Abstract
Reproducibility of results is important in improving the robustness of conclusions drawn from research, particularly in functional magnetic resonance imaging (fMRI). In this study, we aim to replicate a previous study on the neural correlates of face emotion processing above and below awareness level using an independent sample of youth with severe mood dysregulation (SMD) and healthy volunteers (HV). We collected fMRI data in 17 SMD and 20 HV, using an affective priming paradigm with masked (17 ms) and unmasked (187 ms) faces (angry, happy, neutral, blank oval). When processing masked and unmasked angry faces, SMD patients exhibited increased activation in the parahippocampal gyrus (PHG) and superior temporal gyrus relative to HV. When processing masked and unmasked happy faces, SMD patients showed decreased activation in the insula, PHG and thalamus compared with HV. During masked face processing in general across emotions, youth with SMD showed greater ventromedial prefrontal cortex (vmPFC) activation relative to HV. Perturbed activation in emotion processing areas (e.g. insula, PHG, superior temporal gyrus and thalamus) manifests as hyper-sensitivity toward negative emotions and hypo-sensitivity toward positive emotions may be important in the etiology and maintenance of irritability, aggression and depressive symptoms in SMD. vmPFC dysfunction may mediate over-reactivity to face emotions associated with irritability.
Keywords: irritability, severe mood dysregulation, functional neuroimaging, backward masking, face emotion processing
Introduction
Severe, chronic irritability is a common and impairing problem, affecting 3–5% of youth (Brotman et al., 2006; Stringaris and Goodman, 2009), and predicts poor outcomes in adulthood, including depressive and anxiety disorders (Brotman et al., 2006; Stringaris et al., 2009), high suicidality (Pickles et al., 2010) and low income and educational attainment (Stringaris et al., 2009). There has been a recent increase in irritabiltiy research, partly due to the introduction of disruptive mood dysregulation disorder (DMDD) to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) (American Psychiatric Association, 2013). Much of the research support for irritability has been derived from studies on severe mood dysregulation (SMD), a phenotype characterized by severe, chronic irritability (Leibenluft et al., 2003; Roy et al., 2014) and upon which DMDD was based.
SMD youth show deficits in face emotion labeling (Guyer et al., 2007; Rich et al., 2008) and aberrant neural responses when processing emotional faces (Brotman et al., 2010; Thomas et al., 2012, 2013, 2014). This work has primarily focused on consciously perceived face emotions. Only one study has probed automatic, unconscious face emotion processing in irritable youth using functional magnetic resonance imaging (fMRI) (Thomas et al., 2014). However, there are concerns regarding Type I error in fMRI research (Lieberman and Cunningham, 2009; Poldrack and Mumford, 2009; Vul et al., 2009). Replication studies, particularly in fMRI (Lieberman and Cunningham, 2009; Bennett and Miller, 2010), are critical in validating important results and conclusions drawn from prior research.
Previous literature suggests that emotional processing consists of two distinct but simultaneously activated neural pathways; one mediates explicit, conscious processing, while the other mediates implicit, subliminal processing (LeDoux, 1996; Morris et al., 1998; Whalen et al., 1998). To probe these two processes, researchers typically manipulate the duration of stimuli (e.g. <40 ms for implicit processing and longer for explicit processing; Esteves and Ohman, 1993). A common method to assess implicit emotional processing is backward masking, where a prime stimulus (e.g. emotional face) is presented too briefly to reach awareness, followed by a target stimulus (the ‘mask’) that is presented long enough to be identified. Using this method, research in healthy adults and those with anxiety and mood disorders has implicated several brain regions in face processing below the awareness level. Some prominent regions include the amygdala, which plays a central role in rapid, automatic, non-conscious processing of affective visual stimuli (Morris et al., 1998; Whalen et al., 1998; Rauch et al., 2000; Sheline et al., 2001; Killgore and Yurgelun-Todd, 2004; Nomura et al., 2004; Armony et al., 2005; Dannlowski et al., 2007a,b; Bryant et al., 2008; Kugel et al., 2008; Carlson et al., 2009; Sabatini et al., 2009; Suslow et al., 2009, 2010a,b; Duan et al., 2010; Victor et al., 2010; Killgore et al., 2014; Lichev et al., 2015), as well as anterior cingulate cortex (Killgore and Yurgelun-Todd, 2004; Duan et al., 2010; Victor et al., 2012) and inferior frontal gyrus (Nomura et al., 2004; Dannlowski et al., 2007b; Duan et al., 2010; Lichev et al., 2015), which have inhibitory control over amygdala responses. Other regions include structures implicated in face perception, such as the fusiform gyrus (Nomura et al., 2004; Suslow et al., 2009; Lichev et al., 2015), middle occipital gyrus (MOG; Suslow et al., 2009, 2010b), superior temporal gyrus (STG; Sabatini et al., 2009; Suslow et al., 2009, 2010b; Victor et al., 2012; Lichev et al., 2015) and insula (Suslow et al., 2009, 2010b; Killgore et al., 2011; Victor et al., 2012). Among these studies, a generally consistent finding that differentiates adult patients with anxiety and/or mood disorders from healthy volunteers (HV) is that the former exhibited heightened amygdala activation during early, automatic stages of emotional processing (Rauch et al., 2000; Sheline et al., 2001; Bryant et al., 2008; Suslow et al., 2010a; Victor et al., 2010).
In contrast to the large body of literature on masked face emotion processing in adults, there are only a few fMRI studies in youth (Pine et al., 2001; Killgore and Yurgelun-Todd, 2007; Monk et al., 2008; Thomas et al., 2014). Masked face paradigm is important to probe the neural correlates of rapid, automatic emotional processing in irritable youth, as the difficulties to regulate emotion may occur without insight or subjective awareness. Prolonged viewing of emotional faces may obscure perturbed neural dysfunction (e.g. in the amygdala) that occurs during early, rapid processing of emotions (Nomura et al., 2004; Monk et al., 2008). Aberrant automatic processing of emotional faces (e.g. angry, happy faces) may contribute to the mood dysregulation and interpersonal difficulties in irritable youth. Thomas et al. (2014) incorporated masked and unmasked faces in one paradigm and found that youth with SMD showed more activation in the MOG when viewing faces that were not consciously perceived vs consciously perceived, while HV showed the opposite pattern. In addition, youth with SMD exhibited increased activation in the posterior cingulate, STG and MOG when viewing angry faces (regardless of awareness level); youth with SMD also showed increased activation in the STG when viewing happy faces (regardless of awareness level; Thomas et al., 2014). In this study, SMD did not differ from HV in brain activation in response to fearful faces (Thomas et al., 2014).
