Abstract
Inner speech has been implicated in important aspects of normal and atypical cognition, including the development of auditory hallucinations. Studies to date have focused on covert speech elicited by simple word or sentence repetition, while ignoring richer and arguably more psychologically significant varieties of inner speech. This study compared neural activation for inner speech involving conversations (‘dialogic inner speech’) with single-speaker scenarios (‘monologic inner speech’). Inner speech-related activation differences were then compared with activations relating to Theory-of-Mind (ToM) reasoning and visual perspective-taking in a conjunction design. Generation of dialogic (compared with monologic) scenarios was associated with a widespread bilateral network including left and right superior temporal gyri, precuneus, posterior cingulate and left inferior and medial frontal gyri. Activation associated with dialogic scenarios and ToM reasoning overlapped in areas of right posterior temporal cortex previously linked to mental state representation. Implications for understanding verbal cognition in typical and atypical populations are discussed.
Keywords: monologue, dialogue, fMRI, auditory verbal hallucinations, covert speech
Introduction
Inner speech—the experience of silent, verbal thinking—has been implicated in many cognitive functions, including problem-solving, creativity and self-regulation (Morin, 2009; Fernyhough, 2013; Alderson-Day and Fernyhough, 2015a), and disruptions to the ‘internal monologue’ have been linked to varieties of pathology, including hallucinations and depression (Frith, 1992; Nolen-Hoeksema, 2004). Enhanced understanding of inner speech hence has implications for understanding of both typical and atypical cognition. Although interest in inner speech has grown in recent years (Morin et al., 2011; Williams et al., 2012; Fernyhough, 2013), conceptual and methodological challenges have limited what is known about the neural processes underpinning this common experience.
Most neuroimaging studies to date have operationalized inner speech as a unitary phenomenon equivalent to a first-person monologue (Hinke et al., 1993; Simons et al., 2010). Methods of eliciting inner speech have typically involved either subvocal recitation (e.g. covertly repeating ‘You are a x’ in response to a cue; McGuire et al., 1995) or prompting participants to make phonological judgements about words using inner speech (such as which syllable to stress in pronunciation; Aleman et al., 2005). Such studies have shown recruitment during inner speech of areas associated with overt speech production and comprehension, such as left inferior frontal gyrus (IFG), supplementary motor area (SMA) and the superior and middle temporal gyri (McGuire et al., 1996; Shergill et al., 2002; Aleman et al., 2005).
However, inner speech is a complex and varied phenomenon. In behavioural studies, everyday inner speech is often reported to be involved in self-awareness, past and future thinking and emotional reflection (D’Argembeau et al., 2011; Morin et al., 2011), while in cognitive research, inner speech appears to fulfill a variety of mnemonic and regulatory functions (e.g. Emerson and Miyake, 2003; see Alderson-Day and Fernyhough, 2015a, for a review). Vygotsky (1987) posited that inner speech reflects the endpoint of a developmental process in which social dialogues, mediated by language, are internalized as verbal thought. Following from this view, the subjective experience of inner speech will mirror the external experience of communication and often have a dialogic structure (Fernyhough, 1996, 2004), involving the co-articulation of differing perspectives on reality and, in some cases, representation of others’ voices. Evidence for the validity of these distinctions is provided by findings from a self-report instrument, the varieties of inner speech questionnaire (VISQ: McCarthy-Jones and Fernyhough, 2011). Studies with student samples have documented high rates of endorsement (>75%) for inner speech involving dialogue rather than monologue, alongside a number of other phenomenological variations (Alderson-Day et al., 2014; Alderson-Day and Fernyhough, 2015b).
Recognizing this complexity of inner speech, particularly its conversational and social features, is important both for ecological validity (Fernyhough, 2013) and for understanding atypical cognition (Fernyhough, 2004). Auditory verbal hallucinations (AVH) have been proposed to reflect misattributed instances of inner speech (Bentall, 1990; Frith, 1992), but studies inspired by this view have arguably relied on a relatively impoverished, ‘monologic’ view of inner speech. In the context of a growing recognition of social and conversational dimensions of AVH (Bell, 2013; Ford et al., 2014), knowing more about the heterogeneity of inner speech could enhance AVH models (Jones and Fernyhough, 2007).
Almost no data exist on the neural basis of dialogic or conversational inner speech, and what there is has largely focused on imagining words or sentences spoken in other voices (often referred to as ‘auditory verbal imagery’). For example, Shergill et al. (2001) asked participants either to silently rehearse sentences of the form ‘I like x…’ in their own voice (inner speech) or to imagine sentences spoken in another voice in the second or third person (auditory verbal imagery). While sentence repetition was associated with activation of left IFG, superior temporal gyrus (STG), insula and the SMA, imagined speech in another person’s voice recruited a bilateral frontotemporal network, including right IFG, left pre-central gyrus and right STG. Similarly, in an AVH study by Linden et al. (2011), auditory imagery for familiar voices, such as conversations with family members, was associated with bilateral activation in IFG, superior temporal sulcus (STS), SMA and anterior cingulate cortex in healthy participants.
Research on overt conversational processing has also implicated a bilateral network including right frontal and temporal homologues of left-sided language regions. For example, Caplan and Dapretto (2001) compared judgements for logical and contextual violations of conversations in an functional magnetic resonance imaging (fMRI) task. Whereas logic judgements were associated with a left-sided Broca–Wernicke network, judgements about pragmatic context recruited right inferior frontal and middle temporal gyri, along with right prefrontal cortex (PFC). The involvement of right frontotemporal regions in pragmatic language processing is supported by evidence of selective impairments in prosody, humour and figurative language in cases of right-hemisphere damage (Mildner, 2007).
Finally, two recent studies by Yao et al. (2011; 2012) have indicated a specific role for right auditory cortex in the internal representation of other voices. In a study of silent reading, Yao et al. (2011) examined activation of left and right auditory cortex when participants read examples of direct and indirect speech (e.g. ‘The man said ‘I like cricket’’ vs ‘The man said that he likes cricket’). Reading of direct speech was specifically associated with activation in middle and posterior right STS compared with indirect speech. The same areas were also active in a second study (Yao et al., 2012) when participants listened to examples of direct speech read in a monotonous voice, but that was not the case during listening to indirect speech. Yao et al. argued that the activation of these regions during silent reading and listening to monotonous direct speech might reflect an internal simulation of the suprasegmental features of speech, such as tone and prosody.
Taken together, these findings suggest that dialogic forms of inner speech are likely to draw on a range of regions beyond a typical left-sided perisylvian language network, including the right IFG, right middle temporal gyrus (MTG) and the right STG/STS. Following Shergill et al. (2001) and, to a lesser degree, Yao et al. (2011), it could be hypothesized that the involvement of these regions is required for the simulation of other people’s voices to complement one’s own inner speech. On such a view, dialogic inner speech could be conceptualized simply as monologic inner speech plus the phonological representation of other voices, leading to recruitment of voice-selective regions of right temporal cortex.
However, generating an internal conversation requires more than simply mimicking the auditory qualities of the voices involved. First, dialogic inner speech could draw on theory-of-mind (ToM) capacities, requiring not only just the representation of a voice but also the sense and intention of a plausible and realistic interlocutor. If dialogic inner speech utilized such processes, then it should be possible to identify recruitment of typical ToM regions, including medial PFC (mPFC), posterior cingulate/precuneus and the temporoparietal junction (TPJ) area, encompassing posterior STG, angular gyrus and inferior parietal lobule (Spreng et al., 2009). Right TPJ has been associated with ToM in a number of fMRI and positron emission tomography (PET) studies, mostly based on false-belief tasks (Saxe and Powell, 2006), while left TPJ has been linked to mental state representation (Saxe and Kanwisher, 2003) and understanding of communicative intentions (Ciaramidaro et al., 2007). A view of dialogic inner speech as drawing on ToM capacities would suggest that it should be associated with established ToM networks and posterior temporoparietal cortex, in addition to frontotemporal regions associated with voice representation.
