Abstract
Frontal asymmetry in alpha oscillations is assumed to be associated with psychopathology and individual differences in emotional responding. Brain-activity-based feedback is a promising tool for the modulation of cortical activity. Here, we validated a neurofeedback protocol designed to change relative frontal asymmetry based on individual alpha peak frequencies, including real-time average referencing and eye-correction. Participants (N = 60) were randomly assigned to a right, left or placebo neurofeedback group. Results show a difference in trainability between groups, with a linear change in frontal alpha asymmetry over time for the right neurofeedback group during rest. Moreover, the asymmetry changes in the right group were frequency and location specific, even though trainability did not persist at 1 week and 1 month follow-ups. On the behavioral level, subjective stress on the second test day was reduced in the left and placebo neurofeedback groups, but not in the right neurofeedback group. We found individual differences in trainability that were dependent on training group, with participants in the right neurofeedback group being more likely to change their frontal asymmetry in the desired direction. Individual differences in trainability were also reflected in the ability to change frontal asymmetry during the feedback.
Keywords: frontal EEG asymmetry, randomized placebo control design, trainability, specificity, interpretability
Introduction
Frontal asymmetry has been studied extensively in individual differences research on emotional and motivational processes. It refers to the average difference in brain activity between the left and right frontal areas, measured as hemispheric differences in alpha power in electroencephalography (EEG) recordings across several minutes (Harmon-Jones et al., 2010). Activity in the left-frontal hemisphere has been linked to an approach system that is activated when an individual is moving towards goals or experiences positive emotions. Conversely, a right lateralised withdrawal system is involved in negative affect or the motivation to move away from potentially dangerous situations or stimuli (Tomarken et al., 1992; Davidson, 1998; Coan et al., 2006).
Frontal asymmetry has important implications for mental health and stress adaptation (Sullivan and Gratton, 2002; Davidson, 2004). For instance, greater left-sided frontal activity at rest was found to predict greater emotional flexibility (Papousek et al., 2012), better emotion regulation (Jackson et al., 2003), more positive and decreased negative affect (Tomarken et al., 1992), as well as a smaller task-induced cortisol increase (Quaedflieg et al., 2015). Moreover, extreme right frontal asymmetry has been associated with affective disorders such as depression (Thibodeau et al., 2006) and social anxiety disorder (SAD; Moscovitch et al., 2011). In addition, increasing left-sided activity or decreasing right-sided activity with repetitive transcranial magnetic stimulation (rTMS) resulted in improvements in depression (for review see Loo and Mitchell, 2005) and anxiety (for review see Pallanti and Bernardi, 2009) symptoms.
EEG neurofeedback is another method used to modulate cortical activity. The individual is given feedback on his or her current brain activity, based on real-time analysis of EEG signals. In order to induce a specific pattern of brain activity, the individual receives ‘rewarding’ feedback whenever the EEG changes in the preferred direction. Using this positive reinforcement, the desired brain activity is learned through operant conditioning (Heinrich et al., 2007; Harmon-Jones et al., 2010). There has been a recent surge in the use of neurofeedback for the treatment of several kinds of disorders and preliminary studies suggest its clinical efficacy (see for example ADHD: Arns et al., 2014 but see van Dongen-Boomsma et al., 2013; Depression: Choi et al., 2011; Peeters et al., 2014). Even though neurofeedback has often been applied in clinical research, the validation of neurofeedback as a treatment protocol lags behind (Lofthouse et al., 2012; Gruzelier, 2014). To assess the development of self-regulation, three criteria have been proposed (Zoefel et al., 2011). First, the trained frequency needs to change significantly (i.e. trainability). If trainability is successful, the specificity in the trained frequency band and location (i.e. independence) as well as the stability over time has to be assessed. Finally, the behavioural effects of the training need to be determined (i.e. interpretability).
EEG neurofeedback has often been used in attempts at changing frontal asymmetry (i.e. F4-F3). Allen et al. (2001) investigated the effect of five frontal alpha asymmetry neurofeedback sessions on participants’ emotional responses to movies. Training healthy participants to display relative right-sided frontal activity was associated with less approach responses to happy movies. However, they did not investigate the independence or stability of the training. Independence of location of frontal alpha asymmetry neurofeedback training was assessed by Harmon-Jones et al. (2008), who trained participants for two days, and found a specific difference in the change in alpha asymmetry on frontal but not on parietal electrodes for the relative left frontal asymmetry group. Recently, Peeters et al. (2014) investigated the possibility of changing frontal asymmetry in a single neurofeedback session. They reported that it is feasible to change frontal asymmetry in both directions in healthy participants, and that this change is specific in terms of location. All in all, the effectiveness of neurofeedback trainings to change relative frontal asymmetry and mood state is in dire need of a more thorough empirical validation.
In light of these considerations, the present study aimed to further validate and explore the potential of frontal alpha asymmetry neurofeedback. In particular, we compared three frontal asymmetry protocols that were developed to increase relative right-sided frontal alpha asymmetry, to increase relative left-sided frontal alpha asymmetry, or to yield no effects on frontal alpha asymmetry (i.e. the placebo control group). Extending prior studies that examined the effect of neurofeedback on frontal asymmetry, we determined frontal asymmetry for each participant based on individual alpha peak frequency (IAF). The main advantage of this approach is that it controls for the large individual differences in alpha frequency (Klimesch et al., 1993; Doppelmayr et al., 1998) that may impair the trainability of frontal asymmetry based on conventional frequency bands. It was hypothesised that following six days of neurofeedback, participants would display the intended change in frontal asymmetry along with a change in current mood and task-induced subjective and neuroendocrine stress responses. Specifically, participants trained to shift relative frontal alpha power towards the right hemisphere were expected to show decreased negative affect and decreased subjective and neuroendocrine stress responses over time. The opposite pattern of findings was expected for the left group. No changes over time were hypothesized for the placebo group. Additionally, we explored whether gender differences in the effectiveness of neurofeedback training exist. Furthermore, for both the left and right group, it was expected that the frontal alpha asymmetry training would result in changes specifically in the (individual) alpha band and solely at frontal locations. In addition, we explored whether the asymmetry changes would persist up to 1 month following the final neurofeedback session. Finally, based on previous studies demonstrating large individual differences in the ability to learn how to regulate cortical activity (e.g. Hanslmayr et al., 2005; Zoefel et al., 2011; Kouijzer et al., 2013; Dekker et al., 2014), we classified and compared participants who responded well to the training to those who did not or did so to a lesser degree.
Materials and methods
Participants
The present experiment was part of a larger study that investigated the effect of neurofeedback on resilience (Quaedflieg et al., 2015). Right-handed healthy male (n = 30) and female (n = 30) undergraduates (mean age = 20.96 s.d. = 2.82; range: 18–31 years) were recruited via advertisements at Maastricht University. Participants were screened for eligibility using the following exclusion criteria: history of psychiatric, neurologic, cardiovascular or neuroendocrine diseases, heavy smoking (i.e. more than 15 cigarettes/day), medication use known to affect the autonomic nervous system (ANS) or hypothalamic-pituitary-adrenal (HPA) axis, drug use and body mass index (BMI) outside the normal range (i.e. 18–30 kg/m2). For women, the use of oral contraceptives served as an inclusion criterion to reduce variability in cortisol responses resulting from hormonal alterations (e.g. Kudielka et al., 2009). Test protocols were approved by the standing ethics committee of the Faculty of Psychology and Neuroscience, Maastricht University. All participants provided written informed consent and were given a minor incentive (course credits or money) in return for their participation.
