Abstract
In Australia, a conclusive aetiology of Lyme disease-like illness in human patients remains elusive, despite growing numbers of people presenting with symptoms attributed to tick bites. In the present study, we surveyed the microbial communities harboured by human-biting ticks from across Australia to identify bacteria that may contribute to this syndrome. Universal PCR primers were used to amplify the V1-2 hyper-variable region of bacterial 16S rRNA genes in DNA samples from individual Ixodes holocyclus (n = 279), Amblyomma triguttatum (n = 167), Haemaphysalis bancrofti (n = 7), and H. longicornis (n = 7) ticks. The 16S amplicons were sequenced on the Illumina MiSeq platform and analysed in USEARCH, QIIME, and BLAST to assign genus and species-level taxonomies. Nested PCR and Sanger sequencing were used to confirm the NGS data and further analyse novel findings. All 460 ticks were negative for Borrelia spp. by both NGS and nested PCR analysis. Two novel “Candidatus Neoehrlichia” spp. were identified in 12.9% of I. holocyclus ticks. A novel Anaplasma sp. was identified in 1.8% of A. triguttatum ticks, and a novel Ehrlichia sp. was identified in both A. triguttatum (1.2%) ticks and a single I. holocyclus (0.6%) tick. Further phylogenetic analysis of novel “Ca. Neoehrlichia”, Anaplasma and Ehrlichia based on 1,265 bp 16S rRNA gene sequences suggests that these are new species. Determining whether these newly discovered organisms cause disease in humans and animals, like closely related bacteria do abroad, is of public health importance and requires further investigation.
Introduction
Over the last 30 years in Australia there have been reports of an illness in humans, the onset of which has been putatively associated with parasitism by ticks, most frequently the Australian paralysis tick (Ixodes holocyclus) [1]. This undetermined disease usually presents as acute flu-like symptoms including headache, fever, and fatigue that can persist for weeks to months, and may develop into a severe chronic illness that can include, but is not limited to, myalgia, arthralgia, chronic migraine, and a systemic inflammatory syndrome [1, 2]. Similarities between these symptoms and those of Lyme disease have led to the controversial diagnosis by some physicians of Lyme disease in Australian patients [3, 4].
In the northern hemisphere, Lyme disease is caused by the bacteria Borrelia burgdorferi sensu lato and is transmitted by several species of Ixodes ticks, including I. ricinus and I. persulcatus in Europe and Asia, and I. scapularis and I. pacificus in North America, none of which occur in Australia [5, 6]. Borrelia burgdorferi sensu lato is not considered by many physicians to occur in Australia, and over 20 years of scientific effort has failed to find sufficient evidence of B. burgdorferi sensu lato in Australian ticks, wildlife, or humans that did not acquire Borrelia infection overseas [1, 2, 7]. Consequently, there is significant public concern and medical uncertainty over the diagnosis and treatment of a Lyme disease-like illness in Australia, and there is a need for robust scientific inquiry to clarify the aetiology of this illness.
Ixodes holocyclus is the most significant Australian tick species from both a medical and veterinary perspective [8]. It is the tick most commonly found parasitising humans and domestic animals in its enzootic range, which spans coastal areas along almost the entire east coast of Australia and includes many of Australia’s most densely populated regions [9]. Its natural wildlife hosts include a variety of small marsupials such as bandicoots (Isoodon spp. and Perameles spp.) and possums (Trichosurus vulpecula and Pseudocheirus peregrinus) [8]. Ixodes holocyclus causes life-threatening paralysis in domestic animals through envenomation, and in humans it can cause weakness, paralysis, and dermatological and allergic reactions, including mammalian meat allergies [10]. It is also a vector of the human pathogens Rickettsia australis and R. honei, agents of Queensland tick typhus and Flinders Island spotted fever, respectively [11, 12]. On the west coast of Australia, the most common human-biting tick is the ornate kangaroo tick, Amblyomma triguttatum [8], which is a putative host of Coxiella burnetii, the aetiological agent of Q fever, and the spotted fever pathogen R. gravesii [8, 13, 14].
