Abstract
Cytochrome P450 oxidoreductase (POR) is a 2-flavin protein that transfers electrons from NADPH via its FAD and FMN moieties to all microsomal cytochrome P450 enzymes, including steroidogenic and drug-metabolizing P450s. Defects in the POR gene can cause POR deficiency (PORD), manifested clinically by disordered steroidogenesis, genital anomalies and skeletal malformations. We examined the POR mutant A287P, which is the most frequent cause of PORD in patients of European ancestry and partially disrupts most P450 activities in vitro. Flavin content analysis showed that A287P is deficient in FAD and FMN binding, although the mutation site is distant from the binding sites of both flavins. Externally added flavin partially restored the cytochrome c reductase activity of A287P, suggesting that flavin therapy may be useful for this frequent form of PORD. Transient kinetic dissection of the reaction of POR with NADPH and the reduction in cytochrome c by POR using stopped-flow techniques revealed defects in individual electron transfer steps mediated by A287P. A287P had impaired ability to accept electrons from NADPH, but was capable of a fast FMN ➔ cytochrome c electron donation reaction. Thus the reduced rates of P450 activities with A287P may be due to deficient flavin and impaired electron transfer from NADPH.
Keywords: adrenal hyperplasia, cytochrome P450, molecular basis of disease, mutation, steroid
INTRODUCTION
Cytochrome P450 oxidoreductase (POR) is a 78 kDa flavoprotein that contains FAD and FMN moieties in distinct domains (for a review see [1]). POR transfers electrons from NADPH to all 50 human microsomal cytochrome P450 enzymes. These P450s catalyse key reactions in the biosynthesis of steroids, sterols, bile acids and leukotrienes and in xenobiotic metabolism [2]. In addition to cytochrome P450 enzymes, POR also provides electrons to squalene mono-oxygenase [3], fatty acid elongase [4], haem oxygenase [5], cytochrome b5 [6] and sterol reductase [7]. POR deficiency (PORD) is an autosomal recessive form of congenital adrenal hyperplasia [8,9]. Severely affected patients typically have disordered steroidogenesis causing disordered sexual development, and Antley–Bixler skeletal malformation syndrome. PORD impairs activities of the steroidogenic enzymes 17α-hydroxylase/17,20-lyase (P450c17, CYP17A1), 21-hydroxylase (P450c21, CYP21A2) and aromatase (P450aro, CYP19A1) causing glucocorticoid deficiency and disordered sex development. Milder PORD may manifest as infertility, polycystic ovary syndrome and mildly disordered steroidogenesis. The impairment of electron transfer to microsomal CYP26B1, the enzyme that normally degrades retinoic acid, appears to account for the disordered skeletal development in patients with Antley–Bixler skeletal malformation syndrome that may accompany PORD [10].
POR co-localizes with microsomal P450s on the cytoplasmic side of the endoplasmic reticulum, anchored through a 44-residue N-terminal peptide, which is essential for POR–P450 interaction and P450 reduction [11]. POR has three distinct structural domains: an N-terminal domain that binds FMN, a C-terminal domain that binds FAD and NADPH, and a connecting domain that allows conformational changes through its hinge region during electron transfer [12–14]. In each redox cycle, POR receives two electrons from NADPH and passes the electrons one at a time to its redox partner via its FAD and FMN moieties [15].
More than 200 mutations/polymorphisms have been identified in the POR gene, including over 60 in the protein-coding region, most of which are associated with PORD [16]. Despite the extensive study of POR, the underlying mechanism by which these mutations alter the function of POR, and in turn those of P450s, is unclear. The crystallographic structures of rat and human POR predict defective flavin binding for three mutations (Y181D, Y459H and V492E) that occur in residues involved in cofactor binding [12,15,17–21]. However, the precise mechanism of POR dysfunction for most PORD mutations is not readily apparent.
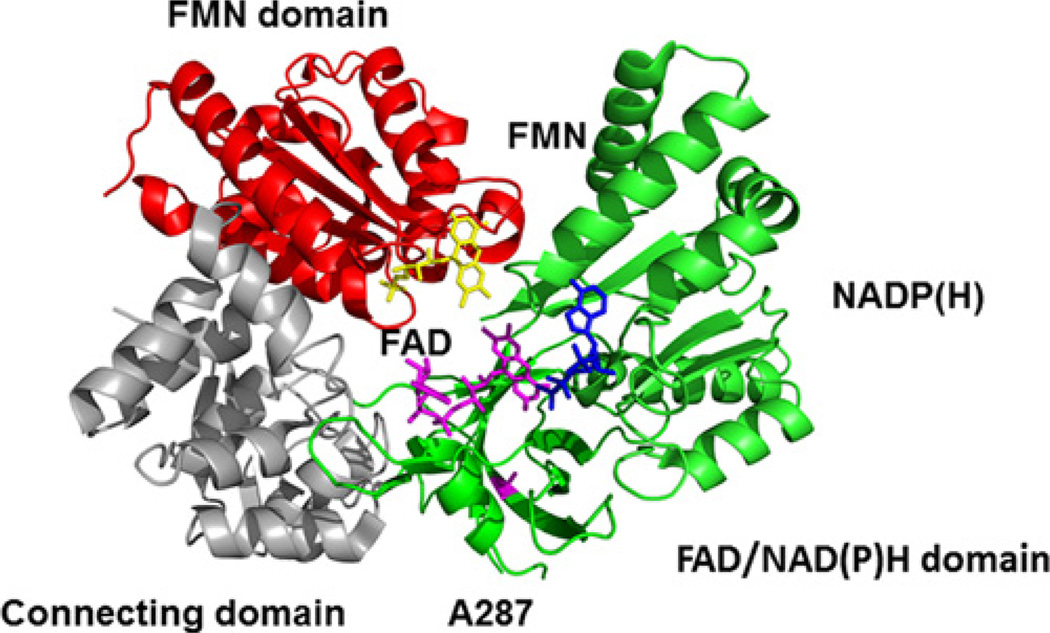
The POR variant A287P is found in ~40% of POR-deficient patients of European ancestry [9]. This mutation causes mild to moderate phenotypes including both skeletal malformation and disordered sex development [8,9,22]. Functional assays of the capacity of the A287P mutant to support various reactions in vitro show that A287P dramatically decreases both 17α-hydroxylase and 17,20-lyase activity of CYP17A1, the activities of several drug-metabolizing P450s and the activity of haem oxygenase [8,9,16,23–29]. In vivo metabolic profiling confirms an association of this mutation with subnormal drug metabolism [30]. Interestingly, A287P does not affect the 21-hydroxylase activity of CYP21A2 or the aromatase activity of CYP19A1 [31,32]. The molecular basis of these effects of A287P remains unclear. Ala287 is located in the FAD/NADPH domain over 15 Å (1 Å = 0.1 nm) away from the closest cofactor (FAD), with no apparent role in POR function (Figure 1). To understand the effect of the A287P mutation on POR function, we used transient (stopped-flow) techniques to characterize the kinetics of electron transfer from NADPH, to its recipient flavin centres and to donate electrons to its classic final acceptor protein cytochrome c. This analysis revealed molecular defects in the individual steps of the POR electron transfer chain when theA287P mutant is substituted for wild-type (WT) POR.
