Abstract
Emotion and cognition are dynamically coupled to bodily arousal: the induction of anger, even unconsciously, can reprioritise neural and physiological resources toward action states that bias cognitive processes. Here we examine behavioural, neural and bodily effects of covert anger processing and its influence on cognition, indexed by lexical decision-making. While recording beat-to-beat blood pressure, the words ANGER or RELAX were presented subliminally just prior to rapid word/non-word reaction-time judgements of letter-strings. Subliminal ANGER primes delayed the time taken to reach rapid lexical decisions, relative to RELAX primes. However, individuals with high trait anger were speeded up by subliminal anger primes. ANGER primes increased systolic blood pressure and the magnitude of this increase predicted reaction time prolongation. Within the brain, ANGER trials evoked an enhancement of activity within dorsal pons and an attenuation of activity within visual occipitotemporal and attentional parietal cortices. Activity within periaqueductal grey matter, occipital and parietal regions increased linearly with evoked blood pressure changes, indicating neural substrates through which covert anger impairs semantic decisions, putatively through its expression as visceral arousal. The behavioural and physiological impact of anger states compromises the efficiency of cognitive processing through action-ready changes in autonomic response that skew regional neural activity.
Keywords: systolic blood pressure, subliminal, lexical decision task, fMRI, emotion
Introduction
Affective states are expressed in body and brain, and these neural and physiological changes can influence subsequent cognitive processes. Different emotion types generally fall into prescribed and distinct patterns of autonomic and central activation (Harrison et al., 2013). These, in turn, can lead to predictable effects on cognition and behaviour, such as enhanced attentional capture and facilitated reaction time. Much research focuses on the effect of emotions such as fear, sadness and happiness, which align with psychopathological states of anxiety, depression and hypomania. However anger remains relatively understudied (in terms of neuronal and physiological mechanisms of action) and it is harder to predict the likely influences of anger on cognition and behaviour.
Exposure to most emotion categories can result in a mirroring, or embodiment, of that emotion in the perceiver (Harrison et al., 2006; Singer and Lamm, 2009; Harrison et al., 2010). Observing anger has the potential to elicit either an anger state, or a ‘complementary’ fear state (Harrison et al., 2013), reflecting the dual response of fight or flight. In accordance with its negative status, (subliminal) anger priming decreases liking judgments of neutral stimuli (Murphy and Zajonc, 1993) and enhances avoidance behaviours (Marsh et al., 2005). Anger nevertheless possesses motivating properties normally expressed by positive emotions and which facilitate behaviour. As a consequence of such observations, anger is also viewed as an approach emotion (van Honk and Schutter, 2006; Carver and Harmon-Jones, 2009; Lewis, 2010). Overt or subliminal anger primes elicited a greater desire to possess associated everyday objects compared to neutral or fear primes (Aarts et al., 2010). Anger is linked to ‘high optimism, positive expectations and experiences of high coping potential’ (Lerner and Keltner, 2001) and, importantly, anger is associated with perceived task ease, a status which again diverges from other negatively valenced emotions, such as sadness, which are associated with greater perceived task difficulty (Gendolla and Silvestrini, 2011).
Both facilitatory and impairing effects of anger thus have the potential to occur, with the direction and the magnitude of anger effects sensitive to context (e.g. obtainable reward, Aarts et al., 2010) and individual differences (e.g. testosterone levels) (Wirth and Schultheiss, 2007).
The physiological expression of anger and hostility has been recognised as important in psychosomatic medicine and health (Smith et al., 2004). Effects of anger on physiological parameters are potentially pathoaetiological in the genesis of cardiovascular disease and hypertension, yet are subject to individual differences. Systolic blood pressure correlates with questionnaire measures of trait anger (Francis et al., 1991). However the process of inducing anger may determine the extent and direction of cardiovascular responses (Kreibig, 2010): Anger elicited in the context of harassment or personalized recall increases cardiac output (Prkachin et al., 2001) along with a shortening of the pre-ejection period, indicating sympathetic effects (Neumann and Waldstein, 2001; Herrald and Tomaka, 2002). In contrast, physiological responses to angry facial expressions presented both supra and subliminally can induce cardiac states more reminiscent of fear, such as heart rate decreases instead of increases, as well as increases in heart rate variability (HRV) and decreases in galvanic skin response (Jonsson and Sonnby-Borgstrom, 2003; Vrana and Gross, 2004; Dimberg and Thunberg, 2007), reflecting potential parasympathetic activation. Subliminal primes displaying the word ANGER have been shown to increase both systolic and diastolic blood pressure, relative to a control group exposed to the subliminal prime RELAX (Hull et al., 2002). Using subliminal priming thus presents a valuable tool to explore the interplay between physiology and brain in an anger context and provides an insight into the mechanisms through which anger may exert effects on cognition and behaviour. Research to date has attempted to delineate the effects of emotions such as fear, happiness and sadness on cognition and behaviour, or study the relationship between blood pressure and neural activity (Coulson et al., 2015). However, no study has specifically investigated the interplay of neural and physiological pathways through which anger influences cognition. Indeed, propensity for anger to be both a motivating emotion and an emotion with negative status renders the consequential effects of anger on attention and reaction time unclear. A greater understanding of anger effects on blood pressure and neural activity, and their subsequent relationship to behaviour, can help provide insight into the mechanisms through which anger may exert its influence. We thus simultaneously monitored physiology (e.g. blood pressure) and brain (e.g. using fMRI) to comprehensively detail the physiological and neural mechanisms through which subliminal ANGER may affect decision making.
Summary of experiments
The experiments were designed to investigate whether subliminal induction of an emotional state, specifically anger, altered cognitive processing, as exemplified by lexical decision-making. On each experimental trial, the participant saw a target letter-string presented on a video monitor and was required to judge if this string was a word or nonsense non-word. Before each presentation of the target letter-string, the participant was ‘emotionally-primed’ by the words ANGER, or RELAX, which were presented subliminally at the point of visual fixation. We checked carefully that the ANGER and RELAX words were subliminal (i.e. not consciously seen by the participants) using a similar laboratory threshold task, described below. Participants performed the main task in the laboratory (Experiment 1) using accuracy and reaction time measures of performance, and during functional neuroimaging (Experiment 2) to test how in the brain emotions can alter decisions. We also measured participants’ blood pressure during task performance to characterise the physiological expression of emotional state, manifestation in the brain and impact on behaviour.