EEG data acquisition
The electroencephalogram (EEG) was recorded using a BrainAmp amplifier and BrainVision Recorder software (BrainProducts, Germany) from 23 Ag/AgCl electrodes (F7, F3, Fz, F4, F8, FC3, FC4, T7, T8, C3, Cz, C4, CP3, CPz, CP4, P7, P3, Pz, P4, P8, O1, Oz, O2) positioned in an elastic cap according to the international 10-20 system, referenced to the left mastoid (A1). Signals at A2 were also recorded for re-referencing to computerized linked mastoids. Signals were sampled continuously at 100 Hz and band-pass filtered (0.01–30 Hz). An electrode at AFz served as ground. Two electrodes at the outer canthi of both eyes recorded horizontal eye movements and two electrodes above and below the left eye recorded vertical eye movements. Scalp-electrode impedance was kept below 5 kΩ to ensure high-quality EEG recordings and homologous scalp electrodes were within 1 kΩ of each other. Participants were shown the raw recording signals to demonstrate common artefacts that occur due to body and eye movements.
Before the start of the first neurofeedback session, the IAF was determined as the dominant frequency rhythm between 5 and 15 Hz at the posterior electrode (Pz) on 3 min of resting eyes closed data (Doppelmayr et al., 1998; Klimesch, 1999). The IAF bandwidth was defined as the IAF ± 0.20 × IAF. The same bandwidth was used for all six training sessions since IAF has been shown to be stable over time (van Boxtel et al., 2012). The frontal alpha asymmetry scores were determined in the IAF band.
Resting frontal asymmetry was measured twice during four minutes, at the beginning and the end of the test or training session, whereby participants focused on a black fixation cross on a grey background on the computer monitor.
Procedure
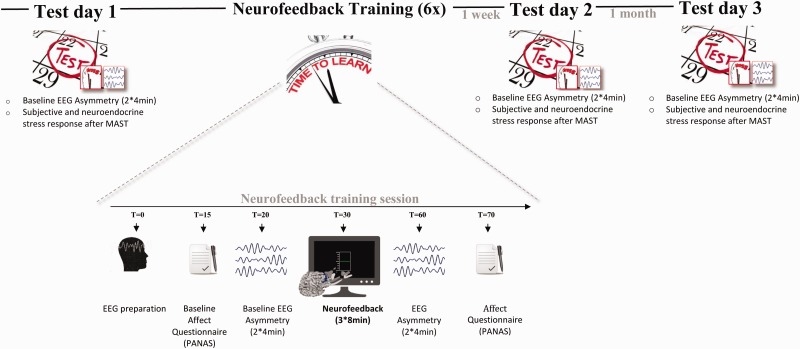
An overview of the experimental procedure is displayed in Figure 1. The experiment consisted of a baseline test day followed by six neurofeedback sessions and two follow-up test days, 1 week and 1 month later. The six neurofeedback sessions were distributed over the course of 2 weeks. All testing took place between 12:30 and 18:00 h to avoid morning fluctuations in the circadian rhythm of cortisol and time-of-day effects on frontal asymmetry (Velo et al., 2012). Participants were instructed to refrain from eating, exercising extensively, or drinking anything but non-sparkling water for 2 h prior to the experimental session. Upon arrival in the laboratory, a bogus saliva sample was taken to increase participants’ honesty in disclosing non-adherence to these instructions (cf. Quaedflieg et al., 2013). Participants were seated in front of a 22-inch widescreen monitor (Philips, the Netherlands) at approximately 56 cm viewing distance.
Fig. 1.
Overview of the study design and neurofeedback training sessions.
Test days
Each test day consisted of a baseline asymmetry measurement after which stress was induced using the Maastricht Acute Stress Test (MAST: Smeets et al., 2012). The MAST consists of a 5 min preparation phase in which the task is explained and a 10 min acute stress phase that includes alternating trials of immersing their hand into ice water (2°C) and counting backwards in steps of 17 starting at 2043 along with social-evaluative pressure (i.e. negative feedback and videotaping). Neuroendocrine and subjective stress responses were measured with synthetic Salivettes (Sarstedt, Etten-Leur, The Netherlands) and 100 mm Visual Analogue scales (VAS). Participants provided saliva samples 20 min after arrival in the lab (tbase), 5 min before (tpre-stress) the MAST and 5 times afterwards (t+0, t+10, t+30, t+40, t+55 min with reference to the end of the stressor). The Area under the curve with respect to increase (AUCi) from the pre-stress sample was calculated as a single measure of the total cortisol concentration in response to the MAST for each participant individually (cf. Pruessner et al., 2003).
Samples were stored at –20°C until cortisol levels were determined by a commercially available luminescence immune assay kit (IBL, Hamburg, Germany). Mean intra- and inter-assay coefficients of variation are typically less than 5%, and the lower and upper detection limits were 0.015 mg/dl (0.41 nmol/l) and 4.0 mg/dl (110.4 nmol/l), respectively.
Training days
Each neurofeedback session consisted of a baseline asymmetry measurement, three neurofeedback blocks and a post-neurofeedback asymmetry measurement (see Figure 1). Current negative affect was assessed using the state version of the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988). The PANAS consists of 20 items divided in two subscales that quantify current positive affect (PA) and negative affect (NA) using 5-point scales (anchors: 1 = very slightly or not at all; 5 = extremely). Higher scores on the NA scale are indicative of higher levels of experienced negative affect.
EEG Neurofeedback training
A double-blind placebo controlled design was applied in which subjects were randomly assigned to one of three frontal alpha asymmetry neurofeedback groups. The power of alpha oscillations is inversely related to brain activity (Pfurtscheller et al., 1996; Goldman et al., 2002; Laufs et al., 2003; Pizzagalli et al., 2005). Thus, a brain that is said to be relative asymmetric to the right has an alpha predominance in the left hemisphere. Participants in the left neurofeedback group received positive feedback if they increased relative right alpha power. Participants in the right neurofeedback group received positive feedback if they increased relative left alpha power. Participants in the placebo group were not fed back their own current asymmetry. Instead, the feedback received by the placebo group was based on neurofeedback training scores extracted from a pilot study with ten participants. The placebo feedback included randomized data from three left and three right neurofeedback sessions and was unique for each participant.