Figure 1. Location of the A287P mutation in POR structure.
Mutation site (magenta), FMN (yellow), FAD (magenta) and NADPH (blue).
EXPERIMENTAL
Materials
Pyridine nucleotides were purchased from Roche Applied Science. All other reagents were purchased from Sigma–Aldrich and were of ACS (American Chemical Society) grade or better. Isocitrate, isocitrate dehydrogenase, equine cytochrome c and FAD and FMN were also from Sigma–Aldrich.
Protein expression and purification
WT human POR lacking the 27 N-terminal residues (N-27) was modified to contain a C-terminal Gly3His6-tag to aid purification and subcloned into a pET22b vector [33]. The A287P mutant was generated by site-directed mutagenesis based on the WT sequence [9]. Both proteins were expressed in Escherichia coli CD41(DE3) cells and purified from bacterial membranes using Ni2+-nitrilotriacetate (Ni-NTA) affinity column chromatography as described in [9,33]. The final protein was analysed by SDS/PAGE (10%gel) and stored at −80°C in 20 mM potassium phosphate buffer (pH 7.4) and 20% glycerol. All biophysical experiments were carried out at 25°C in 20 mM potassium phosphate buffer (pH 7.4) and 20% glycerol.
Determination of flavin content
FAD and FMN were quantified with HPLC/fluorescence detection as described previously with slight modification [34]. Briefly, FAD and FMN were released from purified POR or POR mutant by boiling for 5 min. The denatured protein debris was spun down at 13000g for 10 min. FAD and FMN in the supernatant were analysed in a Waters Alliance 2695 chromatographic system in tandem with a Waters W474 fluorescence detector. Chromatographic separation of FAD and FMN was performed on a C18, 5 µm, 4.6 × 250 mm reverse-phase column (Agilent Zorbax-ODS) eluted with a linear gradient of 10 mM (NH4)2HPO4 (pH 5.5) (solvent A)/methanol (solvent B) at a flow rate of 1 ml/min. Solvent B was changed from 10% to 50% (v/v) over 10 min; and changed from 50% to 10% over 1 min; then kept at 10% for 4 min. FAD and FMN fluorescence was detected by excitation at 450 nm and emission at 520 nm and quantified using standard curves constructed with flavin solutions of known concentration. Flavin content was normalized to protein amount for WT and A287P POR.
Steady-state activity for reduction in cytochrome c
The rate of POR-catalysed reduction in cytochrome c was determined by monitoring the absorbance change at 550 nm (Δε =21.1 mM−1·cm−1) on a BIOTEK Synergy 2 Multimode plate reader. All measurements were carried out in triplicate in 96-well format. The reaction mixture contained 3–12 nM POR, 80 µM cytochrome c, 0.1–200 µM NADPH and an NADPH-regenerating system (10 mM isocitrate, 0.5 unit of isocitrate dehydrogenase and 5 mM MgCl2). Reactions were initiated by adding NADPH and monitored over 5 min; rates were extrapolated from the linear range of the kinetic traces. Plots of initial rate versus concentration of NADPH were fitted to the Michaelis–Menten equation in order to determine values for kcat and Km,NADPH. To determine the effect of the addition of FAD or FMN, rates of reactions were determined at a fixed concentration of NADPH (200 µM) as the concentration of flavins was varied (0.5–100 µM FAD or FMN).
Transient kinetic measurements
Experiments were performed under anaerobic conditions using an Applied Photophysics SX.18 MV stopped-flow spectrometer. All buffers were rendered oxygen-free by evacuation and extensive bubbling with argon before use. Transient reduction in POR by NADPH was conducted under single-turnover conditions where POR and NADPH were delivered from separate syringes in equimolar amounts and changes in A450 and A590 were monitored. Data were fitted to standard single- or double-exponential equations. When a double-exponential equation was used the amplitudes of the fast and slow phases and their associated kobs were reported. For transient reduction in cytochrome c, POR was reduced anaerobically by an equimolar amount of NADPH. This POR–NADPH solution was then rapidly mixed with the cytochrome c solution. Because the POR–NADPH complex was preformed in stoichiometric amounts, changes in A550 would represent a single-turnover event. Final concentrations after mixing were 2.1 µM for WT, 5 µM for A287P and 50 µM for cytochrome c. Kinetic transients were fit to either a single-exponential (eqn 1) or burst-phase equation (eqn 2); where A is the amplitude, kobs is the rate constant for the single exponential, kburst is the rate constant for the exponential burst phase, and kss is the rate constant for the linear steady-state phase.
