Abstract
Diabetes mellitus is a metabolic disorder that increases fracture risk and interferes with bone formation and impairs fracture healing. Type 1 diabetes mellitus (T1DM) and Type 2 diabetes mellitus (T2DM) both increase fracture risk and have several common features that affect bone including hyperglycemia and increased AGE formation, ROS generation, and inflammation. These factors affect both osteoblasts and osteoclasts lead to increased osteoclasts and reduced numbers of osteoblasts and bone formation. In addition to fracture healing, T1DM and T2DM impair bone formation under conditions of perturbation such as bacteria induced periodontal bone loss, which reduces expression of factors that stimulate osteoblasts such as BMPs and growth factors and increase osteoblast apoptosis.
Keywords: diabetes, inflammation, advanced glycation end-products (AGE), oxidative stress, Insulin, Hyperglycemia, osteoblast, osteoclast, fracture healing
Introduction
Diabetes mellitus (DM) is a chronic metabolic disease with high blood glucose levels [1-3]. Diabetes results from deficits in the production of insulin or deficit insulin resistance coupled with insufficient insulin production. Type 1 diabetes mellitus (T1DM) is due to the lack of insulin production by the pancreas and requires daily administration of insulin. It is typically caused by destruction of pancreatic β-cells of autoimmune etiology. Type 2 diabetes mellitus (T2DM) is characterized by the inability to use insulin efficiently, referred to as insulin resistance combined with an inability to produce a sufficient amount of insulin to overcome the insulin resistance. Diabetes mellitus often leads to serious complications that affect the heart, blood vessels, eyes, kidneys, and nerves. It has also been increasingly recognized that diabetes adversely affects bone health.
Insulin receptor signaling activates Ras, which leads to activation of MAP kinases and promotes growth. Insulin induces another intracellular cascade that leads to phosphorylation of insulin receptor substrate 1 (IRS1) and IRS2 and activation of phosphatidylinositide-3-kinase (PI3K), which phosphorylates and activates Akt. One of the effects of Akt is to phosphorylate and deactivate Foxo1; another is to phosphorylate and inhibit glycogen synthase kinase-3β (Gsk3β). FOXO1 is a transcription factor that induces genes that control glycogenolysis and gluconeogenesis and its activity can lead to hyperglycemia. In addition FOXO1 is activated in tissues associated with a number of diabetic complications including soft tissue during wound healing and bone fracture [4, 5]. Insulin resistance may involve reduced expression or phosphorylation of IRS-1/IRS-2 due to various causes including inflammation. Diminished IRS1 and IRS2 activity reduces activation of PI3K but increases MAP kinase activation. Normal expression and function of IRS1 and IRS2 is needed to activate PI3K and Akt. Akt signaling prevents inappropriate activation of FOXO1 and is essential for maintaining homeostasis. Thus, a reduction in insulin signaling leads to reduced Akt and increased FOXO1 activation to promote hyperglycemia. This may contribute to organ failure and diabetic complications due to insulin resistance.
High levels of glucose contribute to diabetic complications by inducing stress at the cellular level, glycating proteins that lead to the formation of advanced glycation endproducts, increasing production of reactive oxygen species, and enhancing expression of cytokines such as tumor necrosis factor [1, 6, 7]. In diabetic humans and animals there is increased production of inflammatory mediators by macrophages in adipose tissue leading to increased systemic inflammation, which among other factors contributes to insulin resistance [8]. Diabetic conditions such as high glucose levels, increased formation of advanced glycation endproducts and increased generation of ROS lead to greater expression of inflammatory cytokines at the local level when tissues are perturbated by events such as wounding.
Diabetes, Inflammation and Bone
Pro-inflammatory mediators including TNF-α, IL-1β, IL-6 and IL-18 are increased locally in diabetes mellitus and are thought to contribute to diabetic complications [7, 9]. Diabetics have difficulty in down regulating inflammation once induced [10, 11]. Increased levels of TNF may limit the capacity of diabetics to down regulate other inflammatory genes and increase apoptosis, which has been shown to reduce bone coupling in diabetic animals [12].
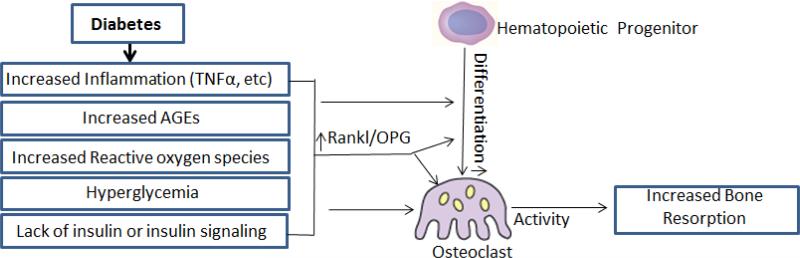
During perturbation diabetes increases and prolongs inflammation, which may lead to enhanced osteoclastogenesis. Diabetes increases osteoclast formation in a number of conditions including periodontal disease, fracture healing and osteoporosis [6, 12, 13]. Diabetes-increased osteoclasts may pertain to situations where bone is challenged by injury or inflammation rather than basal levels. Diabetic animals with periodontitis have higher levels of IL-1β, TNF-α, and prostaglandin E2, which induce and prolong osteoclast mediated resorption [14]. Diabetic rats with periodontitis and T1DM have a 2 to 4-fold increase in the number of osteoclasts and individuals with T1DM have increased levels of IL-17 and IL-23, which promote osteoclast formation through RANKL (Figure 1) [15, 16]. T2DM rats have a 2 to 4-fold increase in osteoclasts induced by periodontal infection compared to infected normoglycemic controls [11, 17, 18]. Similarly, humans with T2DM and periodontitis have significantly increased levels of TNF-α, IL-1β and IL-6 associated with prolonged inflammation and increased lipid peroxidation and dyslipidemia [16, 19, 20]. Diabetes leads to increased RANKL/OPG ratios and TNF levels that contribute to greater bone resorption [11, 21]. In humans, the ratio of RANKL/OPG and TNF levels are increased in poorly controlled diabetics [19, 22]. Fatty acid levels in diabetics may also contribute to increased osteoclastogenesis [23]. The capacity to resolve inflammation is an important aspect of limiting bone resorption as shown by diminished bone loss in animals treated with resolvins [24] or by use of TNF inhibitors [10, 11].
Figure1. Mechanisms of diabetes-increased osteoclastogenesis.