A second key difference between dialogue and monologue concerns their structure and complexity. Generating an internal dialogue involves representational demands that are absent from sentence repetition or subvocal rehearsal. Whereas, in monologue, a single speaker’s voice or perspective is sufficient, in dialogue more than one perspective must be generated, maintained and adopted on an alternating basis (Fernyhough, 2009). Internally simulating a conversation could also involve imagination of setting, spatial position and other details that distinguish interlocutors. Therefore, any differences observed between dialogic and monologic inner speech may not reflect representation of other voices or agents, so much as indexing the requirement to generate and flexibly switch between conversational positions and situations ‘in the mind’s eye’. If dialogic inner speech depended on such skills, it might be expected to recruit areas more typically associated with the generation and control of mental imagery, such as middle frontal gyrus (MFG), precuneus and superior parietal cortex (Zacks, 2007; McNorgan, 2012).
There are therefore reasons to believe that the production of dialogic inner speech will differ from monologic examples of the same process in three ways: recruitment of regions involved in representing other voices, involvement of ToM resources to represent other agents and the activation of brain networks involved in the generation and control of mental imagery. To test these predictions, we employed a new fMRI paradigm for eliciting monologic (i.e. verbal thinking from a single perspective) and dialogic inner speech, so that the neural correlates of the two can be compared.
To investigate the cognitive processes involved in dialogic inner speech, we used a conjunction analysis (Price and Friston, 1997) to compare dialogue-specific activation with two other tasks: a ToM task (Walter et al., 2004) and a novel perspective-switching task. The ToM task was chosen because it included non-verbal scenarios requiring inferences about communication and the representation of other agents’ intentions; in this way, any conjunction between dialogue and ToM should not reflect overlaps in the processing of verbalized language. The perspective-switching task was developed to match the switching and imagery-generation demands of the dialogic task, while avoiding the inclusion of social agents, which feature in many existing perspective-switching tasks. Conjunctions observed between the perspective-switching and dialogic tasks should therefore reflect similarities in structure and task demands, rather than representations of agents and mental states tapped in the ToM task. We predicted that (i) dialogic inner speech—in contrast to a monologic control condition—would activate not only right-hemisphere language homologue regions such as right IFG, MTG and STG but also areas typically associated with ToM processing, such as the TPJ and (ii) any further differences between dialogic and monologic scenarios would overlap with networks associated with perspective switching and mental imagery, such as the MFG or the superior parietal lobule.
Materials and methods
Participants
Twenty-one individuals [6 male; age m(s.d.) = 24.38 (6.73) years] were recruited from university settings. All participants were right-handed, native English speakers with normal or corrected-to-normal vision. No participants reported any history of cardiovascular disease, neurological conditions or head injury. Participants received either course credit or a gift voucher. All procedures were approved by the local university ethics committee.
Scanning materials and procedure
Participants completed three tasks in the scanner: inner speech, ToM and perspective-switching (followed by an anatomical scan). Each task was preceded by a single practice trial. All stimuli were presented using E-Prime 2.0 (Schneider et al., 2002). Participants viewed stimuli by looking upwards at a mirror directed at a monitor (Cambridge Research Systems Ltd. BOLDscreen MR Safe display; 1920 × 1200 resolution, refresh rate 60 Hz) placed behind the scanner bore. Button press responses (all right-handed) were collected using a fiber-optic response button box (Psychology Software Tools).
Inner speech
Participants were presented with a written description of a scenario involving either dialogue or monologue and were asked to generate inner speech in that scenario until they saw a cue to stop. Dialogic scenarios involved conversations and interviews with familiar people (Table 1). Monologic scenarios were matched to dialogic scenarios for their content and setting, but only included a single speaker. Instructions were presented for 10 s, followed by a fixation cross (the cue for inner speech) for 45 s and an intertrial interval of 3–5 s (including a stop signal for 2 s). In total, five dialogic and five monologic scenarios were presented. At the end of the scanning session, participants were asked to rate out of 100 (i) how vividly they imagined the scenarios, (ii) the vividness of any visual imagery they used during the task and (iii) the everyday characteristics of their own inner speech, using the VISQ (McCarthy-Jones and Fernyhough, 2011). The imagery self-ratings were included to check task compliance and to provide a control indicator of how much participants drew on visual (rather than verbal) imagery during the task. The VISQ was included for exploratory analysis of how individual differences in everyday inner speech may have affected task performance and related brain activations. It includes four subscales: dialogic inner speech (items include, e.g. ‘I talk back and forward to myself in my mind about things’), evaluative/motivational inner speech (e.g. ‘I think in inner speech about what I have done, and whether it was right or not’), other people in inner speech (e.g. ‘I experience the voices of other people asking me questions in my head’) and condensed inner speech (e.g. ‘I think to myself in words using brief phrases and single words rather than full sentences’). The VISQ has been shown to have good internal and test–retest reliability (McCarthy-Jones and Fernyhough, 2011; Alderson-Day et al., 2014).
Table 1.
Dialogic and monologic scenarios in the inner-speech task
| Scenario | Dialogic | Monologic |
|---|---|---|
| A visit to your old school | Conversation with a teacher | Making a speech to students |
| A job interview | Talking to the interviewer | Doing a presentation |
| Calling a relative | Conversation with relative | Leaving a voicemail |
| Being in a documentary | Doing an interview | Speaking to camera |
| Meeting the Prime Minister | Interviewing the PM | Suggesting a new law |
Theory-of-mind
Using a cartoon-based ToM task from Walter et al. (2004), participants viewed a sequence of three cartoons depicting a simple story (‘Story’ phase) and were then prompted to choose the logical end of the story from three options (‘Choice’ phase). Stories either required deciphering of actors’ intentions (e.g. pointing to see if a seat was free) or reasoning about physical causality (e.g. a football breaking some bottles). To examine ToM skills relevant to inner speech, the ‘communicative intention’ condition from Walter et al. (2004) was used, as compared with the physical reasoning control condition. ‘Story’ phase images were presented sequentially for 3 s each, followed by the ‘Choice’ phase for 7 s and a jittered intertrial interval of 7–11 s. A total of 10 ToM stories and 10 physical reasoning stories were presented in a random order. Participants indicated which image completed the story (A, B or C) using a button box, and their percentage accuracy was recorded.
Visual perspective switching
The timing and structure of the perspective-switching task was designed to match the inner-speech task. Participants first viewed an instruction page (10 s) describing a visual scene or object and asking them to imagine it from a particular perspective, e.g. ‘Imagine a train viewed from the outside. Try to picture what it looks like in your mind.’ Underneath, this was followed by an instruction to either switch perspective when prompted by a cue (the ‘Switch’ condition) or to maintain the image from single perspective until prompted to stop (the ‘Stick’ condition). In the Switch condition, the instruction page was followed by a 45 s imagery phase, in which every 7 s a cue appeared (either ‘OUTSIDE’ or ‘INSIDE’, 2 s presentation). In the Stick condition, cues appeared with the same regularity but only from one perspective (i.e. only ‘INSIDE’). After scanning, participants rated how vividly they had imagined each scene/object, and how easy they found switching between different viewpoints (rated out of 100).
Mock scanner behavioural task
Production of inner speech is difficult to verify objectively, leaving open the possibility that any differences observed between dialogic and monologic scenarios might not reflect underlying inner speech processes. To explore this further, we ran a post hoc behavioural study in a mock MRI scanner that replicated the layout, conditions and stimulus setup of the 3T scanner used for imaging. A separate set of 20 participants [2 male; age m(s.d.) = 19.65 (1.31) years] attempted the original inner-speech task and then rated a variety of phenomenological characteristics for each dialogic and monologic scenario (see Supplementary Materials for an example response sheet). Specifically, participants rated each scenario for its (i) overall vividness, (ii) presence of inner speech, (iii) presence of visual imagery, (iv) vividness of one’s own voice, (v) vividness of other voices, and (vi) the number of times there was a ‘switch’ in perspective, voice or role (items 1–5 were rated as percentages).