Each neurofeedback session consisted of three neurofeedback training blocks of 8 min. Real-time calculations were done by a filter written for BrainVision Recview (Brain Products, Germany) and included re-referencing to an average A1 and A2 reference, eye blink correction, epoching, transformation to the frequency domain, and asymmetry calculation. Online eye blink correction and re-referencing were performed since eye movement artifacts influence the EEG activity especially at the frontal sites and in the alpha band, and because computerized linked mastoids (i.e. average of A1 + A2) reference has a somewhat superior signal-to-noise ratio compared to Cz as a reference (Hagemann, 2004; Coan et al., 2006). Re-referencing and eye correction were performed with a linear derivation. The eye correction coefficients were determined in every neurofeedback training session using linear regression (Semlitsch et al., 1986) implemented in a plugin written for EEGLAB (Delorme and Makeig, 2004). To compute power density values, corrected data were divided in two sec epochs with 75% overlap and then transformed to the frequency domain using a fast-Fourier transformation (FFT; 100% Hanning window). Asymmetry scores were calculated every 0.5 s in the individual alpha frequency band as log-transformed alpha-power density values, ln (F4) – ln (F3). Positive alpha asymmetry scores indicate greater relative left than right frontal activity; negative alpha asymmetry scores indicate greater relative right than left frontal activity. In order to provide smooth feedback to the participant, the 10 last asymmetry values were included in a linear weighted (i.e. oldest data given a weight of 0.1 and newest a weight of 1) moving average.
The feedback stimulus was presented visually via a PC using Presentation (Neurobehavioral Systems), in the form of a boxplot-like meter. The start position of the line of the meter was always in the middle and represented the baseline asymmetry of the training day. The position of the line provided real-time feedback with regard to the baseline asymmetry and was above the middle if the current relative asymmetry was a shift in the desired direction compared to the baseline of the day. Moreover, the colour of the line provided feedback with regard to the asymmetry measured 0.5 s before and became green if the current asymmetry was a shift in the desired direction compared to the previous measurement. A numerical value that represented overall training performance, and which was continuously upgraded during the 8 min neurofeedback blocks, was displayed below the meter (see Peeters et al., 2014). The increase of the score represented the size of the shift in the asymmetry in the desired direction. It was explained to the participants that the line represented their brain activity at that moment and that this line would turn green if they would have the desired activity. They were instructed to try to earn as many points as they could by keeping the line of the meter green. It was made clear to participants that the total score was unrelated to the compensation that the participants would be receiving. Analyses showed that the average score that was attained, was the same for the left and right neurofeedback training group1.
EEG Offline analysis and statistical analysis
Offline analyses were performed with Vision Analyzer 2.0 (Brain Products, Germany). The offline derivation of frontal asymmetry scores was consistent with previous studies (e.g. Meyer et al., 2014; see for review Allen et al., 2004) and almost identical to the real-time data steps (see 2.4 EEG neurofeedback training). One step was added, namely epochs containing EEG changes exceeding ±75 µV were automatically omitted from averages. On average, 735 epochs of the rest measurements and 701 epochs of the neurofeedback blocks were artefact free. Data from three participants were excluded because less than 30% of artifact-free epochs were retained and data of one other participant were lost due to recording software failure. Thus, the final sample for the EEG analysis consisted of 56 participants (i.e. right group: 20, left group: 18, placebo: 18). Moreover, the subjective stress data of two participants were missing. The final sample for the behavioral analysis consisted of 54 participants (i.e. right group: 19, left group: 17, placebo: 18).
Statistical analyses were performed on z-transformed (i.e. using the mean of rest asymmetry on test day 1 and training 1 and its s.d.) frontal asymmetry scores with repeated measures ANOVAs reporting linear contrasts (Rosnow and Rosenthal, 1989). Follow-up simple effects analyses were performed using one-way ANOVAs. A P-value less than 0.05 was considered statistically significant and a P-value between 0.05 and 0.10 as a statistical trend towards significance. If analyses yielded significant or trend-level findings, ANOVAs were supplemented with Partial Eta Squared () values as a measure of effect size ( of 0.01 indicate small effects, of 0.06 medium effects, and of 0.14 large effects; Fritz et al., 2012).
Results
Trainability was assessed by investigating the effect of the neurofeedback protocol on relative frontal asymmetry in the alpha band during the neurofeedback blocks and at rest over the six training sessions. If trainability was successful, the specificity (i.e. frequency and location) and stability as well as the effect on the behavioural level of the neurofeedback training were assessed. Finally, individual differences in self-regulation were evaluated.
The effectiveness of frontal alpha asymmetry neurofeedback
Trainability
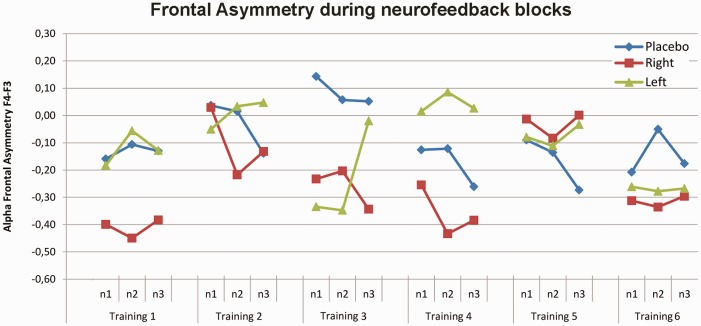
Figure 2 displays the time course of change in relative frontal alpha asymmetry (F4–F3) during the neurofeedback blocks over 6 neurofeedback training sessions. Trainability, defined as a change in alpha-band frontal asymmetry over training days was assessed first. The linear contrasts with training (1–6) and block (blocks 1–3) as linear-subject variables and group (left, right, control) and gender (male, female) as between-subject variables, revealed that training group did not differentially change the relative frontal asymmetry measured over the training days during the feedback (training × group interaction: F(2,50) = 0.32, P = 0.72). Moreover, training had also no effect on frontal asymmetry within sessions measured during the feedback (group × block interaction: F(2,50) = 1.35, P = 0.27; group main: F(2,50) = 0.49, P = 0.62) and was not different between males and females (training × group × gender interaction: F(2,50) = 0.48, P = 0.62).
Fig. 2.
Frontal Alpha Asymmetry (F4-F3) during the neurofeedback blocks over 6 neurofeedback training sessions. Training group did not differentially change the relative frontal asymmetry measured during feedback between day 1 and 6. Positive alpha asymmetry scores indicate greater relative left than right frontal activity, while negative alpha asymmetry scores indicate greater relative right than left frontal activity. Each neurofeedback session consisted of three neurofeedback blocks. Abbreviations: n1-3= neurofeedback training blocks.
