Abstract
Neuroimaging studies on trait inference demonstrated that the ventral medial prefrontal cortex (mPFC) houses neural representations of memory codes for traits . In this study, we investigate the neural code not only of traits, but also of persons who exemplify these traits. We used repetition suppression, which is a rapid suppression of the neuroimaging signal upon repeated presentation of the same stimulus or core stimulus characteristics—in this case, the implied trait and person. Participants inferred familiar person’s traits. At each trial, a critical (target) sentence described a behavior that implied a trait, and was preceded by a (prime) sentence that implied the same trait and portrayed the same person, the same trait but portrayed a different person or did not imply a trait and portrayed a different person. As predicted, we found partly overlapping repetition suppression areas in the ventral mPFC when persons and traits were repeated, indicating that not only traits but also familiar persons have a neural code in the ventral mPFC. We also found a negative correlation between activation when reading about a new person and participants’ social network size, indicating that experience with larger social groups results in less recruitment of a person code.
Keywords: fMRI repetition suppression, person memory code, trait memory code
Introduction
Our closest relatives and friends, what they mean to us and who they are, occupy a special place in our lives and—we speculate—also in our brain. There is a plethora of functional magnetic resonance imaging (fMRI) research suggesting that the medial prefrontal cortex (mPFC) is involved in the processing and integration of person information, as part of the mentalizing network that is recruited when we attribute beliefs, traits or other characteristics to people (Mitchell et al., 2006; Todorov et al., 2007; Ma et al., 2011, 2012b; for a review see Van Overwalle, 2009).
A critical question is whether the mentalizing network subserves specific knowledge of persons and traits in the form of neural representations or ‘memory codes’, independently from other representations related to action components from which a trait is abstracted, or bystanders involved in the action? How can we identify such neural codes? Typical fMRI activation when making social inferences (e.g. trait attribution) confounds the processing of prerequisite processes (e.g. behaviors) and consequential post-processes (e.g. emotions) and hence does not allow to pinpoint the neural population that represents this social knowledge (De Graaf et al., 2012). To identify neural codes, researchers turned to the technique of fMRI ‘repetition suppression’. Repetition suppression or adaptation refers to the observation that repeated presentation of a stimulus (or a concept) reduces fMRI responses relative to a novel stimulus or stimulus characteristics that are variable and irrelevant (Grill-Spector et al., 2006). Repetition suppression has generally been taken as evidence for a neural marker that represents the memory of the stimulus or concept. This memory representation facilitates the activation and processing of the stimulus when repeated and is the location where specific information is stored and processed in the brain. This is consistent with predictive coding (Friston, 2005; Grill-Spector et al., 2006; Gotts et al., 2012) and connectionist models of neural functioning (McClelland and Rumelhart, 1988) that have also been applied to social cognition (Van Rooy et al., 2003; Van Overwalle and Labiouse, 2004; for a review, see Van Overwalle, 2007) since suppression can be seen as a decrease in prediction error to the same stimulus in a memory code.
Repetition suppression effects have been amply demonstrated in many perceptual domains dealing with the perception of color, shape and so on (Grill-Spector et al., 1999; Thompson-Schill et al., 1999; Kourtzi and Kanwisher, 2000; Engel and Furmanski, 2001; Grill-Spector and Malach, 2001; Bedny et al., 2008; Devauchelle et al., 2009; Roggeman et al., 2011; Diana et al., 2012; Josse et al., 2012). In the social domain, fMRI repetition suppression has also been observed during action observation (Ramsey and Hamilton, 2010a,b) and trait inferences of others similar to the self (Jenkins et al., 2008).
More important for this study, Ma et al. (2014a) applied an fMRI repetition suppression paradigm to demonstrate that a memory code representing other’s traits is located in the mPFC. They presented two sentences in which different persons engaged in various behaviors that implied either the same trait, opposite trait (e.g. kind–unkind) or no trait at all. These researchers found robust suppression of activation in the ventral mPFC when a critical trait-implying sentence was preceded by a prior sentence that implied the same or opposite trait, but not when the prior sentence was trait irrelevant. This suppression effect was found nowhere else in the brain. They interpreted this result as evidence for a broad representation of traits in the mPFC, regardless of the trait valence. In a control study, they ruled out the alternative explanation that the suppression effect was a result of valence repetition (Ma et al., 2014b). In another repetition suppression study, Szpunar et al. (2014) asked their participants to imaging a future event in which they interacted with a familiar person. They found reliable fMRI suppression in the ventral mPFC when the same, rather than a different, person was presented in successive sentences, and so identified this region as the location where familiar people are represented. Taken together, recent fMRI research suggests that memory codes of familiar persons and their traits are represented by neural populations in the ventral mPFC.
The idea that our close relatives and friends occupy a privileged place in the brain is not a new one. The social brain hypothesis (Dunbar, 1992, 1998) states that the growth of the neocortex of monkeys, apes and hominids during the evolution is caused by the increasing mental demands imposed by the social complexities involved by the larger groups these species lived in (Dunbar and Shultz, 2007; Gamble et al., 2011). This relation between social network size and brain size is not only observed between, but also within species. Imaging studies with humans confirmed the link between a larger social network and more ventral mPFC volume (Powell et al., 2010), and both are associated with better mentalizing competency suggesting that mentalizing is the social cognitive skill that underpins this relation (Lewis et al., 2011).
The present aim is to replicate and extend the trait repetition suppression study by Ma et al. (2014a) by identifying not only the memory code for traits, but also of familiar persons. Participants read two consecutive sentences describing a behavior performed by a familiar person implying a moral trait. These sentences implied the same or a different trait, and when the same trait was implied, they described the same or a different person. We expect to replicate the finding of Ma et al. (2014a) that repeated presentation of behaviors implying the same trait leads to repetition suppression in the mPFC. Moreover, we also expect that the repeated presentation of the same familiar person leads to repetition suppression in the mPFC. We also want to investigate whether traits and persons are represented in separate or overlapping brain areas. Contrary to Szpunar et al. (2014), we describe specific behaviors rather than imaginary interactions, and so hope to have more control over participants’ mental inferences and reasoning.
We also measured participant’s social network size. In line with the social brain hypotheses (Dunbar, 1992, 1998), we expect a relationship between social network size and activity in an area of the mPFC subserving the memory codes for persons, but less so for trait information.
Methods
Participants
Participants were 44 right-handed individuals (22 women and 22 men) with ages varying from 18 to 29 years (M = 22.86). All participants were native Dutch speaking, reported no abnormal neurological history and had normal or corrected-to-normal vision. We excluded two additional participants due to excessive head movements (more than 10% outlier scans, see below). Informed consent was obtained in a manner approved by the Medical Ethics Committee at the Hospital of University of Ghent, where the study was conducted, and the Free University Brussels. In exchange for their participation, participants were paid 10 euros.
Stimulus material
Participants read sentences describing a behavior performed by a known person that implied a moral trait (nice, friendly, trustworthy, honest, generous and their opposites). The sentences were adapted from other trait repetition suppression studies (Ma et al., 2014a; Van der Cruyssen et al., 2015) so that each behavioral description became applicable to any (known) person (family member or friend). We also constructed new sentences. Like these prior suppression studies, sentences that were new or substantially adapted were pilot tested by rating the applicability and valence of the implied traits using 7-point scales (with respective anchors: 1 = ‘not applicable at all’ and 7 = ‘very applicable’; 1 = ‘very negative’ and 7 = ‘very positive’). Sentences with extreme trait applicability (<3 or >5) and valence ratings (<3 or >5) were retained for the experiment.
Procedure
Participants were instructed to visualize a person performing a behavior and to infer the trait underlying the behavior. Persons were names or nicknames provided by the participant of 5 men and 5 women they were very close to. To remind participants of the task, each trial started by presenting the word ‘trait’ in the middle of the screen during 2 s. After this, two sentences (a prime and a target sentence) were presented.
