Abstract
Communication between the amygdala and other brain regions critically regulates sensitivity to threat, which has been associated with risk for mood and affective disorders. The extent to which these neural pathways are genetically determined or correlate with risk-related personality measures is not fully understood. Using functional magnetic resonance imaging, we evaluated independent and interactive effects of the 5-HTTLPR genotype and neuroticism on amygdala functional connectivity during an emotional faces paradigm in 76 healthy individuals. Functional connectivity between left amygdala and medial prefrontal cortex (mPFC) and between both amygdalae and a cluster including posterior cingulate cortex, precuneus and visual cortex was significantly increased in 5-HTTLPR S′ allele carriers relative to LALA individuals. Neuroticism was negatively correlated with functional connectivity between right amygdala and mPFC and visual cortex, and between both amygdalae and left lateral orbitofrontal (lOFC) and ventrolateral prefrontal cortex (vlPFC). Notably, 5-HTTLPR moderated the association between neuroticism and functional connectivity between both amygdalae and left lOFC/vlPFC, such that S′ carriers exhibited a more negative association relative to LALA individuals. These findings provide novel evidence for both independent and interactive effects of 5-HTTLPR genotype and neuroticism on amygdala communication, which may mediate effects on risk for mood and affective disorders.
Keywords: amygdala, prefrontal cortex, 5-HTTLPR, neuroticism, functional connectivity, fMRI
Introduction
A large corpus of research implicates dysfunction within a corticolimbic circuit encompassing the amygdala and medial prefrontal cortex (mPFC), including the anterior cingulate cortex (ACC), in the pathophysiology of mood and affective disorders (LeDoux, 2000; Mayberg, 2003), including major depression (Groenewold et al., 2013), bipolar disorder (Blond et al., 2012) and panic disorder (Dresler et al., 2013). This circuit critically regulates responses to salient environmental stimuli, including indices of threat (Phillips et al., 2004; Quirk and Mueller, 2008). Communication between the amygdala and additional distributed brain regions also critically shapes sensitivity to threat and related behavioral phenotypes but remains less well studied (Adolphs, 2010; Pessoa and Adolphs, 2010). Thus, a better understanding of the mechanisms that shape this communication and its association with personality features would be beneficial.
The serotonin (5-HT) system is centrally involved in mood and affect in healthy individuals and dysfunction in serotonin signaling is implicated in the pathophysiology of mood and affective disorders (Lucki, 1998; Albert and Benkelfat, 2013). The serotonin transporter (5-HTT) linked polymorphic region (5-HTTLPR) is a commonly studied polymorphism in the promoter region of the 5-HTT gene including a short/long (S/L) allele in conjunction with rs25531, a single nucleotide polymorphism (A/G). In vitro, the LA allele is characterized by increased gene transcription compared with the other ‘S-type’ alleles (S′= SA, SG or LG) (Lesch et al., 1996; Hu et al., 2006). This polymorphism has been used as a model for differences in serotonin signaling affecting neuro-developmental processes or signaling in adulthood (Hariri et al., 2006). Additionally, the S′ allele is considered to be a risk factor for depression in the context of stressful life events (SLE) (McGuffin et al., 2011). Thus, elucidating effects of 5-HTTLPR on amygdala communication would inform models of how it shapes environmental sensitivity and risk for mood and affective disorders.
Although the S′ allele has been associated with increased threat-related amygdala reactivity (Hariri et al., 2002, 2005), subsequent studies and meta-analyses suggest main effects account for <1% of the variation in this measure (Murphy et al., 2013; Bastiaansen et al., 2014). However, given the distributed communication between the amygdala and other brain regions, going beyond reactivity and evaluating 5-HTTLPR effects on measures of amygdala functional connectivity (i.e. measures of correlated brain activity) may provide complementary insight into its effects on brain function and related risk (Meyer-Lindenberg, 2009; Rowe, 2010). In line with this, studies have investigated functional connectivity between amygdala and prefrontal areas and reported that the S allele predicted decreased amygdala-perigenual ACC functional coupling (Pezawas et al., 2005) and increased amygdala-ventromedial PFC (vmPFC) functional coupling (Heinz et al., 2005; Friedel et al., 2009). Psychophysiological interaction (PPI) is a functional connectivity approach that models task-dependent variation in functional connectivity, which is intriguing considering evidence for differential effects of negative and neutral emotional faces on amygdala and mPFC reactivity (Fusar-Poli et al., 2009; Etkin et al., 2011). However, there is a limited understanding about 5-HTTLPR effects on emotional face dependent amygdala-prefrontal functional connectivity in healthy adults and less known about functional connectivity with other brain regions. Thus, an evaluation of these effects would shed light on neurobiological mechanisms shaped by 5-HTTLPR genotype.
The personality trait neuroticism is strongly associated with increased risk for depression (Kendler et al., 2006; Kotov et al., 2010; Klein et al., 2011), increased rumination (Roberts et al., 1998), maladaptive coping strategies and increased vulnerability following stressful events (Cimbolic Gunthert et al., 1999; Campbell-Sills et al., 2006; Shoji et al., 2010). A recent study reported that 5-HTTLPR moderated the association between neuroticism and vulnerability to SLE (Markus, 2013). High neuroticism S′S′ individuals exhibited increased vulnerability, illuminating potential interactive effects of 5-HTTLPR and neuroticism on psychological vulnerability. In the context of neuroimaging, one previous functional magnetic resonance imaging (fMRI) study investigated associations between neuroticism and threat-related amygdala functional connectivity and reported a negative and positive association with mPFC/ACC and dorsomedial prefrontal cortex (dmPFC), respectively (Cremers et al., 2010). However, no study has evaluated the relation between threat-related amygdala functional connectivity and neuroticism in the context of 5-HTTLPR genotype.
Within this study, we sought to further characterize effects of 5-HTTLPR genotype and neuroticism on the engagement of amygdala-related neural pathways in response to threat-related stimuli within a cohort of 76 healthy individuals. Furthermore, by evaluating the extent to which associations with neuroticism depended on genotype, we aimed to identify functional circuits related to characteristics that have been tied to vulnerability to SLE (i.e. S′S′ genotype and high neuroticism). Due to the posited importance of the amygdala-prefrontal circuit, we first evaluated effects of genotype on threat-related connectivity within this region. We then conducted whole-brain analyses to identify distributed networks of amygdala communication associated with 5-HTTLPR genotype and neuroticism.