Methods
Experiment 1: Psychophysiology
Participants
Twenty-one healthy control participants were recruited from advertisements placed around the University and Medical School campus to take part in the laboratory-only physiological investigation. These healthy volunteers (7 men, 14 women; mean age 22.7 years ± s.d. 1.1; all right handed) provided written consent to take part in the study which was approved by the Brighton and Sussex Medical School (BSMS) Research Governance and Ethics Committee. Two participants were excluded due to artefacts within the blood pressure recording. One participant was excluded as an extreme outlier (reaction time differences were greater than 3 s.d. from the mean of the group sample).
Paradigm
Two-hundred neutral seven-letter words were chosen from a selection of word databases (e.g. ASKNEW). Of these, half had a letter substituted to form 100 non-word letter-strings. Thus the total stimulus set comprised 100 words and 100 non-word letter-strings that served as the basis for lexical decisions. During the experiment, these letter-strings were preceded either by the subliminal prime ANGER, or by RELAX to form 50 trials in each condition (50 words preceded by RELAX, 50 non-words preceded by RELAX, 50 words preceded by ANGER, 50 non-words preceded by ANGER). Each prime was presented in blocks of four. This ensured that a minimum of four ANGER or four RELAX trials were presented consecutively. Within each four-prime block, half of trials were word trials and half were non-word trials, the order of which were randomized. In each trial, the ANGER and RELAX primes were rendered subliminal using forward and backward masking (Hull et al., 2002) (see below). This cognitive task was run using Matlab software and was synchronized with physiological recording using Spike2 6.08 software.
Physiological recording
In Experiment 1, blood pressure was recorded using non-invasive, beat-to-beat monitoring via photoplethysmographic technology (Finometer PRO; Finapres 2300, Ohmeda, Eaglewood, CO, USA). An inflatable finger cuff and infrared plethysmograph were fitted to the index finger of the subject’s right hand, allowing measurements of beat-to-beat systolic blood pressure.
Procedure
After a 5 min calibration period, the participant was invited to start the task of 200 experimental trials. Each trial began with a 1500 ms fixation cross, followed by a forward mask of seven scrambled letters (17 ms), the ANGER or RELAX prime (17 ms), a blank screen (17 ms), a backwards mask of seven scrambled letters (50 ms) and a blank screen (100 ms). This rapid series of events was immediately followed by a target word/non-word seven letter-string that remained on the screen until participants made a lexical decision, responding by key-press either ‘1’ to denote a word, or ‘2’ to signify a non-word. Participants were encouraged to answer as quickly and accurately as possible. Lexical decision strings remained on the screen until the participant made their response. Reaction times (RTs) and correctness were recorded. The order in which these blocks were presented was randomized.
Experiment 2: Neuroimaging
Participants
A second group of twenty healthy control participants were recruited from advertisements placed around the Medical School and University campus. One participant was removed from all analyses due to non-compliance with experimental procedures and excessive movement in the scanner, resulting in 9 male and 10 female participants (mean age 24.6 years ± s.d. 5.0; all right handed).
Physiological recording
Blood pressure was recorded using a Portapres device (Finapres Medical Systems) adapted in-house to be fully fMRI compatible (Gray et al., 2009). Due to equipment failure within the scanning environment, complete sets of blood pressure recordings could not be obtained for five participants. An fMRI compatible inflatable finger cuff and infrared plethysmograph were fitted to the thumb of the subject’s left hand, allowing measurements of beat-to-beat systolic blood pressure. This was recorded and synchronized with stimulus presentation and scan volumes using Spike 2 6.08 software.
Paradigm and procedure
The stimuli and experimental protocol were identical to that followed in the psychophysiological lab, with a few minor modifications made to better optimize task design for fMRI. Lexical decisions were now blocked in groups of six by prime (RELAX, ANGER) instead of four. Within each six-prime block, half of trials were word trials and half were non-word trials, the order of which were randomized. In addition, each trial was fixed at a 3500 ms to create block durations of 21 s. Previously, all prime decisions remained on the screen until a lexical decision was made before switching to a fixation cross. To ensure a fixed trial length, this cross remained on screen until a total trial duration of 3500 ms was reached. If no decision was made within the 3500 ms period, the experiment proceeded to the next trial. Participants were warned to not let this occur, and instructed to make their responses as quickly and as accurately as possible.
MRI acquisition and preprocessing
Participants underwent functional magnetic resonance imaging (fMRI) in a Siemens Avanto 1.5 Tesla MRI scanner (Siemens, Erlangen, Germany). Prior to acquisition of the functional dataset, a whole brain T1-weighted structural scan was acquired for each participant (MPRAGE, 0.9 mm3 voxels, 192 slices, 1160/4.24 TR/TE, 300 ms inversion time, 230 × 230 mm2 FOV, 5 min acquisition time). Then, during performance of the experimental task, functional echo-planar datasets sensitive to blood oxygen level dependent contrast were acquired using axial slices (anteriorposterior phase encode direction) tilted 30° from intercommissural plane to minimize T2* signal dropout from orbitofrontal and anterior temporal regions. Thirty-five 3-mm slices with a 0.75 mm interslice gap provided whole-brain coverage with an in-plane resolution of 3 × 3 mm (TE 42 ms, volume TR 2620 ms). Images were preprocessed using SPM 8 (http://www.fil.ion.ucl.ac.uk/spm/). The initial four functional volumes were discarded to allow for equilibration of net magnetization. Images were then spatially realigned and unwarped, and spatially normalized to standard MNI space (Montreal Neurological Institute). Normalized functional scans were then smoothed with an 8 mm Gaussian smoothing kernel. For small volume correction analyses focusing on brainstem, a 15-mm sphere functional ROI for dorsal pons [2 −30 −32] was derived from previous work demonstrating its sensitivity to first order representations of bodily state (Critchley et al., 2001), implicating it in anger processing (Damasio et al., 2000) and core aspects of emotion (Damasio, 2010).