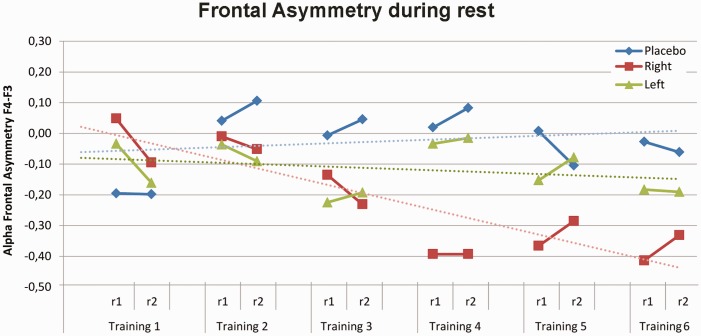
For the frontal asymmetry during rest, the linear contrasts with training (1–6) and measurement (pre, post) as within-subject variables and group (left, right, control) and gender (male, female) as between-subject variables revealed a training main effect (F(1,50) = 4.14, P = 0.05, = 0.08) and a trend-level training × group interaction (F(2,50) = 2.86, P = 0.07, = 0.10), without a gender difference (training × group × gender interaction: F(2,50) = 0.52, P = .60) or measurement (pre-post) effect (measurement main effect F(1,50) = 0.24, P = 0.63; training × group × measurement interaction: F(2,50) = 1.83, P = 0.18). Thus, follow-up analyses were performed across pre and post asymmetry measurements and across gender. The neurofeedback training changed the frontal asymmetry for the right group; the frontal asymmetry became relatively more right sided over training days (training main effect F(1,19) = 10,69 P = 0.004; = 0.36). However, for the left and control group there was no significant main effect of training (left: F(1,17) = 0.65, P = 0.43; control: F(1,17) = 0.23, P = 0.64). Figure 3 displays the time course of change in relative frontal alpha asymmetry (F4–F3) during the rest measurements before and after the neurofeedback blocks over six neurofeedback training sessions per group.
Fig. 3.
Frontal Alpha Asymmetry (F4-F3) during the rest measurements over 6 neurofeedback training sessions. Training group differentially changed the relative frontal asymmetry measured during rest between day 1 and 6 with a significant linear training effect for the right group (dashed red line). Positive alpha asymmetry scores indicate greater relative left than right frontal activity, while negative alpha asymmetry scores indicate greater relative right than left frontal activity. Abbreviations: r1 = asymmetry measurement before the neurofeedback training, r2 = asymmetry measurement after the neurofeedback training.
Independence
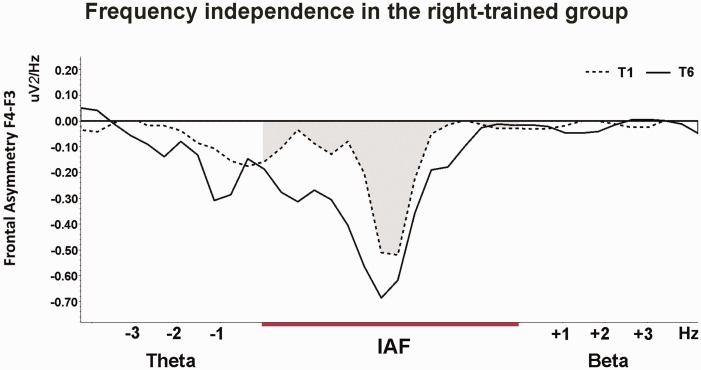
As the trainability analyses revealed that the trained frequency changed significantly in the right group, independence, stability, and interpretability, were subsequently assessed in the right group. The changes in relative frontal alpha asymmetry in the right group were compared to changes in asymmetry in other frequency bands or on other locations to assess the specificity of the frontal alpha asymmetry training. Both the theta and beta frequency bands were defined individually based on the IAF band. Similar to prior studies (e.g. Zoefel et al., 2011) theta was defined as the frequency ranging 3 till 1 Hz below the lower bound of the IAF band. Beta was defined as the band ranging 1–3 Hz above the upper bound of the IAF band. The 1 Hz range below and above IAF was excluded to avoid frequency smearing. Figure 4 displays the frequency spectra of training 1 and 6 for the beta, individual alpha frequency (IAF) and theta band and shows that the influence of the neurofeedback training on the spectra is most pronounced in the trained IAF band. This was corroborated by the linear contrasts with training (1–6) and measurement (pre, post) as within subject variables (training × frequency interaction: F(1,16) = 13.80, P = 0.002; = 0.46). Follow-up analysis indicated that in the right group, frontal asymmetry in both the theta and beta band did not change over the six training sessions (training main effect theta: F(1,17) = 0.05, P = 0.83; Beta: F(1,16) = 0.47, P = 0.51).
Fig. 4.
Frequency independence of the neurofeedback training in the right group. Frequency spectra of the baseline frontal asymmetry (F4-F3) of T1 (dashed lines) and of the baseline asymmetry of T6 (solid lines). The influence of the neurofeedback training on the spectra is most pronounced in the trained IAF band (grey area). There was no significant effect in theta (IAF -1 till -3 Hz) and beta (IAF +1 till +3 Hz) band, thereby fulfilling the independence criterion.
The effect of the neurofeedback training in the right group on different electrode positions was assessed using linear contrasts with training (1–6), location (F4–F3, C4–C3, P4–P3) and measurement (pre, post) as within subject factors. This revealed a trend-level training × location interaction (F(1,18) = 3.29, P = 0.08; = 0.15). A follow-up analysis revealed that the regulation of frontal alpha asymmetry at F4–F3 in the right group was independent of changes in alpha asymmetry on the medial central and parietal locations (training main effect: C4–C3: F(1,19) = 0.001, P = 0.98; P4–P3: F(1,19) = 0.73, P = 0.40). The location specificity of the frontal alpha asymmetry training was further investigated on the adjacent frontal locations F8–F7 and FC4–FC3. The pattern of the alpha asymmetry change was the same in adjacent frontal locations (training × location interaction: F(1,19) = 1.38, P = 0.25; training main effect F(1,19) = 15.36, P = 0.001; = 0.45).
Stability
The stability of the frontal alpha asymmetry training in the right group was assessed 1 week and 1 month after the last neurofeedback training session. Figure 5 shows that the relative frontal alpha asymmetry returned to baseline after the last neurofeedback training session. This was corroborated by the significant main effect of day (F(3,57) = 2.88, P = 0.04; = 0.13). Follow-up planned comparisons confirmed the expected changes in asymmetry between training 1 and 6 (P = 0.002), but the effect did not persist (training 1 vs 1 week: P = 0.60; training 1 vs 1 month P = 0.57).
Fig. 5.
Stability of change in Frontal Alpha Asymmetry (F4-F3) measured during rest (r1) in the right group. Thick lines represent the group mean and thin lines display the individual stability 1 week and 1 month after the last neurofeedback training. Positive alpha asymmetry scores indicate greater relative left than right frontal activity while negative alpha asymmetry scores indicate greater relative right than left frontal activity.
Interpretability
The behavioural effect of the right neurofeedback training was assessed by testing the effect of training group on current mood using baseline corrected NA scores measured on the training days (1–6) and on task-induced subjective and neuroendocrine stress response induced by the MAST on test days (1–3). Table 1 displays the subjective stress response on the test days. Training group seemed to differentially affect the subjective stress response between test day 1 and 2 (test day × group trend-level interaction F(2,48) = 2.34, P = 0.10; = 0.09). Simple-effect analyses revealed that the three training groups did not differ in task-induced subjective stress on test day 1 (F(2,52) = 0.98, P = 0.38), but did differ at trend-level on test day 2 (F(2,52) = 2.49, P = 0.09; = 0.09), with participants in the right neurofeedback group being more stressed than participants in the left group (P = 0.03), while the left and placebo group did not differ and were less stressed on the second test day (P = 0.23). Our interpretation is that the reduction in subjective stress response observed in the left and placebo groups is due to repeated stress induction in the laboratory. The neurofeedback training effect in the right group seemingly counteracted the reduction in subjective stress. No change over training days, nor neurofeedback group effects were found for current negative mood and neuroendocrine stress response (all Ps > 0.65).
