Abstract
A human 3′-5′-exoribonuclease (3′hExo) has recently been identified and shown to be responsible for histone mRNA degradation. Functionally, 3′hExo and a stem–loop binding protein (SLBP) target opposite faces of a unique highly conserved stem–loop RNA scaffold towards the 3′ end of histone mRNA, which is composed of a 6 bp stem and a 4 nt loop, followed by an ACCCA sequence. Its Caenorhabditis elegans homologue, ERI-1, has been shown to degrade small interfering RNA in vitro and to function as a negative regulator of RNA interference in neuronal cells. We have determined the structure of the nuclease domain (Nuc) of 3′hExo complexed with rAMP in the presence of Mg2+ at 1.6 Å resolution. The Nuc domain adopts an α/β globular fold, with four acidic residues coordinating a binuclear metal cluster within the active site, whose topology is related to DEDDh exonuclease family members, despite a very low level of primary sequence identity. The two magnesium cations in the Nuc active site are coordinated to D134, E136, D234 and D298, and together with H293, which can potentially act as a general base, provide a platform for hydrolytic cleavage of bound RNA in the 3′→5′ direction. The bound rAMP is positioned within a deep active-site pocket, with its purine ring close-packed with the hydrophobic F185 and L189 side-chains and its sugar 2′-OH and 3′-OH groups hydrogen bonded to backbone atoms of Nuc. There are striking similarities between the active sites of Nuc and ε186, an Escherichia coli DNA polymerase III proofreading domain, providing a common hydrolytic cleavage mechanism for RNA degradation and DNA editing, respectively.
Keywords: active-site pocket, bound nucleotide conformation, 3′-5′ DEDDh exoribonuclease, hydrolytic cleavage mechanism
Introduction
A human 3′-5′-exoribonuclease (3′hExo) is a recently identified exoribonuclease, that targets human histone mRNAs and degrades them in a 3′→5′ direction.1 Mature metazoan histone mRNAs are not polyadenylated but end instead with a unique, highly conserved stem–loop scaffold, composed of a 6 bp stem and a 4 nt loop, followed by an ACCCA sequence. The stem–loop and flanking sequences are targeted by the stem–loop binding protein (SLBP), which interacts specifically with the 5′-flanking segment of the stem–loop, the lower part of the stem and the loop residues.2 3′hExo is unable to cleave the SLBP-bound mRNA stem–loop, but rather targets the opposite stem–loop face from bound SLBP, through contacts with the upper part of the stem and 3′-flanking segment of the RNA stem–loop.1
Recently, the ERI-1 protein has been identified in a genetic screen for mutants in the Caenorhabditis elegans nervous system with enhanced RNA interference activity.3 The ERI-1 protein exhibits 3′-5′ exonuclease activity, and regulates RNA interference (RNAi) negatively through degradation of the 2 nt 3′-overhangs of small interfering RNAs (siRNAs). Its human orthologue, 3′hExo, also exhibits siRNase activity in vitro.
Both 3′hExo, which is involved in human histone mRNA degradation, and ERI-1, which is involved in C. elegans negative regulation of RNA interference, contain a SAP/SAF-module and a 3′-5′ exonuclease (Nuc) domain. The Nuc domain has been classified as belonging to either a classic DEDD family in 3′hExo1 or a novel DEMD family in ERI-1,3 on the basis of alignment patterns. Exoribonucleases of the DEDD family, which have a characteristic core comprised of four invariant acidic amino acid residues (DEDD) in three separate sequence motifs, are subdivided into DEDDh and DEDDy subfamilies, on the basis of the conservation pattern in motif III, where DEDDh and DEDDy subfamily members contain H-x(4)-D and Y-x(3)-D sequences, respectively.4
Our long-term goal on the 3′hExo project is to solve the structures of the binary complexes of the histone mRNA stem–loop with SLBP and 3′hExo, and the ternary SLBP-3′hExo-mRNA stem–loop complex, towards an improved understanding of factors that control histone mRNA degradation. As a first step, we have attempted to structurally characterize the folding topology of the individual domains of 3′hExo and report here on the crystal structure of the ribo-adenine-5′-monophosphate (rAMP)-bound Nuc domain (segment 123–322) of 3′hExo in the presence of Mg2+ at 1.6 Å resolution. Ribonucleotide 5′-monophosphates (rNMPs) are the products of the 3′-5′ exonuclease hydrolysis reaction and thus are believed to mimic interactions associated with nuclease activity.