Methods
Participants
Data from 76 healthy participants (mean ± SD age: 25.63 ± 5.23, nine females), pooled from a cross-section of studies, were included in this study. All participants completed the same MRI scan session and related questionnaires. Participants were recruited from the Copenhagen region via online advertisements for research projects approved by the Ethics Committee of Copenhagen and Frederiksberg, Denmark (H-1-2010-091, amendments: 28633, 30043; H-1-2010-085, amendments 28641, 33540; (KF)01-2006-20, amendment: 23504, 23830). Inclusion criteria included: (i) 18–50 years of age, (ii) no present or past psychiatric or neurological illness, (iii) no present or past substance or alcohol abuse and (iv) normal physical examination and blood screening results. Informed consent was obtained prior to study participation. All participants had structural MR-images free from abnormalities. Neuroimaging data from this study have been included in previously published studies (Fisher et al., 2014, 2015a,b).
Personality assessment
Participants completed the Danish version of NEO Personality Inventory Revised (NEO-PI-R) self-report personality questionnaire (Costa and McCrae, 1992; Skovdahl-Hansen et al., 2004). NEO-PI-R consists of 240 items and evaluates five overall personality dimensions: neuroticism, extraversion, openness, agreeableness and conscientiousness.
Genotyping
Analysis of the 5-HTTLPR triallelic carrier status was performed on DNA purified from saliva. Samples were collected using the Oragene DNA self-collection kit OG-500 from DNA Genotek. Genotype status for the 5-HTTLPR (SLC6A4; 17q11.1-q12) was performed using a TaqMan 5-exonuclease allelic discrimination assay (Assay-on-Demand, Applied Biosystems, Foster City, CA). The ABI 7500 multiplex polymerase chain reaction (PCR) device (Applied Biosystems) was used for this analysis. Genotyping of the rs25531 A/G single nucleotide polymorphism for determination of ‘triallelic’ 5-HTTLPR status (i.e. LA, LG and S) was determined by PCR amplification from the forward primer 5′-GGCGTTGCCGCTCTGAATGC-3′ and reverse primer 5′-GAGGGACTGAGCTGGACAACCAC-3′. The fragments were then digested by the restriction enzyme MspI and separated by gel electrophoresis.
MRI acquisition
Participants underwent a scan session in a 3T-Trio MRI scanner using an eight-channel head coil (Siemens, Erlangen, Germany) as described in Grady et al. (2013) and Fisher et al. (2014). Blood oxygen level-dependent fMRI images were acquired using a T2*-weighted gradient-echo planar imaging sequence (repetition time = 2500 ms, echo time = 26 ms, flip angle = 76°, in-plane matrix = 64 × 64, in-plane resolution = 3 × 3 mm, number of slices within a whole-brain volume = 41, slice thickness = 3 mm, gap = 0.75 mm). Image acquisition was optimized for signal recovery within orbital frontal cortex by tilting slice orientation from a transverse toward a coronal orientation by ∼30°, and by using a preparation gradient pulse (Deichmann et al., 2003). A total of 312 whole-brain volumes (156 per run) were acquired for each scan session. A high-resolution T1-weighted whole-brain three-dimensional structural magnetic resonance scan was also acquired using a spin-echo sequence, (inversion time = 800 ms, echo time = 3.92 ms, repetition time = 1540 ms, flip angle = 9°, in-plane matrix = 256 × 256, in-plane resolution = 1 × 1 mm, number of slices = 192, slice thickness = 1 mm, no gap).
Emotional faces paradigm
During the fMRI scan session, participants completed a gender matching task on blocks of either fearful, angry or neutral faces from the Karolinska Directed Emotional Faces database (Lundqvist et al., 1998). The task consisted of two runs, totaling 32 blocks of neutral faces, 16 blocks of angry faces and 16 blocks of fearful faces. Stimulus presentations and response recordings were performed using E-prime (Psychological Software Tools, Pittsburgh, PA). For further task description, see Grady et al. (2013), Hornboll et al. (2013) and Fisher et al. (2014).
fMRI data analysis
Functional images were pre-processed in SPM5 (http://www.fil.ion.ucl.ac.uk/spm/), including realignment of functional images to a subject-specific mean functional image and co-registeration with the high-resolution T1-weighted structural image. Functional images were normalized into a standard Montreal Neurological Institute space (MNI space) based on normalization of the T1-weighted structural image determined using VBM5 (http://dbm.neuro.uni-jena.de/vbm/vbm5-for-spm5/) and smoothed with an 8 mm Gaussian kernel. Smoothed functional images were included in single subject general linear models (GLM) including task conditions (i.e. fear, angry and neutral faces) and regressors accounting for head movement, heart beat and respiration. We employed a canonical hemodynamic response function to estimate task condition effects (i.e. beta images), which were used to construct contrast images for our effect of interest [i.e. aversive (fear and angry) vs neutral faces] (Hornboll et al., 2013; Fisher et al., 2014). These contrast images were entered into second-level design matrices to determine main effects of task.
PPI analysis
We evaluated task-dependent variation in functional connectivity using a PPI analysis (Friston et al., 1997; O'Reilly et al., 2012). A PPI effect can be interpreted such that reactivity in a seed region contributes to the reactivity in another region depending on the experimental condition. We defined a left and right amygdala seed (119 and 107 voxels, respectively) based on the functional response to task across all participants within an amygdala ROI defined using the WFU Pickatlas, version 3.0.3 (Maldjian et al., 2003). We created single subject PPI models for estimating threat-related functional connectivity using the generalized PPI (gPPI) toolbox (http://www.nitrc.org/projects/gppi) implemented in SPM8. gPPI is a specific implementation of PPI, which allows for estimating connectivity for more than two experimental conditions (McLaren et al., 2012; Cisler et al., 2014). We created independent PPI models for each participant, for each amygdala seed. Single subject PPI GLMs were generated by first extracting the mean time series across voxels within a seed for each participant. The time series was then deconvolved to estimate neural activity and create a separate interaction term between the deconvolved time series and each task condition, which was subsequently reconvolved with the canonical hemodynamic response function (Gitelman et al., 2003). The seed time series and three reconvolved interaction terms were entered into a single subject PPI GLM including task conditions and regressors as described above. Threat-related differences in functional connectivity were determined by contrasting task-specific beta estimates for respective task-specific interaction terms (i.e. aversive vs neutral faces). These contrast images were then entered into second-level design matrices to determine main effects of functional connectivity and associations with variables of interest (i.e. neuroticism and 5-HTTLPR status).