| (1) |
| (2) |
RESULTS
Spectral properties, flavin content and steady-state kinetics
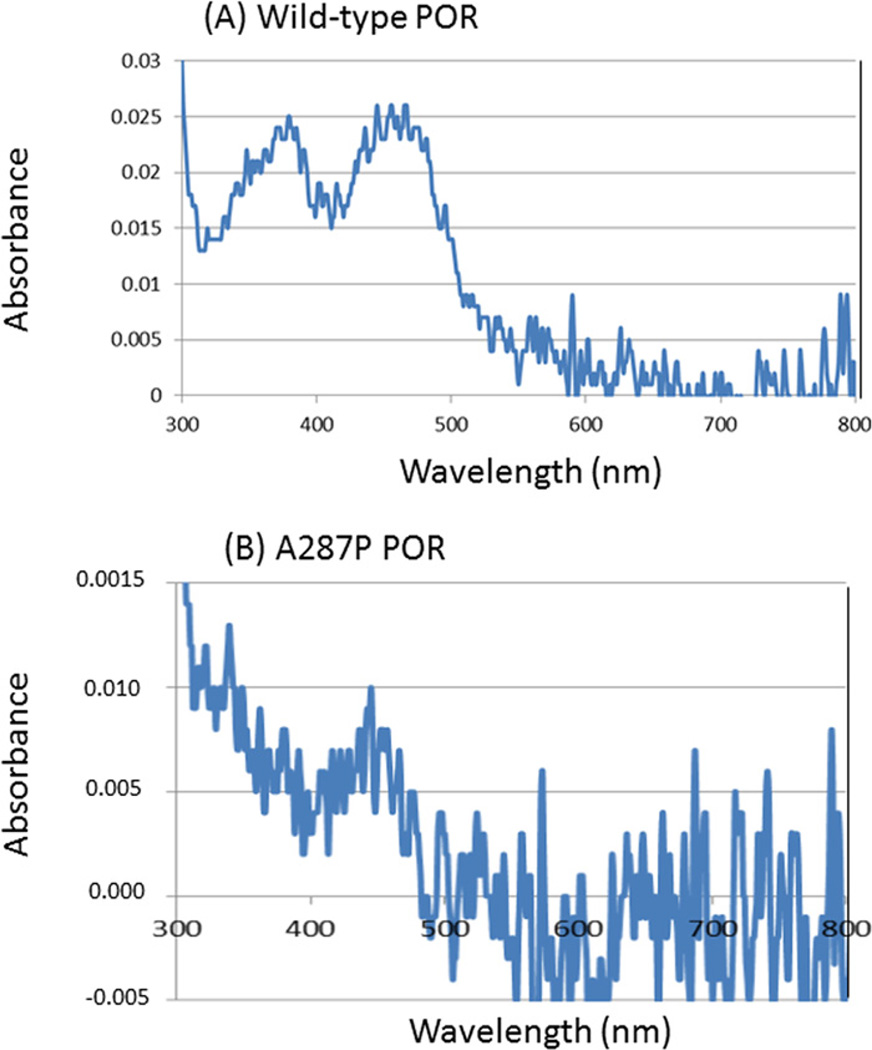
The WT and A287P POR proteins were expressed in E. coli as truncated proteins in which the first 27 N-terminal residues have been deleted (N-27 POR) but contained a C-terminal tag. This form of POR is soluble, but can still anchor in membranes and support P450 reactions. We found that the N-27 POR and the C-terminal tagged construct gave identical steady-state kinetic parameters for the 17α-hydroxylase and 17,20-lyase activities of P450c17 indicating that they are validated POR forms for our experiments (results not shown). The WT and A287P purified proteins were light yellow in colour. Figure 2 shows the visible absorption spectra of the WT and A287P POR. The WT POR exhibited the characteristic flavin absorption peaks near 389 and 452 nm and no significant absorption at 590 nm, consistent with the flavins being in the fully oxidized state. The UV-visible absorbance spectrum of the A287P POR protein differed from the WT POR. The flavin absorption signature could not be resolved indicating lower flavin content compared with the WT protein. Analysis of flavin content by RP-HPLC revealed that, whereas each molecule of the WT POR contained one molecule of FAD and one molecule of FMN, the molar contents of both flavins in the A287P mutant protein were substantially diminished compared with the WT (Table 1). This result was unexpected because structural studies of POR do not suggest that Ala287 is involved in FAD or FMN binding (Figure 1) [1,12].
Figure 2. Visible absorption spectra of the WT and A287P POR proteins.
(A) WT (5 µM), (B) A287P (5 µM) without exogenously added flavin. Spectra were taken using a nanodrop spectrometer.
Table 1.
Flavin content of POR
| FAD (mol/mol of POR) | FMN (mol/mol of POR) | |
|---|---|---|
| WT | 0.96 ± 0.06 | 1.14 ± 0.05 |
| A287P | 0.23 ± 0.03* | 0.37 ± 0.04* |
Measurements were made in triplicate on three different batches of protein samples. The means ± S.E.M. are given.
P ≤ 0.004 compared with WT (Student’s t test).
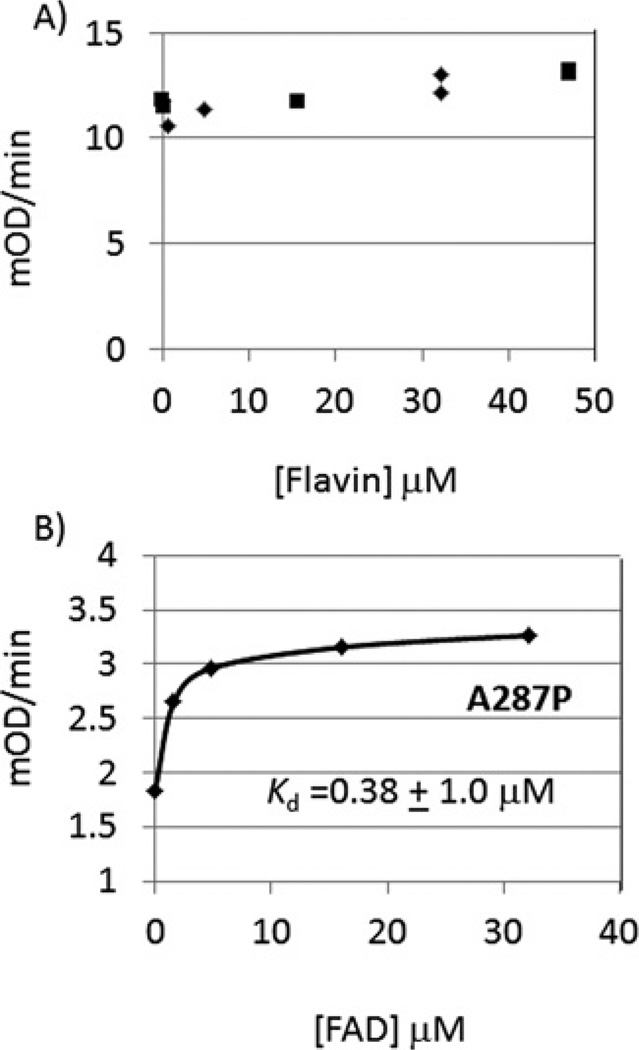
Cytochrome c is used as a surrogate electron recipient since its reduction by POR is a direct measurement of POR activity, unlike assays based on the indirect measurement of a P450 reaction where product formation from P450 turnover is monitored. Consistent with previous results, A287P showed a 9.2-fold reduction in kcat but a similar Km,NADPH for cytochrome c reduction [8,9,16] (Table 2). Addition of FAD and FMN enhanced the kcat for cytochrome c reduction catalysed by A287P up to 3.3-fold, partially rescuing the mutant. By contrast, the addition of FAD or FMN to WT assays had essentially no effect on kcat (0.97- to 1.10-fold change). The Kd for FAD for WT POR could not be estimated since saturation was achieved at the lowest concentration used. By contrast the Kd for FAD was estimated to be <1.0 µM for A287P (Figures 3A and 3B).