Diabetes leads to hyperglycemia, enhanced and prolonged inflammation, formation of AGEs and generation of ROS. This dysregulation as well as reduced insulin signaling may lead to increased osteoclast formation, particularly when bone is challenged by wounding, bacteria induced inflammation or other events that disrupt homeostasis. This dysregulation may lead to an increased RANKL/OPG ratio or affect osteoblasts through other mechanisms to increase bone resorption.
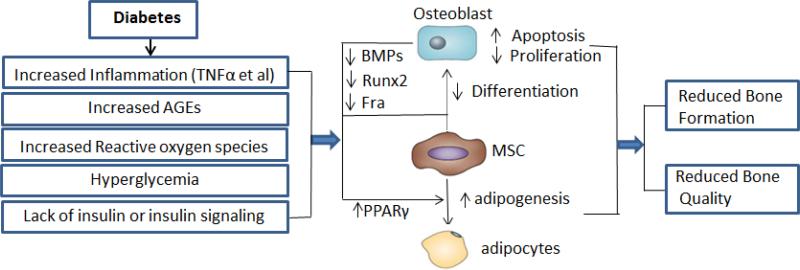
Diabetes decreases osteoblast formation and function and reduces the number of osteoblasts. Bone formation is reduced in diabetics as reflected by reduced levels of osteocalcin in type 2 diabetic patients compared to non-diabetic controls, reflecting a decrease in osteoblast activity, which is inversely related to IL-6 and C reactive protein (CRP) [25]. Rats with type 2 diabetes have decreased expression of BMPs and FGF, reduced osteocalcin expression and reduced bone coupling [12]. These deficits are linked to diabetes-increased inflammation since they are reversed by inhibition of TNF [12]. A mechanism through which this may occur is greater or prolonged expression of TNF in bone of diabetics when stimulated by injury or inflammation that leads to increased nuclear factor-kappa-B activity and reduced expression of fra-1 and runx2 in osteoblasts and reduced expression of mediators that stimulate osteoblast growth and differentiation (Figure 2) [26, 27]. Diabetes-enhanced inflammation may reduce osteoblast numbers through increased apoptosis. Type 1 diabetes increases osteoblast caspase 3 activity and Bax/Bcl-2 ratio mediated by increased levels of TNF-α [28]. Type 2 diabetes also increases the expression of pro-apoptotic genes that affect bone [10].
Figure2.
Mechanisms of diabetes-reduced bone formation. Diabetes leads to hyperglycemia, enhanced and prolonged inflammation, formation of AGEs and generation of ROS. This dysregulation as well as reduced insulin signaling may adversely affect osteoblasts and reduce bone formation particularly when bone is challenged by wounding, bacteria induced inflammation or other events that disrupt homeostasis. The effect of dysregulation may lead to a reduction in BMPs, Runx2 or Fra1, an increase in PPARγ or other mechanisms to reduce bone formation or bone quality.
Diabetes, AGEs and Bone
Elevated levels of glucose enhance protein glycation (nonenzymatic glycosylation), with the formation of advanced glycation end-products (AGEs). AGEs are non-enzymatic chemical modifications of proteins by aldose sugars, formed by the oxidation of products generated during the Maillard reaction. The accumulation of AGEs has been associated with diabetic complications as well as degenerative diseases that occur with aging. AGEs bind to a number of receptors including the receptor for AGEs (RAGE) and stimulate inflammatory cytokines [29]. Diabetes increases formation of AGEs and increases RAGE expression [6]. RAGE signaling activates the transcription factor NF-κB to increase expression of the receptor activator for nuclear factor κ-B ligand (RANKL) [30]. AGEs and hyperglycemia are linked to increased osteoclast formation (Figure 1) [31, 32] and RAGE is expressed in osteoclasts and stimulates osteoclastogenesis [33]. Mice that lack RAGE have decreased bone resorption and increased bone mass [33]. Blockade of RAGE signaling by treatment of mice with soluble RAGE reduces bacteria-induced periodontal bone loss [34]. RAGE also down regulates expression of osteoprotegerin (OPG) to enhance osteoclastogenesis and bone resorption [35]. In addition, AGEs inhibit differentiation of osteoblasts as reflected by reduced expression of alkaline phosphate and collagen 1α1 and inhibited formation of a mineralized matrix [36]. Moreover, there is evidence that AGEs induce osteoblast apoptosis to reduce osteoblast numbers and impair bone formation [37].
Diabetes, ROS and Bone
Under diabetic conditions, various tissues produce reactive oxygen species (ROS) [38, 39]. Oxidative stress is increased in diabetes and contributes to diabetic complications. Superoxide production is increased in the mitochondria as a result of increased glucose levels, which lead to greater inflammation [40, 41]. A primary mechanism is the overproduction of the superoxide anion (O−2) by the mitochondrial electron transport chain. In addition, diabetes causes a reduction in antioxidant levels to increase susceptibility to oxidative stress [42]. There are several sources of ROS in cells including stimulation by AGEs [43], high glucose induced overload of the electron transport chain in mitochondria [40] and the activity of membrane-bound NADPH oxidase [44, 45]. High levels of ROS negatively affect bone [46, 47]. Intracellular H2O2 increases the differentiation and survival of osteoclasts. The formation of reactive oxygen species (ROS) induces RANKL expression and enhances greater osteoclast formation [48]. Hyperglycemia-induced ROS production also increases expression of RAGE, which may contribute to osteoclast formation [49].
The long-term effect of oxidative stress is to reduce bone mass. The importance of protection against oxidative stress was shown by deletion of the transcription factor, forkhead box-O (FOXO). Deletion of FOXO1, FOXO3 and FOXO4 results in reduced expression of antioxidant enzymes and failure to protect against oxidative stress [50]. Interestingly, FOXO1 is induced by RANKL stimulation and has a direct effect in stimulating osteoclast formation [51]. The long-term effects of oxidative stress may be particularly important for long-lived cells such as osteocytes and mesenchymal stem cells. Mesenchymal stem cells play an essential role in bone formation and osteocytes are critical for regulating bone remodeling, particularly in response to mechanical stimulation. The long term impact of oxidative stress on bone maybe mediates through its detrimental effect on these two types of long-lived cells [52]. Since diabetes increases formation of superoxide radicals and inhibits antioxidant defenses its impact on mesenchymal stem cells and osteocytes may be one of the mechanisms through which diabetes impacts the long term health of bone.