We created four conditions (Table 1). All conditions ended with a target sentence that implied a trait (e.g. ‘[Mom] tells her friend the truth’ implying honesty), and were preceded by a different prime sentence depending on the condition. In the Repeated Trait/Repeated Person condition, the prime sentence implied the same trait (e.g. honesty) and referred to the same person (e.g. ‘[Mom] shows the instructor her fault’). In the Repeated Trait/Different Person condition, the prime sentence implied the same trait but involved another person (e.g. ‘[Dad] shows the instructor his fault’). In the No Trait/Different Person condition, the prime implied no trait at all (i.e. the person did not act) and referred to another person (e.g. ‘[Dad] sees blossoms on the trees’). We added a singleton condition in which only a single trait-implying target sentence was presented, to avoid that participants would ignore the prime sentence.
Table 1.
Schematic presentation of the design
| Condition |
Sentences |
Target sentence |
|
|---|---|---|---|
| Trait | Person | Prime sentence | |
| Repeated | Repeated | [Mom] shows the instructor her fault | [Mom] tells her friend the truth |
| Repeated | Different | [Dad] shows the instructor his fault | [Mom] tells her friend the truth |
| No | Different | [Dad] sees blossoms on the trees | [Mom] tells her friend the truth |
| Singleton | – | [Mom] tells her friend the truth | |
Note: The persons between straight brackets were replaced by the names chosen by each individual participant.
There were 20 trials in each condition. Counterbalanced between conditions and participants, we presented one of the four versions of the material to each participant, while all trials were presented in a random order across conditions. Sentences varied between 4 and 7 words and were presented in the middle of the screen during 5.5 s. Prime and target sentences were preceded by a jittered inter-stimulus interval, varying from 2.5 to 4.5 s randomly drawn from a uniform distribution, during which participants viewed passively a fixation crosshair. After reading the target sentence participants rated at their own pace how applicable the implied trait or its opposite was to the person in real life, using one of the four response buttons: 1 = ‘never’, 2 = ‘sometimes’, 3 = ‘often’, and 4 = ‘always’.
After participants left the scanner, we measured their social network size, using the Social Network Questionnaire designed by Lewis et al. (2011). Participants were requested to list the initials of everyone with whom they had some kind of social contact (i) during the last 7 days and (ii) during the last month (i.e. ∼30 days). Contact was defined as some form of interaction, including face-to-face, phone call, email or text messaging, or a letter. Excluded were people who were contacted for professional reasons (e.g. doctor, lawyer, hairdresser, priest, plumber, employer, supervisor, etc.) unless that was considered an interaction of a mainly social nature at the time. Participants could look up a list of names in their phone/address book, and indicated the gender of each contact.
Imaging procedure
Images were collected with a Siemens Magnetom Trio TIM scanner system (Siemens Medical Systems, Erlangen, Germany) using a 32-channel radiofrequency head coil. Stimuli were projected onto a screen at the end of the magnet bore that participants viewed by way of a mirror mounted on the head coil. Stimulus presentation was controlled by E-Prime 2.0 (www.pstnet.com/eprime; Psychology Software Tools) running under Windows XP. Subjects were placed head first and supine in the scanner bore. Subjects were instructed not to move their heads to avoid motion artifacts. Foam cushions were placed within the head coil to minimize head movements. First, a high-resolution anatomical images were acquired using a T1-weighted 3D MPRAGE sequence [TR = 2530 ms, TE = 2.58 ms, TI = 1100 ms, acquisition matrix = 256 × 256 × 176, sagittal FOV = 220 mm, flip angle = 7°, voxel size = 0.9 × 0.86 × 0.86 mm3 (resized to 1 × 1 × 1 mm3)]. Second, whole-brain functional images were collected in a single run using a T2*-weighted gradient echo sequence, sensitive to BOLD contrast (TR = 2000 ms, TE = 35 ms, image matrix = 64 × 64, FOV = 224 mm, flip angle = 80°, slice thickness = 3.0 mm, distance factor = 17%, voxel size = 3.5 × 3.5 × 3.5 mm3, 30 axial slices).
Image processing
The fMRI data were preprocessed and analyzed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK). Data were preprocessed to remove sources of noise and artifact. Functional data were corrected for differences in acquisition time between slices for each whole-brain volume, realigned to correct for head movement, and co-registered with each participant’s anatomical data. The functional data were then transformed into a standard anatomical space (2 mm isotropic voxels) based on the ICBM152 brain template (Montreal Neurological Institute), which approximates Talairach and Tournoux atlas space. Normalized data were then spatially smoothed (6 mm full-width at half-maximum, FWHM) using a Gaussian Kernel. Finally, the preprocessed data were examined, using the Artifact Detection Tool software package (ART; http://web.mit.edu/swg/art/art.pdf; http://www.nitrc.org/projects/artifact_detect), for excessive motion artifacts and for correlations between motion and experimental design, and between global mean signal and experimental design. Outliers were identified in the temporal differences series by assessing between-scan differences (Z-threshold: 3.0 mm, scan to scan movement threshold: 0.5 mm; rotation threshold: 0.02 radians). These outliers were omitted in the analysis by including a single regressor for each outlier. No correlations between motion and experimental design or global signal and experimental design were identified. Six directions of motion parameters from the realignment step as well as outlier time points (defined by ART) were included as nuisance regressors. We used a default high-pass filter of 128 s and serial correlations were accounted for by the default auto-regressive AR(1) model.
Statistical analysis
Analyses of the fMRI data at the first (single participant) level were conducted using the general linear model of SPM8 (Wellcome Department of Cognitive Neurology, London, UK) of which the event-related design was modeled with two regressors for each condition (and only one regressor for the singleton condition), time locked at the presentation of the prime and target sentences and convolved with a canonical hemodynamic response function with event duration set to 0 for all conditions. Six motion parameters from the realignment as well as outlier time points (identified by ART) were included as nuisance regressors. We did not model the response of the participants as a separate regressor.
At the second (group) level, we conducted a whole-brain analysis, thresholded at P < 0.05, family wise error (FWE) corrected without a minimum cluster extent. Specific comparisons between conditions were performed on the parameter estimates associated with each trial type for each subject, using simple t-tests with P < 0.05, FWE corrected. We defined a repetition suppression effect as the prime > target contrast, and specified interactions with this contrast to identify the repetition suppression effect for the Person or Trait. That is, we defined an interaction contrast reflecting the assumption that the repetition suppression (prime > target) contrast would be significant only for the Repeated Trait (or Repeated Person) condition. Specifically, to identify trait suppression, the prime > target contrast was compared in the conditions with vs without Trait repetition, while holding the Person factor constant (e.g. comparing the Repeated Trait/Different Person vs No Trait/Different Person conditions). Likewise, to identify person suppression, the prime > target contrast was compared in the conditions with vs without Person repetition, while holding the Trait factor constant (e.g. comparing the Repeated Trait/Repeated Person vs Repeated Trait/Different Person conditions; see Table 2 for the contrasts specified). To explore whether the brain areas identified are not involved in the complementary process of repetition enhancement which refers to an increase of activation from prime to target, we defined the reverse, target > prime contrast for all experimental conditions and their interactions (see Table 3 for the contrasts specified).
Table 2.