Experiment 2 a: Subliminal threshold check
To verify that all ANGER and RELAX primes were presented below the subjective (and objective) threshold; participants were given an additional series of 32 trials in the psychophysiological laboratory as the final experimental procedure (for participants in Experiment 2 only). All task parameters remained the same, but instead of making word/non-word judgments, participants were now instructed to press ‘1’ if the word/non-word was preceded by ANGER or ‘2’ if it was proceeded by RELAX. After this forced choice decision, their confidence was assessed on a scale of 1 (complete guess) to 50 (complete confidence) using a computerized VAS.
Data analyses
Physiological data processing
Beat-to-beat values of systolic blood pressure (mmHg) recorded during both the laboratory and fMRI experiments were processed as follows: First the heartbeat values were interpolated over time (100 Hz) and smoothed using a Gaussian function (set to 1) implanted in Matlab (MATLAB and Statistics Toolbox Release 2012b, The MathWorks, Inc., Natick, Massachusetts, United States). These created a constant signal over systolic peaks and averaged across potential spike artefacts. Mean systolic blood pressure levels were then derived by averaging systolic levels over each block of trials and the baseline value (defined by the average systolic level from the preceding 2000 ms from block onset) was subtracted to index average evoked blood pressure change. These average block values were then analysed according to emotion prime type to give mean systolic levels separately for blocks containing ANGER versus RELAX primes.
Behavioural data
To limit the effect of outliers, all reaction time (rt) analyses were performed on the reciprocal of the reaction time (1/rt), transforming data so that higher values signify a faster response. Mean correct responses were calculated for each emotion prime condition (total number of correct responses during subliminal anger prime blocks and relax prime blocks). A repeated measures 2 × 2 ANOVA containing prime (ANGER, RELAX) and word status (word, non-word) was performed for reciprocal rt within the psychophysiological study, and, to mirror subsequent fMRI analyses, a targeted within-participants t-test (2-tailed) investigated the main effect of prime during the neuroimaging study behavioural replication. To investigate the relationship between blood pressure change and task performance, blood pressure elevations due to ANGER primes (ANGER – RELAX) were correlated with changes in reciprocal reaction time due to ANGER primes (1/rt for ANGER – RELAX primes). The strength of the correlation was measured using Pearson’s r. For behavioural and blood pressure analyses, effect sizes are reported using Cohen’s d (d for within-participant t-tests), partial eta squared (η2p for ANOVA) (Cohen, 1988).
Trait anger was assessed using the anger dimension of the Buss/Perry Aggression Questionnaire (Buss and Perry, 1992).
Neuroimaging analyses
Functional imaging data were analysed using SPM 8 within a mixed effects design to investigate the influence of ANGER and RELAX primes on brain activity. In individual first-level analyses, neural activity was modelled for blocks containing ANGER primes and contrasted to blocks containing RELAX primes. Blocks were convolved with a canonical haemodynamic response function and regressed in a multiple regression GLM analysis against voxel-wise time-series data from the preprocessed echoplanar images. Six movement regressors derived at pre-processing were included as confounding covariates. Subject-wise contrast images were tested for experimental effects of interest in second-level group analyses focusing on the main effect of prime (ANGER versus RELAX). To specifically isolate the neural substrates through which affective primes impact on behaviour, the degree to which participants were susceptible to the affective primes was calculated (a reciprocal correction was applied to limit the influence of outliers (Whelan, 2008): average 1/rt for ANGER – average 1/rt for RELAX primes). This value was regressed against subject contrast images at the second level, modelled as the only variable of interest. Individual threshold maps were set to P < 0.005, and all results reported were cluster-level corrected using family wise error rate (FWE) P < 0.05.
To investigate neural coupling with systolic blood pressure changes, the average systolic blood pressure level was calculated during each scan volume. These were then convolved to the hemodynamic response and regressed at first level for each participant, along with convolved standard deviation for these measures and raw systolic values. Movement regressors were also included in the model, and contrasts at the second level explicitly modelled positive and negative activity associated with rises and falls in convolved systolic blood pressure levels. Individual threshold maps were again set to P < 0.005 and all results are reported at P < 0.05 cluster level.
As there are known differences between males and females in the reaction to anger (Stemmler and Wacker, 2010) and in neural activation in reaction to emotional manipulations (Sacher et al., 2013), sex was added as a nuisance regressor in all fMRI analyses.
Results
Experiment 1: Psychophysiological laboratory experiment
Behavioural analyses
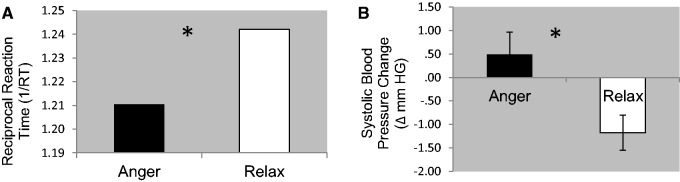
A main effect of prime [F(1,20) = 13.031, P = 0.002; η2p = .395] signified that reaction times for lexical decisions following the subliminal prime ANGER were significantly impaired relative to lexical decisions preceded by the subliminal prime RELAX (Figure 1A). Reaction times were also delayed when making lexical decisions to non-words relative to words, as indicated by a main effect of word type [F(1,20) = 65.960, P < 0.001; η2p = .767]. There was no interaction between prime and word type, thus the ANGER prime impaired reaction time irrespective of whether the lexical decision was in response to a word or a non-word stimulus [η2p = .055].
Fig. 1.
(A) Reciprocal reaction times against prime type: Reaction times for word/non-word decisions were significantly longer when proceeding ANGER primes and faster following RELAX primes. (B) Systolic blood pressure change against prime type: ANGER primes raised blood pressure while RELAX primes reduced it, relative to preceding baseline.