Data analysis
Group-level main effects of task reactivity and PPI were evaluated in SPM8. Two regions of interest were defined using the WFU Pickatlas: a bilateral amygdala ROI and an ACC/mPFC (Brodmann areas 24, 25 and 32, 3D dilation = 1) ROI, which shares direct anatomical connections with the amygdala (Pandya et al., 1981; Barbas, 1995; Ongur and Price, 2000). Main effects of neuroticism and 5-HTTLPR (S′ carriers vs LALA) on reactivity and functional connectivity were evaluated in group level GLMs, including age and sex as covariates. Where statistically significant associations were observed, we extracted mean functional connectivity estimates from those regions for further analysis and visualization. Extracted estimates were evaluated in linear regression models within the statistical package R version 3.0.2 (R Core Team, 2013). Genotype-by-neuroticism interaction effects were tested on these extracted estimates within linear regression models. A significance threshold of q < 0.05, false-discovery rate DFR corrected, was applied to this set of interaction analyses to control type-I error (Benjamini and Hochberg, 2005).
To account for the issue of multiple comparisons with voxel-level analyses, 3dClustSim, a program using a Monte Carlo simulation method within the Analysis of Functional NeuroImages (AFNI) neuroimaging software package (http://www.afni.nimh.nih.gov/afni/), was used to calculate a cluster size unlikely to occur by chance (α < 0.05) at an uncorrected voxel-level threshold of P < 0.01. The cluster extent thresholds required for amygdala, ACC/mPFC and whole-brain search volume were 12, 196 and 555 voxels, respectively.
As a post hoc analysis, we evaluated functional connectivity estimates from our data within ROIs previously associated with 5-HTTLPR genotype (Heinz et al., 2005; Pezawas et al., 2005; Friedel et al., 2009) and neuroticism (Cremers et al., 2010). We extracted mean functional connectivity estimates from a 10 mm radius sphere centered on previously reported peak voxel coordinates.
Results
Demographic information is detailed within Table 1. The personality measure neuroticism did not differ between 5-HTTLPR genotype groups (P = 0.858). Neuroticism scores were similar to those reported within a normative population of young Danish men (Skovdahl-Hansen et al., 2004). There was an overrepresentation of men, 5-HTTLPR LALA and S′S′ individuals, reflecting sex- and genotype-specific recruitment criteria for the studies from which data were drawn.
Table 1.
Demographic information
| S′LA/S′S′ | LALA | P-value | |
|---|---|---|---|
| N | 45 | 31 | — |
| Age | 25.66 ± 5.37 | 25.58 ± 5.11 | 0.946 |
| Sex (male/female) | 40/5 | 27/4 | 0.812 |
| Neuroticism | 74.22 ± 18.67 | 75.10 ± 23.67 | 0.858 |
| Extraversion | 119.29 ± 19.46 | 120.29 ± 19.33 | 0.826 |
LALA: 31, LAS′: 22, S′S′: 23; (S′= S A/LG); P-value corresponds to two-sample t-test or χ2-test (two-tailed). Values reflect mean ± s.d.
Main effects of threat-related reactivity and functional connectivity
Reactivity was statistically significantly increased during aversive vs neutral faces within our amygdala ROI (left amygdala: [−28, −4, −18], z = 6.16, k = 119 voxels, P < 0.05, corrected; right amygdala: [30, −2, −20], z = 7.01, k = 107 voxels, P < 0.05, corrected). We observed distributed whole-brain task-related reactivity including increased response to aversive faces in visual cortex, fusiform gyrus and amygdala (Supplementary data, Table 1). We did not observe a statistically significant main effect of amygdala PPI (i.e. amygdala functional connectivity during aversive faces was not statistically different from functional connectivity during neutral faces).
5-HTTLPR, reactivity and threat-related functional connectivity
We did not observe any statistically significant associations between mean extracted reactivity estimates of amygdala reactivity and 5-HTTLPR (S′ carriers vs LALA: left amygdala [95% confidence interval (CI)]: 0.023 [−0.27, 0.73], P = 0.39; right amygdala [95% CI]: 0.32 [−0.21, 0.86], P = 0.23). Voxel-level analyses of our mPFC ROI and whole brain did not yield any clusters in which 5-HTTLPR genotype was statistically significantly associated with reactivity.
Regarding amygdala PPI, we observed a cluster within our a priori mPFC ROI, wherein S′ carriers showed statistically significantly greater left amygdala functional connectivity ([−8, 40, 0], z = 3.23, k = 284 voxels, P < 0.05, corrected; Table 2). This effect was not statistically significant within our mPFC ROI for right amygdala functional connectivity.
Table 2.
Results of voxel-based PPI analysis
| X, Y, Z | Z-score | Cluster size | |
|---|---|---|---|
| 5-HTTLPR | |||
| Left amygdala seed, S′ carrier > LALA | |||
| Medial prefrontal cortexa | −8, 40, 0 | 3.23 | 284 |
| Medial prefrontal cortex | −12, 46, −18 | 3.81 | 744 |
| Visual cortex, precuneus and PCC | −2, −54, 42 | 4.04 | 2732 |
| Right amygdala seed, S′ carrier > LALA | |||
| Visual cortex, precuneus and PCC | 6, −70, 24 | 4.19 | 3963 |
| Neuroticism | |||
| Left amygdala seed, negative correlation | |||
| Left lOFC, vlPFC and temporal pole | −28, 24, −12 | 4.37 | 1061 |
| Right amygdala seed, negative correlation | |||
| Prefrontal cortex incl. dmPFC, dlPFC and ACC | 26, 18, 30 | 4.49 | 2648 |
| Left lOFC, vlPFC and temporal pole | −46, 20, −18 | 4.19 | 1674 |
| Right parietal lobule | 64, −54, 26 | 4.16 | 714 |
| Visual cortex | −4, −96, 14 | 3.64 | 1322 |
Coordinates reported in Montreal Neurological Institute space. Statistical threshold for search volume: P < 0.01 (voxel-level), cluster > 501 voxels. PCC, posterior cingulate cortex; OFC, orbital frontal cortex; dmPFC, dorsomedial prefrontal cortex; vlPFC, ventrolateral PFC; dlPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex. aRegion-of-interest analysis statistical threshold: P < 0.01 (voxel-level), cluster > 174 voxels.