Here, we modified the task used by Thomas et al. (2014) by removing fearful faces, and thus increasing the number of trials with other faces (e.g. angry, happy faces) and the power of the analyses. We used this modified paradigm to examine masked and unmasked face emotion processing in youth with SMD and HV. Significant contribution of this study to the field includes (i) the examination of automatic, unconscious as well as explicit, conscious face emotion processing in a single paradigm; (ii) an expansion of the scarce literature on automatic, unconscious face emotion processing in irritable youth and (iii) a replication of a previous study with a similar paradigm in an independent sample. As noted above, such replication is rare yet pivotal in neuroimaging and thought leaders in the field have recently expressed concern about the lack of replication among fMRI studies (Lieberman and Cunningham, 2009; Bennett and Miller, 2010; Barch and Yarkoni, 2013; Ioannidis et al., 2014). We hypothesized that there would be awareness-modulated group differences in the MOG (Thomas et al., 2014) and emotion-modulated group differences in the MOG, STG, posterior cingulate, anterior cingulate cortex, posterior insula and inferior parietal lobe (Thomas et al., 2012, 2013, 2014) based on previous research in SMD. Additionally, we hypothesized that youth with SMD, relative to HV, would demonstrate amygdala dysfunction during unmasked (Brotman et al., 2010; Thomas et al., 2012, 2013, 2014) but not masked (Thomas et al., 2014), emotional face processing based on previous research.
Materials and methods
Participants
All participants, aged 8–18 years, were enrolled in an Institutional Review Board-approved study at the National Institute of Mental Health (NIMH). Parents and children gave written informed consent/assent. A total of 47 children (25 SMD and 22 HV) enrolled; 10 subjects were excluded for the following reasons: poor behavioral performance (3 SMD and 2 HV), excessive motion during fMRI acquisition (>3 mm; 2 SMD) and inability to complete the task (3 SMD). The final sample included 17 youth with SMD and 20 HV. Children with SMD were recruited through advertisements to support groups, professional meetings and psychiatrists. HV were drawn from the community through advertisements.
All children were assessed using the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL; Kaufman et al., 1997). The K-SADS-PL, including an additional module for assessing irritability and excessive reactivity to negative stimuli (SMD module), was administered separately to children and parents by masters or doctoral level clinicians with excellent inter-rater reliability (kappa ≥ 0.9 for all diagnoses). Patients met criteria for SMD, with severe and impairing irritability, severe outburst and at least three hyperarousal symptoms (e.g. distractibility, racing thoughts or flight of ideas and intrusiveness) beginning before age 12; symptoms were present for at least 1 year (without 2 months symptom-free periods) and caused impairment in at least two of three settings (home, school and with peers). Hypomanic or manic episodes of 1 day or longer were considered exclusionary, so none of the participants met criteria for bipolar disorder. Comorbid diagnoses are reported in Table 1. For detailed criteria for assessing SMD, please see Leibenluft et al. (2003) and Leibenluft (2011). Although DMDD was not proposed at the time of the data collection, all SMD youth also met criteria for DMDD (i.e. irritable mood and temper outbursts, present before age 10), using retrospectively applied criteria.
Table 1.
Sample characteristics
| Characteristics | SMD | HV | t or χ2 | P |
|---|---|---|---|---|
| (n = 17) | (n = 20) | |||
| Mean ± SD | Mean ± SD | |||
| Age (years) | 14.08 ± 2.79 | 14.95 ± 2.19 | −1.06 | 0.298 |
| Full-scale IQa | 107.19 ± 12.38 | 115.00 ± 13.18 | −1.82 | 0.078 |
| CDRS | 26.65 ± 5.27 | — | — | — |
| PARSa | 12.13 ± 3.61 | — | — | — |
| CGAS (6 months) | 49.41 ± 9.64 | — | — | — |
| Number of medications | 1.65 ± 1.54 | — | — | — |
| Number of co-occurring diagnoses | 1.71 ± 0.99 | — | —— | — |
| N (%) | N (%) | |||
| Male | 13 (76.5) | 10 (50) | 2.74 | 0.098 |
| Co-occurring diagnoses | ||||
| ADHD | 13 (76.5) | — | — | — |
| Major depression | 5 (29.4) | — | — | — |
| Any anxiety disorderb | 11 (64.7) | — | — | — |
| CD | 1 (5.9) | — | — | — |
| Medication at scan | ||||
| Unmedicated | 6 (35.3) | 20 (100) | — | — |
| Atypical antipsychotic | 9 (52.9) | 0 | — | — |
| Lithium | 1 (5.9) | 0 | — | — |
| Antiepileptic | 6 (35.3) | 0 | — | — |
| Antidepressant | 3 (17.6) | 0 | — | — |
| Stimulants | 3 (17.6) | 0 | — | — |
Note. CDRS, Children’s Depression Rating Scale; PARS, Pediatric Anxiety Rating Scale; CD, conduct disorder.
aMissing data for 1 SMD.
bIncludes generalized anxiety disorder, separation anxiety disorder, social phobia, panic disorder, post-traumatic stress disorder and obsessive compulsive disorder.
Other clinician-rated clinical symptoms were assessed in the SMD group using the Children’s Depression Rating Scale (Poznanski et al., 1984) and the Pediatric Anxiety Rating Scale (Research Units on Pediatric Psychopharmacology Anxiety Study Group, 2002) within 48 h of scanning. To evaluate levels of global functioning in children with SMD, clinicians administered the Children’s Global Assessment Scale (CGAS; Shaffer et al., 1983).
HV were medication-free and had no lifetime psychiatric diagnoses and no first-degree relatives with mood disorders, as ascertained by parent interview. Exclusion criteria for all participants were IQ < 70, history of head trauma, neurological disorder, pervasive developmental disorder, unstable medical illness or substance abuse/dependence.
Affective priming paradigm
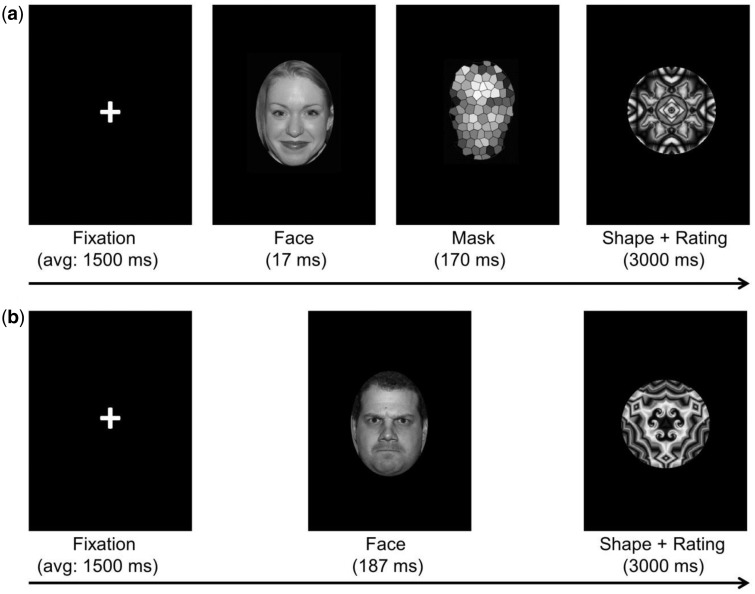
Participants completed a modified affective priming task (Thomas et al., 2014) during fMRI data acquisition. There were four runs in the task: two for the aware condition (with unmasked faces) and two for the non-aware condition (with masked faces). The runs were interleaved, i.e. alternating between aware and non-aware conditions. The order of the runs was counterbalanced across participants. In both the aware and non-aware conditions, participants indicated on a scale from 1 (did not like) to 5 (liked a lot) how much they liked an abstract shape presented for 3000 ms after the masked or unmasked face (Figure 1). This is to ensure participants were attending to the task by providing a behavioral response and also to test whether responding was influenced by the emotional primes. In the aware condition, a fixation cross (1250–1750 ms, average 1500 ms) followed by a face or blank oval (187 ms) was presented before the shape (3000 ms; Figure 1). In the non-aware condition, subjects saw a fixation cross (1250–1750 ms, average 1500 ms), followed by a face or blank oval (17 ms), a scrambled face mask (170 ms) and finally an abstract shape (3000 ms; Figure 1). Each event was 3187 ms long. The face stimuli were angry, happy, neutral or ‘no face’ (blank oval); there were 36 trials for each stimulus under each awareness condition. Stimuli were presented randomly. The duration of the task was approximately 28 min. Prior to scanning, outside the scanner on a desktop computer, participants completed a practice run of eight trials for each awareness condition, using faces not presented during scanning.