Following this, participants also attempted a novel version of the inner-speech task that included articulatory suppression, a commonly used secondary task that is thought to interfere with inner speech use (e.g. Baddeley et al., 1984; Williams et al., 2012). Specifically participants were asked to attempt the inner-speech task again but while repeating a different day of the week, out loud, for the duration of each scenario. The idea of this was to test whether engaging with the inner-speech task really did require use of inner speech to be performed successfully. To minimize effects of repeating the same scenarios, participants were encouraged to modify each situation (i.e. imagine speaking to a different relative) and only had to imagine scenarios for half the original time (22.5 s).
fMRI acquisition
All data were acquired at Durham University Neuroimaging Centre using a 3T Magnetom Trio MRI system (Siemens Medical Systems, Erlangen, Germany) with standard gradients and a 32-channel head coil. T2*-weighted axial echo planar imaging (EPI) scans were acquired parallel to the anterior/posterior commissure line with the following parameters: field of view (FOV) = 212 × 212 mm, flip angle (FA) = 90°, repetition time (TR) = 2160 ms, echo time (TE) = 30 ms, number of slices (NS) = 35, slice thickness (ST) = 3.0 mm, interslice gap = 0.3 mm, matrix size (MS) = 64 × 64. Images for each task were collected as separate runs (280 volumes each per run). For each participant, an anatomical scan was acquired using a high-resolution T1-weighted 3D-sequence (NS: 192; ST: 1 mm; MS: 512 × 512; FOV: 256 × 256 mm; TE: 2.52 ms; TR: 2250 ms; FA 9°).
Data analysis
All analyses were conducted using Statistical Parametric Mapping (SPM), version 8 (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB (2012b) (The Mathworks Inc).
Images were realigned to the first image to correct for head movement. After realignment, the signal measured in each slice was shifted in time relative to the acquisition time of the middle slice using a sinc interpolation to correct for different acquisition times. Volumes were then normalized into standard stereotaxic anatomical MNI-space using the transformation matrix calculated from the first EPI-scan of each subject and the EPI-template. The default settings for normalization in SPM8 with 16 non-linear iterations and the standard EPI-template supplied with SPM8 were used. The normalized data with a resliced voxel size of 3 × 3 × 3mm were smoothed with a 6 mm full width half maximum (FHWM) isotropic Gaussian kernel to accommodate intersubject variation in brain anatomy. The time-series data were high-pass filtered with a high-pass cutoff of 1/128 Hz and first-order autocorrelations of the data were estimated and corrected for. The first four volumes of each run were discarded to allow for equilibrium of the T2 response. Movement parameters from the realignment phase were visually inspected for outliers and included as regressors for single-subject (first level) analyses.
Single-subject analyses were conducted using a general linear model. The inner-speech and perspective-switching tasks were modelled as a block design with an instruction phase (4 volumes) and imagery phase (17 volumes). For the inner-speech task, three conditions were modelled in the analyses: monologic inner speech (17v), dialogic inner speech (17v) and the instruction phase (4v). The perspective-switching task was modelled in an identical way, but with Switch and Stick conditions instead of dialogic and monologic. The expected hemodynamic response at stimulus onset was modelled as a block design, convolved with a canonical hemodynamic response function. Following Walter et al. (2004), the ToM task was modelled as an event-related design with four regressors: ToM-Story, ToM-Choice, Physical-Story and Physical-Choice. Subsequently, parameter estimates of the regressor for each of the different conditions were calculated from the least mean squares fit of the model to the time-series. ‘Story’ and ‘Choice’ regressors on the ToM task were combined within each condition for the generation of contrast images (Walter et al., 2004).
For the inner-speech task, differences between parameter estimates for dialogic and monologic inner speech were tested within-subjects at the individual level, then tested at the group level with a one sample t-test. Comparisons of dialogic and monologic conditions with baseline were also made to provide further information on each condition’s neural correlates. The same procedure was applied for key comparisons on the ToM task and perspective-switching task (ToM Reasoning > Physical Reasoning and Switch > Stick, respectively). The contrasts between dialogic and monologic inner speech, ToM Reasoning and Physical Reasoning and Switch and Stick conditions were then used in a conjunction analysis to assess shared components of each task.
Because differences between dialogic and monologic inner speech were expected to be relatively small, we chose a cluster correction with a higher sensitivity to small sample sizes in comparison to the SPM cluster correction. A cluster extent threshold method (Slotnick et al., 2003; Slotnick and Schacter, 2004) was used to identify groups of contiguous voxels that were active at a value of P < 0.05, corrected for multiple comparisons. A Monte Carlo simulation with 10 000 iterations was used to estimate cluster thresholds based on the voxel-wise probability of a Type 1 error. For a voxel-wise error of P < 0. 01, a cluster of 11 or more voxels was required for P < 0.05, corrected for multiple comparisons. For a voxel-wise error of P < 0.001, clusters of 6 or more voxels were required for P < 0.05, corrected. As the latter criterion has been recommended to avoid false positives (Woo et al., 2014), the results reported later are all significant at P < 0.05 (corrected) based on a voxel-wise error of P < 0.001, unless otherwise stated. MNI voxel positions were converted into equivalent Talairach and Tournoux (1988) co-ordinates in MATLAB for anatomical labelling. All structure and Brodmann areas (BA) were labelled using the Talairach Daemon applet (Lancaster et al., 2000). Brain images were generated using SPM and MRICron (Rorden et al., 2007).
Results
Two participants were excluded from the analyses due to movement during the inner-speech task. Thus, the results later display data from a sample of 19 participants (5 male, age m(s.d.) = 24.63 (7.01) years).
Inner speech
Table 2 displays the contrast between dialogic and monologic inner speech (all clusters at P < 0.05, corr.). Significantly increased activation for dialogic compared with monologic inner speech was evident in STG bilaterally, left inferior and medial frontal gyri and a collection of posterior midline structures, including the left precuneus and right posterior cingulate. The opposite contrast, Monologic > Dialogic inner speech, did not identify any significant activations. Compared with baseline, dialogic inner speech was associated with significantly increased activation in left posterior insula (x = −39; y = −18, z = 7; t = 4.38, P < 0.05, corr.) only. At more liberal threshold levels (when the cluster extent was thresholded based on a voxel-wise error of P < 0.01), both dialogic and monologic inner speech were associated with left-hemisphere activation compared with baseline, including the left IFG, medial frontal gyrus, insula and caudate.
Table 2.
Regions activated significantly more during dialogic inner speech as compared with monologic inner speech (all P < 0.05, corrected, minimal cluster size 6 voxels.)
| BA | x | y | z | t | No. of voxels | |
|---|---|---|---|---|---|---|
| L precuneus | 31 | −15 | −58 | 34 | 7.44 | 566 |
| R superior temporal gyrus | 41 | 50 | −26 | 16 | 6.76 | 128 |
| R superior temporal gyrus | 13 | 42 | −47 | 21 | 6.70 | 16 |
| R superior temporal gyrus | 13 | 48 | −41 | 22 | 6.49 | 22 |
| R cingulate gyrus | 23 | 6 | −17 | 32 | 6.32 | 128 |
| L medial frontal gyrus | 9 | 0 | 35 | 34 | 6.28 | 158 |
| L inferior frontal gyrus | 47 | −24 | 29 | −11 | 5.87 | 14 |
| R posterior cingulate | 30 | 21 | −47 | 12 | 5.49 | 10 |
| R posterior cingulate | 31 | 24 | −58 | 15 | 5.49 | 27 |
| L STG/insula | 13 | −42 | −21 | 5 | 5.43 | 17 |
| L cerebellum | −30 | −48 | −17 | 5.08 | 28 | |
| L middle occipital gyrus | 18 | −27 | −82 | 8 | 4.92 | 8 |
| L thalamus | −21 | −27 | 8 | 4.88 | 13 | |
| L superior temporal gyrus | 13 | −45 | −46 | −17 | 4.59 | 6 |
| R pre-central gyrus | 9 | 36 | 6 | 31 | 4.23 | 11 |
| R middle temporal gyrus | 37 | 48 | −60 | −1 | 4.03 | 6 |
Self-ratings for vividness of inner speech scenarios were high (m = 73.42, s.d. = 13.13). Vivid visual imagery was also reported, although this tended to vary considerably across participants (m = 58.68, s.d. = 27.73, range = 0–100).