Table 2.
Cytochrome c reductase activity and the effect of flavin addition
| Fold change in kcat | |||||
|---|---|---|---|---|---|
| Km, NADPH (µM) | kcat (min−1) | FAD | FMN | FAD and FMN | |
| WT | 2.0 ± 0.3 | 319 ± 3.8 | 0.97 | 1.03 | 1.10 |
| A287P | 1.66 ± 0.29 | 35.8 ± 1.2 | 2.2 | 1.5 | 3.3 |
Each point from the v versus [S] curve was measured in triplicate and iteratively fitted to the Michaelis–Menten equation using GraFit version 5.0 to generate the means ± S.E.M.
Figure 3. Effect of addition of flavins on cytochrome c reductase activity.
Estimates of Kd (FAD) were made by fitting the data to a single-binding site hyperbolic function using GraFit version 5.0. (A) Effect of added FAD (◆) and FMN (■) on cytochrome c reduction catalysed by WT POR; (B) effect of added FAD on cytochrome c reduction catalysed by A287P. For (A) points are from duplicate measurements; for (B) each point was determined in triplicate and a mean ± S.E.M. was computed by an iterative fit to the binding isotherm yielding a Kd of 0.38 ± 1.0 µM, where the large error is probably related to the inaccurate measurement of the residual activity in the mutant without added flavin.
Electron transfer from NADPH to POR
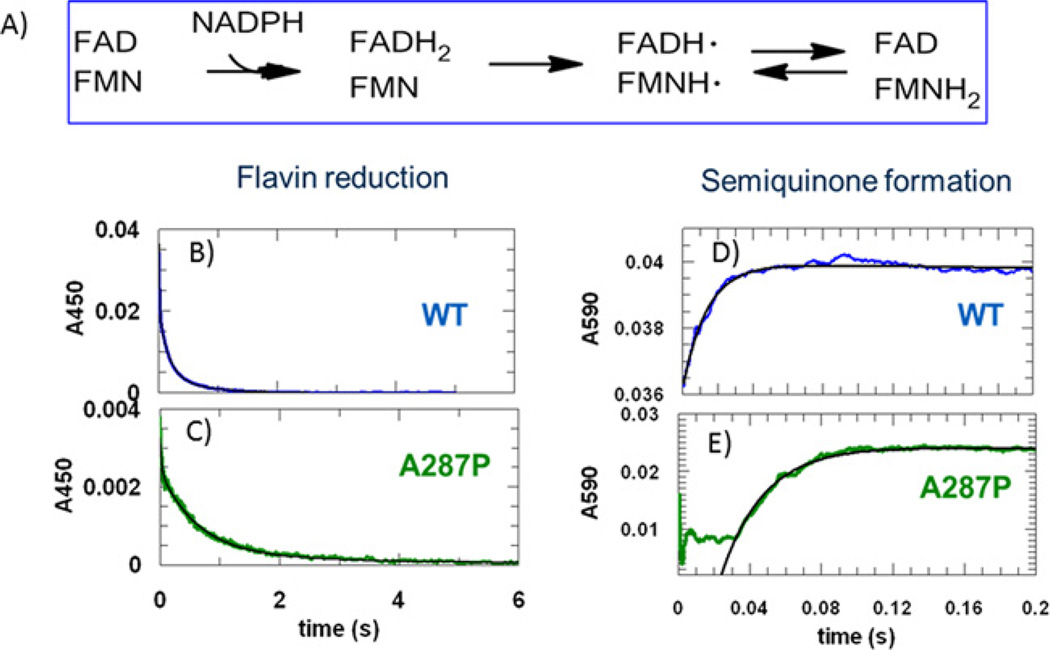
The electron transfer catalysed by POR follows the pathway: NADPH ➔ FAD ➔ FMN ➔ acceptor (Figure 4A), with FMNH2 being the form that transfers electrons to the acceptor proteins [35]. Rapid mixing of POR with NADPH in the stopped-flow instrument under anaerobic single-turnover conditions allows one to discern the steps involved in the half-reaction of POR reduction by NADPH. POR and NADPH solutions (in 1:1 or molar ratios) were rapidly mixed from separate syringes and changes in A450 and A590 were monitored. Decay of the A450 signal reflects NADPH reduction in flavin, which includes FAD reduction and, to a lesser extent, FMN reduction (i.e. electron transfer from FADH2 to FMN to yield FADH•/FMNH• and FAD/FMNH2 forms) (Figures 4B and 4C). The increase in the A590 signal reflects the formation of the blue disemiquione FADH•/FMNH• complex (Figures 4D and 4E). This reaction for WT POR has been studied extensively, mostly using soluble forms of POR that cannot support reactions in membranes [35–40]. Consistent with those previous studies, reduction in flavin by NADPH with the WT occurred rapidly and was biphasic. The amplitude of the fast phase represented 78.4% of the total signal and gave a kobs1 of 68 s−1 whereas the amplitude of the slow phase represented 21.6% of the signal with a kobs2 of 4.7 s−1. The fast phase represents the transfer of electrons from NADPH to FAD and the slow phase represents a subsequent electron transfer event. The increase in absorbance at 590 nm represents the electron transfer from FADH2 to FMN to form the disemiquinone. This event occurred at a higher or equal rate (kobs =82 s−1) to the fast phase observed at 450 nm.
Figure 4. Transient kinetics of POR reduction by one molar equivalent of NADPH.
(A) Electron transfer scheme. (B) and (C) Absorbance decay at 450 nm, reflecting flavin reduction, which includes FAD reduction and also, to a lesser extent, electron transfer from FADH2 to FMN catalysed by WT and A287P POR. (D) and (E) Absorbance increase at 590 nm, reflecting the formation of the blue disemiquinone (FADH•/FMNH•) catalysed by WT and A287P POR. Black lines show best fits to the normalized data. Equal molar amounts of POR and NADPH solutions were rapidly mixed and changes in absorbance at 450 nm and 590 nm were followed using a stopped-flow spectrometer. Final concentrations after mixing were 15.98 µM for WT and 17.7 µM for A287P.