Repetition suppression (prime > target contrast) effects from the whole-brain analysis
| Anatomical label | x | y | z | Voxels | Max t |
|---|---|---|---|---|---|
| Prime > Target | |||||
| Repeated Trait/Repeated Person | |||||
| mPFC | 2 | 44 | −8 | 1238 | 7.79*** |
| mPFC | −10 | 40 | −4 | 6.49*** | |
| mPFC | 0 | 50 | 4 | 6.43*** | |
| Repeated Trait/Different Person | |||||
| mPFC | 0 | 46 | −2 | 664 | 6.85*** |
| mPFC | 10 | 42 | 0 | 5.67*** | |
| mPFC | 14 | 32 | −6 | 5.62** | |
| No Trait/Different Person | |||||
| L parahippocampal gyrus | −28 | −42 | −10 | 54 | 6.29*** |
| L parahippocampal gyrus | −32 | −36 | −14 | 5.87*** | |
| L superior occipital gyrus | −36 | −84 | 30 | 57 | 7.93*** |
| Trait suppression: Interaction of Prime > Target for Trait Repetition > No Trait Repetition | |||||
| Trait Repetitions | |||||
| (across different persons: 0 0 1 −3 1 1) | |||||
| mPFC | 10 | 42 | −2 | t93 | 5.40** |
| mPFC | 0 | 44 | −4 | 5.20** | |
| mPFC | −4 | 36 | 6 | t14 | 4.93* |
| L parahippocampal gyrus | −30 | −34 | −14 | 16 | 5.08* |
| PCC | −10 | −34 | 38 | 12 | 5.05* |
| L superior occipital gyrus | −36 | −82 | 32 | 26 | 5.76*** |
| Person suppression: Interaction of Prime > Target for Person Repetition > No Person Repetition | |||||
| Person Repetitions | |||||
| (across similar traits: 1 −3 1 1 0 0) | |||||
| mPFC | −2 | 46 | −6 | p1967 | 8.04*** |
| mPFC | −8 | 48 | 0 | 7.58*** | |
| mPFC | 6 | 30 | −8 | 6.66*** | |
| R parahippocampal gyrus | 26 | −28 | −12 | 15 | 5.63** |
| PCC | 2 | −58 | 22 | 40 | 5.44** |
| Conjunction of Trait and Person suppression | |||||
| (see two contrasts above) | |||||
| mPFC | 10 | 42 | −2 | 93 | 5.40** |
| mPFC | 0 | 44 | −4 | 5.20** | |
| mPFC | −4 | 36 | 6 | 14 | 4.93* |
Notes: Coordinates refer to the MNI (Montreal Neurological Institute) stereotaxic space. Whole-brain analysis thresholded at P < 0.05, FWE corrected with a voxel extent of ≥10. The contrasts between parentheses refer to the Prime and Target in the Repeated Trait/Repeated Person, Repeated Trait/Different Person, No Trait/Different Person conditions, respectively. PCC = posterior cingulate cortex, L = left, R = right. Superscripts indicate the cluster peaks that show the expected repetition suppression pattern for t trait and p person.
*P < 0.05, **P < 0.01, ***P < 0.001 (FWE peak corrected).
Table 3.
Repetition Enhancement (Target > Prime) contrast effects from the whole-brain analysis
| Anatomical label | x | y | z | Voxels | Max t |
|---|---|---|---|---|---|
| Target > Prime | |||||
| Repeated Trait/Repeated Person | |||||
| R superior frontal gyrus | 30 | 50 | 20 | 126 | 5.54** |
| R middle frontal gyrus | 48 | 28 | 32 | 2388 | 7.94*** |
| L middle frontal gyrus | −44 | 24 | 32 | 8269 | 9.40*** |
| R middle frontal gyrus | 36 | 4 | 58 | 944 | 7.27*** |
| R superior temporal gyrus | 48 | 14 | −20 | 11 | 5.13* |
| L superior temporal gyrus | −48 | −28 | −8 | 828 | 7.08*** |
| Caudate | −16 | −4 | 14 | 598 | 6.27*** |
| Thalamus | 10 | −14 | 4 | 457 | 6.03*** |
| L insula | −30 | −32 | 12 | 10 | 4.99* |
| R middle temporal gyrus | 48 | −36 | −2 | 897 | 7.35*** |
| R inferior parietal lobule | 36 | −54 | 44 | 396 | 7.48*** |
| L superior parietal lobule | −28 | −56 | 42 | 1832 | 8.32*** |
| Precuneus | −6 | −66 | 46 | 16 | 5.11* |
| PCC | −28 | −68 | 6 | 79 | 5.84*** |
| L lingual gyrus | −24 | −72 | −6 | 36 | 5.23** |
| L lingual gyrus | −34 | −72 | −10 | 15 | 5.01* |
| Lingual gyrus | −16 | −86 | −6 | 36 | 5.41** |
| Cuneus | −6 | −94 | 10 | 146 | 5.84*** |
| Cerebellum (Declive) | 12 | −72 | −28 | 564 | 8.38*** |
| Repeated Trait / Different Person | |||||
| L superior frontal gyrus | −34 | 52 | 18 | 147 | 5.71*** |
| R middle frontal gyrus | 48 | 26 | 34 | 595 | 6.51*** |
| L middle frontal gyrus | −44 | 24 | 30 | 2584 | 7.52*** |
| R middle frontal gyrus | 36 | −6 | 52 | 96 | 5.47** |
| R inferior frontal gyrus | 30 | 24 | −4 | 136 | 6.17*** |
| L insula | −28 | 22 | −4 | 177 | 6.42*** |
| Medial frontal gyrus | 8 | 12 | 50 | 541 | 6.29*** |
| R precentral gyrus | 38 | −28 | 58 | 14 | 5.02* |
| R middle temporal gyrus | 50 | −30 | −8 | 116 | 5.46** |
| L middle temporal gyrus | −52 | −40 | 2 | 19 | 5.05* |
| L inferior parietal lobule | −46 | −40 | 46 | 39 | 5.25** |
| R inferior parietal lobule | 36 | −56 | 46 | 335 | 6.81*** |
| L precuneus | −26 | −60 | 50 | 880 | 7.56*** |
| Precuneus | 2 | −66 | 44 | 165 | 5.47** |
| L PCC | −28 | −68 | 6 | 28 | 5.65** |
| L inferior occipital gyrus | −34 | −74 | −6 | 499 | 6.06*** |
| Cerebellum (Declive) | 10 | −68 | −26 | 70 | 5.64** |
| No Trait/Different Person | |||||
| R middle frontal gyrus | 44 | 26 | 30 | 12 | 4.98* |
| L middle frontal gyrus | −38 | −2 | 54 | 6552 | 8.35*** |
| R superior temporal gyrus | 44 | 14 | −28 | 17 | 5.36** |
| R superior temporal gyrus | 52 | 10 | −16 | 12 | 5.06* |
| R precentral gyrus | 62 | −4 | 18 | 40 | 5.64** |
| R precentral gyrus | 36 | −16 | 58 | 443 | 5.73*** |
| L precentral gyrus | −48 | −18 | 30 | 47 | 5.13* |
| Thalamus | −8 | −14 | 6 | 391 | 6.34*** |
| Thalamus | 12 | −14 | 6 | 171 | 5.77*** |
| Cingulate cyrus | −2 | −16 | 28 | 30 | 5.32** |
| R insula | 44 | −30 | −2 | 257 | 6.45*** |
| R supramarginal gyrus | 62 | −52 | 22 | 15 | 5.10* |
| R precuneus | 28 | −60 | 54 | 193 | 5.85*** |
| Precuneus | −2 | −62 | 34 | 4490 | 8.96*** |
| L precuneus | −24 | −72 | 26 | 49 | 5.37** |
| Cuneus | −18 | −92 | 6 | 4161 | 10.62*** |
| Trait enhancement: Interaction of Target > Prime for No Trait Repetition > Trait Repetition | |||||
| Trait Repetitions | |||||
| (across different persons: 0 0 −1 3 −1 −1) | |||||
| L middle frontal gyrus | −28 | 48 | 12 | t306 | 6.61*** |
| R middle frontal gyrus | 46 | 26 | 32 | t830 | 6.92*** |
| L middle frontal gyrus | −44 | 24 | 30 | 2746 | 7.64*** |
| R middle frontal gyrus | 34 | −6 | 52 | 116 | 5.78*** |
| R claustrum | 28 | 24 | −2 | t64 | 5.56** |
| L insula | −28 | 20 | −6 | t110 | 6.43*** |