Diabetes, Hyperglycemia and Bone
Studies on osteoclasts derived from db/db T2DM mice and T2DM patients found that osteoclasts differentiation was enhanced by hyperglycemia, suggesting an increased capacity for bone resorption. This may contribute to increased alveolar bone loss in T2DM patients with periodontitis [31]. High levels of glucose stimulate the generation of reactive oxygen species which in turn can increase osteoclast formation and activity [53, 54]. Since the effect of high glucose often takes several days it is possible that it works indirectly by stimulating increased generation of ROS, increased cytokine expression and formation of AGEs. Increased glucose levels interfere with osteoblast differentiation and osteoblast function reflected by decreased expression of the osteoblast marker genes (Figure 2) [55]. High glucose stimulates production of reactive oxygen species and activation of NF-kB to affect osteoblasts [56]. Hyperglycemia may affect bone through enhanced expression of proinflammatory cytokines such as TNFα, which reduces osteoblast differentiation, osteoblast activity and increases osteoblast apoptosis [57, 58]. High glucose levels reduce expression of the transcription factor RUNX2 and inhibit bone formation [54, 59-61]. Furthermore, it interferes with production of a mineralized matrix [55]. Osteoblast viability is decreased by high glucose. Another mechanism is through increased PPARγ activation that promotes adipogenesis from mesenchymal stem cells at the expense of bone formation to reduce bone mass [62].
Diabetes, Insulin and Bone
Insulin binds to receptors on osteoblasts and stimulates anabolic effects [63]. It is possible that the reduced insulin levels or reduced insulin signaling in osteoblasts negatively affects bone and contributes to reduced bone formation caused by diabetes [64, 65]. Activation of insulin-like substrate1 (IRS-1) affects bone turnover, while activation of IRS-2 shifts the balance of bone formation and resorption towards formation. Insulin stimulates osteoblast proliferation, inactivates p27, and promotes collagen synthesis [66]. In T1DM, the deficiency of insulin and IGF-1 leads to impaired bone formation, abnormal mineralization, abnormal bone microarchitecture, increased fragility of the bone, and reduced peak bone mass [67]. It has been proposed that hyperinsulinia in the early stages of T2DM increases bone mass through effects on bone formation via IRS-1 and IRS-2 surface receptors [68]. Physiological levels of insulin reduce the ability of PTH to activate protein kinase C in osteoblasts [69, 70], suggesting that insulin may be a physiological antagonist of bone resorption. T1DM diabetes and later stages of T2DM reduced insulin signaling may remove a brake on osteoblast-induced osteoclast formation.
Diabetes and Impaired Fracture Healing
Diabetic fracture is a significant co-morbidity of both type I and type II diabetes and is characterized by microarchitectural changes that decrease bone quality [64, 66]. Meta-analysis shows a consistent pattern of increased risk of fracture in men and women and in studies conducted in the United States and Europe. The Nurses’ Health Study with 109,983 women aged 34–59 years and follow up 22 years later indicated that both T1DM and T2DM are both associated with an increased risk of hip fracture [71]. The relative risk of hip fracture is increased 6-7 fold for individuals with T1DM, which is considerably higher than the increased risk (1.4-1.7 fold) in T2DM [72]. The fracture risk of T1DM increases because of a decrease of BMD, which is linked to impaired bone formation that may be linked to a deficiency of insulin and insulin-like growth factor-1 (IGF-1) [73]. T2DM is often characterized by normal or high bone mineral density (BMD). Diabetes may be associated with a reduction of bone strength that is not reflected in the measurement of BMD [74] results in high risk of fracture.
Fracture repair involves formation of a hematoma after injury that generates the production of cytokines and growth factors. This leads to an inflammatory response that is necessary for the recruitment of mesenchymal stem cells [75, 76]. These cells proliferate and differentiate to chondrocytes that form cartilage during the endochondral phase of bone formation. Cells along the periosteum differentiate into osteoblasts to produce new bone. The cartilage mineralizes and mechanically stabilizes the fracture site. Mineralized cartilage is then removed by the action of osteoclasts. Factors important in this process are TNF-α, macrophage colony stimulating factor (MCSF) and RANKL [77]. The transition from cartilage to bone is linked to increased angiogenesis [75]. The last phase is bone remodeling, which involves the action of osteoclasts and osteoblasts to reshape the bone to its final form. Diabetic animals exhibit both decreased and delayed bone formation [78]. Diabetic fracture healing may be caused in part, by reduced growth factor levels as shown by improved healing with application of FGF-2 to the fracture site [79].
Healing of fractures in diabetic patients is prolonged by 87% [80] and has a 3.4 fold higher risk of complications including delayed union, non-union, redislocation or pseudoarthrosis [81, 82]. Clinical studies in humans indicate that diabetes delays fracture healing [82]. A study of spontaneously diabetic animals revealed that diabetic fracture healing was characterized by decreased bone apposition and mineralization [78]. The reparative phase of bone fracture healing is initiated by proliferation and chondroblastic differentiation of periosteal precursor cells resulting in a hyaline cartilage callus around the wounded bone [83]. Imbalances in chondrocyte apoptosis, premature removal of cartilage, reduced osteoblast differentiation and function and alterations in vascularization have been shown to affect the transition from cartilage to bone [84, 85]. Supernormal osteoclast activity disturbs remodeling of the osseous callus [84]. It has been proposed that insulin insufficiency, hyperglycemia and oxidative stress are mechanisms that affect fracture healing in T1DM and T2DM. They may reduce osteoblast differentiation, increase osteoclast activity, and alter apoptosis of chondrocytes and osteoblasts to interfere with fracture healing in diabetic patients [84, 86-88].
Type I collagen is the major protein component in bone. Enzymatic cross-linking between collagen molecules is essential and tightly regulated. Accumulation of AGEs in cortical and trabecular bone increases the stiffness of the collagen network and reduce ductility [89, 90]. These alterations can lead to increased fragility [91]. Non enzymatic glycation causes a significant reduction in propagation fracture toughness and bone [92]. In addition to its structural effects, AGEs can affect the function of bone cells to induce apoptosis, interfere with differentiation and function of osteoblasts and reduce bone mineralization [36, 93].