Blood pressure analyses
Average systolic blood pressure levels were significantly elevated during ANGER prime blocks relative to RELAX prime blocks [t(18) = 2.5 P = 0.02; d = 0.579], in which ANGER primes caused an increase in blood pressure relative to baseline, and RELAX a relative decrease (Figure 1B).
Behaviour and physiological interactions
The relationship between blood pressure reactivity and reaction time was assessed. Changes in blood pressure between ANGER and RELAX priming conditions were analysed against reciprocal reaction time differences between the two priming conditions, separately for each participant. This relationship was significant (r = −0.498, P = 0.038) (Figure 2), demonstrating that ANGER primes were associated with increased reaction times and elevated blood pressure response, relative to RELAX primes.
Fig. 2.
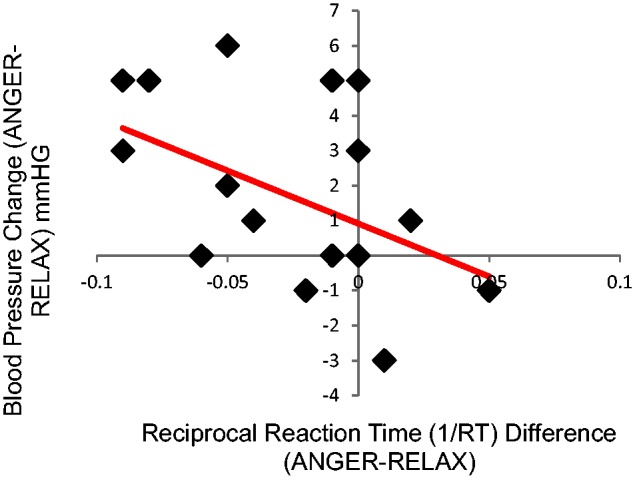
Relationship between systolic blood pressure elevation and augmented RT to ANGER versus RELAX primes. Individuals with greater blood pressure variation between ANGER and RELAX primes also had a larger difference in reaction time for lexical decisions preceded by ANGER versus RELAX primes. Specifically, individuals with greater increases in blood pressure to ANGER versus RELAX primes displayed a greater slowing in reaction time for lexical decisions to ANGER primes.
Experiment 2: Neuroimaging experiment
Behavioural replication
Reaction times for lexical decisions following ANGER primes were significantly slower relative to those following RELAX primes [t(18) = −3.646, P = 0.002; d = −0.898], replicating the previous ANGER impairment effect.
Trait anger
Trait anger was related to the difference in reaction time between ANGER and RELAX trials (r = 0.5, P = 0.047), signifying that individuals high on trait anger were more likely to be sped up by subliminal ANGER primes relative to RELAX primes (despite the group-level propensity for subliminal anger primes to impede reaction time).
Systolic blood pressure replication
Systolic blood pressure was significantly enhanced on trials preceded by an ANGER prime relative to a RELAX prime [t(13) = 2.30, P = 0.038 d = 0.711].
Neuroimaging data
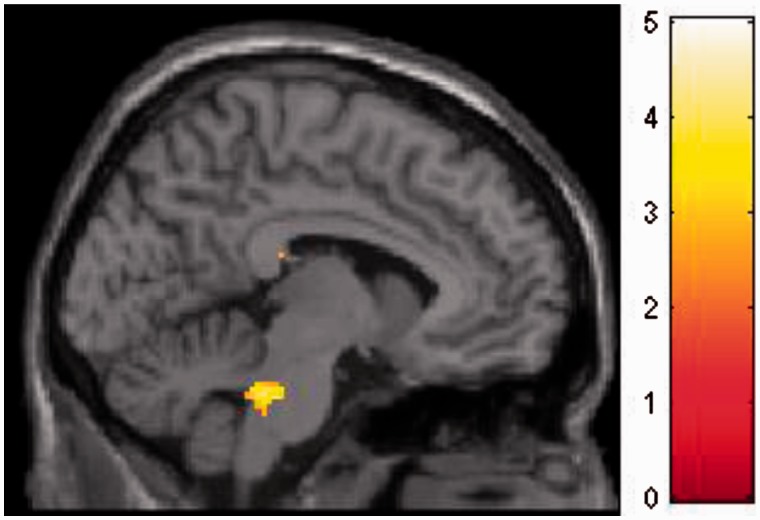
Results demonstrate that ANGER primes relative to RELAX primes activated brainstem, specifically dorsal pons/parabrachial nucleus ([−8 −36 −30], 48 voxels, T = 5.34), an area sensitive to sympathetic arousal (Figure 3). ANGER trials induced hypoactivation of parietal lobe extending into postcentral gyrus and precuneus ([56 −32 −44], 1106 voxels, T = 5.76, P-FWE < 0.001), and visual cortex/cuneus ([24 −88 32], 352 voxels, T = 4.64, P-FWE = 0.019).
Fig. 3.
Main effect of ANGER (vs RELAX) prime in dorsal pons/parabrachial nucleus, an area containing reciprocal relationships with extended central amygdaloid nucleus and hypothalamus, while also encompassing nuclei with ascending noradrenergic projections to midbrain periaqueductal grey and relays of descending autonomic pathways.