Theory-of-mind
The contrast between ToM and physical reasoning was associated with significant activation in anterior and posterior STG bilaterally, along with midline activation centring on left precuneus (Table 3). Although left STG activity separated into anterior and posterior clusters, right STG activation was centred on posterior areas close to the TPJ but evident all along the gyrus. In contrast, physical reasoning compared with ToM reasoning showed significantly greater recruitment of the left anterior lobe of the cerebellum (−27, −48, −12, t = 5.97), right cuneus (15, −82, 5; t = 5.73), right caudate (27, −41, 18; t = 3.88), left post-central gyrus (−45, −28, 36; t = 4.60) and left lingual gyrus (−21, −80, −8; t = 4.10). Performance on the ToM task was acceptable (Accuracy m = 84.21%, s.d. = 10.03%, range = 65–100%).
Table 3.
Regions activated significantly more during theory-of-mind (ToM) reasoning as compared with physical reasoning (all P < 0.05, corr.)
| BA | x | y | z | t | No. of voxels | |
|---|---|---|---|---|---|---|
| L superior temporal gyrus | 38 | −42 | 16 | −20 | 9.13 | 295 |
| R superior temporal gyrus | 13 | 48 | −41 | 20 | 9.00 | 556 |
| L superior temporal gyrus | 39 | −45 | −52 | 23 | 8.44 | 211 |
| L precuneus | 31 | −3 | −52 | 31 | 8.12 | 387 |
| L cerebellum | −9 | −34 | −8 | 5.58 | 12 | |
| R fusiform gyrus | 37 | 42 | −40 | −15 | 5.38 | 10 |
| R medial frontal gyrus | 9 | 6 | 50 | 15 | 5.25 | 8 |
| L parahippocampal gyrus | −30 | −8 | −16 | 5.20 | 21 | |
| L thalamus | −9 | −28 | 0 | 4.49 | 7 | |
| L parahippocampal gyrus | −33 | −11 | −16 | 4.41 | 6 |
Visual perspective switching
Compared with baseline, both the Switch and Stick conditions of the perspective-switching task showed significant activation: the Switch condition was associated with activation of left posterior insula (−45, −7, 1; t = 4.16) and left STG (−31, 1, −14; t = 4.78), while the Stick condition indicated activation of right posterior insula (42, −4, 2, t = 5.14), left MFG (−21, −7, 46; t = 4.77), left IFG (−45, 25, −5, t = 4.25) and right transverse temporal gyrus (33, −27, 13, t = 4.08, all P < 0.05, corr.). However, no significant differences were evident in the direct contrast between the two conditions. Self-ratings for vividness of mental images were again high (m = 76.68, s.d. = 18.00), as were ratings of ease in making shifts in perspective (m = 75.53, s.d. = 21.85).
Conjunctions of inner speech, theory-of-mind and perspective-switching
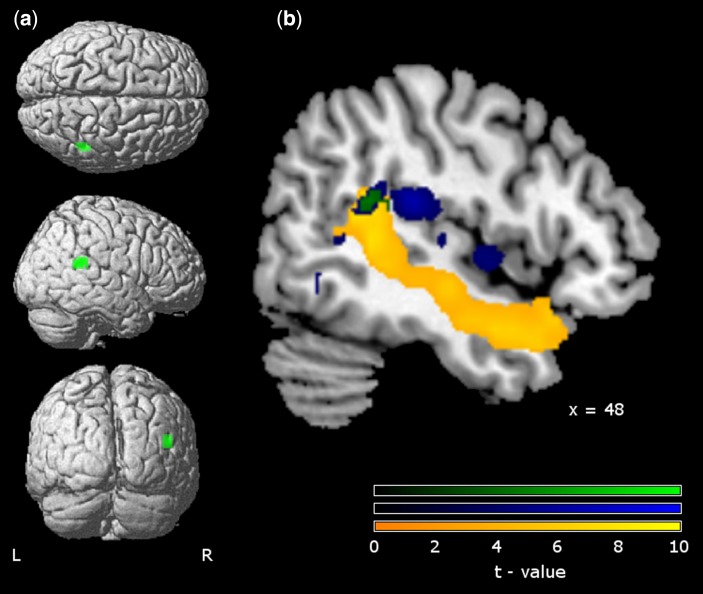
The contrasts between (i) dialogic and monologic inner speech and (ii) ToM and physical reasoning were incorporated into a conjunction analysis. As Figure 1 shows, only one cluster showed significant activation differences for both contrasts, centring on right posterior STG (48, −41, 20, t = 4.59, cluster size = 15, P < 0.05, corr.). Using a voxel-wise error of P < 0.01 for exploratory purposes, overlaps between the two tasks were also evident in right anterior STG, precuneus, right MTG, left paracentral lobule and right fusiform gyrus (all P < 0.05, corr.). When a conjunction analysis was run comparing the Dialogic > Monologic contrast and the Switch > Stick contrast, no significant clusters were observed (all P > 0.05, corr.). Overlaps at the lower significance threshold (voxel-wise P < 0.01) were evident in a ventral cluster encompassing the right posterior cingulate (3, −65, 10), the left cuneus (−12, −68, 7) and smaller clusters in right IFG (33, 31, 5) and left precuneus (−9, −60, 40).
Fig. 1.
Conjunction of dialogic inner speech and theory-of-mind. A cluster in right STG (Fig.1a) was evident for both dialogic inner speech > monologic inner speech and ToM > physical reasoning, rendered here on the standard MNI brain supplied by SPM. Dialogic inner speech (Fig 1b; blue) was evident in right STG, cingulate and frontal gyrus, while ToM (yellow) was associated with extensive right STG activation running posterior to anterior. Their conjunction (green) was at the posterior end of right STG, in the TPJ area. ToM, Theory-of-mind; STG, superior temporal gyrus, all P < 0.05, corr., clusters > 6 voxels.
Individual differences in inner speech
We examined correlations between (i) Dialogic > Monologic inner speech activations and self-report scores for vividness during the task and (ii) Dialogic > Monologic inner speech activations and self-report scores on the VISQ. These analyses revealed very similar activation areas to the group analysis. Self-report scores for vividness of the inner speech scenarios were significantly associated with clusters in right posterior MTG (36, −58, 15; t = 5.47, cluster size = 37) and right cingulate gyrus (6, −23, 35; t = 4.93, cluster size = 9; P < 0.05, corr.). Scores on the Dialogic Inner Speech subscale of the VISQ were associated with a cluster in the same area of right MTG (39, −58, 15; t = 6.57, cluster size = 10), along with two areas of the right precuneus ((i) 15, −67, 26; t = 4.89, cluster size = 11; (ii) 15, −49, 31; t = 4.66, cluster size = 13; all P < 0.05, corr.). No significant associations were observed for self-reported use of visual imagery nor for the other components of the VISQ (evaluative, other people and condensed inner speech).
Generating dialogic and monologic scenarios: the roles of inner speech and imagery processes
Phenomenological ratings from the mock scanner version of the task were used to examine use of inner speech and visual imagery across dialogic and monologic scenarios. As Table 4 indicates, dialogic and monologic scenarios were equivalent in all respects bar vividness of other voices (t = 7.47, df = 19, P < 0.001) and mean number of switches per scenario (t = 5.35, df = 19, P < 0.001), both of which were more common for dialogic inner speech (all P values are Bonferroni corrected). For both dialogic and monologic scenarios, inner speech was present to a significantly greater degree than visual imagery (dialogic: t = 3.21, df = 19, P = 0.036; monologic: t = 3.79, df = 19, P =0.010). As may be expected, vividness for one’s own voice was also stronger on average than vividness of other voices (dialogic: t = 5.95, df = 19, P < 0.001; monologic: t = 11.00, df = 19, P < 0.001).
Table 4.