Insulin acts directly on osteoblasts to increased proliferation, reduce apoptosis, stimulate glucose uptake, increase collagen synthesis and enhance sensitivity to PTH [94, 95]. Insulin also increases chondrocyte proliferation, differentiation, formation of extra cellular matrix [96, 97]. In contrast osteoclast activity in vitro is reduced by insulin [98]. Treatment with systemic insulin reverses impaired fracture healing suggests that insulin signaling plays a necessary role in repair. However the interpretation is limited by the fact that insulin also treats hyperglycemia making it unclear whether the effects are due to the direct effects of insulin on bone cells or the indirect effects due to reversal of hyperglycemia or both [99-101]. Experiments have been performed to study the effect of local insulin application to fracture healing. These studies suggest that insulin has direct effects on the repair process [65]. Local application of insulin restored the deficit in cell proliferation in diabetic animals and improved fracture healing. Histologic and radiographic outcomes of osseous healing in a femoral defect model in diabetic animals were also improved by local insulin delivery with enhanced formation of mineralized tissue at the defect site [102]. In a non-diabetic rat model local insulin accelerated fracture healing but did not alter the final outcome. At early time points application of local insulin increased VEGF expression, enhanced vascularity and increased formation of mineralized tissue and increased mechanical strength at early time points [103].
The complications of diabetes mellitus affect the vasculature in the form of macro- and microangiopathy and wound healing. Both T1DM and T2DM contribute to diabetic macroangiopathy, which leads to greater atherosclerosis and microangiopathy, which contributes to diabetic retinopathy and impaired wound healing [104]. AGEs, defective signal transduction, and an imbalance of matrix metalloproteinases (MMPs) can all lead to the progression of atherosclerosis in major arteries. Increased levels of prothrombotic factors, restricted formation of collateral vessels and increased loss of endothelial cells and pericytes are important aspects of microangiopathy [105]. Moreover, in diabetic wound healing high glucose levels alter the downstream targets of the transcription factor FOXO1 to induce inflammatory mediators instead of TGFβ1, providing an epigenetic explanation for reduced growth factor and increased expression of inflammatory mediators in diabetic wounds [106].
Summary
T1DM and T2DM both increase fracture risk and have several common mechanisms including increased AGE formation, increased ROS generation, and increased inflammation. These factors affect osteoblasts and osteoclasts as summarized in Figures 1 and 2. However there are significant differences whereby T1DM has a greater effect on bone mass and T2DM affects bone quality. Both humans and animal models of T1DM and T2DM display impaired fracture healing but T1DM patients have a greater risk of developing fractures. Moreover, animals with T1DM and T2DM exhibit impaired bone formation under conditions of perturbation such as bacteria induced periodontal bone loss and bone fracture healing.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest Statement
The Authors must submit their disclosure forms
Human and Animal Rights and Informed Consent
This article contains no studies with human or animal subjects performed by the author.
Contributor Information
Hongli Jiao, Department of Periodontics, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA.
E. Xiao, Department of Periodontics, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA Department of Oral and Maxillofacial Surgery, Peking University School and Hospital of Stomatology, Beijing, 100081, China.
Dana T. Graves, Department of Periodontics, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA Department of Oral and Maxillofacial Surgery, Peking University School and Hospital of Stomatology, Beijing, 100081, China.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Moseley KF. Type 2 diabetes and bone fractures. Curr Opin Endocrinol Diabetes Obes. 2012;19(2):128–135. doi: 10.1097/MED.0b013e328350a6e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gong Z, Muzumdar RH. Pancreatic function, type 2 diabetes, and metabolism in aging. International journal of endocrinology. 2012;2012:320482. doi: 10.1155/2012/320482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Yan W, Li X. Impact of diabetes and its treatments on skeletal diseases. Frontiers of medicine. 2013;7(1):81–90. doi: 10.1007/s11684-013-0243-9. [This paper discusses the impact of diabetes on skeletal diseases and shows that both T1DM and T2DM are associated with an increased risk of osteoporosis and fragility fractures. Bone mineral density is reduced in T1DM, whereas patients with T2DM have normal or slightly higher bone density, suggesting impaired bone quality is involved in T2DM.] [DOI] [PubMed] [Google Scholar]
- 4.Hameedaldeen A, Liu J, Batres A, Graves GS, Graves DT. FOXO1, TGF-beta regulation and wound healing. International journal of molecular sciences. 2014;15(9):16257–16269. doi: 10.3390/ijms150916257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Zhou Y, Graves DT. FOXO transcription factors: their clinical significance and regulation. Biomed Res Int. 2014;2014:925350. doi: 10.1155/2014/925350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamagishi S. Role of advanced glycation end products (AGEs) in osteoporosis in diabetes. Curr Drug Targets. 2011;12(14):2096–2102. doi: 10.2174/138945011798829456. [DOI] [PubMed] [Google Scholar]