| Medial frontal gyrus | −6 | 8 | 50 | 779 | 6.39*** |
| R precentral gyrus | 36 | −18 | 58 | 16 | 5.21** |
| L inferior parietal lobule | −44 | −34 | 46 | 17 | 4.94* |
| R inferior parietal lobule | 36 | −56 | 46 | t281 | 6.71*** |
| R middle temporal gyrus | 48 | −36 | −2 | 119 | 5.58** |
| L middle temporal gyrus | −48 | −40 | 0 | 156 | 6.24*** |
| L precuneus | −26 | −60 | 50 | 824 | 7.32*** |
| Precuneus | 0 | −62 | 36 | 234 | 6.42*** |
| R cerebellum (culmen) | 34 | −52 | −30 | 30 | 5.58** |
| Cerebellum (declive) | 10 | −68 | −26 | 66 | 5.50** |
| Person enhancement: Interaction of Target > Prime for No Person Repetition > Person Repetition | |||||
| Person repetitions | |||||
| (across similar traits: −1 3 −1 −1 0 0) | |||||
| L middle frontal gyrus | −44 | 52 | −4 | p827 | 8.61*** |
| L middle frontal gyrus | −46 | 24 | 32 | p2670 | 8.24*** |
| R middle frontal gyrus | 40 | 2 | 60 | p149 | 6.08*** |
| R inferior frontal gyrus | 32 | 26 | −4 | 1297 | 7.32*** |
| L inferior frontal gyrus | −28 | 24 | −6 | p230 | 7.64*** |
| Cingulate gyrus | 8 | 18 | 44 | p1246 | 7.69*** |
| Caudate | 14 | 10 | 2 | 35 | 5.08* |
| Caudate | −16 | −2 | 14 | 214 | 5.84*** |
| Thalamus | 14 | −16 | 14 | 56 | 5.31** |
| L superior temporal gyrus | −48 | −30 | −8 | 397 | 6.31*** |
| R superior temporal gyrus | 54 | −40 | 8 | p 359 | 6.05*** |
| R postcentral gyrus | 46 | −32 | 44 | 15 | 5.15** |
| No gray matter | −28 | −52 | 38 | 955 | 7.12*** |
| R inferior parietal lobule | 36 | −54 | 44 | 67 | 5.72*** |
| Cuneus | −12 | −96 | 16 | 30 | 5.25** |
| Cerebellum (declive) | 12 | −72 | −28 | p346 | 8.59*** |
| Cerebellum (declive) | −12 | −72 | −28 | p19 | 5.23** |
| Conjunction of Trait and Person enhancement | |||||
| (see two contrasts above) | |||||
| L superior frontal gyrus | −34 | 52 | 18 | 172 | 5.93*** |
| R middle frontal gyrus | 46 | 28 | 32 | 346 | 6.72*** |
| L middle frontal gyrus | −44 | 24 | 30 | 2121 | 7.64*** |
| R middle frontal gyrus | 36 | 0 | 58 | 13 | 5.10* |
| R claustrum | 28 | 24 | −2 | 60 | 5.56** |
| L insula | −28 | 20 | −6 | 109 | 6.43*** |
| Medial frontal gyrus | −6 | 10 | 50 | 642 | 6.39*** |
| R middle temporal gyrus | 48 | −36 | −2 | 111 | 5.58** |
| L inferior parietal lobule | −46 | −36 | 46 | 11 | 4.87* |
| R inferior parietal lobule | 36 | −54 | 44 | 66 | 5.72*** |
| L superior temporal gyrus | −48 | −38 | 0 | 121 | 5.84*** |
| L superior parietal lobule | −28 | −56 | 40 | 597 | 6.91*** |
| Cerebellum (declive) | 10 | −68 | −26 | 54 | 5.50** |
Notes: Coordinates refer to the MNI (Montreal Neurological Institute) stereotaxic space. Whole-brain analysis thresholded at P < 0.05, FWE corrected with a voxel extent of ≥10, only coordinates of the highest peak in each cluster are reported (see supplementary table for all peaks). The contrasts between parentheses refer to the Prime and Target in the Repeated Trait/Repeated Person, Repeated Trait/Different Person, and No Trait/Different Person conditions, respectively. PCC = posterior cingulate cortex, L = left, R = right. Superscripts indicate the cluster peaks that show the expected repetition enhancement pattern for t trait and p person.
*P < 0.05, **P < 0.01, ***P < 0.001 (FWE peak corrected).
To further verify whether the brain areas identified in the interaction analysis showed the predicted repetition suppression or enhancement pattern, we computed the percentage signal change. For this, we identified regions of interest (ROIs) as a sphere of 3 mm around the peak coordinates of the interaction contrasts. We then extracted the percentage signal change in these ROIs for each participant using the MarsBar toolbox (http://marsbar.sourceforge.net). We also calculated repetition indexes for each condition, which were defined as the percentage signal change of target minus prime sentences for each condition. These data were further analyzed using t-tests with a threshold of P < 0.05, one sided.
To check for a relationship between percent signal change and social network size, we calculated the correlation of percent signal change and reported amount of contacts for each condition separately. Two participants with a number of reported contacts larger than three standard deviations from the mean network size were excluded from this analysis.
Results
For the analysis of the fMRI data, we used a similar strategy as Ma et al. (2014a) to detect a repetition suppression effect during trait and person processing. We first conducted a whole-brain random effects analysis contrasting prime > target trials in all conditions, and then conducted whole-brain interaction analyses to identify repetition effects. These analyses were followed by an analysis of signal change to verify whether the predicted repetition pattern was present.
Whole-brain analysis
The whole-brain random effects analyses of the prime > target repetition suppression contrast revealed a significant repetition suppression effect in the mPFC for the Repeated Trait/Repeated Person and Repeated Trait/Different Person conditions (Table 2). The repetition interactions revealed a significant repetition suppression effect in the mPFC, for both trait and person. There were other, but smaller significant clusters as well in these interaction contrasts, including the posterior cingulate cortex and parahippocampal gyrus. A conjunction analysis revealed an overlap of trait and person repetition suppression in the mPFC (Table 2).
For enhancement, none of the reverse, target > prime contrast or their interaction contrasts showed repetition effects in the mPFC, but revealed other regions (Table 3).
Region of interest analysis
We computed the percent signal change in ROIs centered around the peak values of the repetition interactions to verify whether the expected repetition pattern was present. We calculated a repetition suppression index by subtracting the percent signal change in the prime sentence from those in the target sentence for every condition and every ROI separately.