Self-reported vividness ratings for dialogic and monologic scenarios in mock scanner conditions
| Dialogic |
Monologic |
||||
|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Sig. | |
| Vividness (overall) | 62.45% | 11.73% | 63.00% | 13.00% | |
| In inner speech? | 70.57% | 19.23% | 72.55% | 22.37% | |
| In visual imagery? | 46.08% | 20.76% | 38.05% | 22.84% | |
| Vividness of own voice | 69.90% | 14.49% | 73.40% | 14.67% | |
| Vividness of other voices | 43.91% | 20.76% | 18.80% | 17.60% | *** |
| Number of switches | 1.65 | 1.07 | 0.40 | 0.59 | *** |
***P < 0.001 (Bonferroni-corrected P values used).
Finally, Table 5 shows mean ratings for dialogic and monologic scenarios combined, compared across the normal and articulatory suppression versions of the task. Articulatory suppression had the effect of lowering vividness ratings for inner speech, one’s own voice and other voices but had no effect on levels of visual imagery (P = 0.999) and number of switches (P = 0.148).
Table 5.
Self-reported vividness ratings for inner speech scenarios in mock scanner under normal conditions and during articulatory suppression
| Normal conditions |
Articulatory suppression |
||||
|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Sig. | |
| Vividness (overall) | 62.73% | 11.93% | 36.01% | 16.54% | *** |
| In inner speech? | 71.56% | 20.18% | 32.12% | 23.38% | *** |
| In visual imagery? | 42.07% | 20.90% | 44.60% | 22.18% | |
| Vividness of own voice | 71.65% | 14.31% | 32.78% | 20.77% | *** |
| Vividness of other voices | 31.36% | 20.90% | 15.88% | 14.12% | ** |
| Number of switches | 1.03 | 0.69 | 0.55 | 0.49 | |
**P < 0.01, ***P < 0.001 (Bonferroni-corrected P values used).
Discussion
This study attempted to examine neural differences between two varieties of internal self-talk: dialogic and monologic inner speech. In line with the hypothesis that generating dialogic scenarios would be associated with recruitment of a network extending beyond the left frontotemporal language regions, dialogue was associated with significantly greater activation, compared with monologue, in the precuneus, posterior cingulate and the right STG (BA13 and BA41), alongside activation in left insula, IFG, STG and cerebellum. Conjunction analysis identified an overlap with ToM reasoning specifically in right posterior STG, although shared substrates with visual perspective-switching could not be fully assessed due to null results in the contrast between switching and single-perspective imagery on that particular task.
The involvement of a left-hemisphere network including IFG, STG and the cerebellum during generation of dialogic scenarios is consistent with prior inner speech studies (Shergill et al., 2001; Simons et al., 2010; Geva et al., 2011) and implies a greater demand on these areas when a dialogue must be produced (in contrast to a monologue). Although the IFG and insula are often implicated in inner-speech tasks (although see Jones, 2009), activations of posterior STG and lateral regions of temporal cortex are observed depending on specific task demands, such as self-monitoring of inner speech rate (Shergill et al., 2002) and phonology (Aleman et al., 2005). The cerebellum, in contrast, has been proposed to support maintenance of verbal working memory (i.e. articulatory rehearsal) via its connections with motor cortex (Marvel and Desmond, 2010).
Although a number of right-hemisphere regions were active during the dialogic condition, there was less evidence to suggest that this involved the specific recruitment of either language region homologues or voice-selective areas. For example, although activation in the right STG was more anterior than in the left STG, and was close to regions that have been previously related to listening to familiar voices (Shah et al., 2001), it actually overlapped more with areas previously associated with spatial rather than auditory processing (Ellison et al., 2004). This suggests that the right-hemisphere differences between the dialogic and monologic conditions were not simply picking out additional voice representation demands (cf. Shergill et al., 2001) but relate instead to other cognitive factors.
The results of conjunction analysis indicated the involvement of social-cognitive processes in dialogic scenarios. Activity in posterior right STG was evident during both dialogic scenarios and ToM reasoning, in a region previously linked to both ToM (Fletcher et al., 1995) and imagery for personal perspectives (Ruby and Decety, 2001). It is also close to sections of right TPJ that have been implicated in representation of other people’s beliefs and states of knowledge (Saxe and Powell, 2006; Sebastian et al., 2012). Along with ToM, right TPJ has been proposed to play a role in managing divided attention and non–ToM-based perspective switching (Mitchell, 2008; Aichhorn et al., 2009), although there is debate as to whether these functions are subserved by the same or separable components of the TPJ (Scholz et al., 2009). Recent research on structural connectivity suggests that TPJ splits into three separate subregions: a dorsal component connecting to lateral anterior PFC, an anterior region connecting to the ventral attentional network and a posterior region connecting to social cognitive areas such as the precuneus and posterior cingulate (Mars et al., 2012). The cluster identified in this study would appear to be located between the latter two putative sub-regions of the TPJ, implicating both social-cognitive and attentional processes.
Apart from right STG, there was evidence (at less conservative significance levels) of functional overlaps between dialogic inner speech and ToM in an area of right MTG that has been previously linked to retrieval of face-word associations (Henke et al., 2003) and reflection on third-person traits (Kjaer et al., 2002). There was also overlap in posterior midline structures, although generally the two processes appeared to involve separate parts of the precuneus and posterior cingulate cortex, with the ToM cluster much closer to the midline. Dialogic inner speech also prompted activation in anterior medial frontal gyrus but ToM reasoning did not (cf. Walter et al., 2004).
The involvement of anterior and posterior midline structures in the contrast between dialogic and monologic conditions may indicate that the default mode network (DMN) is involved in generating internal dialogue (Buckner et al., 2008). ToM, autobiographical memory and resting-state cognition have been proposed to draw on a shared ‘core’ network including mPFC, precuneus, posterior cingulate and TPJ (Spreng et al., 2009). If the dialogic quality of inner speech imbues it (compared with monologic inner speech) with qualities of open-endedness, flexibility and creativity (Fernyhough, 1996, 2009), then it would arguably draw on some of the same introspective processes that the DMN is thought to underpin.
The remaining clusters identified in the contrast between dialogic and monologic inner speech also point to a range of processes associated with DMN functioning. Left precuneus has been associated with the simulation of third-person perspectives (Ruby and Decety, 2001) and episodic memory retrieval (Zysset et al., 2002), while right posterior cingulate has been linked to retrieval of autobiographical memories (Fink et al., 1996; Ryan et al., 2001). One possibility is that dialogic scenarios simply place a greater demand on memory processes, requiring the representation of specific events or people that would otherwise not be needed for generating one’s own voice. This seems unlikely, however, given that the monologic and dialogic scenario pairs were chosen to have the same general content (a school visit, a job interview, etc.), which should have minimized any differences between the conditions in terms of autobiographical memory demands. Alternatively, it may be that the scene construction processes thought to underpin autobiographical memory retrieval (Hassabis and Maguire, 2009) are similar to those recruited in producing a realistic and immersive dialogue. A direct comparison of scene construction, autobiographical memory and inner speech would be required to parse out these possibilities.
The results from the individual differences analysis highlighted a slightly different range of activation foci to the group contrast for dialogic > monologic inner speech: specifically, vividness ratings correlated with activation in the right MTG and cingulate gyrus, while dialogic inner speech (assessed as a general trait) correlated with the same MTG area, plus two sections of right precuneus. This contrasts with the involvement of ‘classic’ inner speech areas (left IFG, STG and cerebellum) and the focus on right STG seen in the group analysis of dialogic vs monologic inner speech.
The lack of correlates in the individual differences analysis in left frontotemporal areas suggests that covert articulation, per se, may not be so important for generating particularly vivid or dialogic scenarios. Nevertheless, the other areas identified in this analysis implicate similar processes and networks to the group analysis. For instance, the right MTG and the two sections of the right precuneus that correlated with dialogic inner speech reports have previously been implicated in theory-of-mind (Atique et al., 2011; Brüne et al., 2011). Other regions identified in this analysis are associated with processes that are also likely to be involved in generating dialogic scenarios. For example, right MTG has been associated with accurate and confident recall (Chua et al., 2006, Giovanello et al., 2010), while the right precuneus has been associated with retrieval of verbal episodic memory (Fernandes et al., 2005), context-rich autobiographical memories (Gilboa et al., 2004) and first-person perspectives memories (sometimes called ‘field’ memories; Nigro and Neisser, 1983; Eich et al., 2009). The activation of cingulate gyrus for vividness ratings, though likely not specific to this process, has been linked previously to a right anterior insula network involved in affective engagement (Touroutoglou et al., 2012). When these results are taken together, it might suggest that the tendency to engage in dialogic inner speech in everyday life does not reflect a trait towards ‘more’ inner speech—understood simply as a greater frequency of covert articulation—but instead indicates a greater tendency to recall and re-engage in previous interactions with others, and perhaps even to use these episodic memories to plan future social interactions.