Fig. 1.
Affective priming paradigm. (a) Non-aware condition. (b) Aware condition.
Post-task assessments
Immediately after the affective priming task and during structural scanning, two tasks were administered to assess whether the awareness manipulation was successful. The order of the two post-tasks was random. In both, participants were shown the faces in the non-aware condition and informed about the presence of the face in the ‘flash’ before the shape. In one post-task, participants were asked to identify the gender of the face. We combined all participants’ accuracy data and ran one-sample t-tests vs chance (50%) for each gender (male, female). In the other post-task, subjects were asked to identify the face emotion (angry, happy or neutral). To examine if any emotion ‘leaked’ from the mask into awareness, we conducted one-sample t-tests on accuracy for angry, happy and neutral faces vs chance (33.3%). These tests were intentionally liberal since the concern here was to identify any evidence of emotion perception in subjects, even at a liberal statistical threshold.
Image acquisition and preprocessing
Data were acquired on a 3T GE scanner with an eight-channel head coil. Structural images used T1-weighted axial acquisition [three-dimensional spoiled-gradient-recall acquisition in the steady state with inversion recovery prep pulse; 256 × 192 matrix, 128 1.2-mm axial slices, 22 cm field of view (FOV)] to allow normalization to standard space (Talairach and Tournoux, 1988). Functional imaging was performed axially using a multi-slice gradient echo-planar sequence (24 cm FOV, 96 × 96 matrix, 38 contiguous 2.6 mm slices, TR = 2300 ms, TE = 25 ms, voxel size = 2.6 × 2.5 × 2.5 mm).
fMRI data were analyzed using Analysis of Functional NeuroImages program (Cox, 1996). The first four volumes in each series were discarded, leaving 704 repetition times per participant. Preprocessing included despiking, slice timing correction, coregistration, spatial smoothing (kernel full width at half maximum = 6), masking, intensity scaling and transformation into Talairach space. Repetitions with motion > 1 mm relative to the preceding repetition were removed from the analysis. Regressors for each emotion in each awareness condition [Emotion (4; angry, happy, neutral, no face) × Awareness (2; aware, non-aware)] were created by convolving stimulus times with a gamma-variate hemodynamic response function. Individual-subject linear regression modeling was performed per voxel, with eight task regressors (Emotion × Awareness) plus one regressor modeling missed trials, a third-order polynomial modeling the baseline drift and six motion parameters. Beta coefficients and t-statistics were calculated for each voxel and regressor. Blank-fixation trials provided a baseline.
Data analysis
Behavioral data
Shape ratings and reaction time (RT) on the affective priming task were compared in two separate (one for rating and the other for RT) Diagnosis (2; SMD, HV) × Emotion (4; angry, happy, neutral, no face) × Awareness (2; aware, non-aware) repeated-measures analyses of variance (ANOVAs), with Emotion and Awareness as within-subject variables and Diagnosis as a between-subject variable.
fMRI data
At the group level, we conducted a whole-brain Diagnosis (2) × Emotion (4) × Awareness (2) ANOVA, using a statistical threshold of P ≤ 0.005, uncorrected, voxel-wise extent threshold of k ≥ 20 (Lieberman and Cunningham, 2009). There were no suprathreshold voxels in the three-way ANOVA. Given our primary interest in differences between SMD and HV, analyses then focused on the two-way ANOVAs that included diagnosis, i.e. Diagnosis × Emotion and Diagnosis × Awareness. For clusters surpassing this threshold, average signal change values were extracted and post-hoc ANOVAs or t-tests were performed in SPSS.
Because masked faces are thought to bypass top–down cortical regulation of the amygdala and elicit amygdala responses (Whalen et al., 1998; Rauch et al., 2000; Killgore et al., 2014), we also conducted ROI analyses on the left and right amygdala defined by the Talairach-Tournoux Daemon. Mean signal intensity was extracted from each ROI for each stimulus type and was submitted to a Diagnosis (2) × Emotion (4) × Awareness (2) ANOVA in SPSS. Post-hoc ANOVAs or t-tests were performed in SPSS to understand significant main effects or interactions. In addition, we examined the association between parent- or child-reported irritability (using the Affective Reactivity Index; Stringaris et al., 2012) and the observed brain activation. Given trend-level between-group differences in gender and IQ, additional post-hoc analyses using SPSS were conducted to examine their effects on the fMRI findings. We also conducted post-hoc analyses to test the effects of medication, comorbidities, functional impairment (CGAS), anxiety symptoms and attention-deficit/hyperactivity disorder (ADHD) on the brain activation.
Results
Demographics
Chi-square analysis or t-tests were used to compare gender distribution, age and IQ between groups. There were marginally significant between-group differences in gender distribution (P = 0.098) and IQ (P = 0.078), i.e. SMD youth had a slightly lower IQ and more males compared with HV. Post-hoc analyses were conducted to examine the effect of these factors on our fMRI findings.
Behavioral data
Rating
There were no significant three-way (i.e. Diagnosis × Emotion × Awareness) or two-way (i.e. Diagnosis × Emotion, Diagnosis × Awareness or Emotion × Awareness) interactions or main effect of Diagnosis. Main effects of Emotion (F3,105 = 5.11, P = 0.007) and Awareness (F1,35 = 9.19, P = 0.005) were significant. Somewhat surprisingly, shapes presented after both angry and neutral faces were liked more than those presented after happy and no face (P ≤ 0.04). Shapes presented in the non-aware condition were liked more than those in the aware condition (P = 0.005).
Reaction time
Similarly, there were no significant three-way and two-way interactions or main effects of Diagnosis and Awareness. Only the main effect of Emotion was significant (F3,105 = 6.56, P = 0.002), i.e. participants responded more slowly to shapes presented after angry or neutral faces than to shapes presented after happy or no face (P ≤ 0.05).
fMRI data
Whole-brain analysis
There were no significant findings from the Diagnosis × Emotion × Awareness interaction. Given our interest in between-group differences, we next examined the two-way interactions of Diagnosis × Emotion and Diagnosis × Awareness.