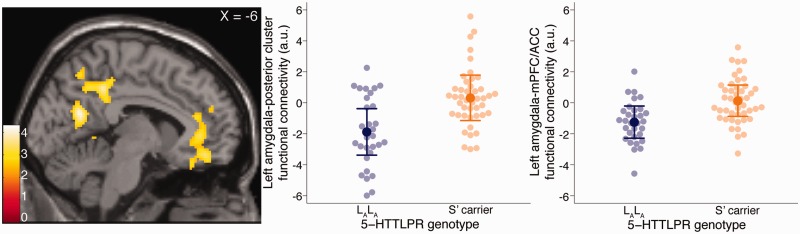
A whole-brain analysis revealed statistically significantly greater left amygdala functional connectivity in S′ carriers with a region including the previously described mPFC/ACC cluster, extending into vmPFC ([−12, 46, −18], z = 3.81, k = 744 voxels, P < 0.05, corrected; Figure 1 and Table 2). Additionally, S′ carriers showed statistically significantly greater right and left amygdala functional connectivity with medial posterior regions including visual areas, precuneus and posterior cingulate cortex (PCC) (left amygdala seed: [−2, −54, 42], z = 4.04, k = 2732 voxels, P < 0.05, corrected; right amygdala seed: [6, −70, 24], z = 4.19, k = 3963 voxels, P < 0.05, corrected; Figure 1 and Table 2).
Fig. 1.
Effects of 5-HTTLPR on amygdala functional connectivity. Left, statistical parametric map showing two regions within which left amygdala functional connectivity was significantly greater in S′ carriers relative to LALA individuals. Color bar represents t-scores. Middle, plot of mean left amygdala functional connectivity with 2732 voxel cluster comprising visual, precuneus and PCC by 5-HTTLPR genotype. Right, plot of mean left amygdala functional connectivity with 744 voxel mPFC/ACC cluster by 5-HTTLPR genotype. Darker points and error bars reflect mean and 95% CI, respectively. Lighter points represent individual functional connectivity estimates, adjusted for effects of age, sex and neuroticism.
Neuroticism, reactivity and threat-related functional connectivity
There were no statistically significant associations between mean extracted estimates of amygdala reactivity and neuroticism (left amygdala [95% CI]: 0.0034 [−0.0092, 0.016], P = 0.59; right amygdala [95% CI]: 0.0059 [−0.0076, 0.019], P = 0.39). Voxel-level analyses of our mPFC ROI and whole-brain analyses did not yield any clusters in which neuroticism was statistically significantly associated with reactivity.
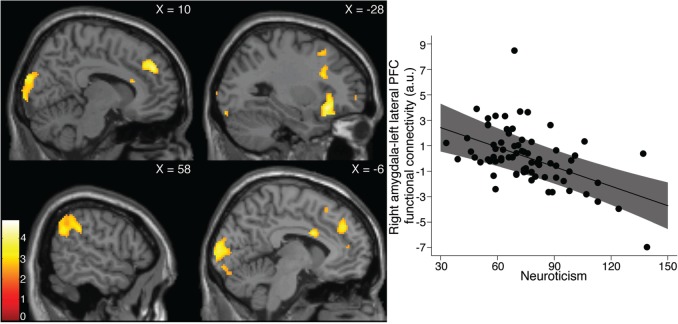
Within our mPFC ROI we did not observe any statistically significant associations between neuroticism scores and amygdala functional connectivity. A whole-brain analysis revealed a statistically significant negative association between neuroticism and left and right amygdala functional connectivity with a cluster including left lateral orbitofrontal cortex (lOFC), ventrolateral prefrontal cortex (vlPFC) and temporal pole (left amygdala: [−28, 24, −12], z = 4.37, k = 1061 voxels, P < 0.05, corrected; right amygdala: [−46, 20, −18], z = 4.19, k = 1674 voxels, P < 0.05, corrected; Figure 2 and Table 2). Further, for right amygdala, we observed statistically significant negative associations in visual cortex ([−4, −96, 14], z = 3.64, k = 1322 voxels, P < 0.05, corrected; Figure 2 and Table 2), a region including dmPFC, dorsolateral prefrontal cortex (dlPFC) and ACC ([26, 18, 30], z = 4.49, k = 2648 voxels, P < 0.05, corrected; Figure 2 and Table 2), and a cluster within right inferior parietal lobule including BA 40 ([64, −54, 26], z = 4.16, k = 714 voxels, P < 0.05, corrected; Figure 2 and Table 2).
Fig. 2.
Negative association between neuroticism and amygdala functional connectivity. Left, statistical parametric maps showing regions within which right amygdala functional connectivity was significantly negatively associated with neuroticism (upper left: dlPFC/dmPFC/ACC and visual cortex; upper right: left lOFC/vlPFC; lower left: right parietal lobule; lower right: dlPFC/dmPFC/ACC and visual cortex). Color bar represents t-scores. Right, representative plot of negative association: mean right amygdala functional connectivity with 1674 voxel cluster including left lOFC/vlPFC significantly negatively associated with neuroticism. Line and shading represents best fit and 95% CI of fit line, respectively. Individual functional connectivity estimates are adjusted for age, sex and 5-HTTLPR genotype.
5-HTTLPR-by-neuroticism effects on threat-related amygdala functional connectivity
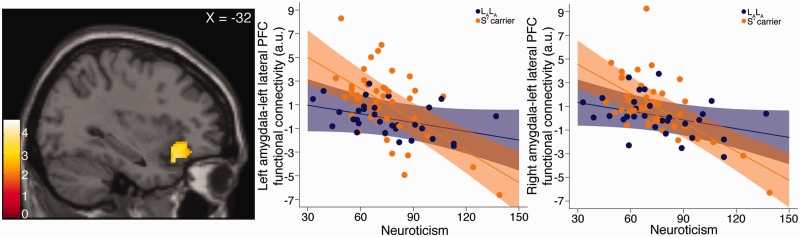
We then evaluated the extent to which 5-HTTLPR genotype moderated the association between neuroticism and threat-related amygdala functional connectivity. Focusing on extracted functional connectivity estimates from the clusters in which we observed a significant effect of 5-HTTLPR or neuroticism, we observed a significant moderation effect on functional connectivity between both amygdalae and left lOFC/vlPFC/temporal pole (interaction effect on extracted cluster connectivity estimate: left amygdala [95% CI]: −0.064 [−0.11, −0.018], puncorr = 0.0067, qFDR = 0.027; right amygdala: −0.057 [−0.097, −0.017], puncorr = 0.006, qFDR = 0.027; Figure 3). Specifically, S′ carriers showed a significantly more negative association between neuroticism scores and functional connectivity compared with LALA individuals.
Fig. 3.