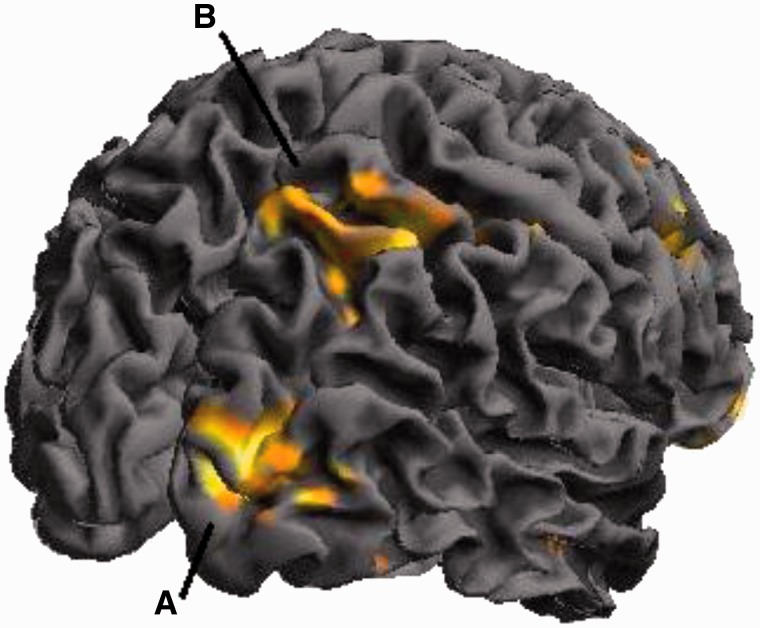
The impairing effect on reaction time of ANGER, relative to RELAX primes was also associated with attenuation of activity across visual areas, including cuneus, middle occipital gyrus and lingual gyrus, also parietal cortices including superior parietal lobule (see Figure 4 and Table 1 for full listing of brain activity inversely correlated with 1/rt).
Fig. 4.
Brain activity inversely correlated with 1/rt: The impairing effect on reaction time of ANGER relative to RELAX primes was also associated with attenuation of activity across visual areas including (A) cluster peaking the middle occipital gyrus [40 −86 8] and (B) right parietal lobe [34 −48 54].
Table 1.
Neural areas correlated with the facilitated RT due to prime
| Brain region | Side | Voxels | T-Score | Coordinates(mm) |
P-FWE | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Middle occipital gyrus | R | 369 | 6.12 | 32 | 56 | −8 | 0.012 |
| Middle occipital gyrus | R | 831 | 6.09 | 40 | −86 | 8 | <0.001 |
| Cuneus | R | 5.82 | 22 | −90 | 6 | ||
| Middle frontal gyrus extending into superior frontal gyrus | R | 384 | 4.89 | 46 | 24 | 30 | 0.009 |
| Parietal lobe | R | 808 | 4.83 | 34 | −48 | 54 | <0.001 |
Note: Reciprocal rt was regressed against voxel-wise activity for the contrast RELAX > ANGER, demonstrating brain areas sensitive to the impairing effect of ANGER primes on speeded reaction time. Table thresholded at P < 0.05 FWE corrected.
When investigating neural activity coupled with elevations in systolic blood pressure, hypoactivation within a number of regions (Table 2).
Table 2.
Neural activation negatively coupled with systolic activation (i.e. hypoactivation with elevations in systolic blood pressure)
| Brain region | Side | Voxels | T-Score | Coordinates(mm) |
P | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Posterior temporal lobe extending into occipital lobe | L | 233 | 6.97 | −30 | −48 | 6 | 0.009 |
| Parietal lobe/Precuneus | R | 168 | 6.14 | 18 | −56 | 42 | 0.023 |
| Parahippocampus extending into amygdala/fusiform gyrus | L | 158 | 4.88 | −26 | −4 | −28 | 0.027 |
| Precentral/Postcentral gyrus | L | 224 | 4.72 | −64 | −2 | 22 | 0.010 |
| Precentral/Postcentral gyrus | R | 306 | 4. 64 | 58 | −8 | 32 | 0.004 |
| Cuneus | R | 180 | 3.85 | 20 | −64 | 20 | 0.013 |
| Parietal lobe/Precuneus | 389 | 3.78 | 0 | −64 | 48 | 0.001 | |
Note: Table thresholded at P < 0.05 cluster corrected.
Cardiac analysis
In Experiment 1 and Experiment 2 respectively, heart rate [t(17) = −0.05, P = 0.96; t(13) = −0.94, P = 0.37] and HRV [t(17) = 0.72, P = 0.48; t(13) = 1.54, P = 0.16] did not differ between the blocks of ANGER and RELAX primes, suggesting the effects on blood pressure, mediated sympathetically, were associated with suppression of the baroreflex.
Subliminal threshold check
Objective threshold
The mean amount of correct responses in a forced choice test (ANGER prime versus RELAX prime) revealed a mean of 18.1 (SEM = 0.79) correct out of 32 trials (16 would be expected by chance). This did not significantly differ using a 1-way t-test.
Subjective threshold
When correlating confidence with accuracy for each subject, all correlations were non-significant, with the exception of one participant. This participant did not differ significantly in behaviour from the other participants as their average reaction times for both lexical decisions preceded by ANGER (638 ms) and RELAX (625 ms) were less than one standard deviation (159 and 156 ms, respectively) from the group means (766 and 749 ms). In any case, exclusion of this participant from the principal analysis of reaction time differences between ANGER and RELAX conditions retained significance using both raw reaction time [(t(17) = 2.74, P = 0.014)] and reciprocal reaction time [t(17) = −3.40, P = 0.003].
Discussion
Our data show that the implicit manipulation of autonomic state using affective primes impacts directly on cognitive processes. Specifically, the subliminal processing of ANGER primes, compared to RELAX primes, impairs the speed with which lexical decisions are made and raises systolic blood pressure. Within the brain, this effect of subliminal ANGER primes was associated with enhanced brainstem activation, yet also engendered a widespread relative attenuation of activity across parietal and occipital cortices which mostly paralleled the reaction-time signatures of cognitive impairment.
The degree to which individuals were susceptible to the deleterious effect of subliminal ANGER primes was predicted by the magnitude of their blood pressure response: the magnitude of increased systolic blood pressure evoked by subliminal ANGER correlated with the magnitude of impaired reaction time across participants. Functional neuroimaging revealed that ANGER primes elicited brain activation in pons, an area implicated in the regulation of sympathetic arousal. In addition, ANGER primes attenuated activity within cortical regions including occipitotemporal areas implicated in visual object representations (including orthographic and semantic) and subsections of parietal lobe (implicated in visual attention). Together, these results suggest two principal mechanisms through which subliminal anger can impede decision making: First, through autonomic cardiovascular arousal, indexed by heightened blood pressure, where the extent of anger induced elevations in systolic blood pressure was proportionally detrimental to the semantic decisions as reflected in reaction times. Second, through attention and visual processing areas, where underlying activity within cuneus, occipital gyrus and parietal cortex, normally present or facilitated during relaxed states, is compromised by the covert processing of ANGER.