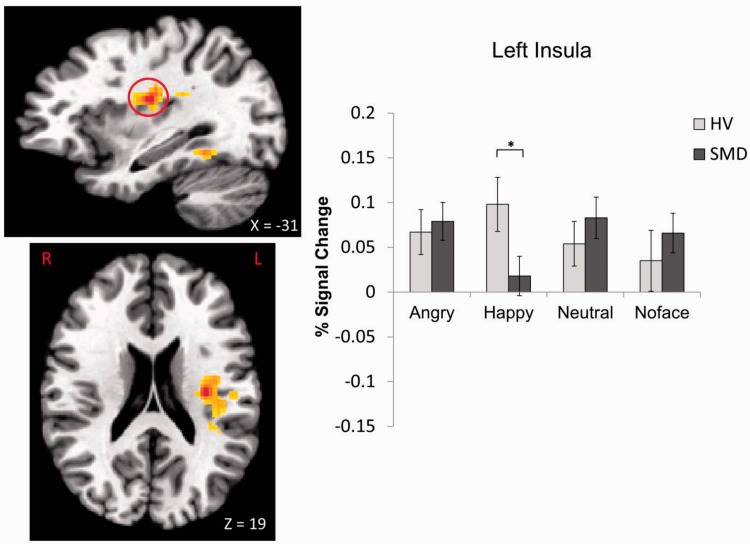
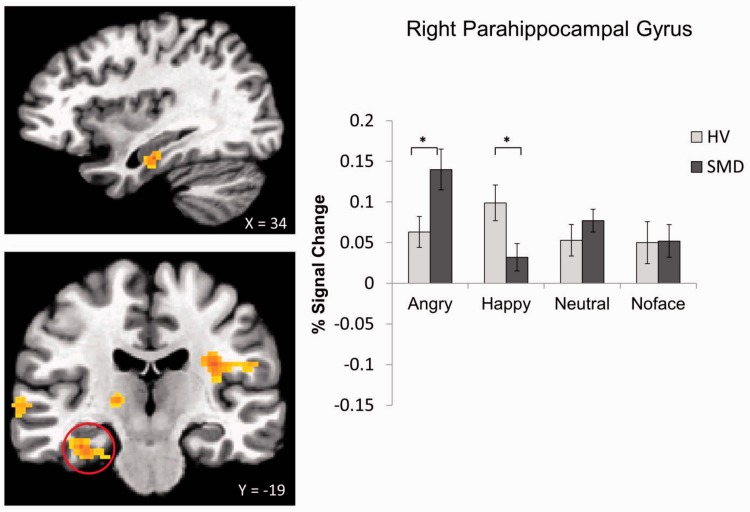
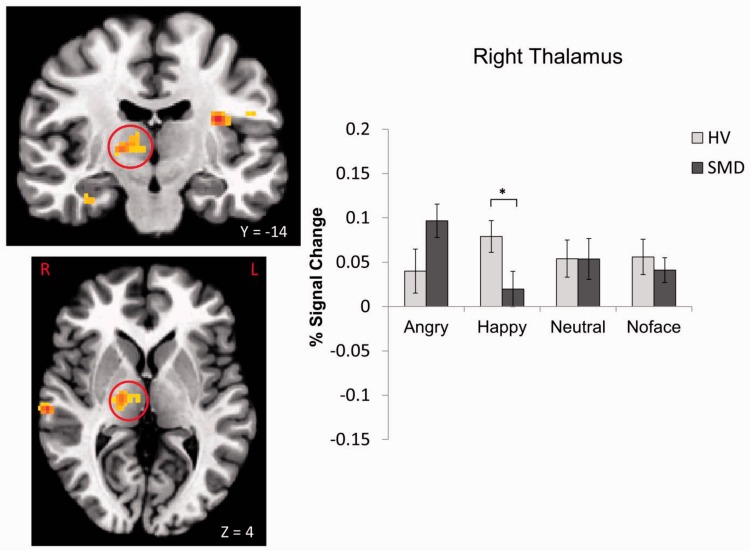
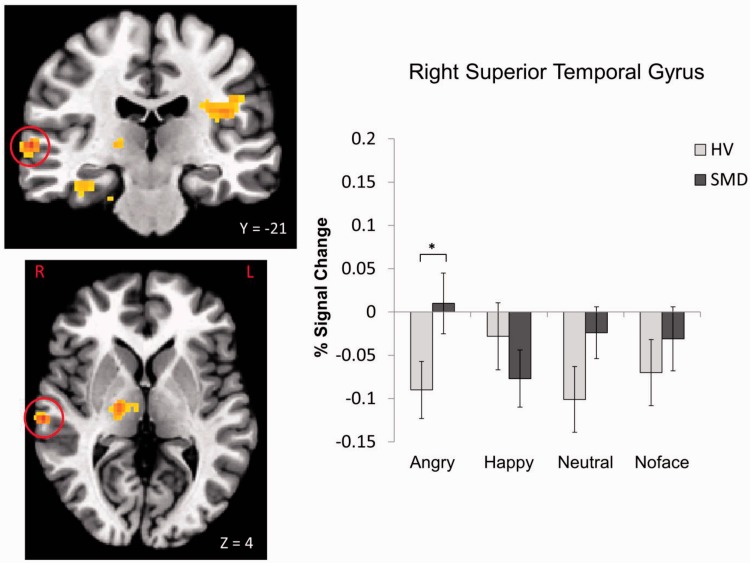
A Diagnosis × Emotion interaction was found in 10 regions, including the left insula, bilateral culmen and parahippocampal gyrus (PHG), left declive, right thalamus, left cerebellar lingual, right STG and right cingulate gyrus (Table 2, Figures 2–5; see Supplementary Figures S1–S6). These were driven primarily by SMD youth showing hypoactivation during processing of happy faces (Figures 2–4) and hyperactivation during processing of angry faces (Figures 3 and 5) compared with HV. There were also within-group differences across emotion types within SMD patients in the insula, PHG, thalamus and STG (P ≤ 0.02); within-group differences in the HV group emerged in the insula and STG (P ≤ 0.03). In general, SMD youth exhibited the highest activation when viewing angry faces and the lowest activation when viewing happy faces compared with other faces, whereas HV youth exhibited the highest activation when viewing happy faces relative to other faces.
Table 2.