Table 1.
Task-induced subjective stress response on the three test days (Mean ± SEM)
| Right | Left | Placebo | ||||
|---|---|---|---|---|---|---|
| Test day 1 | 73.50 | 3.02 | 69.94 | 3.83 | 69.11 | 3.94 |
| Test day 2 | 72.50 | 3.21 | 59.28 | 5.99 | 62.77 | 5.36 |
| Test day 3 | 70.16 | 2.64 | 62.22 | 4.84 | 65.83 | 3.88 |
Individual differences in frontal asymmetry neurofeedback
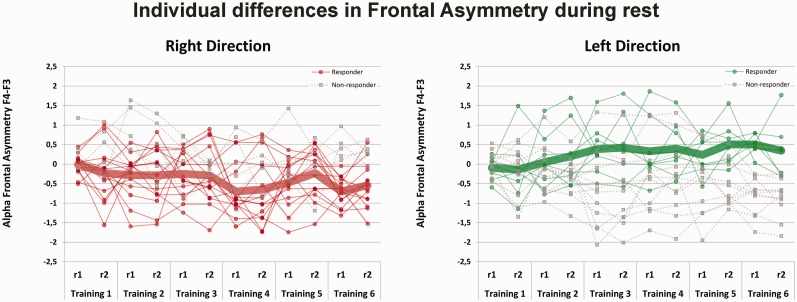
Figure 6 displays the individual differences in frontal asymmetry during the rest measurements across training days as well as the average asymmetry score per group. It has been suggested that not everybody is able to learn how to self-regulate one’s own cortical activity (Hanslmayr et al., 2005; Zoefel et al., 2011; Dekker et al., 2014). We defined responders as participants who produced a shift in relative asymmetry score (difference between T6 and T1) during rest (r1) in the desired direction (i.e. right relative more negative, while left relative more positive alpha asymmetry at T6 than at T1).
Fig. 6.
Individual differences in the Frontal Alpha Asymmetry (F4-F3) scores during rest over the six training sessions per group. Means are displayed separately for responders, i.e. change in frontal asymmetry between T1 and T6 in the desired direction (right more negative while left more positive relative asymmetry; solid thin line) and non-responders (dashed thin line). Thick lines represent the group mean of responders. Positive alpha asymmetry scores indicate greater relative left than right frontal activity while negative alpha asymmetry scores indicate greater relative right than left frontal activity. Abbreviations: r1 = asymmetry measurement during rest before the neurofeedback training, r2 = asymmetry measurement during rest after the neurofeedback training.
Individual differences and neurofeedback group
In the left group, 8 participants were classified as responders and 10 as non-responders. In the right group, 15 participants were responders and 5 were non-responders. This difference in relative proportion of responders per training group was statistically significant (χ2(1) = 3.70, P = 0.05). Based on the odds ratio, participants were 3.8 times more likely to change their frontal asymmetry in the desired direction in the right than in the left training group. This result is consistent with the above results in the group as-a-whole, showing only a significant effect in the right group. There were no differences between males and females with respect to the number of responders per group (right: χ2(1) = 0.61, P = 0.44; Left: χ2(1) = 0.18, P = 0.67).
Individual differences and trainability, interpretability and stability
The validity of the classification into responders and non-responders based on the difference in rest asymmetry over training sessions was assessed on independent measures. A difference between responders and non-responders in changing frontal asymmetry during the feedback was assessed with training (1–5) and block (blocks 1–3) as linear subject variables and group (left, right) and responder (responder, non-responder) as between subject variables. This revealed a trend-level training × responder (F(1,34) = 3.72, P = 0.06; = 0.10) and a group × responder interaction (F(1,34) = 5.55, P = 0.02;; = 0.14). Follow-up analysis revealed a trend-level responder main effect for the right group (F(1,18) = 3.89, P = 0.06; = 0.18) and a trend-level training × responder interaction (F(1,16) = 3.67, P = 0.07; = 0.19) for the left group (see Figure 7) suggesting that responders and non-responders differed in changing frontal asymmetry during the neurofeedback blocks across training sessions. For both groups, no differences between responders and non-responders with regard to subjective stress (test day 1 vs 1 week: day × responder interaction Ps > 0.24) or stability (training 1 vs 1 week: day × responder interaction Ps > 0.21; training 1 vs 1 month: day × responder interaction Ps > 0.11) were found.
Fig. 7.
Individual differences in trainability and interpretability. The classification into responders and non-responders based on the difference in rest asymmetry between training 1 and 6 revealed a difference in the time course of changing relative frontal asymmetry during feedback. The training × responder interaction for the left group is displayed. Positive alpha asymmetry scores indicate greater relative left than right frontal activity while negative alpha asymmetry scores indicate greater relative right than left frontal activity.
Discussion
Frontal alpha asymmetry is assumed to be associated with psychopathology and individual differences in emotional responding. Neurofeedback is a tool that can be used to change frontal alpha asymmetry and could, therefore, prove to be a practical intervention option to increase resilience. The current study assessed the trainability, interpretability, stability, and specificity of a neurofeedback protocol that was designed to change relative frontal alpha asymmetry. The neurofeedback protocol that was developed and evaluated in the current work uses real-time eye-corrected and average mastoid-referenced individual alpha frequency data as the basis for frontal asymmetry feedback. Furthermore, by including a placebo group and follow-up measurements 1 week and 1 month later, the current study extends the knowledge that was accumulated in previous alpha asymmetry neurofeedback studies.
We first assessed the effectiveness of the neurofeedback protocol for the whole sample by evaluating the change in relative frontal asymmetry in the alpha band. For the right group, a linear increase of relative frontal asymmetry during rest over training sessions was found, suggesting transfer of the previous learning experience to the next training. This change in relative asymmetry to the right was only found during the rest measurement and not during the feedback itself. This could be due to the fact that learning from feedback involves high levels of cognitive effort and attention. Importantly, both cognitive effort and outward attention are accompanied by alpha suppression (Klimesch et al., 1998), which may decrease the sensitivity to detect asymmetry changes. Furthermore, it is possible that neurofeedback is more effective in changing tonic rather than phasic alpha asymmetry as tonic alpha changes occur at a slower rate (Hanslmayr et al., 2005). It has been shown that manipulation of alpha oscillations during rest is related to high performance levels and alpha suppression during cognitive tasks (Klimesch et al., 2003).