Anger is an interesting emotion: It is fundamentally negative, yet classified as an ‘approach’ emotion carrying motivation potential (van Honk and Schutter, 2006; Carver and Harmon-Jones, 2009; Lewis, 2010). Anger can facilitate motoric behaviour (Wilkowski and Meier, 2010), yet in our study the implicit viewing of anger raised mean reaction times to semantic decisions indicating an interference effect at the cognitive semantic level, rather than influencing the tuning of motor responses at a more distal level. Notably such interference is also observed with another negative emotion, fear. Processing of fear stimuli prolongs reaction time if they are presented prior to lexical decisions (Calvo and Castillo, 2005) and interferes with performance on a variety of other tasks including Posner cueing paradigms (Fox et al., 2001), Stroop tasks (McKenna and Sharma, 2004) and rapid serial visual presentation paradigms (Barnard et al., 2005). Both fear and anger convey potential threat, hence the extent to which fear interference also varies with anxiety state (Barnard et al., 2005), complements our facilitation effect of anger as a function of trait anger. These findings indicate that manifestations of specific emotions interact with affective priming to facilitate and/or counteract the behavioural impact. Moreover, the interference effect afforded by the subliminal ANGER prime in the majority of participants appears to support the notion that implicit negative emotions or ‘unconscious stress’ may enhance physiological levels in daily life and may even play a role in the stress-health link (Brosschot, 2010; Brosschot et al., 2010). While we observed effects in the majority of participants, future work can determine whether the extent of blood pressure reactivity to emotional primes is influenced by resting blood pressure level.
Meta-analyses suggest motivationally-salient and emotional stimuli increase activation within visual cortices (Phan et al., 2002; Fusar-Poli et al., 2009; Vytal and Hamann, 2010). This effect is coupled to peripheral autonomic (sympathetic) arousal (Critchley et al., 2000; Critchley, 2005). However, in this subliminal priming paradigm, we did not observe a main effect of the (arguably more salient) negative emotion on the level of activation of visual areas. Indeed, we found that for the contrast of ANGER versus RELAX prime conditions, reduced activation of visual areas predicted slower reaction times. This finding is nevertheless in accordance with evidence from a body of event-related potential (ERP) literature for diminished attention and visual activation to anger. In an ERP study, attenuated N400 signal over parietal and occipital regions was observed in children in response to vocal anger (Chronaki et al., 2012), denoting anger-induced suppression of an index of early attentional processing. Similarly for emotion scenes, positive affective stimuli are known also to evoke greater shifts in both oxygenated and deoxygenated haemoglobin within occipital cortices than negative or neutral stimuli (Minati et al., 2009). Our findings in relation to anger (and peripheral autonomic arousal) suggest that the reduced activation in visual areas represents both a central signature of, and a likely basis for, compromised perceptual-cognitive processes reflected behaviourally in reaction time.
A major pathway through which the state of cardiovascular arousal influences brain activity (and associated mental processes) is via arterial baroreceptors. These are stretch-sensitive mechanoreceptors which are engaged in the regulation of arterial blood pressure (Rau et al., 1993; Thrasher, 2002). Increases and decreases in cardiac activity (including blood pressure) excite or inhibit baroreceptors. Stable heart-rate across the task indicates that the baroreflex was suppressed during anger trials (Gianaros et al., 2012). The phasic baroreceptor signalling of the timing and strength of each heartbeat has been viewed over the years as predominantly inhibitory to sensory processes and cortical activity (Lacey and Lacey, 1970; Dworkin et al., 1994). While this inhibition is shown reliably for pain processing; facilitatory effects are present for the processing of certain emotions including fear stimuli (Gray et al., 2012; Garfinkel et al., 2014). Even for pain stimuli concurrent baroreceptor activation is associated with greater amygdala engagement (Gray et al., 2009). Thus the regulatory role of baroreceptors on the brain during blood pressure changes are likely to be more complicated than first anticipated (Minati et al., 2009), potentially incorporating structures isolated in our current paradigm, including visual, attentional and memory networks.
Amygdala and insula have been implicated in stress evoked blood pressure reactivity (Gianaros and Sheu, 2009), and across individuals, those who demonstrate exaggerated blood pressure reactivity also demonstrate enhanced neural activation in a variety of areas implicated in stress-related cardiovascular control, such as posterior cingulate cortex and anterior insula (Gianaros et al., 2007). Attention has been shown to modulate blood pressure reactions and their link to neural activity (Okon-Singer et al., 2014). Interestingly, the study by Okon-Singer et al. (2014), which also implemented beat-to-beat blood pressure within an fMRI scanning environment, suggests areas such as the parietal cortex, parahippocampal gyrus and amygdala are associated with blood pressure reactivity in specific emotional-attentional conditions. These results, in combination with the current findings, point to an involvement of blood pressure reactivity with emotional-attentional systems.
These are the first experiments to investigate how subliminal anger influences semantic decisions via physiological mechanisms in both brain and body. We demonstrate a relationship between systolic blood pressure elevations and impeded reaction time in response to subliminal anger primes, as well as reduced activation in visual and attention networks. Anger possesses the combination of approach and adverse affective properties that typically differentiate positively or negatively valenced emotions. We highlight detrimental effects of anger on a specific relatively ‘high-level’ cognitive process, semantic decision-making; yet we acknowledge and anticipate that anger states will facilitate other types of cognitive and behavioural processes. Future research should thus build upon these findings, to further delineate, in order to predict, the neural and physiological mechanisms through which behavioural and cognitive processes can be both enhanced and impeded by anger.