Significant Diagnosis × Emotion interaction
| Area of activation |
Brodmann area (BA) | Side | Cluster sizea | Talairach coordinatesb |
Analysisc |
|||
|---|---|---|---|---|---|---|---|---|
| x | y | z | F (3, 33) | P | ||||
| Insula | BA 13 | Left | 187 | −31 | −16 | 19 | 8.18 | <0.001 |
| Culmen | Left | 87 | −24 | −36 | −21 | 8.47 | <0.001 | |
| Culmen/PHGs | BA 37 | Left | 60 | −26 | −44 | −11 | 7.65 | <0.001 |
| PHG | Right | 59 | 34 | −19 | −19 | 9.27 | <0.001 | |
| Declive | Left | 52 | −19 | −76 | −19 | 8.63 | <0.001 | |
| Thalamus | Right | 42 | 16 | −14 | 4 | 6.70 | 0.001 | |
| Cerebellar lingual | Left | 37 | −4 | −44 | −11 | 7.26 | <0.001 | |
| STG | BA 21 | Right | 34 | 61 | −21 | 4 | 7.14 | <0.001 |
| Culmen | Right | 33 | 9 | −26 | −21 | 7.42 | <0.001 | |
| Cingulate gyrus | BA 31 | Right | 26 | 14 | −34 | 29 | 7.33 | <0.001 |
aCluster size was determined using a significance threshold of uncorrected P ≤ 0.005 and k ≥ 20.
bCoordinates refer to the voxel with maximum signal intensity.
cStatistics refer to the analysis of the extracted clusters in SPSS.
Fig. 2.
Diagnosis × Emotion interaction in the left insula. *P < 0.05.
Fig. 3.
Diagnosis × Emotion interaction in the right PHG. *P < 0.05.
Fig. 4.
Diagnosis × Emotion interaction in the right thalamus. *P < 0.05.
Fig. 5.
Diagnosis × Emotion interaction in the right STG. *P < 0.05.
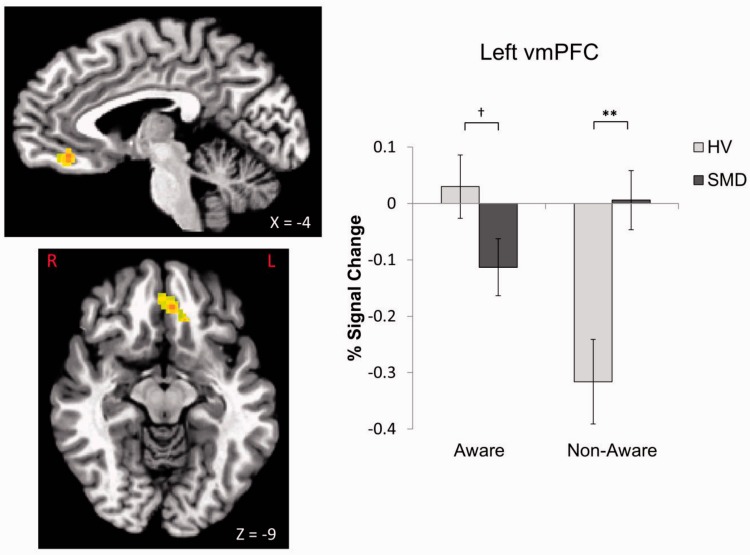
A Diagnosis × Awareness interaction was found in the left ventromedial prefrontal cortex (vmPFC; x = −4, y = 36, z = −9, P = 0.005, k = 65; Figure 6). Relative to HV, youth with SMD exhibited hypoactivation during aware condition at a trend level (t = −1.85, P = 0.07) and hyperactivation during non-aware condition (t = 3.40, P = 0.002; Figure 6). There was a within-group HV difference, with participants showing less vmPFC activation in the non-aware than aware condition (t = 5.04, P < 0.001).
Fig. 6.
Diagnosis × Awareness interaction in the left vmPFC. F1,35 = 18.26, P < 0.001 for Diagnosis × Awareness interaction. †P = 0.07, **P < 0.01.
ROI analysis
In the left amygdala, there were no three-way or two-way interactions or main effects of Diagnosis and Awareness. However, the main effect of Emotion was significant (F3,105 = 5.29, P = 0.003). Angry, happy and neutral faces activated the left amygdala more than ‘no face’ stimuli (P values ≤ 0.02). Similarly, in the right amygdala, only the main effect of Emotion was significant (F3,105 = 5.72, P = 0.002). Angry and happy (and neutral at a trend level, P = 0.075) faces activated the right amygdala more than ‘no face’ stimuli (P ≤ 0.001); angry faces also activated this region more than happy faces (P = 0.02) and neutral faces (at a trend, P = 0.056).
Post-hoc analyses
Irritability symptoms
We conducted bivariate correlation analyses to assess the associations between parent- or child-reported irritability and brain activation. Results showed that parent- or child-reported irritability was negatively correlated with activation in the right thalamus (r = −0.37, P = 0.035) during processing of happy faces and positively correlated with activation in the left vmPFC activation during non-aware condition (r = 0.46, P = 0.007). These findings are largely consistent with results from the categorical findings indicating SMD hypoactivation to happy faces and SMD hyperactivation during non-aware condition.
Effects of covariates
For the whole-brain analyses, the significant two-way interactions of Diagnosis × Emotion in the 10 regions and Diagnosis × Awareness in the left vmPFC all remained significant when covarying IQ and gender (P values ≤ 0.001). For the ROI analyses, the main effect of emotion in the bilateral amygdala also remained significant when covarying IQ and gender (P values ≤ 0.002). There was no association between observed brain activation (e.g. insula, vmPFC and amygdala) and number of medications, number of comorbidities, functional impairment or anxiety symptoms in SMD patients (P values > 0.05). Because of the small number of SMD without ADHD (n = 4), we were not able to compare brain activation between SMD with and without ADHD. Instead, we conducted analyses comparing SMD with ADHD (13 out of 17 SMD) to all HV. All the significant findings in the full sample remained.
Post-tasks
Data from the two post-tasks indicate that participants were unaware of the emotional face prime. On both the post-task gender and emotion identification tasks, accuracy was no better than chance level for any emotion (i.e. 50% for gender identification and 33.3% for emotion identification; P values ≥ 0.16).
Discussion
Using an affective priming task, we compared the neural correlates of aware vs non-aware processing of angry and happy faces in youth with SMD and HV. Given the increasing emphasis on reproducibility of imaging results and the concerns regarding Type I error in fMRI research (Lieberman and Cunningham, 2009; Poldrack and Mumford, 2009; Vul et al., 2009), this study also aimed to replicate a prior study on SMD (Thomas et al., 2014) using a similar paradigm. We found emotion-specific responses in several regions implicated in emotion processing, including the insula, PHG, thalamus and STG. Specifically, in these regions, we found hyperactivation in SMD compared with HV when processing angry faces but hypoactivation when processing happy faces. This is consistent with threat bias and reduced sensitivity to positive affect, linked, respectively, to aggressive and depressive symptoms often observed in irritable youth (Copeland et al., 2013; Leibenluft and Stoddard, 2013). Importantly, STG hyperactivation to angry faces replicated previous findings in an independent sample of youth with SMD (Thomas et al., 2014). In addition, relative to HV, SMD youth exhibited greater vmPFC activation during processing of masked faces, regardless of emotion. Findings with irritability as a dimensional measure, rather than diagnostic status, are largely consistent with these categorical findings.