For task-induced subjective stress responses between test day 1 and 2, we found a reduction in subjective stress on test day 2 in the left and placebo group. Participants in the right group, on the other hand, felt equally stressed on both test days. The absence of a decrease in the right group could result from the neurofeedback training and its shift in relative frontal asymmetry to the right. This interpretation is consistent with the idea that asymmetrical activation of the prefrontal cortex plays a role in adaptive coping, with a right lateralised withdrawal system involved in negative affect (Tomarken et al., 1992; Davidson, 1998; Coan et al., 2006). These results mirror our previous fMRI finding of an association between reduced task-induced subjective stress and enhanced connectivity between the amygdala and left dorsolateral prefrontal cortex during rest (Quaedflieg et al., 2015). No changes in mood were observed over the six training days. Our finding that mood is seemingly unaffected by alpha asymmetry neurofeedback training is well in line with those of previous studies (e.g. Allen et al., 2001; Harmon-Jones et al., 2008), which might imply that unprovoked self-reported mood is not sufficiently sensitive. Several frontal alpha asymmetry neurofeedback studies in patient samples found reduced depressive symptoms (Baehr et al., 1998; Hammond, 2005; Choi et al., 2011; Peeters et al., 2014). Some authors also suggested that in healthy participants, asymmetrical frontal activity acts as a moderator of mood responses when the appropriate emotional stimuli are presented (Coan and Allen, 2004; Coan et al., 2006). This is supported by studies in healthy participants demonstrating that frontal asymmetry is not related to unprovoked affective states (Harmon-Jones and Allen, 1997; Sutton and Davidson, 1997 but see Hagemann et al., 1998). Note in passing that the precise motivational circumstances determining the association with affective responding is not yet fully understood and require further study (Meyer et al., 2014). Training direction did not differentially change the task-induced cortisol response between test day 1 and 2. This corroborates our previous findings of no stress-induced change in frontal asymmetry (Quaedflieg et al., 2015). Moreover, high frequency rTMS intended to change relative frontal activity had no effect on cortisol levels in an unchallenged situation in healthy participants (Baeken et al., 2011). However, that same procedure did change the cortisol response in experimentally stressed females (Baeken et al., 2014), suggesting that the moderating role of baseline frontal alpha activity on the fight-or-flight neuroendocrine response is state-dependent.
The changes in relative frontal alpha asymmetry in the right group occurred independent of other frequency bands and were also found at adjacent frontal locations, but not at central and parietal locations. This confirms the specificity of the results obtained by the current study’s neurofeedback protocol. Similarly, Harmon-Jones et al. (2008) used a two-day alpha frontal asymmetry protocol and found a specific effect between the increase and decrease relative frontal asymmetry group on F4/F3, but not on P4/P3 and, more recently, Peeters et al. (2014) also reported frequency specific effects after one day of frontal alpha asymmetry training. Other studies using alpha amplitude neurofeedback protocols, however, did find changes beyond the target frequency and location (see for example Egner et al., 2004; van Boxtel et al., 2012; Enriquez-Geppert et al., 2014; for a review see Gruzelier, 2014). This suggests that the specificity of the training depends on the type of alpha neurofeedback protocol used (e.g. asymmetry vs amplitude training). The changes in the right group did not persist at 1 week and 1 month follow-up measurements. While such a return to baseline has also been reported by previous studies (Allen et al., 2001; Peeters et al., 2014), it disagrees with findings of Choi et al. (2011) showing a stable effect of left-sided frontal asymmetry training after 1 month in depressive patients. Our data suggests that although we can effectively train healthy people to display more relative right-sided asymmetry, these effects seem to be short-lived.
In line with previous studies using different alpha neurofeedback protocols (Dekker et al., 2014; upper alpha: Hanslmayr et al., 2005; Zoefel et al., 2011), the current data show that not all participants learned from the neurofeedback how to modulate their cortical activity. Trained in the left group, 44% of the participants demonstrated a less negative rest asymmetry score while after training in the right group 75% demonstrated a more negative rest asymmetry score. These data support Allen et al.’s (2001) observation that it is easier to increase right- rather than left-relative frontal asymmetry in healthy participants using EEG neurofeedback. We assessed the validity of the classification into responders and non-responders based on the difference in rest asymmetry between training 1 and 6 based in terms of trainability during the neurofeedback, interpretability, and stability. While we did not find an effect of the neurofeedback training on relative frontal asymmetry during the feedback for the whole group analysis, the resting-EEG-based classification did differentiate responders from non-responders during the feedback training. The predictive value of changes in resting alpha oscillations for the learning ability of alpha neurofeedback was also shown in the study of Wan et al. (2014). Previous alpha neurofeedback studies defined their own responder classification criteria and did not assess the validity of the employed classification on independent measures. Our finding that individual differences in trainability is dependent on training direction, with participants in the right direction being more likely to change their frontal asymmetry in the desired direction, indicates that it is important to make a responder vs non-responder distinction. By doing so, the current and future studies may gain further insight in the processes involved in training self-regulation. Also, for therapeutic applications it is important to unambiguously define a training criterion. A responder criterion would enable to determine the end point of the training or could help to identify participants who do not respond to neurofeedback treatment as early as possible so that no additional and unnecessary training is imposed upon the unresponsive participants.
It has been suggested that in order to be truly effective, feedback should be contingent on brain activity alone. This has implications for the methodological design of the neurofeedback training. For example, since eye movement artifacts influence the EEG activity, especially at the frontal sites and in the alpha band, it is important to correct them real-time when using a frontal alpha asymmetry neurofeedback training protocol (Sherlin et al., 2011; Huster et al., 2014). Moreover, computerized linked mastoids (i.e. average of A1+A2) reference has a somewhat superior signal-to-noise ratio compared to Cz, which has been used in previous frontal alpha asymmetry neurofeedback protocols (Hagemann, 2004; Coan et al., 2006). The current neurofeedback protocol calculated real-time averages of both references and corrected eye blinks online and, thus, represents an important step forward to making instrumental conditioning of brain rhythms more specific.
In sum, individual frontal alpha frequency neurofeedback resulted in a change in relative frontal asymmetry at rest in participants in the right group, and this change in relative frontal alpha asymmetry seemed to affect subjective stress. Moreover, we found that the trainability in the right group was specific with regard to frequency band and location. Individual differences in trainability dependent on training group were found, with participants in the right group being more likely to change their frontal asymmetry in the desired direction. The individual differences in trainability were also reflected in the ability to change frontal asymmetry during the feedback. Whether the current neurofeedback is also capable of eliciting reliable effects at the behavioral level, which would be especially useful in clinical populations, remains open to further empirical testing.
Acknowledgements
We are especially thankful to Jacco Ronner for the neurofeedback software development and to Marijke Hekkenberg, Loran van der Hoeven and Jonathan O’Keeffe for their help in collecting the data.
Footnotes
1 Linear contrasts with training (6 levels) and group (left, right) revealed a non-significant interaction effect (training × group F(1,35) = 0.93, P = 0.34).
Funding
This work was supported by the Netherlands Organization for Scientific Research (NWO) [grant number 056-25-011] to [T.S.].
Conflict of interest. NWO had no further role in the study design; in the collection, analysis and interpretation of the data; in the writing of the report; and in the decision to submit the paper for publication.