Results and Discussion
Crystal structure calculation
We were able to grow diffracting crystals of the rAMP-bound Nuc domain in space group P6122. The initial model of Nuc complexed with rAMP was constructed on the basis of a data set collected on the selenomethionyl-labeled analog of the protein at the peak anomalous wavelength (Table 1). There is one molecule in the asymmetric unit, with continuous electron density traceable along the entire length of the 200 residue protein and for bound rAMP. This structure was then used as a model to refine the structure of the data set collected at the remote wavelength at 1.60 Å resolution (Table 1). An analysis of the final refined structure by PROCHECK showed that 95.6% of the residues were in the most-favored region of the Ramachandran plot, with the remaining residues in additionally allowed regions. Double conformations were assigned for S188, T202, S236 and Q274. This model was also used to determine structures of Nuc crystallized in the presence of deoxy-adenine-5′-monophosphate (dAMP) or deoxy-thymidine-5′-monophosphate (dTMP).
Table 1.
Summary of crystallographic data collection and refinement
| Peak | Remote | |
|---|---|---|
| A. Data collection | ||
| Beamline | SBC-19 | X4A |
| E (keV) | 12.664 | 12.858 |
| Resolution (Å) | 20–1.84 (1.87–1.84) | 20–1.60 (1.63–1.60) |
| Observations | 969,243 | 3,646,480 |
| Unique reflections | 26,863 (1311) | 40,933 (2003) |
| Completeness (%) | 99.9 (100) | 99.5 (100) |
| I/σ(I) | 55.6 (8.7) | 20.6 (5.4) |
| Rmergea | 0.099 (0.408) | 0.134 (0.504) |
| Space group | P6122 | |
| Cell dimensions | ||
| a=b (Å) | 101.6 | |
| c (Å) | 100.4 | |
| α=β (deg.) | 90 | |
| γ (deg.) | 120 | |
| B. Refinement | ||
| Resolution (Å) | 20.0–1.59 | |
| Completeness (%) | 99.29 | |
| Reflections | 38,822 | |
| R-factorb/ | 0.174 | |
| Rfree | 0.207 | |
| Mean B-factor (Å2) | 22.7 | |
| RMSD from ideality | ||
| Bond lengths (Å) | 0.02 | |
| Bond angles (deg.) | 1.35 | |
Rsym = Σ |I − 〈I〉|/Σ |I|
R – factor = Σ |Fo − Fc|/Σ |Fo|
Global fold
Nuc is composed of a six-stranded, twisted β-sheet, which is bracketed by nine α-helices (Figure 1(A)). The order of β strands is 321456, with β strands 2 and 6 aligned antiparallel to the remaining β strands. Helices α3, α4 and α5 cover the convex face of the twisted β-sheet, while the remaining α-helices are positioned along the opposite side of the β-sheet.
Figure 1.
The structure of rAMP-bound Nuc. (A) β-Strands are labeled from β1 to β6, while α-helices are labeled α1 to α8 on proceeding from the N to C terminus within the sequence. The bound rAMP is presented in a stick representation and is colored by atom type. The side-chains of C139 and C186 are shown. All the Figures were drawn by PyMol (DeLano, W.L. The PyMOL Molecular Graphics System on http://www.pymol.org). (B) Potential electrostatics surface of Nuc is colored from blue (basic) to red (acidic), while bound rAMP is shown in yellow. (C) Ribbon diagram of Nuc with B-factor colored from blue (low) to red (high). Nuc in (B) and (C) are in the same orientation.