Youth with SMD, relative to HV, appeared to be hypersensitive to angry faces (masked or unmasked), manifest as increased activation in the PHG and STG. This is true whether the angry faces were perceived consciously or not. This hypersensitivity to angry faces has potentially important implications for SMD and DMDD, as severely irritable youth often show reactive aggression which may arise from possessing a hostile attribution bias and/or attention bias toward threat (e.g. angry faces). Indeed, previous behavioral research has demonstrated that youth with SMD exhibit an attention bias toward angry faces (Hommer et al., 2014). Similarly, adolescents with conduct disorder show deficits in the recognition of angry faces (Fairchild et al., 2009), and changing the perception of face emotions from anger toward happiness may reduce aggressive behavior (Penton-Voak et al., 2013). However, attention bias toward threat is not specific to irritability or disruptive behavior, as it is one of the most replicated pathophysiological findings in anxiety disorders (Bar-Haim et al., 2007). Heightened activation in the PHG and/or STG in response to angry faces has been reported previously in youth with SMD (Thomas et al., 2014), generalized social phobia (Stein et al., 2002) and pediatric bipolar disorder during euthymia (Pavuluri et al., 2007). Although our post-hoc analyses suggested that the brain activation in these regions was not driven by anxiety symptoms, whether the neural sensitivity to threat, e.g. angry faces, is a shared neural mechanism across these mood and anxiety disorders remains to be empirically tested.
In contrast, when viewing happy faces (masked or unmasked), youth with SMD, relative to HV, showed decreased activation in areas such as the insula and thalamus that are highly connected to the amygdala. Insular cortex plays a critical role in mapping visceral states that are associated with emotional experience, giving rise to conscious feelings and interoceptive awareness (Craig, 2003; Singer et al., 2009). Thalamus, on the other hand, is a key area for relaying sensory information and regulation of arousal (Schiff, 2008; Suslow et al., 2010b). Both areas are important for emotional processing and regulation. Indeed, reduced thalamus activation to happy faces may down-regulate the flow of information to the cortex and make the happy faces less salient, thus suggesting a decreased sensitivity to positive stimuli in SMD youth. Given the large body of literature supporting a diminished sensitivity to positive affect and stimuli in depression (for a review, see Forbes and Dahl, 2005) as well as the longitudinal link between chronic irritability and depressive disorders (Brotman et al., 2006; Stringaris et al., 2009), the extent to which hypoactivation in temporal and limbic regions represents a shared or distinct neural marker in SMD and depressive disorders needs to be determined. This finding in SMD, on the other hand, contrasts with the literature on bipolar disorder during mixed states (euthymic, hypo/manic, depressed or mixed) suggesting increased responsiveness to happy faces (Phillips and Vieta, 2007; Passarotti et al., 2011) and thus provides additional evidence to support that severe, chronic irritability is not a developmental presentation of bipolar disorder (Leibenluft, 2011).
In addition to emotion-specific findings, there were non-emotion-specific group differences in activation in the non-aware condition. SMD youth exhibited greater vmPFC activation compared with HV during the non-aware condition. Further, HV, but not SMD, youth showed differential vmPFC activation between the two awareness conditions. These findings suggest that in youth with SMD, vmPFC failed to go ‘offline’ even when the stimulus was presented so briefly that it was difficult to perceive. vmPFC plays a key role in generating affective meaning (Roy et al., 2012), regulating limbic activation in response to emotional stimuli (Etkin et al., 2011), and flexibly updating signal value (Viviani, 2014). Thus, vmPFC dysfunction may mediate over-reactivity to face emotions and face-like stimuli. Moreover, aberrant vmPFC activity may contribute to deficits in ‘context-sensitive regulation’ (Ochsner, 2008) often observed in SMD. Interestingly, our findings, although without emotion specificity, share similarity to a previous study in which greater activation in the orbitofrontal cortex was found in adults with depression than in controls during masked sad vs neutral faces (Victor et al., 2012). Such similarity is noteworthy given the longitudinal associations between severe, chronic irritability and major depressive disorders (Brotman et al., 2006; Stringaris et al., 2009).
Unlike previous research on unmasked face processing in youth with SMD (Brotman et al., 2010; Thomas et al., 2012, 2013), this study did not find between-group differences in amygdala activation. It should be noted that in a similar paradigm, Thomas et al. (2014) also did not find amygdala dysfunction in SMD. However, there was a significant main effect of emotion in this study, i.e. emotional faces activated the amygdala more than did no face stimuli, suggesting the effectiveness of our masked and unmasked faces in eliciting amygdala responses. Nonetheless, this null finding may be attributable to several reasons, including Type II error and the discrepancy in paradigms across studies. For example, in previous studies where youth with SMD differed from HV in amygdala activation (Brotman et al., 2010; Thomas et al., 2012, 2013), face emotions were consciously processed and displayed for a longer duration (>2500 ms) than in the present study (17 ms for masked faces and 187 ms for unmasked faces). Also, in prior studies, participants were asked to explicitly label emotions or implicitly process emotions rather than indicate how much they liked an abstract shape. Thus, the involvement of amygdala in masked and unmasked face processing in irritable youth remains to be determined pending future work with a larger sample.
Our findings in youth with SMD are relevant and likely to inform future research on DMDD; both SMD and DMDD are characterized by severe irritability and temper outbursts that are developmentally inappropriate and out of proportion to the situation. As described earlier, all our SMD youth in this sample, indeed, met criteria for DMDD. Of note, SMD criteria also require symptoms of hyperarousal (e.g. distractibility, racing thoughts or flight of ideas and intrusiveness), whereas DMDD criteria do not. As a result, our SMD sample is enriched for hyperarousal symptoms, and ADHD may be over-represented compared with studies that recruit youth with DMDD rather than SMD. Nonetheless, given the frequent association between ADHD and irritability (Shaw et al., 2014), we anticipate that many youth with DMDD will also meet criteria for ADHD, even though that is not required for the diagnosis. Thus, while it is unclear whether our finding would generalize to youth with DMDD without hyperarousal symptoms or ADHD, heightened sensitivity toward negative emotions and diminished sensitivity toward positive emotions may be a hallmark pathophysiological feature of irritability.
Several limitations of this study merit comment. First, most SMD youth (>65%) had co-occurring diagnoses (e.g. ADHD and anxiety disorders) and were medicated. Our sample size is not large enough to allow for comparisons of the neural activation between SMD youth with vs without comorbidities or medicated vs unmedicated. However, post-hoc analyses indicate no significant association between brain activation and number of medications, number of comorbidities or anxiety symptoms in SMD youth. Also, fMRI findings remain largely the same when restricting analyses to SMD with ADHD and all HV. Nonetheless, future work with a larger sample is needed to rule out the contribution of medications and comorbidities to our findings and to examine symptoms dimensionally across groups (Insel et al., 2010). Moreover, we did not find significant three-way interaction of Diagnosis × Emotion × Awareness. Whether this is due to a lack of power or true null finding should be clarified in future research, although this negative finding is also reported in a previous study with a similar paradigm (Thomas et al., 2014).