- 7.Graves DT, Kayal RA. Diabetic complications and dysregulated innate immunity. Front Biosci. 2008;13:1227–1239. doi: 10.2741/2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz NG, Sousa LP, Sousa MO, Pietrani NT, Fernandes AP, Gomes KB. The linkage between inflammation and Type 2 diabetes mellitus. Diabetes research and clinical practice. 2013;99(2):85–92. doi: 10.1016/j.diabres.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 9•.Nikolajczyk BS, Jagannathan-Bogdan M, Shin H, Gyurko R. State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun. 2011;12(4):239–250. doi: 10.1038/gene.2011.14. [This paper summarizes that the cytokines secreted by multiple immune and non-immune cell types are dominant regulators of the pathological inflammation that characterizes and promotes T2D. These cytokines also be thought to contribute to diabetic complications.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andriankaja OM, Galicia J, Dong G, Xiao W, Alawi F, Graves DT. Gene Expression Dynamics during Diabetic Periodontitis. J Dent Res. 2012;91(12):1160–1165. doi: 10.1177/0022034512465292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Pacios S, Kang J, Galicia J, et al. Diabetes aggravates periodontitis by limiting repair through enhanced inflammation. Faseb J. 2012;26(4):1423–1430. doi: 10.1096/fj.11-196279. [This paper shows that diabetes prolongs inflammation and osteoclastogenesis in periodontitis and through TNF limits the normal reparative process by negatively modulating factors that stimulate bone formation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pacios S, Andriankaja O, Kang J, et al. Bacterial infection increases periodontal bone loss in diabetic rats through enhanced apoptosis. Am J Pathol. 2013;183(6):1928–1935. doi: 10.1016/j.ajpath.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.Alblowi J, Tian C, Siqueira MF, et al. Chemokine expression is upregulated in chondrocytes in diabetic fracture healing. Bone. 2013;53(1):294–300. doi: 10.1016/j.bone.2012.12.006. [This paper points to the importance of TNF-α as a mechanism for diabetes enhanced chemokine expression by chondrocytes, which may contribute to the accelerated loss of cartilage observed in diabetic fracture healing. Moreover, in vitro results of this study point to FOXO1 as a potentially important transcription factor in mediating this effect.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alblowi J, Kayal RA, Siqueria M, et al. High levels of tumor necrosis factor-alpha contribute to accelerated loss of cartilage in diabetic fracture healing. Am J Pathol. 2009;175(4):1574–1585. doi: 10.2353/ajpath.2009.090148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva JA, Lopes Ferrucci D, Peroni LA, et al. Periodontal disease-associated compensatory expression of osteoprotegerin is lost in type 1 diabetes mellitus and correlates with alveolar bone destruction by regulating osteoclastogenesis. Cells, tissues, organs. 2012;196(2):137–150. doi: 10.1159/000330879. [DOI] [PubMed] [Google Scholar]
- 16.Silva JA, Ferrucci DL, Peroni LA, et al. Sequential IL-23 and IL-17 and increased Mmp8 and Mmp14 expression characterize the progression of an experimental model of periodontal disease in type 1 diabetes. Journal of cellular physiology. 2012;227(6):2441–2450. doi: 10.1002/jcp.22979. [DOI] [PubMed] [Google Scholar]
- 17.Kang J, de Brito Bezerra B, Pacios S, et al. Aggregatibacter actinomycetemcomitans infection enhances apoptosis in vivo through a caspase-3-dependent mechanism in experimental periodontitis. Infect Immun. 2012;80(6):2247–2256. doi: 10.1128/IAI.06371-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu R, Bal HS, Desta T, et al. Diabetes enhances periodontal bone loss through enhanced resorption and diminished bone formation. J Dent Res. 2006;85(6):510–514. doi: 10.1177/154405910608500606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bastos AS, Graves DT, Loureiro AP, et al. Lipid Peroxidation Is Associated with the Severity of Periodontal Disease and Local Inflammatory Markers in Patients with Type 2 Diabetes. The Journal of clinical endocrinology and metabolism. 2012 doi: 10.1210/jc.2011-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duarte PM, de Oliveira MC, Tambeli CH, Parada CA, Casati MZ, Nociti FH., Jr. Overexpression of interleukin-1beta and interleukin-6 may play an important role in periodontal breakdown in type 2 diabetic patients. Journal of periodontal research. 2007;42(4):377–381. doi: 10.1111/j.1600-0765.2006.00961.x. [DOI] [PubMed] [Google Scholar]
- 21.Mahamed DA, Marleau A, Alnaeeli M, et al. G(-) anaerobes-reactive CD4+ T-cells trigger RANKL-mediated enhanced alveolar bone loss in diabetic NOD mice. Diabetes. 2005;54(5):1477–1486. doi: 10.2337/diabetes.54.5.1477. [DOI] [PubMed] [Google Scholar]
- 22.Santos VR, Lima JA, Goncalves TE, et al. Receptor activator of nuclear factor-kappa B ligand/osteoprotegerin ratio in sites of chronic periodontitis of subjects with poorly and well-controlled type 2 diabetes. Journal of periodontology. 2010;81(10):1455–1465. doi: 10.1902/jop.2010.100125. [DOI] [PubMed] [Google Scholar]
- 23.Drosatos-Tampakaki Z, Drosatos K, Siegelin Y, et al. Palmitic acid and DGAT1 deficiency enhance osteoclastogenesis, while oleic acid-induced triglyceride formation prevents it. J Bone Miner Res. 2014;29(5):1183–1195. doi: 10.1002/jbmr.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasturk H, Kantarci A, Goguet-Surmenian E, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179(10):7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 25.Sarkar PD, Choudhury AB. Relationships between serum osteocalcin levels versus blood glucose, insulin resistance and markers of systemic inflammation in central Indian type 2 diabetic patients. European review for medical and pharmacological sciences. 2013;17(12):1631–1635. [PubMed] [Google Scholar]
- 26.Chang J, Wang Z, Tang E, et al. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15(6):682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu H, Kraut D, Gerstenfeld L, Graves D. Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology. 2003;144:346–352. doi: 10.1210/en.2002-220072. [DOI] [PubMed] [Google Scholar]
- 28••.Coe LM, Irwin R, Lippner D, McCabe LR. The bone marrow microenvironment contributes to type I diabetes induced osteoblast death. Journal of cellular physiology. 2011;226(2):477–483. doi: 10.1002/jcp.22357. [The findings in this paper implicate the bone marrow microenvironment and TNFα in mediating osteoblast death and contributing to type I diabetic bone loss.] [DOI] [PubMed] [Google Scholar]
- 29.Vlassara H, Striker GE. Advanced glycation endproducts in diabetes and diabetic complications. Endocrinology and metabolism clinics of North America. 2013;42(4):697–719. doi: 10.1016/j.ecl.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Xie J, Mendez JD, Mendez-Valenzuela V, Aguilar-Hernandez MM. Cellular signalling of the receptor for advanced glycation end products (RAGE) Cellular signalling. 2013;25(11):2185–2197. doi: 10.1016/j.cellsig.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Catalfamo DL, Britten TM, Storch DL, Calderon NL, Sorenson HL, Wallet SM. Hyperglycemia induced and intrinsic alterations in type 2 diabetes-derived osteoclast function. Oral Dis. 2013;19(3):303–312. doi: 10.1111/odi.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyata T, Kawai R, Taketomi S, Sprague SM. Possible involvement of advanced glycation end-products in bone resorption. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1996;11(Suppl 5):54–57. doi: 10.1093/ndt/11.supp5.54. [DOI] [PubMed] [Google Scholar]