The side-chains of C139 at the end of β1′ and C186 in α1 are in very close proximity in the folded structure of Nuc, suggestive of the existence of a disulfide bridge in the native protein, but one that would be broken (lower left corner, Figure 1(A), middle panel) under the reductive conditions used for protein purification and crystallization. The corresponding residues in ERI-1 are C160 and C206, suggestive of conservation of this potential disulfide-bridge across species.
Comparison with other proteins
The protein structural fold classification, SCOP,5 categorizes the α/β topology adopted by Nuc to belong to the ribonuclease H-like superfamily. This superfamily comprises seven families: ribonuclease H; DnaQ-like 3′-5′ exonuclease; catalytic domain of retroviral integrase; core domain of mu transposase; transposase inhibitor; catalytic domain of mitochondrial resolvase; and RuvC resolvase. The ribonuclease H family includes RNases, whereas the DnaQ-like 3′-5′ exonuclease family includes DNases.
The ribonuclease H family includes the known structures of RNase HI from prokaryocytes (PDB code 1G15, RNase HI from Escherichia coli),6 RNase HII from archaeon (PDB code 1I39, RNase II from Archaeoglobus fulgidus)7 and HIV RNase H (PDB code1N6Q and 1N5Y, the reverse transcriptase domain from HIV type 1).8 However, the sequence alignment and the structural comparison indicate that Nuc does not belong to this family (data not shown).
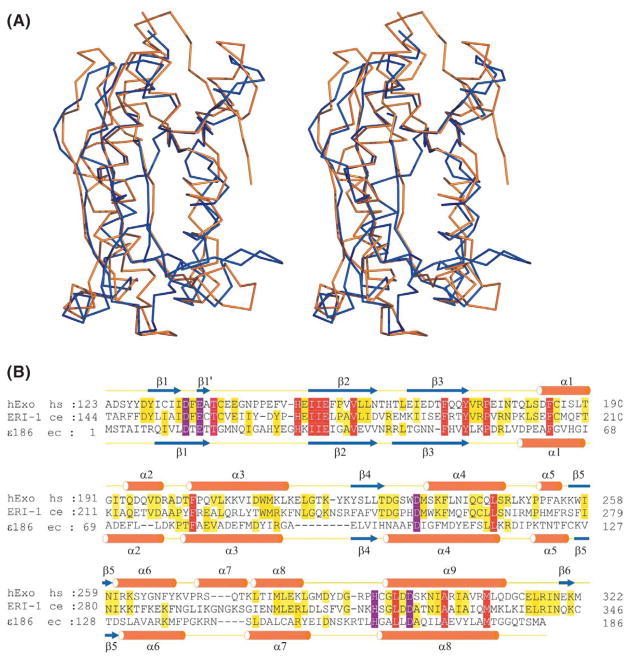
Although 3′hExo has been reported to be an exoribonuclease,1,3 a wider search of the database has established that the structure of its Nuc domain is more similar to members of the DnaQ-like 3′-5′ exonuclease family, which mostly include the exodeoxyribonuclease domains of archeal, phage and prokaryotic DNA polymerases. The structure of the proofreading domain of E. coli DNA polymerase III (ε186), a DnaQ-like 3′-5′ exonuclease family member, has been determined bound to dTMP, the nucleotide products of 3′-5′ DNA exonuclease, in the presence of divalent manganese cations, at pH 5.8 and pH 8.5.9 Although the level of sequence similarity between Nuc and ε186 is below 12%, they actually exhibit a very similar fold with root-mean-square deviation (RMSD) for Cα atoms of 2.8 Å (stereo pair in Figure 2(A)). Most remarkably, the structure-based alignment establishes that Nuc and ε186 adopt similar DEDDh active-site topologies (Figure 2(B)).