Limitations
One-key limitation in interpreting the present results is the extent to which the inner-speech task actually elicited inner speech. Participants were prompted to generate dialogic and monologic scenarios in inner speech, but they may have varied in their ability to do so, or may have drawn on other forms of simulation (such as visual imagery). Similar imagery-generation paradigms have been criticized in related fields (e.g. auditory imagery; Zatorre and Halpern, 2005) and in general it is preferable to include an objective test of inner speech use, such as paradigms that require participants to make rhyming judgements (Geva et al., 2011) or to assess metric stress (Aleman et al., 2005).
To address this limitation, we gathered behavioural data from a mock scanner task in which a separate set of participants reported on their imagery processes for each scenario used during scanning. Scenario stimuli generally prompted high levels of inner speech compared with visual imagery across both dialogic and monologic scenarios, while both kinds of scenario proved difficult to generate (in the sense of leading to post-scan reports of vivid auditory imagery) when inner speech was blocked via articulatory suppression (repeating days of the week). Additional corroboration of the paradigm was provided by the individual differences analysis of inner speech scores, which implicated broadly similar brain regions (right posterior temporal and midline structures) and similar processes (Theory-of-Mind, autobiographical recall) to the main dialogic–monologic contrast.
Taken together, these data at least partly address the concern that participants did not engage in inner speech in producing dialogic and monologic scenarios. Nevertheless, the results presented here need to be replicated alongside a battery of other inner speech measures that do not rely on participants’ self-reports (Aleman et al., 2005), to fully assess the extent to which our new paradigm elicits dialogic and monologic inner speech. The individual difference correlates in particular require replication in a much larger sample than tested here.
A second limitation is that the perspective-switching task did not produce consistent activation maps that could be used in the conjunction analysis, thus limiting the assessment of whether the dialogicality of inner speech depends purely on demands associated with generating and coordinating mental imagery. A novel imagery task was deployed here to match the structure and timing of the inner-speech task but it is possible that a different task with similar demand characteristics would have provided a better control. For instance, mental rotation tasks involve demands to generate and flexibly manipulate mental images, and are consistently associated with activation in a network of frontoparietal regions (McNorgan, 2012).
Implications for psychopathology
Notwithstanding these caveats, the results presented here could have important implications for understanding inner speech in both typical and atypical populations. Although the involvement of ToM-related networks in internal dialogue is perhaps unsurprising, our conjunction analysis findings align with the view that articulating different perspectives may be an important feature of more complex forms of inner speech (Fernyhough, 1996). Abnormalities in the interplay between inner speech and ToM networks may thus explain some important findings in atypical groups.
As a first example, dominant models of AVH explain the phenomenon in terms of misattributed inner speech but struggle to explain why these hallucinations are distinctly experienced in another person’s voice (Jones and Fernyhough, 2007). Previous work has already suggested that dialogic conceptions of inner speech may account for the presence of the voices of others in one’s head (Fernyhough, 2004). Our present study extends this by showing commonalities between many of the neural regions activated during AVH (such as left STG, left insula, left IFG) and those strongly activated during dialogic inner speech (Jardri et al., 2011; Kühn and Gallinat, 2012). Future studies should test the proposal that findings from AVH research can be accounted for by dialogic inner speech occurring in conjunction with altered activity in other neural areas, such as the SMA (McGuire et al., 1995; Raij and Riekki, 2012), causing it to be experienced as non–self-produced. Our study also implies that neuroscientific studies of AVH need to consider social-cognitive networks alongside speech processing to fully understand how such hallucinations occur (see also Bell, 2013).
As a second example, atypical ToM has for a long time been considered a core feature of autism spectrum disorder (Baron-Cohen et al., 1985), but differences in inner speech in autism have only been studied relatively recently (Whitehouse et al., 2006; Wallace et al., 2009; Holland and Low, 2010). Early experience in autism is characterized by delays in language development and significant difficulties with social and communicative interaction (WHO, 1993). If inner speech is shaped by communicative experience—as a Vygotskian approach would suggest—then qualitative differences in the inner speech of people on the autistic spectrum may also be expected (Fernyhough, 1996; Williams et al., 2012). The data presented here are consistent with the idea that there are important interconnections between atypical ToM skills and atypical inner speech, which may mutually inform one another over the course of development. The direction of this relationship remains to be explored: on the one hand, problems with ToM could cause a qualitatively different experience of inner speech in autism; on the other hand, a lack of conversational or communicative inner speech might impact ToM development through limiting opportunities for dialogic interaction with others (Fernyhough, 2008).
In conclusion, we have presented the first neuroimaging study of some important varieties of inner speech, focusing on the contrast between dialogic and monologic forms of self-talk. Our findings provide initial support for the idea that forms of inner speech exist which can be both phenomenologically and neurologically distinguished from the silent commentary of a single inner voice. The data presented here suggest that generating silent dialogues draws on a wider network than classical regions associated with language production and comprehension, including recruitment of a core part of the ToM network. Further work is needed to disambiguate (i) the exact processes shared between dialogic inner speech and ToM, (ii) the involvement of the DMN in this conjunction and (iii) relative contributions of inner speech and forms of mental imagery to creating vivid inner dialogues.
Supplementary Material
Acknowledgements
The authors thank Henrik Walter and Angela Ciaramidaro for providing test materials for the Theory-of-Mind task. They also thank Jon Simons for his helpful comments on the article.
Funding
This work was supported by the Wellcome Trust (WT098455). S.M.J. was supported by an Australian Research Council Discovery Early Career Researcher Award (DE140101077).