Funding
This work was supported by the Brighton and Sussex Medical School IRP programme and via a donation from the Dr. Mortimer and Theresa Sackler Foundation, which supports the Sackler Centre for Consciousness Science at the University of Sussex. H.D.C. and S.N.G. are supported by an ERC Advanced Grant awarded to HDC (CCFIB AG 234150).
Conflict of interest. None declared.
References
- Aarts H., Ruys K.I., Veling H., et al. (2010). The art of anger: reward context turns avoidance responses to anger-related objects into approach. Psychological Science , 21, 1406–10. [DOI] [PubMed] [Google Scholar]
- Barnard P.J., Ramponi C., Battye G., Mackintosh B. (2005). Anxiety and the deployment of visual attention over time. Visual Cognition , 12, 181–211. [Google Scholar]
- Brosschot J.F. (2010). Markers of chronic stress: prolonged physiological activation and (un)conscious perseverative cognition. Neuroscience and Biobehavioral Reviews , 35, 46–50. [DOI] [PubMed] [Google Scholar]
- Brosschot J.F., Verkuil B., Thayer J.F. (2010). Conscious and unconscious perseverative cognition: is a large part of prolonged physiological activity due to unconscious stress? Journal of Psychosomatic Research , 69, 407–16. [DOI] [PubMed] [Google Scholar]
- Buss A.H., Perry M. (1992). The aggression questionnaire. Journal of Personality and Social Psychology , 63, 452–9. [DOI] [PubMed] [Google Scholar]
- Calvo M.G., Castillo M.D. (2005). Processing of threat-related information outside the focus of visual attention. Spanish Journal of Psychology , 8, 3–11. [DOI] [PubMed] [Google Scholar]
- Carver C.S., Harmon-Jones E. (2009). Anger is an approach-related affect: evidence and implications. Psychological Bulletin , 135, 183–204. [DOI] [PubMed] [Google Scholar]
- Chronaki G., Broyd S., Garner M., Hadwin J.A., Thompson M.J.J., Sonuga-Barke E.J.S. (2012). Isolating N400 as neural marker of vocal anger processing in 6-11-year old children. Developmental Cognitive Neuroscience , 2, 268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (1988). Stastical Power Analysis for the Behavioural Sciences, 2nd edn Hillsdale, NJ: Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Coulson J.M., Murphy K., Harris A.D., Fjodorova M., Cockcroft J.R., Wise R.G. (2015). Correlation between baseline blood pressure and the brainstem FMRI response to isometric forearm contraction in human volunteers: a pilot study. Journal of Human Hypertension , 29(7), 449–55. [DOI] [PubMed] [Google Scholar]
- Critchley H.D. (2005). Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology , 493, 154–66. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Elliott R., Mathias C.J., Dolan R.J. (2000). Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. Journal of Neuroscience , 20, 3033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H.D., Mathias C.T., Dolan R.J. (2001). Neuroanatomical basis for first- and second-order representations of bodily states. Nature Neuroscience , 4, 207–12. [DOI] [PubMed] [Google Scholar]
- Damasio A. (2010). Self Comes to Mind: Constructing the Conscious Brain. New York: Pantheon Books. [Google Scholar]
- Damasio A.R., Grabowski T.J., Bechara A., et al. (2000). Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience , 3, 1049–56. [DOI] [PubMed] [Google Scholar]
- Dimberg U., Thunberg M. (2007). Speech anxiety and rapid emotional reactions to angry and happy facial expressions. Scandinavian Journal of Psychology , 48, 321–8. [DOI] [PubMed] [Google Scholar]
- Dworkin B.R., Elbert T., Rau H., et al. (1994). Central Effects of baroreceptor activation in humans—attenuation of skeletal reflexes and pain perception. Proceedings of the National Academy of Sciences of the United States of America , 91, 6329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E., Russo R., Bowles R., Dutton K. (2001). Do threatening stimuli draw or hold visual attention in subclinical anxiety? Journal of Experimental Psychology-General , 130, 681–700. [PMC free article] [PubMed] [Google Scholar]
- Francis R.A., Ernst F.A., Nevels H., Lemeh C.A. (1991). The relationship of blood-pressure to a brief measure of anger during routine health screening. Journal of the National Medical Association , 83, 601–4. [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F., et al. (2009). Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience , 34, 418–32. [PMC free article] [PubMed] [Google Scholar]
- Garfinkel S.N., Minati L., Gray M.A., Seth A.K., Dolan R.J., Critchley H.D. (2014). Fear from the heart: sensitivity to fear stimuli depends on individual heartbeats. Journal of Neuroscience , 34, 6573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendolla G.H.E., Silvestrini N. (2011). Smiles make it easier and so do frowns: masked affective stimuli influence mental effort. Emotion , 11, 320–8. [DOI] [PubMed] [Google Scholar]
- Gianaros P.J., Jennings J.R., Sheu L.K., Derbyshire S.W.G., Matthews K.A. (2007). Heightened functional neural activation to psychological stress covaries with exaggerated blood pressure reactivity. Hypertension , 49, 134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros P.J., Onyewuenyi I.C., Sheu L.K., Christie I.C., Critchley H.D. (2012). Brain systems for baroreflex suppression during stress in humans. Human Brain Mapping , 33, 1700–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros P.J., Sheu L.K. (2009). A review of neuroimaging studies of stressor-evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease risk. Neuroimage , 47, 922–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M.A., Beacher F.D., Minati L., et al. (2012). Emotional appraisal is influenced by cardiac afferent information. Emotion , 12, 180–91. [DOI] [PubMed] [Google Scholar]