- 33.Ding KH, Wang ZZ, Hamrick MW, et al. Disordered osteoclast formation in RAGE-deficient mouse establishes an essential role for RAGE in diabetes related bone loss. Biochem Biophys Res Commun. 2006;340(4):1091–1097. doi: 10.1016/j.bbrc.2005.12.107. [DOI] [PubMed] [Google Scholar]
- 34.Lalla E, Lamster IB, Feit M, et al. Blockade of RAGE suppresses periodontitis-associated bone loss in diabetic mice. J Clin Invest. 2000;105(8):1117–1124. doi: 10.1172/JCI8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamster IB. Diabetes and oral health. What's their relationship? Diabetes self-management. 2012;29(3):30, 32–34. [PubMed] [Google Scholar]
- 36.Sanguineti R, Storace D, Monacelli F, Federici A, Odetti P. Pentosidine effects on human osteoblasts in vitro. Ann N Y Acad Sci. 2008;1126:166–172. doi: 10.1196/annals.1433.044. [DOI] [PubMed] [Google Scholar]
- 37.Alikhani M, Alikhani Z, Boyd C, et al. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone. 2007;40(2):345–353. doi: 10.1016/j.bone.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48(1):1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Dandona P, Thusu K, Cook S, et al. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996;347(8999):444–445. doi: 10.1016/s0140-6736(96)90013-6. [DOI] [PubMed] [Google Scholar]
- 40.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pitocco D, Zaccardi F, Di Stasio E, et al. Oxidative stress, nitric oxide, and diabetes. Rev Diabet Stud. 2010;7(1):15–25. doi: 10.1900/RDS.2010.7.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niedowicz DM, Daleke DL. The role of oxidative stress in diabetic complications. Cell biochemistry and biophysics. 2005;43(2):289–330. doi: 10.1385/CBB:43:2:289. [DOI] [PubMed] [Google Scholar]
- 43.Sakurai T, Tsuchiya S. Superoxide production from nonenzymatically glycated protein. FEBS Lett. 1988;236(2):406–410. doi: 10.1016/0014-5793(88)80066-8. [DOI] [PubMed] [Google Scholar]
- 44.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. The American journal of cardiology. 2003;91(3A):7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 45.Mohazzab KM, Kaminski PM, Wolin MS. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. The American journal of physiology. 1994;266(6 Pt 2):H2568–2572. doi: 10.1152/ajpheart.1994.266.6.H2568. [DOI] [PubMed] [Google Scholar]
- 46•.Morikawa D, Norikawa D, Nojiri H, et al. Cytoplasmic reactive oxygen species and SOD1 regulate bone mass during mechanical unloading. Journal of Bone and Mineral Research. 2013;28(11):2368–80. doi: 10.1002/jbmr.1981. [The results of this paper indicate that mechanical unloading, in part, regulates bone mass via intracellular ROS generation and the Sod1 expression, suggesting that activating Sod1 may be a preventive strategy for ameliorating mechanical unloading-induced bone loss.] [DOI] [PubMed] [Google Scholar]
- 47.Omori K, Ohira T, Uchida Y, et al. Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase. J Leukoc Biol. 2008;84(1):292–301. doi: 10.1189/jlb.1207832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ha H, Kwak HB, Lee SW, et al. Reactive oxygen species mediate RANK signaling in osteoclasts. Experimental cell research. 2004;301(2):119–127. doi: 10.1016/j.yexcr.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 49.Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59(1):249–255. doi: 10.2337/db09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Bartell SM, Kim HN, Ambrogini E, et al. FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nature communications. 2014;5:3773. doi: 10.1038/ncomms4773. [This paper shows that intracellular H2O2 accumulation is a critical and purposeful adaptation for the differentiation and survival of osteoclasts and this is achieved,at least in part, by downregulating the H2O2-inactivating enzyme catalase. Catalase downregulation results from the repression of the transcriptional activity of FoxO1, 3 and 4 by RANKL, the indispensable signal for the generation of osteoclasts, via an Akt-mediated mechanism.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Dong G, Jeon HH, et al. FOXO1 Mediates RANKL-Induced Osteoclast Formation and Activity. J Immunol. 2015;194(6):2878–2887. doi: 10.4049/jimmunol.1402211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almeida M, O'Brien CA. Basic biology of skeletal aging: role of stress response pathways. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68(10):1197–1208. doi: 10.1093/gerona/glt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fraser JH, Helfrich MH, Wallace HM, Ralston SH. Hydrogen peroxide, but not superoxide, stimulates bone resorption in mouse calvariae. Bone. 1996;19(3):223–226. doi: 10.1016/8756-3282(96)00177-9. [DOI] [PubMed] [Google Scholar]
- 54••.Garcia-Hernandez A, Arzate H, Gil-Chavarria I, Rojo R, Moreno-Fierros L. High glucose concentrations alter the biomineralization process in human osteoblastic cells. Bone. 2012;50(1):276–288. doi: 10.1016/j.bone.2011.10.032. [This paper shows that a high concentration of extracellular glucose probably acts as an endogenous factor that alters biomineralization by increasing the amount but reducing the mineral quality. It also suggests that in osteoblastic cells, high glucose could regulate the expression of proinflammatory cytokines by crosstalk between signaling pathways, such as PKC-MAPK with ROS or increased AGEs and RAGEs.] [DOI] [PubMed] [Google Scholar]