The 5-HTTLPR genotype moderates the association between neuroticism and amygdala functional connectivity with lOFC/vlPFC/temporal pole. Left, statistical parametric map showing 1061 voxel cluster within which left amygdala functional connectivity was negatively associated with neuroticism. Middle, 5-HTTLPR significantly moderated this association such that it was more negative for S′ carriers compared with LALA individuals. Right, a similar interaction effect was observed for right amygdala functional connectivity with the left lOFC/vlPFC/temporal pole cluster that was significantly negatively associated with neuroticism (i.e. 1674 voxel cluster noted in upper right parametric map in Figure 2). Color bar represents t-scores. Lines and shading represent best fit and 95% CI on respective regression lines. Points represent individual mean functional connectivity estimates from respective clusters, adjusted for age, sex and main effects of 5-HTTLPR and neuroticism.
Post hoc comparison with previous studies
We compared our results with findings from previous studies investigating effects of 5-HTTLPR (Heinz et al., 2005; Pezawas et al., 2005; Friedel et al., 2009) and neuroticism (Cremers et al., 2010) on amygdala functional connectivity (Supplementary data, Table 2). We observed that S′ carriers had higher left amygdala PPI with a subgenual ACC region reported in Pezawas et al. (2005). We observed a statistically significantly negative association between neuroticism and right amygdala PPI with one of two dmPFC regions reported by Cremers et al. (2010) to be positively correlated with neuroticism.
Discussion
In this study, we evaluated associations of 5-HTTLPR genotype and neuroticism with amygdala reactivity and functional connectivity. Although we did not observe a significant effect of 5-HTTLPR genotype nor neuroticism on reactivity, we identified a cluster in mPFC/ACC with which left amygdala functional connectivity estimates were significantly increased in S′ carriers relative to LALA individuals during aversive (angry and fear) relative to neutral faces. A similar association was observed for functional connectivity between both amygdalae and medial posterior areas including precuneus, PCC and visual cortex. Regarding neuroticism, we observed a negative association with functional connectivity between right amygdala and regions within visual cortex, mPFC/ACC, dmPFC and right inferior parietal lobule. Further, a similar association was observed between both amygdalae and left lOFC/vlPFC/temporal pole. Interestingly, we observed a genotype-by-neuroticism interaction effect in left lOFC/vlPFC/temporal pole, such that neuroticism and functional connectivity were more negatively correlated in S′ carriers relative to LALA individuals. These intriguing findings further implicate the 5-HTTLPR polymorphism and neuroticism in affective and social brain function and indicate that increased vulnerability in S′ carriers and high neuroticism individuals may stem from variation in these distributed neural pathways.
5-HTTLPR S′ carriers relative to LALA individuals showed higher amygdala threat-related functional connectivity with mPFC/ACC. Previous studies have reported decreased and increased seemingly task-independent functional coupling in S carriers relative to LL individuals between the amygdala and ACC and ventromedial PFC, respectively (Heinz et al., 2005; Pezawas et al., 2005). The effect of 5-HTTLPR genotype on amygdala-prefrontal PPI that we observed includes subgenual ACC and vmPFC, which spatially overlaps the region described in Pezawas et al. (2005), encompassing a circuit strongly implicated in fear-related behavior and mood and affective disorders (LeDoux, 2000; Mayberg, 2003). Thus, our findings reinforce a role for this polymorphism in shaping threat-related corticolimbic circuit function. Although our post hoc analyses did not demonstrate clear genotype effects within previously described regions, we express caution in directly comparing these effects because of methodological differences in how functional connectivity was determined. Our findings suggest that S′ carriers are shifted toward heightened engagement of this amygdala-mPFC circuit in response to threat-related stimuli, which is consistent with the S′ allele being linked to increased sensitivity to negative environmental stimuli (Pergamin-Hight et al., 2012). Notably, the heightened connectivity of this circuit in S′ carriers may not be a risk factor on its own, but instead dependent on personality traits (e.g. neuroticism) and environment (e.g. SLE) (Markus, 2013). Our findings reinforce this circuit as a key neural pathway affected by 5-HTTLPR genotype.
More broadly, the amygdala plays a central role in orchestrating cortical processing of salient environmental stimuli, including visual cortices with which it shares direct anatomical projections (Iwai and Yukie, 1987; Amaral et al., 2003; Price, 2003; Vuilleumier, 2005; Pessoa and Adolphs, 2010; Pourtois et al., 2013). This motivated our whole-brain analysis of amygdala functional connectivity, which is relatively less well studied. We found that S′ carriers showed increased engagement of these pathways including greater bilateral amygdala connectivity with visual cortices, including parts of fusiform gyrus, a key face-processing brain region (Haxby et al., 2000). This suggests that heightened communication between amygdala and visual areas in response to socially relevant facial expressions may be an important neurobiological mechanism affected by 5-HTTLPR genotype (Surguladze et al., 2008; Pergamin-Hight et al., 2012). We hypothesize that these genetically driven biases in distributed amygdala communication capture relevant neurobiological mechanisms through which 5-HTTLPR genotype can affect risk for neuropsychiatric illness.
We found that neuroticism was negatively associated with functional connectivity between both right and left amygdala seeds and left lOFC/vlPFC/temporal pole. Additionally, neuroticism was negatively associated with functional connectivity between right amygdala and early visual areas, dmPFC and a region in the right inferior parietal lobule, including BA 40, a region found to habituate in response to repeatedly presented faces (Feinstein et al., 2002). Lateral OFC/vlPFC, encompassing BA47/12, has been tied to social behavior, including emotion regulation and reward-avoidance behavior (Kringelbach and Rolls, 2003, 2004; Ochsner et al., 2004; Rolls, 2004; Lieberman et al., 2007; Beesdo et al., 2009). Furthermore, lateral and posterior OFC share anatomical connections with the amygdala as well as visual areas, including BA 17, 18 and 19 (Amaral and Price, 1984; Barbas, 1988; Carmichael and Price, 1995). In a clinical context, left lOFC/vlPFC is recruited less efficiently in major depression disorder patients compared with healthy individuals (Greening et al., 2014). Complementary to this, our investigation identified decreased amygdala-left lOFC/vlPFC communication as correlated with neuroticism, further implicating this neural pathway in negative affect.