Despite these limitations, our findings partially replicate the study by Thomas et al. (2014) and suggest that youth with SMD may show emotion-specific neural dysfunction in emotion processing areas (e.g. insula, PHG, STG and thalamus) across awareness level. Hyper-sensitivity toward negative emotions (e.g. angry faces) and hypo-sensitivity toward positive emotions (e.g. happy faces) may be important in the etiology and maintenance of irritable, aggressive and depressive symptoms in SMD. In addition, youth with SMD showed vmPFC dysfunction during rapid, automatic processing of face stimuli that were presented below awareness level. If replicated, our findings may have important implications for the treatment of SMD and DMDD. For instance, activation in the emotion processing areas such as insula, PHG, may be used as biomarkers to monitor treatment response. Further, given that irritability is a core feature of SMD and DMDD and a cross-cutting symptom in many pediatric disorders, future research examining the neural correlates of face processing using categorical (i.e. diagnosis) and dimensional (i.e. irritability) approaches would be highly informative.
Supplementary Material
Acknowledgements
We thank the staff of the Emotion and Development Branch at the NIMH and the patients and their parents for their participation in this study.
Funding
This research was supported by the Intramural Research Program of the National Institute of Mental Health (NIMH), National Institutes of Health (NIH; ZIAMH002786) and it was conducted under NIH Clinical Study Protocol 02-M-0021 (ClinicalTrials.gov ID: NCT00025935).
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th edn Washington, DC: American Psychiatric Association. [Google Scholar]
- Armony J.L., Corbo V., Clement M.H., Brunet A. (2005). Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. American Journal of Psychiatry, 162, 1961–3. [DOI] [PubMed] [Google Scholar]
- Barch D.M., Yarkoni T. (2013). Introduction to the special issue on reliability and replication in cognitive and affective neuroscience research. Cognitive Affective and Behavioral Neuroscience , 13, 687–9. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Lamy D., Pergamin L., Bakermans-Kranenburg M.J., van I.M.H. (2007). Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin, 133, 1–24. [DOI] [PubMed] [Google Scholar]
- Bennett C.M., Miller M.B. (2010). How reliable are the results from functional magnetic resonance imaging? Annals of the New York Academy of Sciences , 1191, 133–55. [DOI] [PubMed] [Google Scholar]
- Brotman M.A., Schmajuk M., Rich B.A., et al. (2006). Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biological Psychiatry, 60, 991–7. [DOI] [PubMed] [Google Scholar]
- Brotman M.A., Rich B.A., Guyer A.E., et al. (2010). Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. American Journal of Psychiatry, 167, 61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R.A., Kemp A.H., Felmingham K.L., et al. (2008). Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Human Brain Mapping, 29, 517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland W.E., Angold A., Costello E.J., Egger H. (2013). Prevalence, comorbidity, and correlates of DSM-5 proposed disruptive mood dysregulation disorder. American Journal of Psychiatry, 170, 173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J.M., Reinke K.S., Habib R. (2009). A left amygdala mediated network for rapid orienting to masked fearful faces. Neuropsychologia, 47, 1386–9. [DOI] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–73. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2003). Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology, 13, 500–5. [DOI] [PubMed] [Google Scholar]
- Dannlowski U., Ohrmann P., Bauer J., et al. (2007a). Amygdala reactivity to masked negative faces is associated with automatic judgmental bias in major depression: a 3 T fMRI study. Journal of Psychiatry and Neuroscience, 32, 423–9. [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U., Ohrmann P., Bauer J., Kugel H., Arolt V., Heindel W., Suslow T. (2007b). Amygdala reactivity predicts automatic negative evaluations for facial emotions. Psychiatry Research, 154, 13–20. [DOI] [PubMed] [Google Scholar]
- Duan X., Dai Q., Gong Q., Chen H. (2010). Neural mechanism of unconscious perception of surprised facial expression. Neuroimage, 52, 401–7. [DOI] [PubMed] [Google Scholar]
- Esteves F., Ohman A. (1993). Masking the face: recognition of emotional facial expressions as a function of the parameters of backward masking. Scandinavian Journal of Psychology, 34, 1–18. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Science, 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G., Van Goozen S.H.M., Calder A.J., Stollery S.J., Goodyer I.M. (2009). Deficits in facial expression recognition in male adolescents with early-onset or adolesence-onset conduct disorder. Journal of Child Psychology and Psychiatry and Allied Disciplines , 50, 627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E.E., Dahl R.E. (2005). Neural systems of positive affect: relevance to understanding child and adolescent depression? Development and Psychopathology, 17, 827–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., McClure E.B., Adler A.D., et al. (2007). Specificity of facial expression labeling deficits in childhood psychopathology. Journal of Child Psychology and Psychiatry and Allied Disciplines, 48, 863–71. [DOI] [PubMed] [Google Scholar]
- Hommer R.E., Meyer A., Stoddard J., et al. (2014). Attention bias to threat faces in severe mood dysregulation. Depression and Anxiety, 31, 559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M., et al. (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry, 167, 748–51. [DOI] [PubMed] [Google Scholar]
- Ioannidis J.P.A., Munafò M.R., Fusar-Poli P., Nosek B.A., David S.P. (2014). Publication and other reporting biases in cognitive sciences: detection, prevalence, and prevention. Trends in Cognitive Science , 18, 235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., et al. (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 980–8. [DOI] [PubMed] [Google Scholar]
- Killgore W.D., Yurgelun-Todd D.A. (2004). Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. Neuroimage, 21, 1215–23. [DOI] [PubMed] [Google Scholar]
- Killgore W.D., Yurgelun-Todd D.A. (2007). Unconscious processing of facial affect in children and adolescents. Social Neuroscience, 2, 28–47. [DOI] [PubMed] [Google Scholar]
- Killgore W.D., Britton J.C., Price L.M., Gold A.L., Deckersbach T., Rauch S.L. (2011). Neural correlates of anxiety sensitivity during masked presentation of affective faces. Depression and Anxiety, 28, 243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore W.D., Britton J.C., Schwab Z.J., et al. (2014). Cortico-limbic responses to masked affective faces across PTSD, panic disorder, and specific phobia. Depression and Anxiety, 31, 150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugel H., Eichmann M., Dannlowski U., et al. (2008). Alexithymic features and automatic amygdala reactivity to facial emotion. Neuroscience Letters, 435, 40–4. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. (1996). The Emotional Brain: The Mysterious Underpinnings of Emotional Life. New York: Simon and Schuster. [Google Scholar]
- Leibenluft E. (2011). Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. American Journal of Psychiatry, 168, 129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E., Stoddard J. (2013). The developmental psychopathology of irritability. Development and Psychopathology, 25, 1473–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E., Blair R.J., Charney D.S., Pine D.S. (2003). Irritability in pediatric mania and other childhood psychopathology. Annals of the New York Academy of Sciences, 1008, 201–18. [DOI] [PubMed] [Google Scholar]