The kinetic behaviour of A287P POR differed from WT POR, resulting in significant changes in rate constants and amplitudes (Table 3). The amplitude of the fast phase at 450 nm was largely eliminated since this now only comprised 18.6% of the total signal but the kobs1 was comparable with that of WT POR. By contrast the amplitude of the slow phase at 450 nm now comprised 81.4% of the total signal and the kobs2 was reduced 3-fold. By contrast the associated rate constant at 590 nm was largely intact yielding a kobs of 42 s−1, but there was a consistent lag phase of 20 ms. The majority of the A450 decay occurred with a kobs2 of 1.6 s−1 with A287P, suggesting a much slower electron transfer from NADPH to FAD. The fast phase of A287P decay at 450 nm, which had a small amplitude and the same rate constant as that observed for the increase in absorbance at 590 nm, was assigned to represent the transfer of electrons from FADH2 to FMN to form the disemiquinone. The absolute change in amplitude at A450 for WT was 4.2-fold higher than for A287P (Table 3). The lag phase observed at 590 nm is consistent with a slow reduction in FAD. These differences indicate that the transfer of electrons from NADPH to FAD is largely impeded, but transfer of electrons from FADH2 to FMN and formation of the disemquinone occurs largely unimpeded in the A287P POR mutant.
Table 3.
Apparent rate constants for POR reduction by NADPH
| 450 nm | 590 nm | ||||
|---|---|---|---|---|---|
| A1 (%) | kobs1 (s−1) | A2 (%) | kobs2 (s−1) | kobs1 (s−1) | |
| WT | 78.4 ± 0.1 | 68.0 ± 0.5 | 21.6 ± 0.5 | 4.7 ± 0.10 | 82.4 ± 1 |
| A287P | 18.6 ± 1.0 | 41.9 ± 2.5 | 81.4 ± 4.0 | 1.6 ± 0.1 | 41.4 ± 0.5 |
Means ± S.E.M. for three traces. The final concentrations were 15.0 µM for WT and 14.1 µM for A287P.
Electron transfer to the terminal acceptor cytochrome c
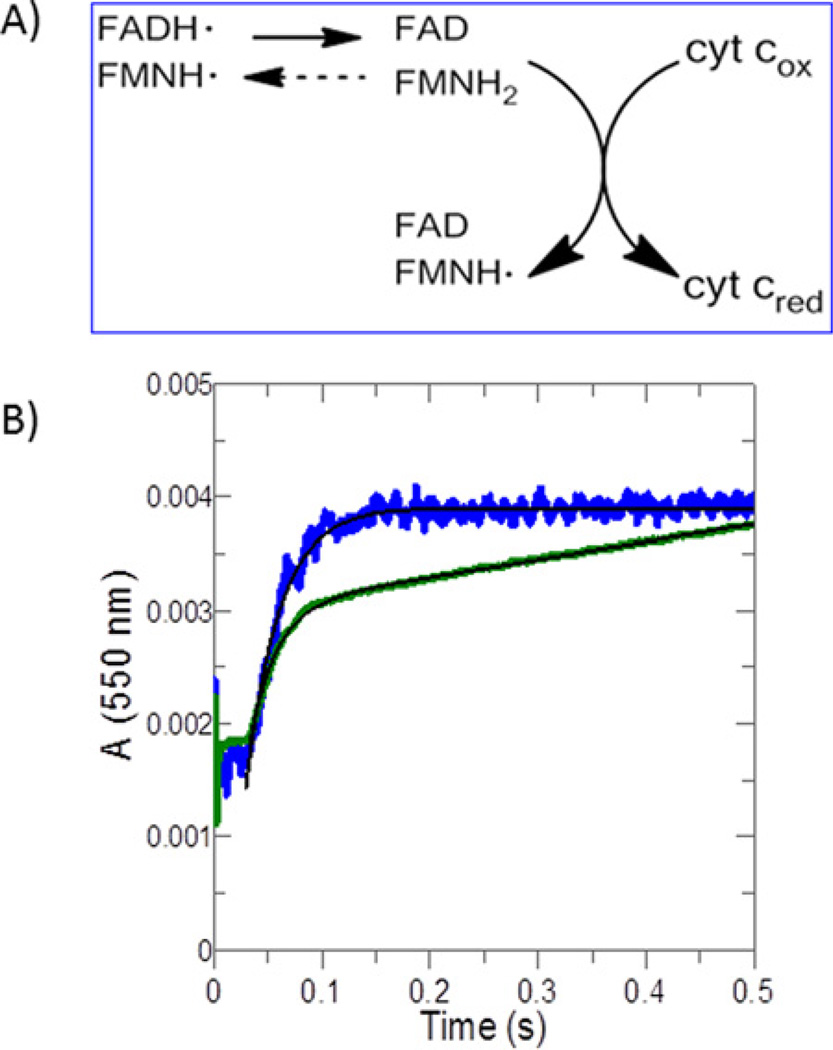
To monitor FMN ➔ cytochrome c electron transfer directly, a solution of cytochrome c was mixed rapidly with reduced POR under single-turnover conditions and the reduction in the haem was monitored by the increase at A550. In these experiments WT POR was stoichiometrically reduced to the two-electron reduced state by a molar equivalent of NADPH, i.e. to yield the catalytically competent FAD/FMNH2 form and the FADH•/FMNH• form of POR, which are in equilibrium. When POR is mixed with cytochrome c, the following steps would occur: (i) formation of a POR–cytochrome c complex; (ii) interflavin electron transfer to convert FADH•/FMNH• to FAD/FMNH2; (iii) electron transfer from FMNH2 to cytochrome c. Our set-up allows us to bypass the steps leading to the formation of FAD/FMNH2 and FADH•/FMNH• forms and directly monitor the events leading to the formation of the reduced cytochrome c. Changes in the POR– cytochrome c interaction rate, in the electron transfer rates and in the equilibrium between FAD/FMNH2 and FADH•/FMNH• caused by the A287P mutation can be detected from the changes in amplitudes and rate constants (Table 4). This set-up is especially useful for A287P, which has impaired reduction in FAD, as slow reduction in flavin would mask the effect of the mutation on electron donation to cytochrome c in steady-state assays. Thus the events monitored are FAD reduction, interflavin electron transfer and electron transfer from FMNH2 to cytochrome c. The rates of these three events control the spectral change observed at 550 nm. The effect of the mutation on the actual FMNH2 ➔ haem transfer can be determined if the step is rate-limiting.