Recently, a survey of bacteria harboured by I. holocyclus ticks from New South Wales (NSW), Australia, using bacterial 16S rRNA gene (16S) profiling, identified four novel candidate pathogens, including a relapsing fever group Borrelia sp., an Anaplasma sp., and two novel “Candidatus Neoehrlichia” species [7]. Phylogenetic analysis of 300 bp 16S rRNA gene sequences from these bacteria revealed that the Borrelia and “Ca. Neoehrlichia” were closely related to the known human tick-borne pathogens B. duttonii and “Ca. N. mikurensis”, respectively [7], which share some clinical similarities to those described by patients suffering Lyme disease-like illness in Australia [15, 16]. The novel Anaplasma sp. was closely related to the tick-borne pathogen of cattle, A. bovis [7]. None of these candidate pathogens had been described previously in Australia.
The present study was designed in order to better understand the range and genetic diversity of microorganisms potentially transmitted to humans by ticks in Australia. As previously described [7], next-generation sequencing (NGS) and bioinformatics tools were used to profile bacterial populations within ticks removed from people around Australia. Additionally, species-specific PCR assays, Sanger sequencing, and Bayesian phylogenetic reconstructions were implemented to further analyse and confirm results obtained by NGS.
Methods
Ethics statement
This research complies with the Australian Code for the ResponsibleConduct of Research, 2007, and was approved by the Murdoch UniversityHuman Research Ethics Committee (Permit No. 2011–005). All tickcollections were opportunistic and were volunteered by people who hadeither removed the ticks from themselves, or had them removed by a medicalprofessional during outpatient treatment. Participants provided writtendocumented consent to participate in this study, and the consent procedurewas approved by the Murdoch University Human Research Ethics Committee(Permit No. 2011–005).
Tick collection and identification
A total of 460 individual ticks were collected from patients attending the outpatient clinic at the Mona Vale Hospital (Mona Vale, NSW, n = 63), or solicited through media coverage and word-of-mouth (n = 397) from people experiencing tick-bite within Australia between 2013 and 2015. Information about the geographical location (Fig 1) and the date of the tick bite was obtained, and all ticks were confirmed (by medical history or questionnaire) to be actively blood feeding on humans at the time of removal. Ticks were preserved in 70% ethanol immediately after removal and morphologically identified into species, instar, and sex, at the Department of Medical Entomology, Westmead Hospital, or at Murdoch University, using standard keys [8, 17]. Tick specimens were then stored in 70% ethanol at 4°C until molecular analysis.
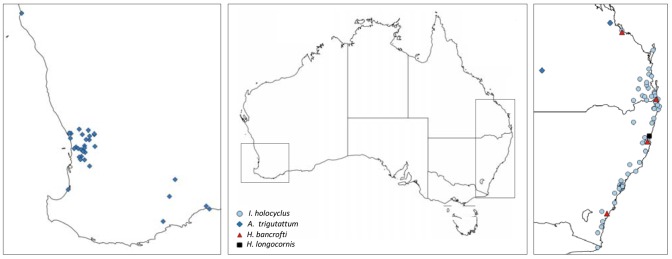
Fig 1. Geographic origin of I. holocyclus, A. triguttatum, and Haemaphysalis ticks used in this study.
Centre, map of Australia; Left, inset of south-west Western Australia; Right, inset of Australian east coast.
DNA extraction
Total genomic DNA was extracted from individual ticks using the QIAGEN DNeasy Blood and Tissue Kit (QIAGEN, Germany) following the manufacturer’s recommendations (QIAGEN Supplementary Protocol: Purification of total DNA from insects). Before DNA extraction, the external surface of ticks was decontaminated in 10% hypochlorite solution, washed in sterile and DNA-free PBS, and 70% ethanol, and air-dried. Ticks were then frozen in liquid nitrogen for 1 minute, and homogenised by shaking with a 5 mm steel bead at 40 Hz for 1 minute. Extraction reagent blanks (EXB) (n = 20) were performed in parallel with all DNA extractions in order to establish background bacterial populations. All DNA extractions were performed in a physical isolation hood to minimise contamination by researchers and the environment, and sterile and DNA-free equipment was used for all procedures.