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Aichhorn M., Perner J., Weiss B., Kronbichler M., Staffen W., Ladurner G. (2009). Temporo-parietal junction activity in theory-of-mind tasks: falseness, beliefs, or attention. Journal of Cognitive Neuroscience, 21(6), 1179–92. [DOI] [PubMed] [Google Scholar]
- Alderson-Day B., Fernyhough C. (2015a). Inner speech: development, cognitive functions, phenomenology, and neurobiology. Psychological Bulletin, 141, 931–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson-Day B., Fernyhough C. (2015b). Relations among trait and state measures of inner speech quality using smartphone app experience sampling. Frontiers in Psychology, 6, 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson-Day B., McCarthy-Jones S., Bedford S., Collins H., Dunne H., Rooke C., et al. (2014). Shot through with voices: dissociation mediates the relationship between varieties of inner speech and auditory hallucination proneness. Consciousness and Cognition, 27, 288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman A., Formisano E., Koppenhagen H., Hagoort P., de Haan E.H.F., Kahn R.S. (2005). The functional neuroanatomy of metrical stress evaluation of perceived and imagined spoken words. Cerebral Cortex, 15(2), 221–8. [DOI] [PubMed] [Google Scholar]
- Atique B., Erb M., Gharabaghi A., Grodd W., Anders S. (2011). Task-specific activity and connectivity within the mentalizing network during emotion and intention mentalizing. Neuroimage, 55(4), 1899–911. [DOI] [PubMed] [Google Scholar]
- Baddeley A., Lewis V., Vallar G. (1984). Exploring the articulatory loop. The Quarterly Journal of Experimental Psychology Section A, 36(2), 233–52. [Google Scholar]
- Baron-Cohen S., Leslie A.M., Frith U. (1985). Does the autistic child have a “theory of mind”? Cognition, 21(1), 37–46. [DOI] [PubMed] [Google Scholar]
- Bell V. (2013). A community of one: social cognition and auditory verbal hallucinations. PLoS Biology, 11(12), e1001723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentall R.P. (1990). The illusion of reality: a review and integration of psychological research on hallucinations. Psychological Bulletin, 107(1), 82–95. [DOI] [PubMed] [Google Scholar]
- Brüne M., Özgürdal S., Ansorge N., von Reventlow H.G., Peters S., Nicolas V., et al. (2011). An fMRI study of “theory of mind” in at-risk states of psychosis: comparison with manifest schizophrenia and healthy controls. Neuroimage, 55(1), 329–37. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. (2008). The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Caplan R., Dapretto M. (2001). Making sense during conversation: an fMRI study. Neuroreport, 12(16), 3625–32. [DOI] [PubMed] [Google Scholar]
- Ciaramidaro A., Adenzato M., Enrici I., Erk S., Pia L., Bara B.G., et al. (2007). The intentional network: how the brain reads varieties of intentions. Neuropsychologia, 45(13), 3105–13. [DOI] [PubMed] [Google Scholar]
- Chua E.F., Schacter D.L., Rand-Giovannetti E., Sperling R.A. (2006). Understanding metamemory: neural correlates of the cognitive process and subjective level of confidence in recognition memory. Neuroimage, 29(4), 1150–60. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A., Renaud O., Van der Linden M. (2011). Frequency, characteristics and functions of future-oriented thoughts in daily life. Applied Cognitive Psychology, 25(1), 96–103. [Google Scholar]
- Eich E., Nelson A.L., Leghari M.A., Handy T.C. (2009). Neural systems mediating field and observer memories. Neuropsychologia, 47(11), 2239–51. [DOI] [PubMed] [Google Scholar]
- Ellison A., Schindler I., Pattison L.L., Milner A.D. (2004). An exploration of the role of the superior temporal gyrus in visual search and spatial perception using TMS. Brain, 127(10), 2307–15. [DOI] [PubMed] [Google Scholar]
- Emerson M.J., Miyake A. (2003). The role of inner speech in task switching: a dual-task investigation. Journal of Memory and Language, 48(1), 148–68. [Google Scholar]
- Fernandes M.A., Moscovitch M., Ziegler M., Grady C. (2005). Brain regions associated with successful and unsuccessful retrieval of verbal episodic memory as revealed by divided attention. Neuropsychologia, 43(8), 1115–27. [DOI] [PubMed] [Google Scholar]
- Fernyhough C. (1996). The dialogic mind: a dialogic approach to the higher mental functions. New Ideas in Psychology, 14(1), 47–62. [Google Scholar]
- Fernyhough C. (2004). Alien voices and inner dialogue: towards a developmental account of auditory verbal hallucinations. New Ideas in Psychology, 22(1), 49–68. [Google Scholar]
- Fernyhough C. (2008). Getting Vygotskian about theory of mind: mediation, dialogue, and the development of social understanding. Developmental Review, 28(2), 225–62. [Google Scholar]
- Fernyhough C. (2009). Dialogic thinking. In Winsler A., Fernyhough C., Montero I. editors. Private Speech, Executive Functioning, and the Development of Verbal Self-Regulation, pp. 42–52, Cambridge: Cambridge University Press. [Google Scholar]
- Fernyhough C. (2013). Inner speech. In Pashler H., editor. The Encyclopedia of the Mind, Vol. 9, pp. 418–20, Thousand Oaks, CA: SAGE Publications. [Google Scholar]
- Fink G.R., Markowitsch H.J., Reinkemeier M., Bruckbauer T., Kessler J., Heiss W.D. (1996). Cerebral representation of one’s own past: neural networks involved in autobiographical memory. Journal of Neuroscience, 16(13), 4275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P.C., Happé F., Frith U., Baker S.C., Dolan R.J., Frackowiak R.S.J., et al. (1995). Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition, 57(2), 109–28. [DOI] [PubMed] [Google Scholar]
- Ford J.M., Morris S.E., Hoffman R.E., Sommer I., Waters F., McCarthy-Jones S., et al. (2014). Studying hallucinations within the NIMH RDoC framework. Schizophrenia Bulletin, 40(Suppl. 4), S295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C. (1992). The Cognitive Neuropsychology of Schizophrenia. Lawrence Erlbaum: Hove. [Google Scholar]
- Geva S., Jones P.S., Crinion J.T., Price C.J., Baron J.-C., Warburton E.A. (2011). The neural correlates of inner speech defined by voxel-based lesion-symptom mapping. Brain, 134(Pt 10), 3071–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A., Winocur G., Grady C.L., Hevenor S.J., Moscovitch M. (2004). Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cerebral Cortex, 14(11), 1214–25. [DOI] [PubMed] [Google Scholar]
- Giovanello K.S., Kensinger E.A., Wong A.T., Schacter D.L. (2010). Age-related neural changes during memory conjunction errors. Journal of Cognitive Neuroscience, 22(7), 1348–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D., Maguire E.A. (2009). The construction system of the brain. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1521), 1263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke K., Mondadori C.R.A., Treyer V., Nitsch R.M., Buck A., Hock C. (2003). Nonconscious formation and reactivation of semantic associations by way of the medial temporal lobe. Neuropsychologia, 41(8), 863–76. [DOI] [PubMed] [Google Scholar]
- Hinke R.M., Hu X., Stillman A.E., Kim S.G., Merkle H., Salmi R., et al. (1993). Functional magnetic resonance imaging of Broca’s area during internal speech. Neuroreport, 4(6), 675–8. [DOI] [PubMed] [Google Scholar]
- Holland L., Low J. (2010). Do children with autism use inner speech and visuospatial resources for the service of executive control? Evidence from suppression in dual tasks. British Journal of Developmental Psychology, 28(2), 369–91. [DOI] [PubMed] [Google Scholar]
- Jardri R., Pouchet A., Pins D., Thomas P. (2011). Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. The American Journal of Psychiatry, 168(1), 73–81. [DOI] [PubMed] [Google Scholar]
- Jones S. (2009). The neuropsychology of covert and overt speech: implications for the study of private speech in children and adults. In Winsler A., Fernyhough C., Montero I., editors. Private Speech, Executive Functioning, and the Development of Verbal Self-Regulation, pp. 69–80, Cambridge: Cambridge University Press. [Google Scholar]
- Jones S.R., Fernyhough C. (2007). Neural correlates of inner speech and auditory verbal hallucinations: a critical review and theoretical integration. Clinical Psychology Review, 27, 140–54. [DOI] [PubMed] [Google Scholar]
- Kjaer T.W., Nowak M., Lou H.C. (2002). Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage, 17(2), 1080–6. [PubMed] [Google Scholar]
- Kühn S., Gallinat J. (2012). Quantitative meta-analysis on state and trait aspects of auditory verbal hallucinations in schizophrenia. Schizophrenia Bulletin, 38(4), 779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Woldorff M.G., Parsons L.M., Liotti M., Freitas C.S., Rainey L., et al. (2000). Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping, 10(3), 120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden D.E.J., Thornton K., Kuswanto C.N., Johnston S.J., van de Ven V., Jackson M.C. (2011). The brain’s voices: comparing nonclinical auditory hallucinations and imagery. Cerebral Cortex, 21(2), 330–7. [DOI] [PubMed] [Google Scholar]