Table 4.
Apparent rate constants for cytochrome c reduction
| A1 (%) | kobs1 (s−1) | kburst (s−1) | A2 (%) | kss (s−1) | |
|---|---|---|---|---|---|
| WT | 100 | 28.1 | N/A | N/A | N/A |
| A287P | 70 | N/A | 41.7 | 30 | 0.2 |
Values are taken from three traces that were superimposed. The final concentrations were 15.98 µM for WT and 17.7 µM for A287P.
Reduction in cytochrome c by WT POR under these single-turnover conditions gave a kinetic transient that fitted to a single exponential yielding a kobs of 28 s−1, which is substantially higher than the kcat of 319 min−1 suggesting that electron transfer before the terminal acceptor step is the slow event. By contrast the A287P POR showed burst-phase kinetic behaviour yielding a kburst of 41.7 s−1, followed by a low linear rate of ~0.2 s−1 (Figure 5, Table 4) which is unexpected under single-turnover conditions. Interpretation of these data are complicated because POR may exist in several different reduced states. However, the burst-phase kinetic behaviour is consistent with the low flavin content in A287P since the experimental set-up would allow for more than one turnover of cytochrome c with the mutant. The rate of the linear portion of the trace is limited by the slow reduction in POR by NADPH in subsequent turnovers. This interpretation suggests that A287P is capable of fast electron flow from FAD/FMNH2 to the haem of cytochrome c.
Figure 5. Transient kinetics of cytochrome c reduction catalysed by reduced POR.
(A) Electron transfer events being monitored. (B) POR was reduced by equimolar NADPH anaerobically and then used to monitor the reduction of excess cytochrome c. Absorbance increase at 550 nm reflects cytochrome c reduction (Δε =21.1 · mM−1 · cm−1). Data were normalized to enzyme concentrations. Black lines show data fitting to single-exponential (WT, blue) and burst-phase (A287P, green equations). The final concentrations were 15.98 µM for WT and 17.7 µM for A287P.
DISCUSSION
A287P is the most frequent mutation causing PORD among Caucasians, occurring in ~40%of patients [9,22]. This mutation can impair POR activity to transfer electrons to CYP1A2, CYP2D6, CYP2C19, CYP3A4 and CYP17, whereas it has no effect on CYP19 and CYP21 [8,9,16,22,24,27,29,41–43]. The underlying mechanism of the differential effects remains unclear. We now describe the effect of this mutation on flavin binding and on individual steps in the transfer of electrons from NADPH to its surrogate recipient, cytochrome c. A287P is defective in flavin content and in reaction with NADPH, but is capable of a fast electron transfer to acceptors.
The deficient flavin content of A287P is unexpected because the crystallographic structure of POR indicates that Ala287 is not near the flavin-binding site, and Ala287 does not appear to play a direct role in POR activity [1,12]. The substitution of a proline for an alanine in a β-sheet of the FAD-binding domain suggests disruption of a β-sheet. Loss of FMN binding has been demonstrated for the POR mutant Y181D [21] and diminished FAD binding for the mutants Y459H and V492E [20]. In each case, the effect of deficient binding of one flavin had a modest effect on the binding of the other. However, with A287P, the binding of both FAD and FMN are diminished to a similar extent (Table 1). Thus, Ala287 may have a role in maintaining the structural integrity of POR.
The decreased content of FMN in A287P explains the observation that external FMN may enhance A287P activity [44]. Aside from hormonal replacement therapy and surgery for the skeletal defects, there are no treatments for PORD. The ability of exogenously added FAD and FMN to restore some activity to A287P suggests that prenatal flavin therapy may ameliorate the phenotype in A287P PORD patients. Kinetic data showed that more FADH•/FMNH• was formedwhenA287P POR reacted with NADPH than when the WT reacted, suggesting there was a shift in equilibrium favouring FADH•/FMNH• over FAD/FMNH2 in the 2e− reduced state. Thus the A287P mutation affects the chemical environment of the FMN moiety. Other POR mutants may also affect flavin redox potentials. In previous reports [20,21], WT POR lacking 66 N-terminal residues (N-66 POR) harboured an FMN semiquinone that was stable in air, whereas the flavins in our N-27 WT protein are fully oxidized.
The electron transfer activity of POR involves multiple conformational changes. While the initial intermolecular electron transfer from NADPH to the FAD requires the FAD domain to rotate away to an open conformation, intramolecular electron transfer from the FAD to the FMN requires that the two flavin domains come into close apposition in a more closed conformation [13–15]. Severe impairment of the flavin reduction step but not of the interflavin transfer or of the FMN ➔ haem transfer by the A287P mutant suggests that the effect of the mutation may also be on NADPH binding or the proper alignment between NADPH and FAD.
ACKNOWLEDGEMENTS
We thank Dr Duanpen Sandee and Ms Izabella Damm for preparation and purification of POR and Dr Li Zhang for help with HPLC.
FUNDING
This work is supported by a pilot-project from the Center of Excellence in Environmental Toxicology [grant number NIH P30-ES013508 (to Y.J.)]; by NIH grant P30-ES013508 (awarded to TMP) and by NIH grant R01-GM073020 (to W.L.M.)].
Abbreviations
- POR
cytochrome P450 oxidoreductase
- PORD
POR deficiency
- WT
wild-type
Footnotes
AUTHOR CONTRIBUTION
Yi Jin designed the study, performed the transient kinetic studies, analysed the data and wrote the first draft of the manuscript. Yi Jin died on 1 August 2014. Mo Chen performed further data analysis and interpretation and edited versions of the manuscript. Walter L. Miller provided WT and mutant POR for the study, advised Yi Jin on experiments and edited drafts of the manuscript. Trevor M. Penning oversaw the project, provided scientific guidance and input into result interpretation and revised the manuscript.