Figure 2.
Structural comparison of the folded topologies of Nuc, ERI-1 and ε186. (A) Ternary structure comparison of Nuc (orange) and ε186 (blue). Nuc is shown in the same orientation as in Figure 1(A), left panel. (B) Primary and secondary structural alignment of Nuc, ERI-1 and ε186. The exonuclease domain of 3′hExo (123 to 322 of NP699163, Nuc), ERI-1 (144 to 346 of AAK39278) and ε186 are aligned on the basis of sequence conservation (Nuc versus ERI-1, sequence identity 39%) and following secondary structure similarity (Nuc versus ε186, sequence identity 13%). Besides five critical (DEDDh) residues, which are colored purple, the identical residues throughout the three sequences are colored red, while the semi-identical residues are colored yellow.
We observe good superposition of the β1 to β4 strand segments within the global structures of Nuc and ε186, while the largest difference is associated with the relative α-helical orientation, especially for helices α1, α3, α4 and α6 (Figure 2(A)). An additional difference is that residues R272 to T275 form a short α-helix (α7) in Nuc, while their counterparts in ε186 form a flank loop (Figures 1(A) and 2). Compared with the structures of DnaQ-like 3′-5′ exonuclease family members, such as ε186 (PDB codes 1J53 and 1J54)9 and exonuclease I (PDB code 1FXX),10 Nuc has one extra β6 strand that is aligned antiparallel relative to β5. Furthermore, in Nuc, due to the presence of P155 in the opposite strand (β2), the first β-strand is interrupted near its center and subdivided into β1 and β1′ (Figures 1(A) and 2(B)). This proline residue is conserved as P175 in ERI-1.
Active site and catalytic mechanism
One bound rAMP molecule is positioned towards the bottom of a deep cavity accessed from the front face of the Nuc scaffold. This cavity is surrounded by helices α1, α7, and α8, and the loop between β1′ and β2 (β1′-β2), thereby leaving an opening between α7 and β1′-β2 (Figures 1 and 3(A)). The rAMP molecule can be positioned readily into the electron density map (stereo pair in Figure 3(B)).
Figure 3.
Stereo views looking into the binding cavity of rAMP-bound Nuc. Overview (A) and close-up view (B) of the rAMP binding pocket within Nuc, with hydrogen bonds defining the divalent cation coordination sites (in A) and defining intermolecular contacts (in B) indicated by broken lines. The 2Fo−Fc map contoured at 1.0 is shown in B.
The conserved acidic DEDD (D134, E136, D234 and D298) residues in Nuc form a negative patch within the active site and coordinate two magnesium ions, which are designated as MgA and MgB (Figures 1(B), 3(A) and 4(A)). These two coordinated magnesium ions and the imidazole ring of conserved H293 are in direct contact with the monophosphate of rAMP (Figures 3(A) and 4(A)). MgA adopts a non-canonical penta-coordination pattern involving three side-chain oxygen atoms from D134, E136 and D298, and two non-bridging oxygen atoms from rAMP, while MgB adopts a classic hexa-coordination pattern with oxygen atoms from D134 (dual coordination), D234, a non-bridging oxygen atom of rAMP and two solvent water molecules, thereby adopting an approximate tetragonal bi-pyramidal geometry (Figure 4(A)).
Figure 4.
Active sites and proposed hydrolysis mechanism. The active sites of (A) Nuc and (B) ε186. Two conformations of dTMP bound to ε186 are indicated by different occupancy (in B). (C) The proposed hydrolysis mechanism of Nuc. The coordination between DEDDh residues, rAMP and divalent ions is indicated by broken lines. The remaining residues are omitted for clarity.