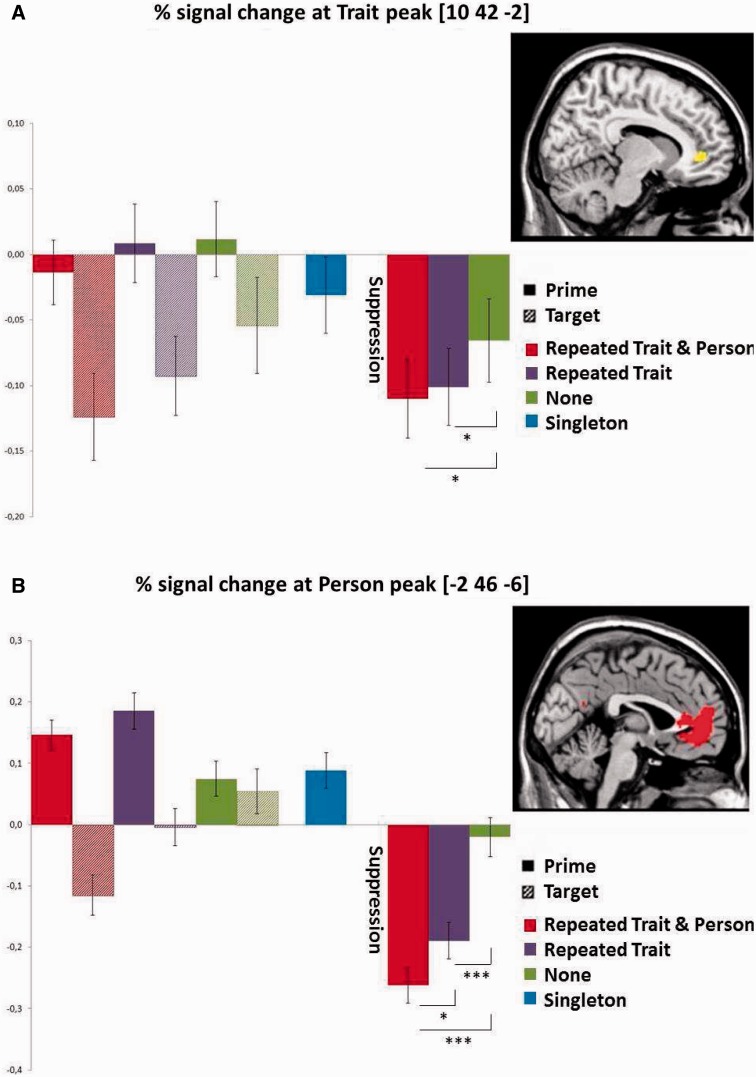
With respect to trait suppression, the analysis at the peak of the mPFC (Figure 1A) revealed as predicted, that the suppression index did not differ between the two conditions involving trait repetition (i.e. Repeated Trait/Repeated Person = Repeated Trait/Different Person), but that these two conditions differed significantly from the No Trait/Different Person condition (P < 0.05). Importantly, the same tests for the other clusters in the trait suppression interaction were not significant, indicating that only the mPFC showed the predicted trait suppression effect.
Fig. 1.
Percentage signal change at the Trait and Person suppression interaction. The left side of each graph shows prime target pairs, the right side the suppression index. The inset on the far right shows the area revealed in the whole-brain analysis thresholded at P < 0.05, FWE corrected, from which the signal change was extracted. (A) Trait suppression is identical in the first two (Repeated Trait) conditions, and differs from the third (No trait) condition. (B) Person suppression is strongest for the first condition (Repeated Person) and differs from the second and third conditions (Different Person). *P < 0.05, ***P < 0.001 (one sided).
With respect to person suppression, the percent signal change analysis of the suppression index revealed the predicted difference between the conditions with or without person repetition (i.e. Repeated Trait/Repeated Person > Repeated Trait/Different Person at P < 0.05; Figure 2A). Moreover, activation in the No Trait/Different Person condition differed from these two conditions (P < 0.001). Importantly, the same tests for the other clusters in the person suppression interaction were not significant, indicating that only the mPFC showed the predicted person suppression effect.
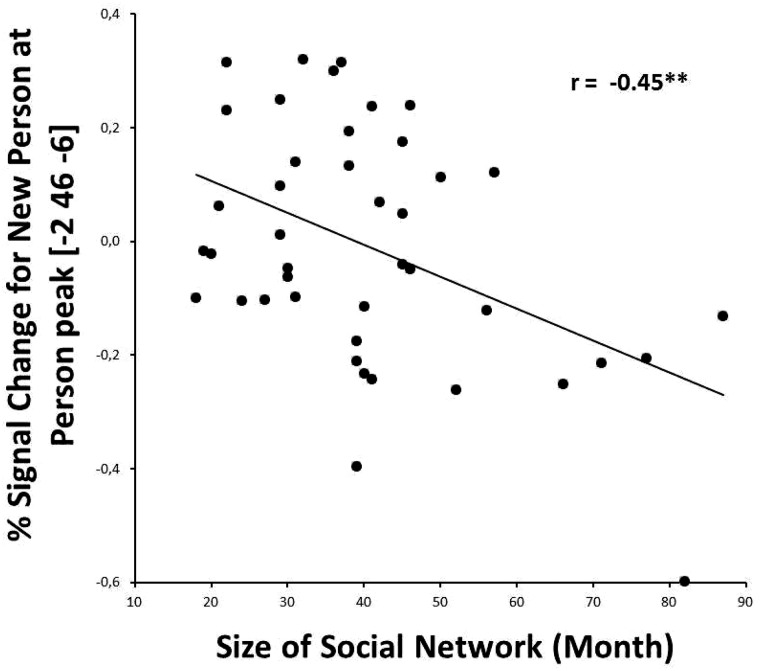
Fig. 2.
Negative correlation between Social Network Size and activation at the Person suppression interaction when processing information about a different person, suggesting that participants with a larger social network invest less mental effort in processing another (familiar) person. Note that the same correlation at the Trait suppression interaction was not significant. **P < 0.01 (two sided).
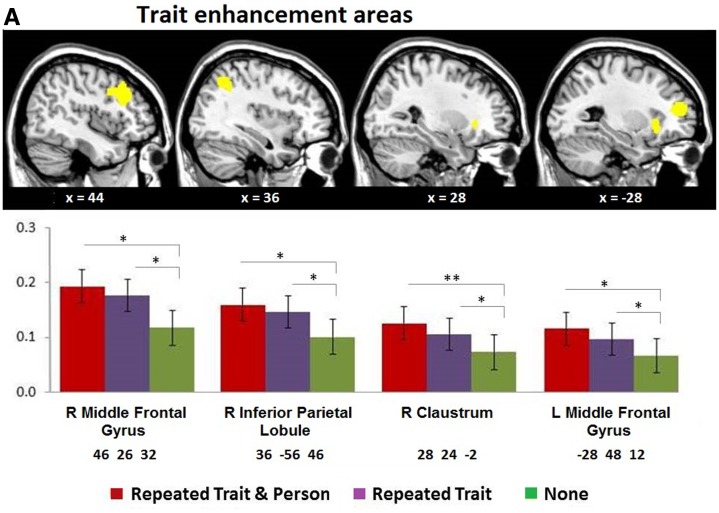
The analysis at the enhancement peaks revealed trait and person enhancement effects (Table 3 and Figure 3). Note that none were located in the mPFC. With respect to trait enhancement, the analysis at the peak of the left and right middle frontal gyrus, right claustrum, left insula and right inferior parietal lobule revealed that the suppression index did not differ between the two conditions involving trait repetition (i.e. Repeated Trait/Repeated Person = Repeated Trait/Different Person), but that these two conditions differed significantly from the No Trait/Different Person condition (Figure 3A).