Bacterial 16S rRNA gene profiling
The V1-2 hyper-variable region (250–320 bp) of bacterial 16S rRNA genes in tick DNA samples were PCR amplified using the primers 27F-Y and 338R as previously described [7]. These PCR assays for I. holocyclus DNA samples also included 10 μM of a “Ca. Midichloria mitochondrii”-specific blocking primer [7], in order to inhibit the amplification of 16S sequences from this highly abundant endosymbiotic bacterium. No-template (NT) and EXB controls were included in all PCR runs.
Amplicon library preparation was performed according to recommended protocols (Illumina Demonstrated Protocol: 16S Metagenomic Sequencing Library Preparation) with exceptions. Individual uniquely indexed libraries were normalised to equimolar concentrations with AxyPrep Mag PCR Normaliser beads (Axygen, USA) following the manufacturer’s recommendations, before pooling in equimolar amounts. Up to 96 uniquely indexed libraries were pooled per sequencing run, which were performed on an Illumina MiSeq using 500-cycle V2 chemistry (250 bp paired-end reads) following the manufacturer’s recommendations. No-template and EXB controls were also sequenced to establish background bacterial populations. All pre-PCR and post-PCR procedures were performed in physically separated laboratories to minimise amplicon contamination.
Next Generation Sequencing Analysis
Sequences were first subjected to quality control procedures as previously described [7], with exceptions. Paired-end reads were merged using USEARCH v8.0.1623 [18] with a minimum overlap length of 50 bp and no gaps allowed in the merged alignments. Primer sequences and distal bases were trimmed from the ends of reads in Geneious v8.1.6 (Biomatters, New Zealand) [19] and reads shorter than the minimum previously reported length of the bacterial 16S V1-2 amplicon (< 250 bp) were removed. Singleton sequences (per sample) and sequences with a > 1% error rate were removed from the dataset using USEARCH v8.0.1623 [18]. Operational taxonomic units (OTUs) were created by clustering sequences at 97% similarity with the UPARSE algorithm [20], and taxonomy was assigned to OTUs in QIIME [21] by aligning to the GreenGenes 16S database (August 2013 release) [22] using the UCLUST algorithm [18] with default parameters. OTUs taxonomically assigned to the family or genus-level were used for further analysis. OTUs that were present in EXB and NT controls were removed from all samples in order to eliminate potentially contaminating and background bacteria.
Following OTU analysis to assign genus level taxonomy to 16S sequences, BLAST was used to resolve the species identity of families and genera that have medical or veterinary significance, or contain members that are known, or proposed, arthropod endosymbionts or pathogens. Species-level taxonomy was only inferred when the query matched 16S sequences from only one species with a ≥ 99% pairwise identity over ≥ 99% the length of the query sequence. Bacterial genera that were deemed not of medical or veterinary significance, known or proposed arthropod endosymbionts, or otherwise previously associated with ticks, and that were detected in less than the mean prevalence of all taxa, are herein not mentioned.
Anaplasmataceae, Borrelia, and Rickettsia-specific PCR and Sanger sequencing
In order to gain more informative phylogenetic data and to verify NGS results, species-specific PCRs were used to further confirm (or refute) the occurrence of: Borrelia spp., Anaplasmataceae species (except Wolbachia spp.), and spotted fever and typhus group Rickettsia species in ticks. The Borrelia-specific assay targeted a 441 bp region of the chromosomal flagellin gene (flaB) and consisted of two nested PCRs, the primary reaction with primers flaB-280F and flaB-RL, and the nested reaction with primers flaB-LL and flaB-737R [23, 24], and verified previously in our laboratory to reliably amplify B. burgdorferi sensu lato, and relapsing fever group Borrelia spp. from tick specimens. The presence of Anaplasmataceae species in ticks was confirmed using a nested PCR assay targeting a 1.3 kb region of the 16S rRNA gene of Anaplasmataceae species (except Wolbachia spp.). The primary PCR contained the primers EC9 and EC12A [25, 26] and the nested reaction contained primers A17a and IS58-1345R [27]. The presence of spotted fever and typhus group Rickettsia species was confirmed with a qPCR assay using the primers CS-F and CS-R, and hydrolysis probe CS-P, as previously described [28].