References
- Allen J.J., Harmon-Jones E., Cavender J.H. (2001). Manipulation of frontal EEG asymmetry through biofeedback alters self-reported emotional responses and facial EMG. Psychophysiology , 38, 685–93. [PubMed] [Google Scholar]
- Allen J.J.B., Coan J.A., Nazarian M. (2004). Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology , 67, 183–218. [DOI] [PubMed] [Google Scholar]
- Arns M., Heinrich H., Strehl U. (2014). Evaluation of neurofeedback in ADHD: The long and winding road. Biological Psychology , 95, 108–15. [DOI] [PubMed] [Google Scholar]
- Baehr E., Rosenfeld J.P., Baehr R., Earnest C. (1998). Comparison of two EEG asymmetry indices in depressed patients vs. normal controls . International Journal of Psychophysiology , 31, 89–92. [DOI] [PubMed] [Google Scholar]
- Baeken C., Vanderhasselt M.A., De Raedt R. (2011). Baseline ‘state anxiety' influences HPA-axis sensitivity to one sham-controlled HF-rTMS session applied to the right dorsolateral prefrontal cortex. Psychoneuroendocrinology , 36, 60–7. [DOI] [PubMed] [Google Scholar]
- Baeken C., Vanderhasselt M.A., Remue J., et al. (2014). One left dorsolateral prefrontal cortical HF-rTMS session attenuates HPA-system sensitivity to critical feedback in healthy females. Neuropsychologia , 57, 112–21. [DOI] [PubMed] [Google Scholar]
- Choi S.W., Chi S.E., Chung S.Y., et al. (2011). Is alpha wave neurofeedback effective with randomized clinical trials in depression? A pilot study. Neuropsychobiology , 63, 43–51. [DOI] [PubMed] [Google Scholar]
- Coan J.A., Allen J.J.B. (2004). Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology , 67, 7–49. [DOI] [PubMed] [Google Scholar]
- Coan J.A., Allen J.J.B., McKnight P.E. (2006). A capability model of individual differences in frontal EEG asymmetry. Biological Psychology , 72, 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.J. (1998). Affective style and affective disorders: Perspectives from affective neuroscience. Cognition & Emotion , 12, 307–30. [Google Scholar]
- Davidson R.J. (2004). What does the prefrontal cortex “do” in affect: perspectives on frontal EEG asymmetry research. Biological Psychology , 67, 219–33. [DOI] [PubMed] [Google Scholar]
- Dekker M.K., Sitskoorn M.M., Denissen A.J., van Boxtel G.J. (2014). The time-course of alpha neurofeedback training effects in healthy participants. Biological Psychology , 95, 70–3. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods , 134, 9–21. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M., Klimesch W., Pachinger T., Ripper B. (1998). Individual differences in brain dynamics: important implications for the calculation of event-related band power. Biological Cybernetics , 79, 49–57. [DOI] [PubMed] [Google Scholar]
- Egner T., Zech T.F., Gruzelier J.H. (2004). The effects of neurofeedback training on the spectral topography of the electroencephalogram. Clinical Neurophysiology , 115, 2452–60. [DOI] [PubMed] [Google Scholar]
- Enriquez-Geppert S., Huster R.J., Scharfenort R., Mokom Z.N., Zimmermann J., Herrmann C.S. (2014). Modulation of frontal-midline theta by neurofeedback. Biological Psychology , 95, 59–69. [DOI] [PubMed] [Google Scholar]
- Fritz C.O., Morris P.E., Richler J.J. (2012). Effect size estimates: current use, calculations, and interpretation. Journal of Experimental Psychology-General , 141, 2–18. [DOI] [PubMed] [Google Scholar]
- Goldman R.I., Stern J.M., Engel J., Cohen M.S. (2002). Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport , 13, 2487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruzelier J.H. (2014). EEG-neurofeedback for optimising performance. III: a review of methodological and theoretical considerations. Neuroscience and Biobehavioral Reviews , 44, 159–82. [DOI] [PubMed] [Google Scholar]
- Hagemann D. (2004). Individual differences in anterior EEG asymmetry: methodological problems and solutions. Biological Psychology , 67, 157–82. [DOI] [PubMed] [Google Scholar]
- Hagemann D., Naumann E., Becker G., Maier S., Bartussek D. (1998). Frontal brain asymmetry and affective style: a conceptual replication. Psychophysiology , 35, 372–88. [PubMed] [Google Scholar]
- Hammond D.C. (2005). Neurofeedback treatment of depression and anxiety. Journal of Adult Development , 12, 131–7. [Google Scholar]
- Hanslmayr S., Sauseng P., Doppelmayr M., Schabus M., Klimesch W. (2005). Increasing individual upper alpha power by neurofeedback improves cognitive performance in human subjects. Applied Psychophysiology Biofeedback , 30, 1–10. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Allen J.J.B. (1997). Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology , 106, 159–63. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Gable P.A., Peterson C.K. (2010). The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biological Psychology , 84, 451–62. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Harmon-Jones C., Fearn M., Sigelman J.D., Johnson P. (2008). Left frontal cortical activation and spreading of alternatives: Tests of the action-based model of dissonance. Journal of Personality and Social Psychology , 94, 1–15. [DOI] [PubMed] [Google Scholar]
- Heinrich H., Gevensleben H., Strehl U. (2007). Annotation: neurofeedback—train your brain to train behaviour. Journal of Child Psychology and Psychiatry , 48, 3–16. [DOI] [PubMed] [Google Scholar]
- Huster R.J., Mokom Z.N., Enriquez-Geppert S., Herrmann C.S. (2014). Brain-computer interfaces for EEG neurofeedback: peculiarities and solutions. International Journal of Psychophysiology , 91, 36–45. [DOI] [PubMed] [Google Scholar]
- Jackson D.C., Mueller C.J., Dolski I., et al. (2003). Now you feel it, now you don't: Frontal brain electrical asymmetry and individual differences in emotion regulation. Psychological Science , 14, 612–7. [DOI] [PubMed] [Google Scholar]
- Kemp A.H., Griffiths K., Felmingham K.L., et al. (2010). Disorder specificity despite comorbidity: Resting EEG alpha asymmetry in major depressive disorder and post-traumatic stress disorder. Biological Psychology , 85, 350–4. [DOI] [PubMed] [Google Scholar]
- Klimesch W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research Reviews , 29, 169–95. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Doppelmayr M., Russegger H., Pachinger T., Schwaiger J. (1998). Induced alpha band power changes in the human EEG and attention. Neuroscience Letters , 244, 73–6. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Sauseng P., Gerloff C. (2003). Enhancing cognitive performance with repetitive transcranial magnetic stimulation at human individual alpha frequency. European Journal of Neuroscience , 17, 1129–33. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Schimke H., Pfurtscheller G. (1993). Alpha frequency, cognitive load and memory performance. Brain Topography , 5, 241–51. [DOI] [PubMed] [Google Scholar]