Two partly occupied dTMPs were positioned into the final model of the ε186 structure with two bound manganese ions at pH 5.8,9 a pH value similar to the condition used for Nuc (pH 5.6) (Figure 4(B)). One major conformation (85%) has the same binding pattern as that found in the rAMP–Nuc complex, while the other conformation corresponds to the pattern in the complex structure of ε186 and dTMP determined at pH 8.5,9 a pH value optimal for ε186 activity. A hydrolysis reaction mechanism has been proposed for ε186,9 based on the structures of the dTMP–ε186 complexes at pH 5.8 and 8.5.
There are differences in the divalent cation B coordination between Nuc and the major ε186 conformation. In Nuc, MgB is hexa-coordinated by the side-chain oxygen atoms of D134 (dual coordination) and D234, two solvent water molecules and one oxygen atom of rAMP, while in ε186, MnB is coordinated by side-chain oxygen atom of D12, four solvent water molecules and dTMP. Since the function of MgB/MnB most likely involves coordination and orientation of the terminal phosphate group of the RNA/DNA chain, rather than catalysis of the hydrolysis reaction, the minor difference in the coordination pattern of MgB/MnB is unlikely to impact on enzyme function. Given that the divalent cation MgA-involved binding patterns and coordination geometries are identical in both structures, Nuc is likely to adopt the same mechanism of phosphodiester bond cleavage proposed previously for ε186.9
In this mechanism, the DEDD residues in Nuc coordinate two magnesium ions, and thereby assist in the binding and orientation of the terminal phosphate group of RNA (Figure 4(C)). A MgA-coordinated water molecule is deprotonated by H293, thereby nucleophilically attacking the last phosphorus atom and breaking the phosphodiester bond of the terminal residue of the oligonucleotide chain. E136 is likely to be involved in deprotonation of the five-coordinate phosphorane (intermediate state), thereby facilitating the collapse to products. The unusual MgA coordination pattern most likely plays a critical role within the transition state through stabilization of the de-protonated water (OH−).
Bound rAMP
Two intermolecular hydrogen bonds are observed between ribose sugar O3′ and O2′ and the amino nitrogen and carboxy oxygen atoms of A137, respectively (Figure 3(B)). The former hydrogen bond was observed also in the ε186 structure between O3′ of dTMP and amino nitrogen atom of T15.9 The second hydrogen bond is likely critical for substrate recognition, since we were unable to trace the electron density for nucleotides in the dAMP-Nuc and dTMP-Nuc crystals (data now shown), which most likely reflect a lower affinity of Nuc for dNMPs. The side-chain of C139, which is adjacent to the rAMP binding pocket, is likely to form a disulfide bond with C186 (see above). Formation of this disulfide bond can be achieved without distortion of the local protein structure, and hence is unlikely to impact on the architecture of the nucleotide-binding pocket and should not interfere with rAMP complex formation.
One face of the purine ring of rAMP is involved in strong stacking with two hydrophobic residues, F185 and L189, in the Nuc complex (Figure 3(A)), with their counterparts in ERI-1 being F205 and F209. There are no further interactions involving the purine ring of rAMP within the nucleotide binding pocket, consistent with Nuc’s sequence-independent exoribonuclease activity.
Interaction with RNAs
Besides accommodating the bound rAMP within its large and deep cavity, the Nuc binding pocket still retains enough space for potentially accommodating the penultimate nucleotide from the end of the RNA chain (Figures 1(B) and 3(A)). W233 and F238 could pack with the penultimate base, while R261, R272 and K276 introduce a basic patch on the surface for binding to the phosphate backbone of the bound RNA (Figures 1(B) and 3(A)). Helix α7 is relatively flexible, in contrast to other segments of Nuc (Figure 1(C)), and could undergo local conformational changes upon complex formation with single-stranded RNA.
However, Nuc appears to lack a binding surface/pocket that could potentially accommodate RNAs longer than dinucleotides. In the absence of the SAP module, the Nuc domain appears to lose its in vitro binding capacity towards both histone mRNA stem–loop and siRNA (data not shown). SAP (named after SAF-box, Acinus, and PIAS), is a putative DNA/RNA-binding module located close to the N or C termini of the functional domain of proteins involved in transcription, repair, RNA processing and apoptotic chromatin degradation.11 This compact module is composed of two α-helices enriched with hydrophobic, polar and bulky amino acid residues and connected by a fixed-length linker. In 3′hExo, the SAP module (residues 80–111) is just to the N terminus of the Nuc domain (residues 123–322).