REFERENCES
- 1.Pandey AV, Fluck CE. NADPH P450 oxidoreductase: structure, function, and pathology of diseases. Pharmacol. Ther. 2013;138:229–254. doi: 10.1016/j.pharmthera.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Nebert DW, Wikvall K, Miller WL. Human cytochromes P450 in health and disease. Philos. Trans. R. Soc. Lond. Biol. Sci. 2013;368:20120431. doi: 10.1098/rstb.2012.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ono T, Bloch K. Solubilization and partial characterization of rat liver squalene epoxidase. J. Biol. Chem. 1975;250:1571–1579. [PubMed] [Google Scholar]
- 4.Ilan Z, Ilan R, Cinti DL. Evidence for a new physiological role of hepatic NADPH:ferricytochrome (P-450) oxidoreductase. Direct electron input to the microsomal fatty acid chain elongation system. J. Biol. Chem. 1981;256:10066–10072. [PubMed] [Google Scholar]
- 5.Schacter BA, Nelson EB, Marver HS, Masters BS. Immunochemical evidence for an association of heme oxygenase with the microsomal electron transport system. J. Biol. Chem. 1972;247:3601–3607. [PubMed] [Google Scholar]
- 6.Enoch HG, Strittmatter P. Cytochrome b5 reduction by NADPH-cytochrome P-450 reductase. J. Biol. Chem. 1979;254:8976–8981. [PubMed] [Google Scholar]
- 7.Nishino H, Ishibashi T. Evidence for requirement of NADPH-cytochrome P450 oxidoreductase in the microsomal NADPH-sterol Delta7-reductase system. Arch. Biochem. Biophys. 2000;374:293–298. doi: 10.1006/abbi.1999.1602. [DOI] [PubMed] [Google Scholar]
- 8.Flück CE, Tajima T, Pandey AV, Arlt W, Okuhara K, Verge C, Jabs EW, Mendonca BB, Fujieda K, Miller WL. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat. Genet. 2004;36:228–230. doi: 10.1038/ng1300. [DOI] [PubMed] [Google Scholar]
- 9.Huang N, Pandey AV, Agrawal V, Reardon W, Lapunzina PD, Mowat D, Jabs EW, van Vliet G, Sack J, Flück CE, Miller WL. Diversity and function of mutations in P450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am. J. Hum. Genet. 2005;76:729–749. doi: 10.1086/429417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laue K, Pogoda HM, Daniel PB, van Haeringen A, Alanay Y, von Ameln S, Rachwalski M, Morgan T, Gray MJ, Breuning MH, et al. Craniosynostosis and multiple skeletal anomalies in humans and zebrafish result from a defect in the localized degradation of retinoic acid. Am. J. Hum. Genet. 2011;89:595–606. doi: 10.1016/j.ajhg.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black SD, Coon MJ. Structural features of liver microsomal NADPH-cytochrome P-450 reductase. J. Biol. Chem. 1982;257:5929–5938. [PubMed] [Google Scholar]
- 12.Wang M, Roberts DL, Paschke R, Shea TM, Masters BS, Kim JJ. Three-dimensional structure of NADPH-cytochrome P450 reductase: prototype for FMN-and FAD-containing enzymes. Proc. Natl. Acad. Sci. USA. 1997;94:8411–8416. doi: 10.1073/pnas.94.16.8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis J, Gutierrez A, Barsukov IL, Huang WC, Grossmann JG, Roberts GC. Domain motion in cytochrome P450 reductase: conformational equilibria revealed by NMR and small-angle X-ray scattering. J. Biol. Chem. 2009;284:36628–36637. doi: 10.1074/jbc.M109.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenner M, Ellis J, Huang WC, Raven EL, Roberts GCK, Oldham NJ. Detection of a protein conformational equilibrium by electrospray ionisation-ion mobility-mass spectrometry. Angew. Chem. Int. Ed. Engl. 2011;50:8291–8294. doi: 10.1002/anie.201101077. [DOI] [PubMed] [Google Scholar]
- 15.Xia C, Hamdane D, Shen AL, Choi V, Kasper CB, Pearl NM, Zhang H, Im SC, Waskell L, Kim JJ. Conformational changes of NADPH-cytochrome P450 oxidoreductase are essential for catalysis and cofactor binding. J. Biol. Chem. 2011;286:16246–16260. doi: 10.1074/jbc.M111.230532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang N, Agrawal V, Giacomini KM, Miller WL. Genetics of P450 oxidoreductase: sequence variation in 842 individuals of four ethnicities and activities of 15 missense mutants. Proc. Natl. Acad. Sci. USA. 2008;105:1733–1738. doi: 10.1073/pnas.0711621105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia C, Panda SP, Marohnic CC, Martasek P, Masters BS, Kim JJ. Structural basis for human NADPH-cytochrome P450 oxidoreductase deficiency. Proc. Natl. Acad. Sci. USA. 2011;108:13486–13491. doi: 10.1073/pnas.1106632108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamdane D, Xia C, Im SC, Zhang H, Kim JJ, Waskell L. Structure and function of an NADPH-cytochrome P450 oxidoreductase in an open conformation capable of reducing cytochrome P450. J. Biol. Chem. 2009;284:11374–11384. doi: 10.1074/jbc.M807868200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubbard PA, Shen AL, Paschke R, Kasper CB, Kim JJ. NADPH-cytochrome P450 oxidoreductase. Structural basis for hydride and electron transfer. J. Biol. Chem. 2001;276:29163–29170. doi: 10.1074/jbc.M101731200. [DOI] [PubMed] [Google Scholar]
- 20.Marohnic CC, Panda SP, Martasek P, Masters BS. Diminished FAD binding in the Y459H and V492E Antley–Bixler syndrome mutants of human cytochrome P450 reductase. J. Biol. Chem. 2006;281:35975–35982. doi: 10.1074/jbc.M607095200. [DOI] [PubMed] [Google Scholar]
- 21.Marohnic CC, Panda SP, McCammon K, Rueff J, Masters BS, Kranendonk M. Human cytochrome P450 oxidoreductase deficiency caused by the Y181D mutation: molecular consequences and rescue of defect. Drug Metab. Dispos. 2010;38:332–340. doi: 10.1124/dmd.109.030445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krone N, Reisch N, Idkowiak J, Dhir V, Ivison HE, Hughes BA, Rose IT, O’Neil DM, Vijzelaar R, Smith MJ, et al. Genotype-phenotype analysis in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. J. Clin. Endocrinol. Metab. 2012;97:E257–E267. doi: 10.1210/jc.2011-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flück CE, Miller WL. P450 oxidoreductase deficiency: a new form of congenital adrenal hyperplasia. Curr. Opin. Pediatr. 2006;18:435–441. doi: 10.1097/01.mop.0000236395.71956.5c. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal V, Huang N, Miller WL. Pharmacogenetics of P450 oxidoreductase. Effect of sequence variants on activities of CYP1A2 and CYP2C19. Pharmacogenet. Genomics. 2008;18:569–576. doi: 10.1097/FPC.0b013e32830054ac. [DOI] [PubMed] [Google Scholar]