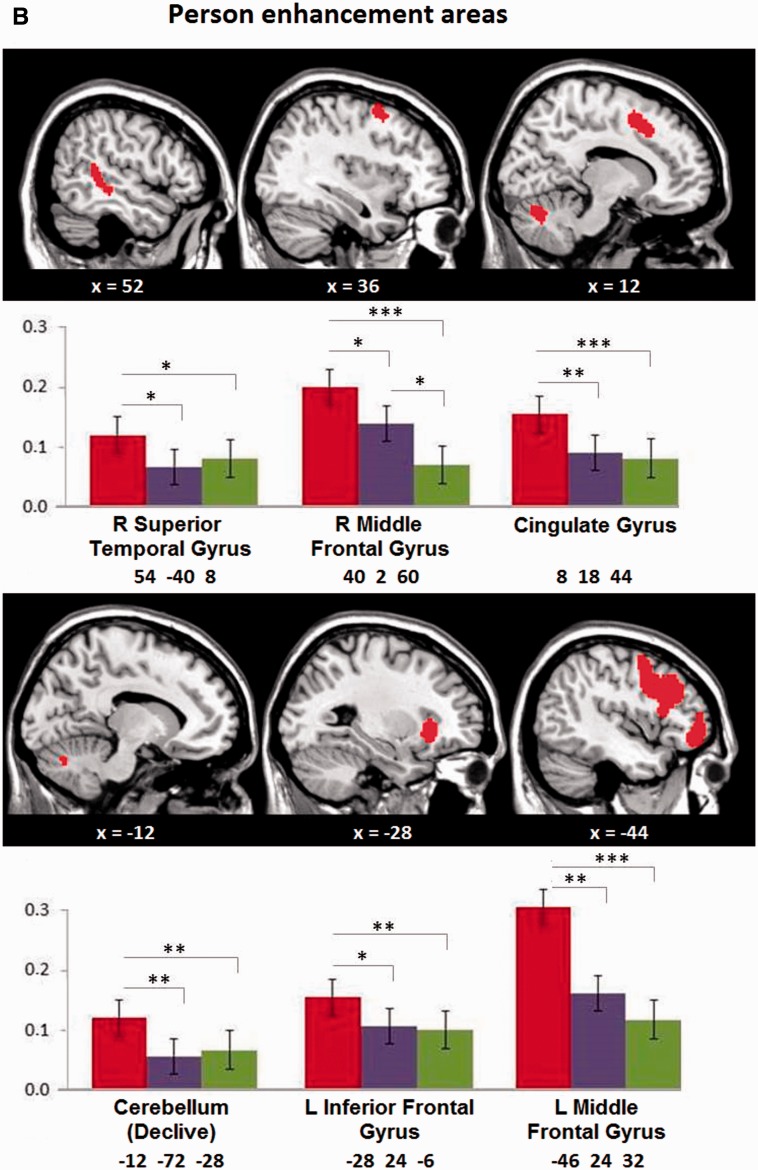
Fig. 3.
(A) Trait and (B) Person repetition enhancement areas. The top row shows areas revealed in the whole-brain analysis thresholded at P < 0.05, FWE corrected. The bottom row shows the corresponding enhancement pattern from the percentage signal change analysis of the largest cluster in each slice (with MNI coordinates). Note that trait enhancement shows no difference between the first two (Repeated Trait) conditions, while person enhancement differs between them. *P < 0.05, **P < 0.01, ***P < 0.001 (one sided).
With respect to person enhancement, the enhancement indexes revealed differences between conditions with or without person repetition (i.e. Repeated Trait/Repeated Person > Repeated Trait/Different Person; Figure 3B) in the bilateral middle frontal gyrus, left inferior frontal gyrus, cingulate gyrus, right superior temporal gyrus and cerebellum (declive). Note that only in the right middle frontal gyrus activation in these two conditions differed from the No Trait/Different Person condition (Figure 3B).
Correlation with social network size
We investigated the relationship between the percent signal change of target sentences and the number of reported social contacts. The mean number of reported social contacts was 25.52 (s.d. = 14.48) for the last week and 40.69 (s.d. = 16.74) for the last month. For the person suppression ROI, we found a significant negative correlation between reported social contacts in the last month and the Repeated Trait/Different Person condition (r = −0.45, P < 0.01; Figure 2 and Table 4). For the trait suppression ROI, as predicted, there was no significant correlation between the number of reported social contacts and the percent signal change, for any condition. For enhancement, we found several negative correlations between percentage signal change and reported social contacts in the last week (Table 4). For various trait enhancement ROIs, there were negative correlations with activation in conditions where traits are repeated (Repeated Trait/Repeated Person and Repeated Trait/Different Person). Likewise, for various person enhancement ROIs, there were negative correlations with activation in person repetition conditions (Repeated Trait/Repeated Person).
Table 4.
Correlations between social network size and activation at Repetition Suppression and Enhancement areas in some relevant conditions
| Anatomical label | x | y | z | Repeated Trait/Repeated Person | Repeated Trait/Different Person |
|---|---|---|---|---|---|
| Person suppression and social network size (month) | |||||
| Person repetition | |||||
| mPFC | −2 | 46 | −6 | – | −0.45** |
| Trait enhancement and social network size (week) | |||||
| Trait Repetition | |||||
| R middle frontal gyrus | 46 | 26 | 32 | −0.32* | −0.31* |
| R inferior parietal lobule | 36 | −56 | 46 | −0.26* | −0.27* |
| R middle temporal gyrus | 48 | −36 | −2 | −0.32* | −0.38** |
| Person enhancement and social network size (week) | |||||
| Person Repetition | |||||
| Cingulate gyrus | 8 | 18 | 44 | −0.25* | – |
| Caudate | 14 | 10 | 2 | −0.28* | – |
| R inferior parietal lobule | 36 | −54 | 44 | −0.29* | – |
Notes: Areas and coordinates refer to Tables 2 and 3. Cell entries in the last two columns are correlations. Only peaks that show a significant correlation between the percentage signal change at the target sentence and social network size of the last week (for enhancement) or month (for suppression) in the relevant conditions are reported. mPFC = medial prefrontal cortex, R = right.
*P < 0.05, **P < 0.01 (one tailed, uncorrected)
Discussion
A broad range of fMRI research suggests that the mentalizing network, and more specifically the mPFC, is involved in the integration of temporary actions and mental states into more abstract and enduring person knowledge such as traits and other characteristics (Mitchell et al., 2006; Todorov et al., 2007; Ma et al., 2011, 2012a). In this study, we argued and demonstrated that the mPFC is not only involved in the processing of person information, but also stores specific knowledge of traits and familiar persons in the form of neural representations that we call memory codes. To uncover these memory codes, we turned to a fMRI repetition design and predicted that repeated processing of a core stimulus (such as a trait or person) suppresses activation in the neural population that stores information about this specific stimulus relative to other superficial and variable stimulus characteristics.
Trait and person memory codes
The present findings confirm and extend the earlier works by Ma et al. (2014a) and Szpunar et al. (2014). Using a similar fMRI suppression design, Ma et al. (2014a) found strong evidence for memory codes in the ventral mPFC for traits implied in behavioral descriptions. In addition, Szpunar et al. (2014) found support for repetition suppression in the mPFC when imaging an interaction with familiar persons. This study extended both studies and demonstrates that both persons and their traits implied in behavioral descriptions are represented at the same time in distinct, but overlapping, neural codes in the ventral mPFC. This may indicate that trait and person memory codes are closely interrelated. Thus, if we associate specific traits strongly with a person (e.g. my mother is very kind), perhaps these traits become a part of the neural representation of the person. This seems plausible, as our participants knew the characteristics of the persons very well because they were family members or close friends. Hence, it is possible that person processing automatically led to the activation of associated trait representations.
Hassabis et al. (2014) put forward a similar idea that trait representations are neurally combined to a more holistic representation of persons if we get to know them better. This view is consistent with connectionist models of brain functioning, which assume that high-level constructs such as traits and persons are represented by neural populations at a distinct layer of the brain (McClelland and Rumelhart, 1988). The notion of a layer does not mean that these neural populations are located in tightly constrained or strictly hierarchical locations. It is more likely that these neural representations are distributed, that is, the neural codes for persons are linked with their major characteristics that might be represented at different locations, although they process information as one functional unit. Given the close relationships between persons and their traits, it does not come as a surprise that they are represented close to each other on the cortical surface because that is computationally more efficient. Taken together, given that traits are abstract representation of concrete behaviors, and given that person and trait representations are closely related, we suggest that the mPFC is a hub region, representing different types of social abstractions linked with other brain areas representing more concrete (behavioral) information.