Our finding of a negative association between neuroticism and right amygdala-mPFC/ACC functional connectivity is consistent with a previously reported association (Cremers et al., 2010). However, our post hoc analyses indicate that the mPFC/ACC regions wherein we observed a significant effect do not overlap those reported by Cremers et al. (2010). These authors also reported a positive association with dmPFC. We did not observe this positive association, instead we observed a negative association. Our post hoc analysis replicating their model (i.e. including extraversion, excluding 5-HTTLPR genotype) did not substantively change our results (Supplementary data, Table 2). Although it is possible that methodological differences may explain this discrepancy [e.g. Cremers et al. (2010) included relatively more females, employed an event-related gender-matching design and contrasted angry and fearful faces separately with neutral faces], it is not clear why these would contribute to differences in observed effects. Thus, our findings reinforce a negative association between neuroticism and amygdala functional connectivity with mPFC/ACC and extend this observation to include left lOFC/vlPFC. Further evaluation is necessary to better understand the association between neuroticism and amygdala-dmPFC PPI.
Interestingly, we found that 5-HTTLPR genotype moderated the association between neuroticism and bilateral amygdala functional connectivity within left lOFC/vlPFC/temporal pole such that S′ carriers with higher neuroticism scores had more negative functional connectivity relative to LALA individuals. Although the S allele has been reported to be associated with anxiety-related traits (Lesch et al., 1996; Schinka et al., 2004; Sen et al., 2004), recent large studies have reported a non-significant association between 5-HTTLPR genotype and neuroticism-like traits, consistent with our sample (Munafo et al., 2005, 2009; Willis-Owen et al., 2005; Middeldorp et al., 2007; Terracciano et al., 2009; Minelli et al., 2011). Intriguingly, a recent study found that only in high neuroticism individuals did 5-HTTLPR genotype moderate the association between SLE and depressive symptoms (Markus, 2013). In light of this, we speculate that decreased amygdala communication with left lOFC/vlPFC/temporal pole may represent an important neural pathway underlying related risk in the context of adverse life events.
There are important limitations to consider with our study. Although our PPI analysis implicates relevant distributed neural pathways, it cannot determine directionality of signaling. We did not observe a statistically significant main effect of functional connectivity (i.e. aversive vs neutral faces), which is consistent with recent PPI studies (Cremers et al., 2010; Passamonti et al., 2012). This observation emphasizes the need for fMRI paradigms that more effectively engage amygdala-prefrontal and other distributed circuits, as this would make more robust such functional connectivity analyses. Given this non-significant main effect and the inherently exploratory nature of voxel-level analyses, we cannot rule out that our observed significant effects are free of false positives. That notwithstanding, our observed 5-HTTLPR effect within our a priori mPFC ROI and additional associations within known visual, face and threat processing regions lend construct validity to our findings. For these reasons, it is important that other investigators seek to replicate our finding. Our study included mostly males, and future studies would help to more precisely determine potential sex-differences in these neural pathways. The effect size of the 5-HTTLPR on threat-related amygdala functional connectivity is not well known and made more difficult by multiple methods for determining connectivity. Despite our attempts to relate our findings with previous studies, these comparisons should be interpreted with caution and emphasize a difficulty in synthesizing findings across functional connectivity studies. A 5-HTTLPR effect on neuroticism could introduce collinearity into our model, as these were each predictors. However, the nearly identical mean neuroticism scores of the genotype groups suggest that this bias is unlikely to have affected our current analysis.
Conclusion
In summary, we observed independent and interactive effects of 5-HTTLPR genotype and neuroticism on threat-related amygdala functional connectivity within regions critically involved in threat processing, emotion regulation and visual processing. These findings benefit our understanding of genetic sources of variability in threat-related neural pathways and highlight novel aspects of brain function, which may mediate 5-HTTLPR and neuroticism-related risk for mood and affective disorders.
Supplementary Material
Acknowledgements
We thank Sussi Larsen, Julian Macoveanu, and Pernille Iversen for assistance in data collection and Peter Jensen for assistance in data management. We thank the Hasholt-Nørremølle laboratory in the Section of Neurogenetics at the Department of Cellular and Molecular Medicine, University of Copenhagen, for their assistance with genotyping. We would like to acknowledge the Simon Spies Foundation for the donation of the Siemens Trio MRI scanner.
Funding
Collection of data that was included in the study was supported by the Lundbeck Foundation Center of Excellence Cimbi [R90-A7722]. M.K.M. was supported by a scholarship from CIMBI. B.M.M. was supported by a scholarship from Brain, Mind and Medicines. S.B.A. was supported by a scholarship from the Lundbeck Foundation [R78-A6881] and Danish Council for Independent Research [11-107187]. H.R.S. was supported by a Grant of Excellence ContAct from the Lundbeck Foundation [R59-A5399]. P.M.F. was supported by a grant from the Danish Council for Independent Research [11-115790].
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Adolphs R. (2010). What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences, 1191, 42–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert P.R., Benkelfat C. (2013). The neurobiology of depression—revisiting the serotonin hypothesis. II. Genetic, epigenetic and clinical studies. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences, 368(1615), 20120535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D.G., Behniea H., Kelly J.L. (2003). Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience, 118(4), 1099–120. [DOI] [PubMed] [Google Scholar]
- Amaral D.G., Price J.L. (1984). Amygdalo-cortical projections in the monkey (Macaca fascicularis). The Journal of Comparative Neurology, 230(4), 465–96. [DOI] [PubMed] [Google Scholar]
- Barbas H. (1988). Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. The Journal of Comparative Neurology, 276(3), 313–42. [DOI] [PubMed] [Google Scholar]
- Barbas H. (1995). Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neuroscience and Biobehavioral Reviews, 19(3), 499–510. [DOI] [PubMed] [Google Scholar]
- Bastiaansen J.A., Servaas M.N., Marsman J.B., et al. (2014). Filling the gap: relationship between the serotonin-transporter-linked polymorphic region and amygdala activation. Psychological Science, 25(11), 2058–66. [DOI] [PubMed] [Google Scholar]