3′hExo degrades histone mRNA and siRNA along the 3′-5′ direction. Therefore, we propose that the SAP module of 3′hExo could potentially cooperate with the nuclease domain (Nuc) by binding to the stem segment of histone mRNA stem–loop or the duplex segment of siRNA, leaving the 3′-overhanging ends positioned within the Nuc active site. Nuc has a well-organized cavity capable of accommodating the 3′-terminal segment of ssRNA.
Materials and Methods
Gene cloning, protein expression and purification
The DNA fragment for Nuc (3′hExo segment 123–322) was generated by overlap-PCR and inserted into a pGBO vector12 between NdeI and EcoRI cleavage sites. The strain BL21 (λDE3) harboring the expression plasmid overproduced GBO-Nuc on induction with 0.3 mM IPTG at 20 °C. The fusion protein was purified by standard Ni2+-chelating protocol and the GBO tag was removed by thrombin digestion, leaving GSHM residues on the N terminus of Nuc.12 After heparin and gel-filtration chromatography, the sample was finally concentrated to 20 mg/ml in 25 mM Hepes(pH 7.5), 500 mM NaCl, 5 mM rAMP (or 5 mM dAMP, 5 mM dTMP) and 20 mM MgCl2.
Crystallization and data collection
Crystals were grown by the hanging-drop, vapor-diffusion method at 4 °C. The crystallization drop was made by mixing 1 μl of protein sample and 1 μl of well solution (2.0 M ammonium sulfate, 0.1 M Na citrate at pH 5.6 and 0.2 M sodium potassium tartrate). The data set on the selenomethionine-labeled Nuc crystal was collected at 100 K with crystals cryoprotected by using 20% (v/v) glycerol and 80% well solution. Two data sets were collected, at Advanced Photon Source, Beamline 19BM, Argonne National Laboratory and, and at the National Synchrotron Light Source, Beamline X4A, Brookhaven National Laboratory, respectively (Table 1).
Structure determination
The crystal structure of Nuc was determined by the single-wavelength anomalous dispersion (SAD) method. Six of seven selenium sites (except for N-terminal methionine residue) were identified and refined by Solve13/Resolve14 with the data set collected on the peak anomalous wavelength. On the basis of the resulting electron density map calculated after maximum-likelihood density modification by Solve/Resolve, an initial model was constructed by ARP/wARP.15 This model served as a starting point for refinement of the data set collected at the remote wavelength. rAMP was next fit into the additional density. Solvent atoms were built by ARP/wARP.15 The model was refitted manually by program O16 and refined finally by Refmac5.17 During the later stages of refinement, two magnesium ions were identified from their cation-binding patterns.
Protein Data Bank accession code
The structure of Nuc has been deposited in Protein Data Bank with accession code 1W0H.
Acknowledgments
This research was funded by NIHGM 073618. We thank Dr Yuren Yuan, Dr Randy Alkire and Xiaochun Yang for advice during X-ray data collection and processing. Use of the Advanced Photon Source beamline SBC-19 at the Argonne National Laboratory is supported by the U.S. Department of Energy, Office of Energy Research, under contract no. W-31-109-ENG-38. Use of the National Synchrotron Light Source beamline X4A at the Brookhaven National Laboratory is supported by the U.S. Department of Energy, Division of Materials Sciences and Division of Chemical Sciences, under contract no. DE-AC02-98CH10886.