- 25.Miller WL, Huang N, Agrawal V, Giacomini KM. Genetic variation in human P450 oxidoreductase. Mol. Cell. Endocrinol. 2009;300:180–184. doi: 10.1016/j.mce.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Sahakitrungruang T, Huang N, Tee MK, Agrawal V, Russell WE, Crock P, Murphy N, Migeon CJ, Miller WL. Clinical, genetic and enzymatic characterization of P450 oxidoreductase deficiency in four patients. J. Clin. Endocrinol. Metab. 2009;94:4992–5000. doi: 10.1210/jc.2009-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agrawal V, Choi JH, Giacomini KM, Miller WL. Substrate-specific modulation of CYP3A4 activity by genetic variants of cytochrome P450 oxidoreductase (POR) Pharmacogenet. Genomics. 2010;20:611–618. doi: 10.1097/FPC.0b013e32833e0cb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller WL, Agrawal V, Sandee D, Tee MK, Huang N, Choi JH, Morrissey K, Giacomini KM. Consequences of POR mutations and polymorphisms. Mol. Cell. Endocrinol. 2011;336:174–179. doi: 10.1016/j.mce.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandee D, Morrissey K, Agrawal V, Tam HK, Kramer MA, Tracy TS, Giacomini KM, Miller WL. Effects of genetic variants of P450 oxidoreductase on catalysis by CYP2D6 in vitro. Pharmacogenet. Genomics. 2010;20:677–686. doi: 10.1097/FPC.0b013e32833f4f9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomalik-Scharte D, Maiter D, Kirchheiner J, Ivison HE, Fuhr U, Arlt W. Impaired hepatic drug and steroid metabolism in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. Eur. J. Endocrinol. 2010;163:919–924. doi: 10.1530/EJE-10-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhir V, Ivison HE, Krone N, Shackleton CHL, Doherty AJ, Stewart PM, Arlt W. Differential inhibition of CYP17A1 and CYP21A2 activities by the P450 oxidoreductase mutant A287P. Mol. Endocrinol. 2007;21:1958–1968. doi: 10.1210/me.2007-0066. [DOI] [PubMed] [Google Scholar]
- 32.Pandey AV, Kempna P, Hofer G, Mullis PE, Flück CE. Modulation of human CYP19A1 activity by mutant NADPH P450 oxidoreductase. Mol. Endocrinol. 2007;21:2579–2593. doi: 10.1210/me.2007-0245. [DOI] [PubMed] [Google Scholar]
- 33.Sandee D, Miller WL. High yield expression of a catalytically active membrane-bound protein: human P450 oxidoreductase. Endocrinology. 2011;152:2904–2908. doi: 10.1210/en.2011-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klatt P, Schmidt K, Werner ER, Mayer B. Determination of nitric oxide synthase cofactors: heme, FAD, FMN, and tetrahydrobiopterin. Methods Enzymol. 1996;268:358–365. doi: 10.1016/s0076-6879(96)68038-0. [DOI] [PubMed] [Google Scholar]
- 35.Vermilion JL, Ballou DP, Massey V, Coon MJ. Separate roles for FMN and FAD in catalysis by liver microsomal NADPH-cytochrome P-450 reductase. J. Biol. Chem. 1981;256:266–277. [PubMed] [Google Scholar]
- 36.Vermilion JL, Coon MJ. Purified liver microsomal NADPH-cytochrome P-450 reductase. Spectral characterization of oxidation-reduction states. J. Biol. Chem. 1978;253:2694–2704. [PubMed] [Google Scholar]
- 37.Yasukochi Y, Peterson JA, Masters BSS. NADPH-cytochrome c (P-450) reductase. Spectrophotometric and stopped flow kinetic studies on the formation of reduced flavoprotein intermediates. J. Biol. Chem. 1979;254:7097–7104. [PubMed] [Google Scholar]
- 38.Oprian DD, Coon MJ. Oxidation-reduction states of FMN and FAD in NADPH-cytochrome P-450 reductase during reduction by NADPH. J. Biol. Chem. 1982;257:8935–8944. [PubMed] [Google Scholar]
- 39.Shen AL, Sem DS, Kasper CB. Mechanistic studies on the reductive half-reaction of NADPH-cytochrome P450 oxidoreductase. J. Biol. Chem. 1999;274:5391–5398. doi: 10.1074/jbc.274.9.5391. [DOI] [PubMed] [Google Scholar]
- 40.Gutierrez A, Munro AW, Grunau A, Wolf CR, Scrutton NS, Roberts GCK. Interflavin electron transfer in human cytochrome P450 reductase is enhanced by coenzyme binding. Relaxation kinetic studies with coenzyme analogues. Eur. J. Biochem. 2003;270:2612–2621. doi: 10.1046/j.1432-1033.2003.03633.x. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Pan LQ, Naranmandura H, Zeng S, Chen SQ. Influence of various polymorphic variants of cytochrome P450 oxidoreductase (POR) on drug metabolic activity of CYP3A4 and CYP2B6. PLoS One. 2012;7:e38495. doi: 10.1371/journal.pone.0038495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marohnic CC, Huber WJ, III, Connick JP, Reed JR, McCammon K, Satya P, Martásek P, Backes WL, Masters BSS. Mutations of human cytochrome P450 reductase differentially modulate heme oxygenase-1 activity and oligomerization. Arch. Biochem. Biophys. 2011;513:42–50. doi: 10.1016/j.abb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subramanian M, Agrawal V, Sandee D, Tam HK, Miller WL, Tracy TS. Effect of P450 oxidoreductase variants on metabolism of model substrates mediated by CYP2C9.1, CYP2C9.2 and CYP2C9.3. Pharmacogenet. Genomics. 2012;22:590–597. doi: 10.1097/FPC.0b013e3283544062. [DOI] [PubMed] [Google Scholar]
- 44.Nicolo C, Fluck CE, Mullis PM, Pandey AV. Restoration of mutant cytochrome P450 reductase activity by external flavin. Mol. Cell. Endocrinol. 2010;321:245–252. doi: 10.1016/j.mce.2010.02.024. [DOI] [PubMed] [Google Scholar]