- Beesdo K., Lau J.Y., Guyer A.E., et al. (2009). Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of General Psychiatry, 66(3), 275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (2005). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B, 57, 289–300. [Google Scholar]
- Blond B.N., Fredericks C.A., Blumberg H.P. (2012). Functional neuroanatomy of bipolar disorder: structure, function, and connectivity in an amygdala-anterior paralimbic neural system. Bipolar Disorders, 14(4), 340–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills L., Cohan S.L., Stein M.B. (2006). Relationship of resilience to personality, coping, and psychiatric symptoms in young adults. Behaviour Research and Therapy, 44(4), 585–99. [DOI] [PubMed] [Google Scholar]
- Carmichael S.T., Price J.L. (1995). Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. The Journal of Comparative Neurology, 363(4), 615–41. [DOI] [PubMed] [Google Scholar]
- Cimbolic Gunthert K., Cohen L.H., Armeli S. (1999). The role of neuroticism in daily stress and coping. Journal of Personality and Social Psychology, 77(5), 1087–100. [DOI] [PubMed] [Google Scholar]
- Cisler J.M., Bush K., Steele J.S. (2014). A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. Neuroimage, 84, 1042–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa P.T., Jr, McCrae R.R. (1992). Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) Professional Manual . Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Cremers H.R., Demenescu L.R., Aleman A., et al. (2010). Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. Neuroimage, 49(1), 963–70. [DOI] [PubMed] [Google Scholar]
- Deichmann R., Gottfried J.A., Hutton C., Turner R. (2003). Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage, 19(2 Pt 1), 430–41. [DOI] [PubMed] [Google Scholar]
- Dresler T., Guhn A., Tupak S.V., et al. (2013). Revise the revised? New dimensions of the neuroanatomical hypothesis of panic disorder. Journal of Neural Transmission, 120(1), 3–29. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein J.S., Goldin P.R., Stein M.B., Brown G.G., Paulus M.P. (2002). Habituation of attentional networks during emotion processing. Neuroreport, 13(10), 1255–8. [DOI] [PubMed] [Google Scholar]
- Fisher P.M., Grady C.L., Madsen M.K., Strother S.C., Knudsen G.M. (2015a). 5-HTTLPR differentially predicts brain network responses to emotional faces. Human Brain Mapping, 30(10), 22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P.M., Haahr M.E., Jensen C.G., Frokjaer V.G., Siebner H.R., Knudsen G.M. (2015b). Fluctuations in [C]SB207145 PET binding associated with change in threat-related amygdala reactivity in humans. Neuropsychopharmacology, 6(10), 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P.M., Madsen M.K., Mc Mahon B., et al. (2014). Three-week bright-light intervention has dose-related effects on threat-related corticolimbic reactivity and functional coupling. Biological Psychiatry, 76(4), 332–9. [DOI] [PubMed] [Google Scholar]
- Friedel E., Schlagenhauf F., Sterzer P., et al. (2009). 5-HTT genotype effect on prefrontal-amygdala coupling differs between major depression and controls. Psychopharmacology, 205(2), 261–71. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. (1997). Psychophysiological and modulatory interactions in neuroimaging. Neuroimage, 6(3), 218–29. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F., et al. (2009). Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience, 34(6), 418–32. [PMC free article] [PubMed] [Google Scholar]
- Gitelman D.R., Penny W.D., Ashburner J., Friston K.J. (2003). Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage, 19(1), 200–7. [DOI] [PubMed] [Google Scholar]
- Grady C.L., Siebner H.R., Hornboll B., Macoveanu J., Paulson O.B., Knudsen G.M. (2013). Acute pharmacologically induced shifts in serotonin availability abolish emotion-selective responses to negative face emotions in distinct brain networks. European Neuropsychopharmacology, 23(5), 368–78. [DOI] [PubMed] [Google Scholar]
- Greening S.G., Osuch E.A., Williamson P.C., Mitchell D.G. (2014). The neural correlates of regulating positive and negative emotions in medication-free major depression. Social Cognitive and Affective Neuroscience, 9(5), 628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewold N.A., Opmeer E.M., de Jonge P., Aleman A., Costafreda S. G. (2013). Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neuroscience & Biobehavioral Reviews, 37(2), 152–63. [DOI] [PubMed] [Google Scholar]
- Hariri A.R., Drabant E.M., Munoz K.E., et al. (2005). A susceptibility gene for affective disorders and the response of the human amygdala. Archives of General Psychiatry, 62(2), 146–52. [DOI] [PubMed] [Google Scholar]
- Hariri A.R., Drabant E.M., Weinberger D.R. (2006). Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biological Psychiatry, 59(10), 888–97. [DOI] [PubMed] [Google Scholar]
- Hariri A.R., Mattay V.S., Tessitore A., et al. (2002). Serotonin transporter genetic variation and the response of the human amygdala. Science, 297(5580), 400–3. [DOI] [PubMed] [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. (2000). The distributed human neural system for face perception. Trends in Cognitive Sciences, 4(6), 223–33. [DOI] [PubMed] [Google Scholar]
- Heinz A., Braus D.F., Smolka M.N., et al. (2005). Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nature Neuroscience, 8(1), 20–1. [DOI] [PubMed] [Google Scholar]
- Hornboll B., Macoveanu J., Rowe J., et al. (2013). Acute serotonin 2A receptor blocking alters the processing of fearful faces in the orbitofrontal cortex and amygdala. Journal of Psychopharmacology, 27(10), 903–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X.Z., Lipsky R.H., Zhu G., et al. (2006). Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. The American Journal of Human Genetics, 78(5), 815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai E., Yukie M. (1987). Amygdalofugal and amygdalopetal connections with modality-specific visual cortical areas in macaques (Macaca fuscata, M. mulatta, and M. fascicularis). The Journal of Comparative Neurology, 261(3), 362–87. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Gatz M., Gardner C.O., Pedersen N.L. (2006). Personality and major depression: a Swedish longitudinal, population-based twin study. Archives of General Psychiatry, 63(10), 1113–20. [DOI] [PubMed] [Google Scholar]
- Klein D.N., Kotov R., Bufferd S.J. (2011). Personality and depression: explanatory models and review of the evidence. Annual Review of Clinical Psychology, 7, 269–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R., Gamez W., Schmidt F., Watson D. (2010). Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychological Bulletin, 136(5), 768–821. [DOI] [PubMed] [Google Scholar]
- Kringelbach M.L., Rolls E.T. (2003). Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage, 20(2), 1371–83. [DOI] [PubMed] [Google Scholar]
- Kringelbach M.L., Rolls E.T. (2004). The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology, 72(5), 341–72. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23, 155–84. [DOI] [PubMed] [Google Scholar]
- Lesch K.P., Bengel D., Heils A., et al. (1996). Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science, 274(5292), 1527–31. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Eisenberger N.I., Crockett M.J., Tom S.M., Pfeifer J. H., Way B.M. (2007). Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science, 18(5), 421–8. [DOI] [PubMed] [Google Scholar]