Abbreviations used
- 3′hExo
human 3′-5′-exoribo-nuclease
- SLBP
stem–loop binding protein
- RNAi
RNA interference
- siRNA
small interfering RNA
- Nuc
3′-5′ exonuclease
References
- 1.Dominski Z, Yang XC, Kaygun H, Dadlez M, Marzluff WF. A 3′ exonuclease that specifically interacts with the 3′ end of histone mRNA. Mol Cell. 2003;12:295–305. doi: 10.1016/s1097-2765(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 2.Battle DJ, Doudna JA. The stem–loop binding protein forms a highly stable and specific complex with the 3′ stem–loop of histone mRNAs. RNA. 2001;7:123–132. doi: 10.1017/s1355838201001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy S, Wang D, Ruvkun G. A conserved siRNA-degrading RNase negatively regulates RNA interference in C. elegans. Nature. 2004;427:645–649. doi: 10.1038/nature02302. [DOI] [PubMed] [Google Scholar]
- 4.Zuo Y, Deutscher MP. Exoribonuclease superfamilies: structural analysis and phylogenetic distribution. Nucl Acids Res. 2001;29:101710–101726. doi: 10.1093/nar/29.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murzin AG, Brenner SE, Hubbard T, Chothia C. SCOP: a structural classification of proteins database for the investigation of sequences and structures. J Mol Biol. 1995;247:536–540. doi: 10.1006/jmbi.1995.0159. [DOI] [PubMed] [Google Scholar]
- 6.Goedken ER, Marqusee S. Co-crystal of Escherichia coli RNase HI with Mn2+ ions reveals two divalent metals bound in the active site. J Biol Chem. 2001;276:7266–7271. doi: 10.1074/jbc.M009626200. [DOI] [PubMed] [Google Scholar]
- 7.Chapados BR, Chai Q, Hosfield DJ, Qiu J, Shen B, Tainer JA. Structural biochemistry of a type 2 RNase H: RNA primer recognition and removal during DNA replication. J Mol Biol. 2001;307:541–556. doi: 10.1006/jmbi.2001.4494. [DOI] [PubMed] [Google Scholar]
- 8.Sarafianos SG, Clark AD, Jr, Das K, Tuske S, Birktoft JJ, Ilankumaran P, et al. Structures of HIV-1 reverse transcriptase with pre- and post-translocation AZTMP-terminated DNA. EMBO J. 2002;21:6614–6624. doi: 10.1093/emboj/cdf637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamdan S, Carr PD, Brown SE, Ollis DL, Dixon NE. Structural basis for proofreading during replication of the Escherichia coli chromosome. Structure (Camb) 2002;10:535–546. doi: 10.1016/s0969-2126(02)00738-4. [DOI] [PubMed] [Google Scholar]
- 10.Breyer WA, Matthews BW. Structure of Escherichia coli exonuclease I suggests how processivity is achieved. Nature Struct Biol. 2000;7:1125–1128. doi: 10.1038/81978. [DOI] [PubMed] [Google Scholar]
- 11.Aravind L, Koonin EV. SAP—a putative DNA-binding motif involved in chromosomal organization. Trends Biochem Sci. 2000;25:112–114. doi: 10.1016/s0968-0004(99)01537-6. [DOI] [PubMed] [Google Scholar]
- 12.Cheng Y, Patel DJ. An efficient system for small protein expression and refolding. Biochem Biophys Res Commun. 2004;317:401–405. doi: 10.1016/j.bbrc.2004.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terwilliger TC, Berendzen J. Automated MAD and MIR structure solution. Acta Crystallog sect D. 1999;55:849–861. doi: 10.1107/S0907444999000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terwilliger TC. Maximum-likelihood density modification. Acta Crystallog sect D. 2000;56:965–972. doi: 10.1107/S0907444900005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perrakis A, Morris R, Lamzin VS. Automated protein model building combined with iterative structure refinement. Nature Struct Biol. 1999;6:458–463. doi: 10.1038/8263. [DOI] [PubMed] [Google Scholar]
- 16.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallog sect A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 17.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallog sect D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]