- Lichev V., Sacher J., Ihme K., et al. (2015). Automatic emotion processing as a function of trait emotional awareness: an fMRI study. Social Cognitive and Affective Neuroscience, 10, 680–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. (2009). Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience, 4, 423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C.S., Telzer E.H., Mogg K., et al. (2008). Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry, 65, 568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.S., Ohman A., Dolan R.J. (1998). Conscious and unconscious emotional learning in the human amygdala. Nature, 393, 467–70. [DOI] [PubMed] [Google Scholar]
- Nomura M., Ohira H., Haneda K., Iidaka T., Sadato N., Okada T., Yonekura Y. (2004). Functional association of the amygdala and ventral prefrontal cortex during cognitive evaluation of facial expressions primed by masked angry faces: an event-related fMRI study. Neuroimage, 21, 352–63. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N. (2008). The social-emotional processing stream: five core constructs and their translational potential for schizophrenia and beyond. Biological Psychiatry, 64, 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti A.M., Sweeney J.A., Pavuluri M.N. (2011). Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology (Berlin), 216, 485–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri M.N., O'Connor M.M., Harral E., Sweeney J.A. (2007). Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biological Psychiatry, 62, 158–67. [DOI] [PubMed] [Google Scholar]
- Penton-Voak I.S., Thomas J., Gage S.H., McMurran M., McDonald S., Munafò M.R. (2013). Increasing recognition of happiness in ambiguous facial expressions reduces anger and aggressive behavior. Psychological Science , 24, 688–97. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Vieta E. (2007). Identifying functional neuroimaging biomarkers of bipolar disorder: toward DSM-V. Schizophrenia Bulletin, 33, 893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles A., Aglan A., Collishaw S., Messer J., Rutter M., Maughan B. (2010). Predictors of suicidality across the life span: the Isle of Wight study. Psychological Medicine , 40, 1453–66. [DOI] [PubMed] [Google Scholar]
- Pine D.S., Grun J., Zarahn E., et al. (2001). Cortical brain regions engaged by masked emotional faces in adolescents and adults: An fMRI study. Emotion, 1, 137–47. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A., Mumford J.A. (2009). Independence in ROI analysis: where is the voodoo? Social Cognitive and Affective Neuroscience, 4, 208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski E.O., Grossman J.A., Buchsbaum Y., Banegas M., Freeman L., Gibbons R. (1984). Preliminary studies of the reliability and validity of the children's depression rating scale. Journal of the American Academy of Child Psychiatry, 23, 191–7. [DOI] [PubMed] [Google Scholar]
- Rauch S.L., Whalen P.J., Shin L.M., et al. (2000). Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry, 47, 769–76. [DOI] [PubMed] [Google Scholar]
- Research Units on Pediatric Psychopharmacology Anxiety Study Group. (2002). The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. Journal of the American Academy of Child and Adolescent Psychiatry, 41, 1061–9. [DOI] [PubMed] [Google Scholar]
- Rich B.A., Grimley M.E., Schmajuk M., Blair K.S., Blair R.J., Leibenluft E. (2008). Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Development and Psychopathology, 20, 529–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M., Shohamy D., Wager T.D. (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Science, 16, 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A.K., Lopes V., Klein R.G. (2014). Disruptive mood dysregulation disorder: a new diagnostic approach to chronic irritability in youth. American Journal of Psychiatry, 171, 918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini E., Della Penna S., Franciotti R., et al. (2009). Brain structures activated by overt and covert emotional visual stimuli. Brain Research Bulletin, 79, 258–64. [DOI] [PubMed] [Google Scholar]
- Schiff N.D. (2008). Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Annals of the New York Academy of Sciences, 1129, 105–18. [DOI] [PubMed] [Google Scholar]
- Shaffer D., Gould M.S., Brasic J., Ambrosini P., Fisher P., Bird H., Aluwahlia S. (1983). A children's global assessment scale (CGAS). Archives of General Psychiatry, 40, 1228–31. [DOI] [PubMed] [Google Scholar]
- Shaw P., Stringaris A., Nigg J., Leibenluft E. (2014). Emotion dysregulation in attention deficit hyperactivity disorder. American Journal of Psychiatry , 171, 276–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Barch D.M., Donnelly J.M., Ollinger J.M., Snyder A.Z., Mintun M.A. (2001). Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry, 50, 651–8. [DOI] [PubMed] [Google Scholar]
- Singer T., Critchley H.D., Preuschoff K. (2009). A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Science, 13, 334–40. [DOI] [PubMed] [Google Scholar]
- Stein M.B., Goldin P.R., Sareen J., Zorrilla L.T., Brown G.G. (2002). Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry, 59, 1027–34. [DOI] [PubMed] [Google Scholar]
- Stringaris A., Goodman R. (2009). Mood lability and psychopathology in youth. Psychological Medicine , 39, 1237–45. [DOI] [PubMed] [Google Scholar]
- Stringaris A., Cohen P., Pine D.S., Leibenluft E. (2009). Adult outcomes of youth irritability: a 20-year prospective community-based study. American Journal of Psychiatry, 166, 1048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A., Goodman R., Ferdinando S., et al. (2012). The Affective Reactivity Index: a concise irritability scale for clinical and research settings. Journal of Child Psychology and Psychiatry and Allied Disciplines , 53, 1109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslow T., Kugel H., Rauch A.V., et al. (2009). Attachment avoidance modulates neural response to masked facial emotion. Human Brain Mapping, 30, 3553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslow T., Konrad C., Kugel H., et al. (2010a). Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biological Psychiatry, 67, 155–60. [DOI] [PubMed] [Google Scholar]
- Suslow T., Kugel H., Reber H., et al. (2010b). Automatic brain response to facial emotion as a function of implicitly and explicitly measured extraversion. Neuroscience, 167, 111–23. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. (1988). Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme. [Google Scholar]
- Thomas L.A., Brotman M.A., Muhrer E.J., et al. (2012). Parametric modulation of neural activity by emotion in youth with bipolar disorder, youth with severe mood dysregulation, and healthy volunteers. Archives of General Psychiatry, 69, 1257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L.A., Kim P., Bones B.L., et al. (2013). Elevated amygdala responses to emotional faces in youths with chronic irritability or bipolar disorder. Neuroimage Clinical, 2, 637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L.A., Brotman M.A., Bones B.L., Chen G., Rosen B.H., Pine D.S., Leibenluft E. (2014). Neural circuitry of masked emotional face processing in youth with bipolar disorder, severe mood dysregulation, and healthy volunteers. Developmental Cognitive Neuroscience, 8, 110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor T.A., Furey M.L., Fromm S.J., Ohman A., Drevets W.C. (2010). Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Archives of General Psychiatry, 67, 1128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor T.A., Furey M.L., Fromm S.J., Bellgowan P.S., Ohman A., Drevets W.C. (2012). The extended functional neuroanatomy of emotional processing biases for masked faces in major depressive disorder. PLoS One, 7, e46439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani R. (2014). Neural correlates of emotion regulation in the ventral prefrontal cortex and the encoding of subjective value and economic utility. Frontiers in Psychiatry, 5, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E., Harris C.R., Winkielman P., Pashler H. (2009). Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspectives on Psychological Science, 4, 274–90. [DOI] [PubMed] [Google Scholar]
- Whalen P.J., Rauch S.L., Etcoff N.L., McInerney S.C., Lee M.B., Jenike M.A. (1998). Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience, 18, 411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.