- 55.Bai XC, Lu D, Bai J, et al. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun. 2004;314(1):197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 56.McCarthy AD, Etcheverry SB, Bruzzone L, Lettieri G, Barrio DA, Cortizo AM. Nonenzymatic glycosylation of a type I collagen matrix: effects on osteoblastic development and oxidative stress. BMC Cell Biol. 2001;2:16. doi: 10.1186/1471-2121-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lechleitner M, Koch T, Herold M, Dzien A, Hoppichler F. Tumour necrosis factor-alpha plasma level in patients with type 1 diabetes mellitus and its association with glycaemic control and cardiovascular risk factors. Journal of internal medicine. 2000;248(1):67–76. doi: 10.1046/j.1365-2796.2000.00705.x. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez Y, Herrera MT, Soldevila G, et al. High glucose concentrations induce TNF-alpha production through the down-regulation of CD33 in primary human monocytes. BMC Immunol. 2012;13:19. doi: 10.1186/1471-2172-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu YY, Yu T, Zhang XH, et al. 1,25(OH)2D3 inhibits the deleterious effects induced by high glucose on osteoblasts through undercarboxylated osteocalcin and insulin signaling. The Journal of steroid biochemistry and molecular biology. 2012;132(1-2):112–119. doi: 10.1016/j.jsbmb.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Ogawa N, Yamaguchi T, Yano S, Yamauchi M, Yamamoto M, Sugimoto T. The combination of high glucose and advanced glycation end-products (AGEs) inhibits the mineralization of osteoblastic MC3T3-E1 cells through glucose-induced increase in the receptor for AGEs. Horm Metab Res. 2007;39(12):871–875. doi: 10.1055/s-2007-991157. [DOI] [PubMed] [Google Scholar]
- 61.Gopalakrishnan V, Vignesh RC, Arunakaran J, Aruldhas MM, Srinivasan N. Effects of glucose and its modulation by insulin and estradiol on BMSC differentiation into osteoblastic lineages. Biochem Cell Biol. 2006;84(1):93–101. doi: 10.1139/o05-163. [DOI] [PubMed] [Google Scholar]
- 62.Lecka-Czernik B, Gubrij I, Moerman EJ, et al. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. J Cell Biochem. 1999;74(3):357–371. [PubMed] [Google Scholar]
- 63.Thomas DM, Hards DK, Rogers SD, Ng KW, Best JD. Insulin receptor expression in bone. J Bone Miner Res. 1996;11(9):1312–1320. doi: 10.1002/jbmr.5650110916. [DOI] [PubMed] [Google Scholar]
- 64.Thrailkill KM, Lumpkin CK, Jr., Bunn RC, Kemp SF, Fowlkes JL. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. American journal of physiology Endocrinology and metabolism. 2005;289(5):E735–745. doi: 10.1152/ajpendo.00159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gandhi A, Beam HA, O'Connor JP, Parsons JR, Lin SS. The effects of local insulin delivery on diabetic fracture healing. Bone. 2005;37(4):482–490. doi: 10.1016/j.bone.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 66•.Nyman JS, Even JL, Jo CH, et al. Increasing duration of type 1 diabetes perturbs the strength-structure relationship and increases brittleness of bone. Bone. 2011;48(4):733–740. doi: 10.1016/j.bone.2010.12.016. [This paper shows that in in a mouse model of T1DM, systemic insulin deficiency thus the lack of insulin signaling in osteoblasts can affect bone formation and architecture, thereby increasing risk of fracture.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Valerio G, del Puente A, Esposito-del Puente A, Buono P, Mozzillo E, Franzese A. The lumbar bone mineral density is affected by long-term poor metabolic control in adolescents with type 1 diabetes mellitus. Hormone research. 2002;58(6):266–272. doi: 10.1159/000066441. [DOI] [PubMed] [Google Scholar]
- 68.Rakel A, Sheehy O, Rahme E, LeLorier J. Osteoporosis among patients with type 1 and type 2 diabetes. Diabetes & metabolism. 2008;34(3):193–205. doi: 10.1016/j.diabet.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 69.Iida-Klein A, Hahn TJ. Insulin acutely suppresses parathyroid hormone second messenger generation in UMR-106-01 osteoblast-like cells: differential effects on phospholipase C and adenylate cyclase activation. Endocrinology. 1991;129(2):1016–1024. doi: 10.1210/endo-129-2-1016. [DOI] [PubMed] [Google Scholar]
- 70.Iida-Klein A, Varlotta V, Hahn TJ. Protein kinase C activity in UMR-106-01 cells: effects of parathyroid hormone and insulin. J Bone Miner Res. 1989;4(5):767–774. doi: 10.1002/jbmr.5650040517. [DOI] [PubMed] [Google Scholar]
- 71.Janghorbani M, Feskanich D, Willett WC, Hu F. Prospective study of diabetes and risk of hip fracture: the Nurses’ Health Study. Diabetes care. 2006;29(7):1573–1578. doi: 10.2337/dc06-0440. [DOI] [PubMed] [Google Scholar]
- 72.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. American journal of epidemiology. 2007;166(5):495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- 73.Moyer-Mileur LJ, Slater H, Jordan KC, Murray MA. IGF-1 and IGF-binding proteins and bone mass, geometry, and strength: relation to metabolic control in adolescent girls with type 1 diabetes. J Bone Miner Res. 2008;23(12):1884–1891. doi: 10.1359/jbmr.080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2007;18(4):427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 75.Ai-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res. 2008;87(2):107–118. doi: 10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gerstenfeld LC, Wronski TJ, Hollinger JO, Einhorn TA. Application of histomorphometric methods to the study of bone repair. J Bone Miner Res. 2005;20(10):1715–1722. doi: 10.1359/JBMR.050702. [DOI] [PubMed] [Google Scholar]
- 77.Gerstenfeld LC, Cho TJ, Kon T, et al. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res. 2003;18(9):1584–1592. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 78.Follak N, Kloting I, Merk H. Influence of diabetic metabolic state on fracture healing in spontaneously diabetic rats. Diabetes Metab Res Rev. 2005;21(3):288–296. doi: 10.1002/dmrr.537. [DOI] [PubMed] [Google Scholar]
- 79.Kawaguchi H, Kurokawa T, Hanada K, et al. Stimulation of fracture repair by recombinatn human basic fibroblast growth factor in normal and streptozotocin-diabetic rats. Endocrinology. 1994;135:774–781. doi: 10.1210/endo.135.2.8033826. [DOI] [PubMed] [Google Scholar]
- 80.Loder RT. The influence of diabetes mellitus on the healing of closed fractures. Clinical orthopaedics and related research. 1988;(232):210–216. [PubMed] [Google Scholar]
- 81.Folk JW, Starr AJ, Early JS. Early wound complications of operative treatment of calcaneus fractures: analysis of 190 fractures. Journal of orthopaedic trauma. 1999;13(5):369–372. doi: 10.1097/00005131-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 82.Retzepi M, Donos N. The effect of diabetes mellitus on osseous healing. Clinical oral implants research. 2010;21(7):673–681. doi: 10.1111/j.1600-0501.2010.01923.x. [DOI] [PubMed] [Google Scholar]
- 83.Ketenjian AY, Jafri AM, Arsenis C. Studies on the mechanism of callus cartilage differentiation and calcification during fracture healing. The Orthopedic clinics of North America. 1978;9(1):43–65. [PubMed] [Google Scholar]