- Lucki I. (1998). The spectrum of behaviors influenced by serotonin. Biological Psychiatry, 44(3), 151–62. [DOI] [PubMed] [Google Scholar]
- Lundqvist D., Flykt A., Ohman A. (1998). The Karolinska Directed Emotional Faces-KDEF. Stockholm: Department of Clinical Neuroscience, Psychology Section, Karolinska Institutet. [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19(3), 1233–9. [DOI] [PubMed] [Google Scholar]
- Markus C.R. (2013). Interaction between the 5-HTTLPR genotype, impact of stressful life events, and trait neuroticism on depressive symptoms in healthy volunteers. Psychiatric Genetics, 23(3), 108–16. [DOI] [PubMed] [Google Scholar]
- Mayberg H.S. (2003). Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. British Medical Bulletin, 65, 193–207. [DOI] [PubMed] [Google Scholar]
- McGuffin P., Alsabban S., Uher R. (2011). The truth about genetic variation in the serotonin transporter gene and response to stress and medication. The British Journal of Psychiatry, 198(6), 424–7. [DOI] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage, 61(4), 1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A. (2009). Neural connectivity as an intermediate phenotype: brain networks under genetic control. Human Brain Mapping, 30(7), 1938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp C.M., de Geus E.J., Beem A.L., et al. (2007). Family based association analyses between the serotonin transporter gene polymorphism (5-HTTLPR) and neuroticism, anxiety and depression. Behavior Genetics, 37(2), 294–301. [DOI] [PubMed] [Google Scholar]
- Minelli A., Bonvicini C., Scassellati C., Sartori R., Gennarelli M. (2011). The influence of psychiatric screening in healthy populations selection: a new study and meta-analysis of functional 5-HTTLPR and rs25531 polymorphisms and anxiety-related personality traits. BMC.Psychiatry, 11, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo M.R., Clark T., Flint J. (2005). Does measurement instrument moderate the association between the serotonin transporter gene and anxiety-related personality traits? A meta-analysis. Molecular Psychiatry, 10(4), 415–9. [DOI] [PubMed] [Google Scholar]
- Munafo M.R., Freimer N.B., Ng W., et al. (2009). 5-HTTLPR genotype and anxiety-related personality traits: a meta-analysis and new data. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 150B(2), 271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S.E., Norbury R., Godlewska B.R., et al. (2013). The effect of the serotonin transporter polymorphism (5-HTTLPR) on amygdala function: a meta-analysis. Molecular Psychiatry, 18(4), 512–20. [DOI] [PubMed] [Google Scholar]
- O'Reilly J.X., Woolrich M.W., Behrens T.E., Smith S.M., Johansen-Berg H. (2012). Tools of the trade: psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience, 7(5), 604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., et al. (2004). For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage, 23(2), 483–99. [DOI] [PubMed] [Google Scholar]
- Ongur D., Price J. L. (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex, 10(3), 206–19. [DOI] [PubMed] [Google Scholar]
- Pandya D.N., van Hoesen G.W., Mesulam M.M. (1981). Efferent connections of the cingulate gyrus in the rhesus monkey. Experimental Brain Research, 42(3-4), 319–30. [DOI] [PubMed] [Google Scholar]
- Passamonti L., Crockett M.J., Apergis-Schoute A.M., et al. (2012). Effects of acute tryptophan depletion on prefrontal-amygdala connectivity while viewing facial signals of aggression. Biological Psychiatry, 71(1), 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergamin-Hight L., Bakermans-Kranenburg M.J., van Ijzendoorn M.H., Bar-Haim Y. (2012). Variations in the promoter region of the serotonin transporter gene and biased attention for emotional information: a meta-analysis. Biological Psychiatry, 71(4), 373–9. [DOI] [PubMed] [Google Scholar]
- Pessoa L., Adolphs R. (2010). Emotion processing and the amygdala: from a ‘low road' to ‘many roads' of evaluating biological significance. Nature Reviews Neuroscience, 11(11), 773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L., Meyer-Lindenberg A., Drabant E.M., et al. (2005). 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience, 8(6), 828–34. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Williams L.M., Heining M., et al. (2004). Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage, 21(4), 1484–96. [DOI] [PubMed] [Google Scholar]
- Pourtois G., Schettino A., Vuilleumier P. (2013). Brain mechanisms for emotional influences on perception and attention: what is magic and what is not. Biological Psychology, 92(3), 492–512. [DOI] [PubMed] [Google Scholar]
- Price J.L. (2003). Comparative aspects of amygdala connectivity. Annals of the New York Acadademy of Sciences, 985, 50–8. [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Mueller D. (2008). Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology, 33(1), 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2013). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Roberts J.E., Gilbon E., Gotlib I.H. (1998). Ruminative response style and vulnerability to episodes of dysphoria: gender, neuroticism, and episode duration. Cognitive Therapy and Research, 22(4), 401–23. [Google Scholar]
- Rolls E.T. (2004). The functions of the orbitofrontal cortex. Brain and Cognition, 55(1), 11–29. [DOI] [PubMed] [Google Scholar]
- Rowe J.B. (2010). Connectivity analysis is essential to understand neurological disorders. Frontiers in Systems Neuroscience, 4, doi:10.3389/fnsys.2010.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinka J.A., Busch R.M., Robichaux-Keene N. (2004). A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Molecular Psychiatry, 9(2), 197–202. [DOI] [PubMed] [Google Scholar]
- Sen S., Burmeister M., Ghosh D. (2004). Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 127B(1), 85–9. [DOI] [PubMed] [Google Scholar]
- Shoji K., Harrigan J.A., Woll S.B., Miller S.A. (2010). Interactions among situations, neuroticism, and appraisals in coping strategy choice. Personality and Individual Differences, 48(3), 270–6. [Google Scholar]
- Skovdahl-Hansen H., Mortensen E.L., Schiotz H.K. (2004). Dokumentation for den danske udgave af NEO PI-R og NEO PI-R Kort Version. Copenhagen: Dansk Psykologisk Forlag. [Google Scholar]
- Surguladze S.A., Elkin A., Ecker C., et al. (2008). Genetic variation in the serotonin transporter modulates neural system-wide response to fearful faces. Genes, Brain and Behavior, 7(5), 543–51. [DOI] [PubMed] [Google Scholar]
- Terracciano A., Balaci L., Thayer J., et al. (2009). Variants of the serotonin transporter gene and NEO-PI-R Neuroticism: no association in the BLSA and SardiNIA samples. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 150B(8), 1070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P. (2005). How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences, 9(12), 585–94. [DOI] [PubMed] [Google Scholar]
- Willis-Owen S.A., Turri M.G., Munafo M.R., et al. (2005). The serotonin transporter length polymorphism, neuroticism, and depression: a comprehensive assessment of association. Biological Psychiatry, 58(6), 451–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.