A limitation of the present design is that it was not fully factorial. A full factorial design with an additional condition in which only a person, but not a trait, is repeated might have been more appropriate for separating trait and person codes. This condition was not included in this study because we wanted to ensure that persons were perceived as possessing trait characteristics and not merely performing unsystematic behaviors. Future research should investigate how familiar or even unknown persons with or without information about their trait characteristics are processed. It is likely that person codes are distributed across the brain, with trait-related codes residing in the mPFC and other information (e.g. face) coded elsewhere.
Repetition enhancement
To ensure that the mPFC was exclusively involved in repetition suppression, we examined the complementary repetition enhancement process. An increase in activation is assumed to indicate that more mental effort is necessary to process a repeated stimulus or stimulus characteristic. As expected, there were no enhancement effects in the mPFC, which confirms the exclusive involvement of mPFC in repetition suppression when traits and persons are processed.
However, we did find enhancement effects for traits and persons in some other brain areas (Figure 3). As can be seen, repetition enhancement areas are more extensive than suppression areas. This should not come as a surprise since the suppression method precisely attempts to isolate a narrowly defined area involved in the processing and encoding of a given stimulus only, excluding all concomitant pre- and post-processing. This is not the case for enhancement. Although we did not have specific hypotheses about repetition enhancement effects, the enhancement interaction contrasts (Table 3) reveal that the great majority of trait and person enhancement areas are part of the executive control network as defined in the cerebrum by Yeo et al. (2011) and in the cerebellum by Buckner et al., (2011). Among the major areas revealed in our study, the bilateral PFC is generally assumed to be involved in working memory (Forbes and Grafman, 2010; Barbey and Patterson, 2011; Van Overwalle, 2011; Meyer and Lieberman, 2012). This area is connected to the mPFC and is recruited during social mentalizing when information needs to be integrated or compared (Ma et al., 2012a), or when working memory demands increase (Van Overwalle, 2011; Meyer and Lieberman, 2012). Also part of this control network is the inferior frontal gyrus, known to be recruited when general abstract concepts are processed (Wang et al., 2010). The claustrum and cingulate gyrus have been identified as multiple information integration centers (Botvinick et al., 1999; Mathur, 2014). Collectively, we can interpret activation in these executive control areas as an increase in mental effort necessary for the integration, comparison and construction of mental representations when information about the person or trait is repeated. Given the systematic involvement of the executive control network in enhancement but not of attention networks, alternative interpretations such as differences in attention allocation are less likely.
Nevertheless, there is a related explanation in terms of differential processing of prime and target sentences. Participants may ignore the trait or person information in the prime sentences, even though 25% of the trials (the singleton condition) invited participants to make a judgment of a person’s trait after the first sentence. Hence, it is possible that participants exerted more effort during the target sentences as they could be sure that a question would follow it. In contrast, for the prime sentence a question may or may not have appeared. This may have resulted in increased processing of the target sentence, not for the integration of the prime information as noted above, but because the target sentence was deemed more relevant.
Social network size
We measured participants’ social network size, and found a negative correlation between social network size of the last month and mPFC activation when processing information about a new person. This negative correlation suggests that processing information about another person requires less activation when the observer’s social network is larger. A possible explanation for this correlation is that more social experience and capacities as revealed by a larger social network lead to more efficient processing of novel persons. Note that this correlation was heavily dependent on its exact location at the peak coordinate of the whole-brain interaction effect. Moving away at a mere 2 mm distance from this peak resulted in similar, but less pronounced correlations. The present correlational results should thus be interpreted with caution and need confirmation by future research. As expected, there was no significant relationship between social network size and activation in the area underpinning trait codes.
Of interest is that we found several negative correlations between number of social contacts during the last week and various trait and person enhancement areas (Table 4). This suggests that less recruitment of the executive control network is necessary when new information is processed for participants with a larger social network size and presumably more efficient social skills. It is interesting to note that most enhancement correlations were revealed with social contacts during the last week, whereas the suppression correlation was found with social contacts during the last month. It seems intuitive plausible that memory representations are related to social skills on the longer term, while enhancement is related to social efficiency on the shorter term. However, these speculations await confirmation from future research.
Future research
An unresolved question is how general or abstract representations of traits and persons are? Research by Ma et al. (2014a) seems to suggest that this representation might encompass quite a broad meaning because traits with opposite valence are represented in the same neural population in the mPFC. Our person suppression area is substantially larger than the trait area (Figure 1). This smaller trait area could be due to the use of a relatively small range of traits that are conceptually similar (e.g. friendly and nice). On the other hand, the larger person area might result from the fact that close friends and family members can differ substantially, and might possess a large range of distinct characteristics. Future research should investigate whether distinct moral traits (e.g. kind, diligent, generous) share a general representation, or are identifiable as distinct neural codes. Future research should also explore to what extent person codes can be distinguished from each other. A critical question is whether repetition suppression is sensitive enough to reveal such distinct locations for different traits (e.g. agreeable vs extraversion) or persons. Perhaps an alternative technique known as multi-voxel pattern analysis is more sensitive, because it identifies distinct patterns of activation, rather than distinct locations. In this analysis, the pattern of activation elicited by a whole brain area is analyzed in order to recover as much as possible the original stimulus that activated them (Davis and Poldrack, 2013). This technique allows to identify with more micro-level detail the code itself, that is, the precise pattern of activation and deactivation distributed among a large array of voxels that represent the content of the code.
Note that we identified trait and person memory codes in the ventral part of the mPFC which is an area involved in the processing of trait-related information about the self and close others. In contrast, the dorsal part of the mPFC is considered to be related to the processing of trait information of unfamiliar people (Van Overwalle, 2009). It is still an open question whether the mPFC contains memory codes of less known or unfamiliar persons.
Conclusion
In order to better understand the organizational principles of the brain, it is necessary to improve our knowledge about the processing and storage of core constructs and their relation to one another. In the current research, we turned to fMRI repetition suppression to identify neural representations in the mPFC. Traditional fMRI research already demonstrated that traits and persons are processed in this area. We provide evidence for the storage of specific trait and person knowledge in the form of memory codes. We interpret our results as confirming the idea that the mPFC is a hub area that aggregates abstract and highly connected mental representations of persons and traits.
