B. Mechanisms of mindfulness. Journal of Clinical Psychology. 2006;62:373–86. doi: 10.1002/jclp.20237. [DOI] [PubMed] [Google Scholar]
- Simmons AN, Fitzpatrick S, Strigo IA, et al. Altered insula activation in anticipation of changing emotional states: neural mechanisms underlying cognitive flexibility in Special Operations Forces personnel. Neuroreport. 2012;23:234–9. doi: 10.1097/WNR.0b013e3283503275. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of the Sciences United States of America. 2008;105:12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley EA. Mindfulness-based Mind Fitness Training (MMFT): An Approach for Enhancing Performance and Building Resilience in High Stress Contexts. In: Le A, Ngnoumen CT, Langer EJ, editors. The Wiley-Blackwell Handbook of Mindfulness. London: Wiley-Blackwell; 2014. pp. 964–85. [Google Scholar]
- Tang YY, Ma Y, Wang J, et al. Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of the Sciences United States of America. 2007;104:17152–6. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Rothbart MK, Posner MI. Neural correlates of establishing, maintaining, and switching brain states. Trends in Cognitive Sciences. 2012;16:330–7. doi: 10.1016/j.tics.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VA, Grant J, Daneault V, et al. Impact of mindfulness on the neural responses to emotional pictures in experienced and beginner meditators. Neuroimage. 2011;57:1524–33. doi: 10.1016/j.neuroimage.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Terasawa Y, Shibata M, Moriguchi Y, Umeda S. Anterior insular cortex mediates bodily sensibility and social anxiety. Social Cognitive and Affective Neuroscience. 2013;8:259–66. doi: 10.1093/scan/nss108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom NJ, Johnson DC, Flagan T, et al. Detecting emotion in others: increased insula and decreased medial prefrontal cortex activation during emotion processing in elite adventure racers. Social Cognitive and Affective Neuroscience. 2012;9(2):225–31. doi: 10.1093/scan/nss127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugade MM, Fredrickson BL. Resilient individuals use positive emotions to bounce back from negative emotional experiences. Journal of Personality and Social Psychology. 2004;86:320–33. doi: 10.1037/0022-3514.86.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & Behavior. 2002;77:477–82. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Proctor SP, Amoroso P, Kane R, Heeren T, White RF. Neuropsychological outcomes of army personnel following deployment to the Iraq war. JAMA: The Journal of the American Medical Association. 2006;296:519–29. doi: 10.1001/jama.296.5.519. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Chan PY, Bradley MM, Lang PJ, Davenport PW. The impact of anxiety on the neural processing of respiratory sensations. Neuroimage. 2011;55:247–52. doi: 10.1016/j.neuroimage.2010.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh CE, Fredrickson BL, Taylor SF. Adapting to life's slings and arrows: Individual differences in resilience when recovering from an anticipated threat. Journal of Research in Personality. 2008a;42:1031–46. doi: 10.1016/j.jrp.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh CE, Wager TD, Fredrickson BL, Noll DC, Taylor SF. The neural correlates of trait resilience when anticipating and recovering from threat. Social Cognitive and Affective Neuroscience. 2008b;3:322–32. doi: 10.1093/scan/nsn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk-Sormaz H. Meditation can reduce habitual responding. Alternative Therapies in Health and Medicine. 2005;11:42–58. [PubMed] [Google Scholar]
- Wolever RQ, Bobinet KJ, McCabe K, et al. Effective and viable mind-body stress reduction in the workplace: a randomized controlled trial. Journal of Occupational Health Psychology. 2012;17:246–58. doi: 10.1037/a0027278. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Johnson SK, Diamond BJ, David Z, Goolkasian P. Mindfulness meditation improves cognition: evidence of brief mental training. Consciousness and Cognition. 2010;19:597–605. doi: 10.1016/j.concog.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. Journal of Neuroscience. 2011;31:5540–8. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]