- Gray M.A., Rylander K., Harrison N.A., Wallin B.G., Critchley H.D. (2009). Following one's heart: cardiac rhythms gate central initiation of sympathetic reflexes. Journal of Neuroscience , 29, 1817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison N.A., Gray M.A., Gianaros P.J., Critchley H.D. (2010). The embodiment of emotional feelings in the brain. Journal of Neuroscience , 30, 12878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison N.A., Kreibig S.D., Critchley H.D. editors. (2013). A Two-Way Road: Efferent and Afferent Pathways of Autonomic Activity in Emotion. New York: Cambridge University Press. [Google Scholar]
- Harrison N.A., Singer T., Rotshtein P., Dolan R.J., Critchley H.D. (2006). Pupillary contagion: central mechanisms engaged in sadness processing. Social Cognitive and Affective Neuroscience , 1, 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrald M.M., Tomaka J. (2002). Patterns of emotion-specific appraisal, coping, and cardiovascular reactivity during an ongoing emotional episode. Journal of Personality and Social Psychology , 83, 434–50. [PubMed] [Google Scholar]
- Hull J.G., Slone L.B., Meteyer K.B., Matthews A.R. (2002). The nonconsciousness of self-consciousness. Journal of Personality and Social Psychology , 83, 406–24. [DOI] [PubMed] [Google Scholar]
- Jonsson P., Sonnby-Borgstrom M. (2003). The effects of pictures of emotional faces on tonic and phasic autonomic cardiac control in women and men. Biological Psychology , 62, 157–73. [DOI] [PubMed] [Google Scholar]
- Kreibig S.D. (2010). Autonomic nervous system activity in emotion: a review. Biological Psychology , 84, 394–421. [DOI] [PubMed] [Google Scholar]
- Lacey I., Lacey B.C. (1970). Some autonomic-central nervous system interrelationships. In: Black P., editors. Physiological Correlates of Emotion. New York: Academic Press, 205–22. [Google Scholar]
- Lerner J.S., Keltner D. (2001). Fear, anger, and risk. Journal of Personality and Social Psychology , 81, 146–59. [DOI] [PubMed] [Google Scholar]
- Lewis M. (2010). The development of anger. In: Potegal M., Stemmler G., Spielberger C., editors. International Handbook of Anger. New York: Springer-Verlag, pp. 177–91. [Google Scholar]
- Marsh A.A., Ambady N., Kleck R.E. (2005). The effects of fear and anger facial expressions on approach- and avoidance-related behaviors. Emotion , 5, 119–24. [DOI] [PubMed] [Google Scholar]
- McKenna F.P., Sharma D. (2004). Reversing the emotional stroop effect reveals that it is not what it seems: the role of fast and slow components. Journal of Experimental Psychology-Learning Memory and Cognition , 30, 382–92. [DOI] [PubMed] [Google Scholar]
- Minati L., Jones C.L., Gray M.A., Medford N., Harrison N.A., Critchley H.D. (2009). Emotional modulation of visual cortex activity: a functional near-infrared spectroscopy study. Neuroreport , 20, 1344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S.T., Zajonc R.B. (1993). Affect, cognition, and awareness—affective priming with optimal and suboptimal stimulus exposures. Journal of Personality and Social Psychology , 64, 723–39. [DOI] [PubMed] [Google Scholar]
- Neumann S.A., Waldstein S.R. (2001). Similar patterns of cardiovascular response during emotional activation as a function of affective valence and arousal and gender. Journal of Psychosomatic Research , 50, 245–53. [DOI] [PubMed] [Google Scholar]
- Okon-Singer H., Mehnert J., Hoyer J., et al. (2014). Neural control of vascular reactions: impact of emotion and attention. Journal of Neuroscience , 34, 4251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Wager T., Taylor S.F., Liberzon I. (2002). Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage , 16, 331–48. [DOI] [PubMed] [Google Scholar]
- Prkachin K.M., Mills D.E., Zwaal C., Husted J. (2001). Comparison of hemodynamic responses, to social and nonsocial stress: evaluation of an anger interview. Psychophysiology , 38, 879–85. [DOI] [PubMed] [Google Scholar]
- Rau H., Pauli P., Brody S., Elbert T., Birbaumer N. (1993). Baroreceptor stimulation alters Cortical cctivity. Psychophysiology , 30, 322–5. [DOI] [PubMed] [Google Scholar]
- Sacher J., Okon-Singer H., Villringer A. (2013). Evidence from neuroimaging for the role of the menstrual cycle in the interplay of emotion and cognition. Frontiers in Human Neuroscience , 7, 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T., Lamm C. (2009). The social neuroscience of empathy. Year in Cognitive Neuroscience , 1156, 81–96. [DOI] [PubMed] [Google Scholar]
- Smith T.W., Glazer K., Ruiz J.M., Gallo L.C. (2004). Hostility, anger, aggressiveness, and coronary heart disease: an interpersonal perspective on personality, emotion, and health. Journal of Personality , 72, 1217–70. [DOI] [PubMed] [Google Scholar]
- Stemmler G., Wacker J. (2010). Personality, emotion, and individual differences in physiological responses. Biological Psychology , 84, 541–551. [DOI] [PubMed] [Google Scholar]
- Thrasher T.N. (2002). Unloading arterial baroreceptors causes neurogenic hypertension. American Journal of Physiology-Regulatory Integrative and Comparative Physiology , 282, R1044–53. [DOI] [PubMed] [Google Scholar]
- van Honk J., Schutter D.J.L.G. (2006). From affective valence to motivational direction—The frontal asymmetry of emotion revised. Psychological Science , 17, 963–5. [DOI] [PubMed] [Google Scholar]
- Vrana S.R., Gross D. (2004). Reactions to facial expressions: effects of social context and speech anxiety on responses to neutral, anger, and joy expressions. Biological Psychology , 66, 63–78. [DOI] [PubMed] [Google Scholar]
- Vytal K., Hamann S. (2010). Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. Journal of Cognitive Neuroscience , 22, 2864–85. [DOI] [PubMed] [Google Scholar]
- Whelan R. (2008). Effective analysis of reaction time data. Psychological Record , 58, 475–82. [Google Scholar]
- Wilkowski B.M., Meier B.P. (2010). Bring it on: angry facial expressions potentiate approach-motivated motor behavior. Journal of Personality and Social Psychology , 98, 201–10. [DOI] [PubMed] [Google Scholar]
- Wirth M.M., Schultheiss O.C. (2007). Basal testosterone moderates responses to anger faces in humans. Physiology & Behavior , 90, 496–505. [DOI] [PubMed] [Google Scholar]