- 84.Kayal RA, Siqueira M, Alblowi J, et al. TNF-alpha mediates diabetes-enhanced chondrocyte apoptosis during fracture healing and stimulates chondrocyte apoptosis Through FOXO1. J Bone Miner Res. 2010;25(7):1604–1615. doi: 10.1002/jbmr.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85•.Bahney CS, Hu DP, Miclau T, 3rd, Marcucio RS. The multifaceted role of the vasculature in endochondral fracture repair. Frontiers in endocrinology. 2015;6:4. doi: 10.3389/fendo.2015.00004. [This paper discusses the multifaceted role of the vasculature during fracture repair and shows that alterations in vascularization can affect the transition from cartilage to bone during fracture repair.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Botushanov NP, Orbetzova MM. Bone mineral density and fracture risk in patients with type 1 and type 2 diabetes mellitus. Folia medica. 2009;51(4):12–17. [PubMed] [Google Scholar]
- 87.Stolzing A, Sellers D, Llewelyn O, Scutt A. Diabetes Induced Changes in Rat Mesenchymal Stem Cells. Cells, tissues, organs. 2010;191(6):453–465. doi: 10.1159/000281826. [DOI] [PubMed] [Google Scholar]
- 88.Sheweita SA, Khoshhal KI. Calcium metabolism and oxidative stress in bone fractures: role of antioxidants. Current drug metabolism. 2007;8(5):519–525. doi: 10.2174/138920007780866852. [DOI] [PubMed] [Google Scholar]
- 89.Tang SY, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007;40(4):1144–1151. doi: 10.1016/j.bone.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28(2):195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 91•.Tang SY, Vashishth D. Non-enzymatic glycation alters microdamage formation in human cancellous bone. Bone. 2010;46(1):148–154. doi: 10.1016/j.bone.2009.09.003. [By using a novel microCT technique to characterize and quantify microdamage, this study shows that the accumulation of AGEs in the bone matrix significantly alters the quantity and morphology of microdamage production and results in reduced fracture resistance.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92•.Tang SY, Vashishth D. The relative contributions of non-enzymatic glycation and cortical porosity on the fracture toughness of aging bone. Journal of biomechanics. 2011;44(2):330–336. doi: 10.1016/j.jbiomech.2010.10.016. [The paper investigates the contribution of AGEs on the fracture toughness of human bone and finds that they cause a 52% reduction in propagation fracture toughness.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93•.Okazaki K, Yamaguchi T, Tanaka K, et al. Advanced glycation end products (AGEs), but not high glucose, inhibit the osteoblastic differentiation of mouse stromal ST2 cells through the suppression of osterix expression, and inhibit cell growth and increasing cell apoptosis. Calcified tissue international. 2012;91(4):286–296. doi: 10.1007/s00223-012-9641-2. [This paper suggests that AGE2 and AGE3 may inhibit the osteoblastic differentiation of stromal cells by decreasing osterix expression and partly by increasing RAGE expression, as well as inhibiting cell growth and increasing cell apoptosis.] [DOI] [PubMed] [Google Scholar]
- 94.Rosen DM, Luben RA. Multiple hormonal mechanisms for the control of collagen synthesis in an osteoblast-like cell line, MMB-1. Endocrinology. 1983;112(3):992–999. doi: 10.1210/endo-112-3-992. [DOI] [PubMed] [Google Scholar]
- 95.Hill PA, Tumber A, Meikle MC. Multiple extracellular signals promote osteoblast survival and apoptosis. Endocrinology. 1997;138(9):3849–3858. doi: 10.1210/endo.138.9.5370. [DOI] [PubMed] [Google Scholar]
- 96.Shukunami C, Ishizeki K, Atsumi T, Ohta Y, Suzuki F, Hiraki Y. Cellular hypertrophy and calcification of embryonal carcinoma-derived chondrogenic cell line ATDC5 in vitro. J Bone Miner Res. 1997;12(8):1174–1188. doi: 10.1359/jbmr.1997.12.8.1174. [DOI] [PubMed] [Google Scholar]
- 97.Iwata K, Asawa Y, Fujihara Y, et al. The effects of rapid- or intermediate-acting insulin on the proliferation and differentiation of cultured chondrocytes. Current aging science. 2010;3(1):26–33. doi: 10.2174/1874609811003010026. [DOI] [PubMed] [Google Scholar]
- 98.Watford M, Mapes RE. Hormonal and acid-base regulation of phosphoenolpyruvate carboxykinase mRNA levels in rat kidney. Archives of biochemistry and biophysics. 1990;282(2):399–403. doi: 10.1016/0003-9861(90)90135-l. [DOI] [PubMed] [Google Scholar]
- 99.Fujii H, Hamada Y, Fukagawa M. Bone formation in spontaneously diabetic Torii-newly established model of non-obese type 2 diabetes rats. Bone. 2008;42(2):372–379. doi: 10.1016/j.bone.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 100.Hamada Y, Kitazawa S, Kitazawa R, Fujii H, Kasuga M, Fukagawa M. Histomorphometric analysis of diabetic osteopenia in streptozotocin-induced diabetic mice: a possible role of oxidative stress. Bone. 2007;40(5):1408–1414. doi: 10.1016/j.bone.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 101.Kayal RA, Alblowi J, McKenzie E, et al. Diabetes Causes the Accelerated Loss of Cartilage During Fracture Repair Which is Reversed by Insulin Treatment. Bone. 2009;44(2):357–363. doi: 10.1016/j.bone.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dedania J, Borzio R, Paglia D, et al. Role of local insulin augmentation upon allograft incorporation in a rat femoral defect model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2011;29(1):92–99. doi: 10.1002/jor.21205. [DOI] [PubMed] [Google Scholar]
- 103.Paglia DN, Wey A, Breitbart EA, et al. Effects of local insulin delivery on subperiosteal angiogenesis and mineralized tissue formation during fracture healing. Journal of orthopaedic research. 2013;31(5):783–791. doi: 10.1002/jor.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ackermann PW, Hart DA. Influence of Comorbidities: Neuropathy, Vasculopathy, and Diabetes on Healing Response Quality. Advances in Wound Care. 2013;2(8):410–421. doi: 10.1089/wound.2012.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Orasanu G, Plutzky J. The Continuum of Diabetic Vascular Disease: From Macro- to Micro- Journal of American College of Cardiology. 2009;53(5 Suppl):S35–S42. doi: 10.1016/j.jacc.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106•.Zhang C, Ponugoti B, Tian C, Xu F, Tarapore R, Batres A, Alsadun S, Lim J, Dong G, Graves DT. FOXO1 Differentially Regulates Both Normal and Diabetic Wound Healing. Journal of Cell Biology. 2015;209(2):289–303. doi: 10.1083/jcb.201409032. [The manuscript demonstrates that the transcription factor FOXO1 switches from inducing a pro- wound response an anti-healing transcription factor when cells are exposed to high glucose. It provides an epigenetic explanation for diabetes-impaired healing.] [DOI] [PMC free article] [PubMed] [Google Scholar]