- Kouijzer M.E., van Schie H.T., Gerrits B.J., Buitelaar J.K., de Moor J.M. (2013). Is EEG-biofeedback an effective treatment in autism spectrum disorders? A randomized controlled trial. Applied Psychophysiology Biofeedback , 38, 17–28. [DOI] [PubMed] [Google Scholar]
- Kudielka B.M., Hellhammer D.H., Wust S. (2009). Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology , 34, 2–18. [DOI] [PubMed] [Google Scholar]
- Laufs H., Kleinschmidt A., Beyerle A., et al. (2003). EEG-correlated fMRI of human alpha activity. Neuroimage , 19, 1463–76. [DOI] [PubMed] [Google Scholar]
- Lofthouse N., Arnold L.E., Hersch S., Hurt E., DeBeus R. (2012). A review of neurofeedback treatment for pediatric ADHD. Journal of Attention Disorders , 16, 351–72. [DOI] [PubMed] [Google Scholar]
- Loo C.K., Mitchell P.B. (2005). A review of the efficacy of transcranial magnetic stimulation (TMS) treatment for depression, and current and future strategies to optimize efficacy. Journal of Affective Disorders , 88, 255–67. [DOI] [PubMed] [Google Scholar]
- Meyer T., Quaedflieg C.W.E.M., Giesbrecht T., Meijer E.H., Abiad S., Smeets T. (2014). Frontal EEG asymmetry as predictor of physiological responses to aversive memories. Psychophysiology , 51, 853–65. [DOI] [PubMed] [Google Scholar]
- Moscovitch D.A., Santesso D.L., Miskovic V., McCabe R.E., Antony M.M., Schmidt L.A. (2011). Frontal EEG asymmetry and symptom response to cognitive behavioral therapy in patients with social anxiety disorder. Biological Psychology , 87, 379–85. [DOI] [PubMed] [Google Scholar]
- Papousek I., Reiser E.M., Weber B., Freudenthaler H.H., Schulter G. (2012). Frontal brain asymmetry and affective flexibility in an emotional contagion paradigm. Psychophysiology , 49, 489–98. [DOI] [PubMed] [Google Scholar]
- Pallanti S., Bernardi S. (2009). Neurobiology of repeated transcranial magnetic stimulation in the treatment of anxiety: a critical review. International Clinical Psychopharmacology , 24, 163–73. [DOI] [PubMed] [Google Scholar]
- Peeters F., Oehlen M., Ronner J., van Os J., Lousberg R. (2014). Neurofeedback as a treatment for major depressive disorder—a pilot study. PLos One , 9, e91837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters F., Ronner J., Bodar L., van Os J., Lousberg R. (2014). Validation of a neurofeedback paradigm: manipulating frontal EEG alpha-activity and its impact on mood. International Journal of Psychophysiology , 93, 116–20. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G., Stancak A., Neuper C. (1996). Event-related synchronization (ERS) in the alpha band—an electrophysiological correlate of cortical idling: a review. International Journal of Psychophysiology , 24, 39–46. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A., Sherwood R.J., Henriques J.B., Davidson R.J. (2005). Frontal brain asymmetry and reward responsiveness—a source-localization study. Psychological Science , 16, 805–13. [DOI] [PubMed] [Google Scholar]
- Pruessner J.C., Kirschbaum C., Meinlschmid G., Hellhammer D.H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology , 28, 916–31. [DOI] [PubMed] [Google Scholar]
- Quaedflieg C.W.E.M., Meyer T., Smeets T. (2013). The imaging Maastricht Acute Stress Test (iMAST): a neuroimaging compatible psychophysiological stressor. Psychophysiology , 50, 758–66. [DOI] [PubMed] [Google Scholar]
- Quaedflieg C.W.E.M., Meyer T., Smulders F.T.Y., Smeets T. (2015). The functional role of individual-alpha based frontal asymmetry in stress responding. Biological Psychology , 104, 75–81. [DOI] [PubMed] [Google Scholar]
- Quaedflieg C.W.E.M., van de Ven V., Meyer T., et al. (2015). Temporal dynamics of stress-induced alternations of intrinsic amygdala connectivity and neuroendocrine levels. PLos One , 10, e0124141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosnow R.L., Rosenthal R. (1989). Statistical procedures and the justification of knowledge in psychological science. American Psychologist , 44, 1276–84. [Google Scholar]
- Semlitsch H.V., Anderer P., Schuster P., Presslich O. (1986). A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology , 23, 695–703. [DOI] [PubMed] [Google Scholar]
- Sherlin L., Arns M., Lubar J., et al. (2011). Neurofeedback and basic learning theory: implications for research and practice. Journal of Neurotherapy: Investigations in Neuromodulation, Neurofeedback and Applied Neuroscience , 15, 292–304. [Google Scholar]
- Smeets T., Cornelisse S., Quaedflieg C.W.E.M., Meyer T., Jelicic M., Merckelbach H. (2012). Introducing the Maastricht Acute Stress Test (MAST): a quick and non-invasive approach to elicit robust autonomic and glucocorticoid stress responses. Psychoneuroendocrinology , 37, 1998–2008. [DOI] [PubMed] [Google Scholar]
- Sutton S.K., Davidson R.J. (1997). Prefrontal brain asymmetry: a biological substrate of the behavioral approach and inhibition systems. Psychological Science , 8, 204–10. [Google Scholar]
- Sullivan RM, Gratton A. (2002). Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology, 27, 99–114. [DOI] [PubMed] [Google Scholar]
- Thibodeau R., Jorgensen R.S., Kim S. (2006). Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. Journal of Abnormal Psychology , 115, 715–29. [DOI] [PubMed] [Google Scholar]
- Tomarken A.J., Davidson R.J., Wheeler R.E., Doss R.C. (1992). Individual differences in anterior brain asymmetry and fundamental dimensions of emotion. Journal of Personality and Social Psychology , 62, 676–87. [DOI] [PubMed] [Google Scholar]
- van Boxtel G.J., Denissen A.J., Jager M., et al. (2012). A novel self-guided approach to alpha activity training. International Journal of Psychophysiology , 83, 282–94. [DOI] [PubMed] [Google Scholar]
- van Dongen-Boomsma M., Vollebregt M.A., Slaats-Willemse D., Buitelaar J.K. (2013). A randomized placebo-controlled trial of electroencephalographic (EEG) neurofeedback in children with attention-deficit/hyperactivity disorder. Journal of Clinical Psychiatry , 74, 821–7. [DOI] [PubMed] [Google Scholar]
- Velo J.R., Stewart J.L., Hasler B.P., Towers D.N., Allen J.J.B. (2012). Should it matter when we record? Time of year and time of day as factors influencing frontal EEG asymmetry. Biological Psychology , 91, 283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan F., Nan W., Vai M.I., Rosa A. (2014). Resting alpha activity predicts learning ability in alpha neurofeedback. Frontiers in Human Neuroscience , 8, 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect—the Panas Scales. Journal of Personality and Social Psychology , 54, 1063–70. [DOI] [PubMed] [Google Scholar]
- Zoefel B., Huster R.J., Herrmann C.S. (2011). Neurofeedback training of the upper alpha frequency band in EEG improves cognitive performance. Neuroimage , 54, 1427–31. [DOI] [PubMed] [Google Scholar]