- Mars R.B., Sallet J., Schüffelgen U., Jbabdi S., Toni I., Rushworth M.F.S. (2012). Connectivity-based subdivisions of the human right “temporoparietal junction area”: evidence for different areas participating in different cortical networks. Cerebral Cortex, 22(8), 1894–903. [DOI] [PubMed] [Google Scholar]
- Marvel C.L., Desmond J.E. (2010). Functional topography of the cerebellum in verbal working memory. Neuropsychology Review, 20(3), 271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy-Jones S., Fernyhough C. (2011). The varieties of inner speech: links between quality of inner speech and psychopathological variables in a sample of young adults. Consciousness and Cognition, 20, 1586–93. [DOI] [PubMed] [Google Scholar]
- McGuire P.K., David A.S., Murray R.M., Frackowiak R.S.J., Frith C.D., Wright I., et al. (1995). Abnormal monitoring of inner speech: a physiological basis for auditory hallucinations. The Lancet, 346(8975), 596–600. [DOI] [PubMed] [Google Scholar]
- McGuire P.K., Silbersweig D.A., Murray R.M., David A.S., Frackowiak R.S., Frith C.D. (1996). Functional anatomy of inner speech and auditory verbal imagery. Psychological Medicine, 26(1), 29–38. [DOI] [PubMed] [Google Scholar]
- McNorgan C. (2012). A meta-analytic review of multisensory imagery identifies the neural correlates of modality-specific and modality-general imagery. Frontiers in Human Neuroscience, 6, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner V. (2007). The Cognitive Neuroscience of Human Communication, 1st edn, New York: Psychology Press. [Google Scholar]
- Mitchell J.P. (2008). Activity in right temporo-parietal junction is not selective for theory-of-mind. Cerebral Cortex, 18(2), 262–71. [DOI] [PubMed] [Google Scholar]
- Morin A. (2009). Inner speech. In Bayne T., Cleeremans A., Wilken P., editors. Oxford Companion to Consciousness, pp. 380–2, Oxford: OUP. [Google Scholar]
- Morin A., Uttl B., Hamper B. (2011). Self-reported frequency, content, and functions of inner speech. Procedia - Social and Behavioral Sciences, 30, 1714–8. [Google Scholar]
- Nigro G., Neisser U. (1983). Point of view in personal memories. Cognitive Psychology, 15(4), 467–82. [Google Scholar]
- Nolen-Hoeksema S. (2004). The response styles theory. In Papageorgiou C., Wells A., editors. Depressive Rumination: Nature, Theory and Treatment, pp. 107–24, Chichester: Wiley. [Google Scholar]
- Price C.J., Friston K.J. (1997). Cognitive conjunction: a new approach to brain activation experiments. Neuroimage, 5(4), 261–70. [DOI] [PubMed] [Google Scholar]
- Raij T.T., Riekki T.J.J. (2012). Poor supplementary motor area activation differentiates auditory verbal hallucination from imagining the hallucination. Neuroimage: Clinical, 1(1), 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C., Karnath H.-O., Bonilha L. (2007). Improving lesion-symptom mapping. Journal of Cognitive Neuroscience, 19(7), 1081–8. [DOI] [PubMed] [Google Scholar]
- Ruby P., Decety J. (2001). Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nature Neuroscience, 4(5), 546–50. [DOI] [PubMed] [Google Scholar]
- Ryan L., Nadel L., Keil K., Putnam K., Schnyer D., Trouard T., et al. (2001). Hippocampal complex and retrieval of recent and very remote autobiographical memories: evidence from functional magnetic resonance imaging in neurologically intact people. Hippocampus, 11(6), 707–14. [DOI] [PubMed] [Google Scholar]
- Saxe R., Kanwisher N. (2003). People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind.” Neuroimage, 19(4), 1835–42. [DOI] [PubMed] [Google Scholar]
- Saxe R., Powell L.J. (2006). It’s the thought that counts: specific brain regions for one component of theory of mind. Psychological Science, 17(8), 692–9. [DOI] [PubMed] [Google Scholar]
- Schneider W., Eschman A., Zuccolotto A. (2002). E-Prime Reference Guide. Pittsburgh: Psychology Software Tools, Inc. [Google Scholar]
- Scholz J., Triantafyllou C., Whitfield-Gabrieli S., Brown E.N., Saxe R. (2009). Distinct regions of right temporo-parietal junction are selective for theory of mind and exogenous attention. PLoS One, 4(3), e4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C.L., Fontaine N.M.G., Bird G., Blakemore S.-J., Brito S.A.D., McCrory E.J. P., et al. (2012). Neural processing associated with cognitive and affective theory of mind in adolescents and adults. Social Cognitive and Affective Neuroscience, 7(1), 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N.J., Marshall J.C., Zafiris O., Schwab A., Zilles K., Markowitsch H.J., et al. (2001). The neural correlates of person familiarity: a functional magnetic resonance imaging study with clinical implications. Brain, 124(4), 804–15. [DOI] [PubMed] [Google Scholar]
- Shergill S.S., Brammer M.J., Fukuda R., Bullmore E., Amaro E., Jr, Murray R.M., et al. (2002). Modulation of activity in temporal cortex during generation of inner speech. Human Brain Mapping, 16(4), 219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill S.S., Bullmore E.T., Brammer M.J., Williams S.C., Murray R.M., McGuire P.K. (2001). A functional study of auditory verbal imagery. Psychological Medicine, 31(2), 241–53. [DOI] [PubMed] [Google Scholar]
- Simons C.J.P., Tracy D.K., Sanghera K.K., O’Daly O., Gilleen J., Dominguez M.-G., et al. (2010). Functional magnetic resonance imaging of inner speech in schizophrenia. Biological Psychiatry, 67(3), 232–7. [DOI] [PubMed] [Google Scholar]
- Slotnick S.D., Moo L.R., Segal J.B., Hart J., Jr. (2003). Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research, 17(1), 75–82. [DOI] [PubMed] [Google Scholar]
- Slotnick S.D., Schacter D.L. (2004). A sensory signature that distinguishes true from false memories. Nature Neuroscience, 7(6), 664–72. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Mar R.A., Kim A.S.N. (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience, 21(3), 489–510. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. (1988). Co-planar Stereotaxic Atlas of the Human Brain: 3-dimensional Proportional System. Thieme Medical Pub, New York. [Google Scholar]
- Touroutoglou A., Hollenbeck M., Dickerson B.C., Barrett L.F. (2012). Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage, 60(4), 1947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vygotsky L.S. (1987). Thinking and Speech. The Collected Works of Lev Vygotsky, Vol. 1, New York: Plenum Press. [Google Scholar]
- Wallace G.L., Anderson M., Happé F. (2009). Brief report: information processing speed is intact in autism but not correlated with measured intelligence. Journal of Autism and Developmental Disorders, 39(5), 809–14. [DOI] [PubMed] [Google Scholar]
- Walter H., Adenzato M., Ciaramidaro A., Enrici I., Pia L., Bara B. (2004). Understanding intentions in social interaction: the role of the anterior paracingulate cortex. Journal of Cognitive Neuroscience, 16(10), 1854–63. [DOI] [PubMed] [Google Scholar]
- Whitehouse A.J., Maybery M.T., Durkin K. (2006). Inner speech impairments in autism. Journal of Child Psychology and Psychiatry, 47(8), 857–65. [DOI] [PubMed] [Google Scholar]
- WHO. (1993). The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva: World Health Organization. [Google Scholar]
- Williams D., Bowler D.M., Jarrold C. (2012). Inner speech is used to mediate short-term memory, but not planning, among intellectually high-functioning adults with autism spectrum disorder. Development and Psychopathology, 24(01), 225–39. [DOI] [PubMed] [Google Scholar]
- Woo C.-W., Krishnan A., Wager T.D. (2014). Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage, 91, 412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao B., Belin P., Scheepers C. (2011). Silent reading of direct versus indirect speech activates voice-selective areas in the auditory cortex. Journal of Cognitive Neuroscience, 23(10), 3146–52. [DOI] [PubMed] [Google Scholar]
- Yao B., Belin P., Scheepers C. (2012). Brain “talks over” boring quotes: top-down activation of voice-selective areas while listening to monotonous direct speech quotations. Neuroimage, 60(3), 1832–42. [DOI] [PubMed] [Google Scholar]
- Zacks J.M. (2007). Neuroimaging studies of mental rotation: a meta-analysis and review. Journal of Cognitive Neuroscience, 20(1), 1–19. [DOI] [PubMed] [Google Scholar]
- Zatorre R.J., Halpern A.R. (2005). Mental concerts: musical imagery and auditory cortex. Neuron, 47(1), 9–12. [DOI] [PubMed] [Google Scholar]
- Zysset S., Huber O., Ferstl E., von Cramon D.Y. (2002). The anterior frontomedian cortex and evaluative judgment: an fMRI study. Neuroimage, 15(4), 983–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.