Funding
SRP15 project funded by the Vrije Universiteit Brussel.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Barbey A.K., Patterson R. (2011). Architecture of explanatory inference in the human prefrontal cortex. Frontiers in Psychology , 2, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M., McGill M., Thompson-Schill S.L. (2008). Semantic adaptation and competition during word comprehension. Cerebral Cortex , 18(11), 2574–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M., Nystrom L.E., Fissell K., Carter C.S., Cohen J.D. (1999). Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature , 402(6758), 179–81. [DOI] [PubMed] [Google Scholar]
- Buckner R., Krienen F., Castellanos A., Diaz J.C., Yeo B.T. (2011). The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology , 106, 2322–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T., Poldrack R.A. (2013). Measuring neural representations with fMRI: practices and pitfalls. Annals of the New York Academy of Sciences , 1296, 108–34. [DOI] [PubMed] [Google Scholar]
- De Graaf T.A., Hsieh P.J., Sack A.T. (2012). The “correlates” in neural correlates of consciousness. Neuroscience and Biobehavioral Reviews , 36(1), 191–7. [DOI] [PubMed] [Google Scholar]
- Devauchelle A.D., Oppenheim C., Rizzi L., Dehaene S., Pallier C. (2009). Sentence syntax and content in the human temporal lobe: an fMRI adaptation study in auditory and visual modalities. Journal of Cognitive Neuroscience , 21(5), 1000–12. [DOI] [PubMed] [Google Scholar]
- Diana R.A., Yonelinas A.P., Ranganath C. (2012). Adaptation to cognitive context and item information in the medial temporal lobes. Neuropsychologia , 50(13), 3062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar R.I.M. (1992). Neocortex size as a constraint on group size in primates. Journal of Human Evolution , 20, 469–93. [Google Scholar]
- Dunbar R.I.M. (1998). The social brain hypothesis. Evolutionary Anthropology , 6(5), 178–90. [Google Scholar]
- Dunbar R.I.M., Shultz S. (2007). Evolution in the social brain. Science , 317(5843), 1344–7. [DOI] [PubMed] [Google Scholar]
- Engel S.A., Furmanski C.S. (2001). Selective adaptation to color contrast in human primary visual cortex. The Journal of Neuroscience , 21(11), 3949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes C.E., Grafman J. (2010). The role of the human prefrontal cortex in social cognition and moral judgment. Annual Review of Neuroscience , 33, 299–324. [DOI] [PubMed] [Google Scholar]
- Friston K. (2005). A theory of cortical responses. Philosophical Transactions of the Royal Society of London: Series B, Biological Sciences , 360(1456), 815–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble C., Gowlett J., Dunbar R. (2011). The social brain and the shape of the palaeolithic. Cambridge Archaeological Journal , 21(1), 115–36. [Google Scholar]
- Gotts S.J., Chow C.C., Martin A. (2012). Repetition priming and repetition suppression: multiple mechanisms in need of testing. Cognitive Neuroscience , 3(3–4), 250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K., Malach R. (2001). fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychologica , 107(1–3), 293–321. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K., Henson R., Martin A. (2006). Repetition and the brain: neural models of stimulus-specific effects. Trends in Cognitive Sciences , 10(1), 14–23. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K., Kushnir T., Edelman S., Avidan G., Itzchak Y., Malach R. (1999). Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron , 24(1), 187–203. [DOI] [PubMed] [Google Scholar]
- Hassabis D., Spreng R.N., Rusu A.A., Robbins C.A., Mar R.A., Schacter D.L. (2014). Imagine all the people: how the brain creates and uses personality models to predict behavior. Cerebral Cortex , 24(8), 1979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins A.C., Macrae C.N., Mitchell J.P. (2008). Repetition suppression of ventromedial prefrontal activity during judgments of self and others. Proceedings of the National Academy of Sciences of the United States of America , 105(11), 4507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse G., Joseph S., Bertasi E., Giraud A.L. (2012). The brain’s dorsal route for speech represents word meaning: evidence from gesture. PLoS One , 7(9), e46108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzi Z., Kanwisher N. (2000). Cortical regions involved in perceiving object shape. The Journal of Neuroscience , 20(9), 3310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P.A., Rezaie R., Brown R., Roberts N., Dunbar R.I.M. (2011). Ventromedial prefrontal volume predicts understanding of others and social network size. NeuroImage , 57(4), 1624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N., Baetens K., Vandekerckhove M., Kestemont J., Fias W., Van Overwalle F. (2014a). Traits are represented in the medial prefrontal cortex: an fMRI adaptation study. Social Cognitive and Affective Neuroscience , 9(8), 1185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N., Baetens K., Vandekerckhove M., Van der Cruyssen L., Van Overwalle F. (2014b). Dissociation of a trait and a valence representation in the mPFC. Social Cognitive and Affective Neuroscience , 9(10), 1506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N., Vandekerckhove M., Baetens K., Van Overwalle F., Seurinck R., Fias W. (2012a). Inconsistencies in spontaneous and intentional trait inferences. Social Cognitive and Affective Neuroscience , 7(8), 937–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N., Vandekerckhove M., Van Hoeck N., Van Overwalle F. (2012b). Distinct recruitment of temporo-parietal junction and medial prefrontal cortex in behavior understanding and trait identification. Social Neuroscience , 7(6), 591–605. [DOI] [PubMed] [Google Scholar]
- Ma N., Vandekerckhove M., Van Overwalle F., Seurinck R., Fias W. (2011). Spontaneous and intentional trait inferences recruit a common mentalizing network to a different degree: spontaneous inferences activate only its core areas. Social Neuroscience , 6(2), 123–38. [DOI] [PubMed] [Google Scholar]
- Mathur B.N. (2014). The claustrum in review. Frontiers in Systems Neuroscience , 8(April), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland J.L., Rumelhart D.E. (1988). Explorations in Parallel Distributed Processing: A Handbook of Models, Programs and Exercises. Cambridge, MA: Bradford. [Google Scholar]
- Meyer M.L., Lieberman M.D. (2012). Social working memory: neurocognitive networks and directions for future research. Frontiers in Psychology , 3(December), 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.P., Cloutier J., Banaji M.R., Macrae C.N. (2006). Medial prefrontal dissociations during processing of trait diagnostic and nondiagnostic person information. Social Cognitive and Affective Neuroscience , 1(1), 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J.L., Lewis P.A., Dunbar R.I.M., García-Fiñana M., Roberts N. (2010). Orbital prefrontal cortex volume correlates with social cognitive competence. Neuropsychologia , 48(12), 3554–62. [DOI] [PubMed] [Google Scholar]
- Ramsey R., Hamilton A.F.D.C. (2010a). Triangles have goals too: understanding action representation in left aIPS. Neuropsychologia , 48(9), 2773–6. [DOI] [PubMed] [Google Scholar]
- Ramsey R., Hamilton A.F.D.C. (2010b). Understanding actors and object-goals in the human brain. NeuroImage , 50(3), 1142–7. [DOI] [PubMed] [Google Scholar]
- Roggeman C., Santens S., Fias W., Verguts T. (2011). Stages of nonsymbolic number processing in occipitoparietal cortex disentangled by FMRI adaptation. The Journal of Neuroscience , 31(19), 7168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar K.K., St Jacques P.L., Robbins C.A., Wig G.S., Schacter D.L. (2014). Repetition-related reductions in neural activity reveal component processes of mental simulation. Social Cognitive and Affective Neuroscience , 9(5), 712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill S.L., D’Esposito M., Kan I.P. (1999). Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron , 23(3), 513–22. [DOI] [PubMed] [Google Scholar]
- Todorov A., Gobbini M.I., Evans K.K., Haxby J.V. (2007). Spontaneous retrieval of affective person knowledge in face perception. Neuropsychologia , 45(1), 163–73. [DOI] [PubMed] [Google Scholar]
- Van der Cruyssen L., Heleven E., Ma N., Vandekerckhove M., Van Overwalle F. (2015). Distinct neural correlates of social categories and personality traits. NeuroImage, 104, 336–46. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. (2007). Social Connectionism: A Reader and Handbook for Simulations. New York: Psychology Press. [Google Scholar]
- Van Overwalle F. (2009). Social cognition and the brain: a meta-analysis. Human Brain Mapping , 30(3), 829–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F. (2011). A dissociation between social mentalizing and general reasoning. NeuroImage , 54(2), 1589–99. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F., Labiouse C. (2004). A recurrent connectionist model of person impression formation. Personality and Social Psychology Review , 8(1), 28–61. [DOI] [PubMed] [Google Scholar]
- Van Rooy D., Van Overwalle F., Vanhoomissen T., Labiouse C., French R. (2003). A recurrent connectionist model of group biases. Psychological Review , 110(3), 536–63. [DOI] [PubMed] [Google Scholar]
- Wang J., Conder J.A., Blitzer D.N., Shinkareva S.V. (2010). Neural representation of abstract and concrete concepts: a meta-analysis of neuroimaging studies. Human Brain Mapping , 31(10), 1459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T.T., Krienen F.M., Sepulcre J., et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology , 106(3), 1125–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.