Borrelia and Anaplasmataceae-specific primary PCRs contained 2 μl of tick DNA and the nested reaction used 1 μl of the primary PCR product as a template. PCRs contained PCR buffer, 2.5 mM MgCl2, 1 mM dNTPs, 0.01 mg BSA (Fisher Biotech, Australia), 1.25 U Perfect Taq Polymerase (5 Prime, Germany), and 400 nM of each primer, in a total volume of 25 μl. All PCRs included NT controls and positive controls (B. afzelii or “Ca. N. mikurensis” from I. ricinus ticks, and R. australis from culture). All positive PCR products were electrophoresed in 2% agarose gels stained with GelRed (Biotium, USA), visualised under UV light, purified with the QIAquick gel extraction kit (QIAGEN, Germany), and sequenced with both forward and reverse PCR primers on an ABI 3730 96 Capillary Sequences using Big dye v3.1 terminators (Life Technologies, USA).
Anaplasmataceae 16S phylogenetic analysis
Phylogenetic analysis was conducted on 1,265bp 16S sequences obtained from the Anaplasmataceae-specific nested PCR on I. holocyclus and A. triguttatum samples, and additional Anaplasmataceae 16S sequences retrieved from GenBank. Sequences were aligned with MAFFT [29] and the gapped alignment was refined with MUSCLE [30]. The most suitable nucleotide substitution model was assessed in MEGA6 [31] and selected based on the Bayesian Information Criterion. Bayesian phylogenetic analysis was performed with the MrBayes software [32] using the HKY85 substitution model and a discrete Gamma distribution with 5 categories, a total chain length of 1,100,000, burn-in length of 100,000, and subsampling every 200 iterations.
Results
Bacterial 16S rRNA gene community profiling
The tick species collected from people while attached and feeding included I. holocyclus (n = 279), A. triguttatum (n = 167), Haemaphysalis bancrofti (n = 7), and H. longicornis (n = 7) (Table 1). Ixodes holocyclus ticks were received from almost the entirety of its enzootic range along the east coast of Australia from Gladstone, Queensland (QLD) to Mallacoota, Victoria (Fig 1). Amblyomma triguttatum ticks were primarily collected from southwest Western Australia (WA), including many semi-rural and rural areas surrounding Perth, as far north as Kalbarri, WA, and southeast at Hopetoun, WA (Fig 1). Amblyomma triguttatum ticks were also received from Rockhampton and Charleville, QLD (Fig 1). Haemaphysalis longicornis were collected from only a single location; Urunga, NSW, and H. bancrofti was collected from four locations, Gladstone, QLD, Currumbin, QLD, Mollymook, NSW, and Tamban, NSW (Fig 1).
Table 1. Summary of sample size, NGS coverage, and taxonomic diversity of tick species and life stages.
| Tick Species | Instar/Sex | Number of samples | Total number of sequences | Mean sequences per sample | Number of bacterial genera a |
|---|---|---|---|---|---|
| I. holocyclus | Females | 167 | 16,196,861 | 96,987.2 | 27 |
| Male | 49 | 4,427,177 | 90,350.5 | 19 | |
| Nymphs | 63 | 3,188,402 | 50,609.5 | 22 | |
| A. triguttatum | Female | 40 | 1,787,788 | 44,694.7 | 19 |
| Male | 24 | 538,571 | 22,440.4 | 16 | |
| Nymph | 103 | 3,032,534 | 29,442.1 | 18 | |
| H. bancrofti | Male | 1 | 13,284 | 13,284.0 | 16 |
| Nymph | 6 | 145,742 | 24,290.3 | 15 | |
| H. longicornis | Female | 3 | 72,635 | 24211.7 | 20 |
| Nymph | 4 | 101,927 | 25,481.7 | 18 | |
| NT and EXB Controls | 25 | 945,238 | 37,809.5 | 41 |
a For tick samples only genera that were found in more than the mean prevalence of all taxa are shown
After NGS quality control procedures, 30,450,159 16S sequences from 460 tick samples and 25 NT and EXB control samples were used for analysis (Table 1). A total of 41 bacterial genera that were found in NT and EXB controls were removed from the dataset as background bacteria. All of the background taxa were either ubiquitous environmental or human-associated commensal bacterial genera that to the best of our knowledge have never been associated with tick-borne human or veterinary disease.
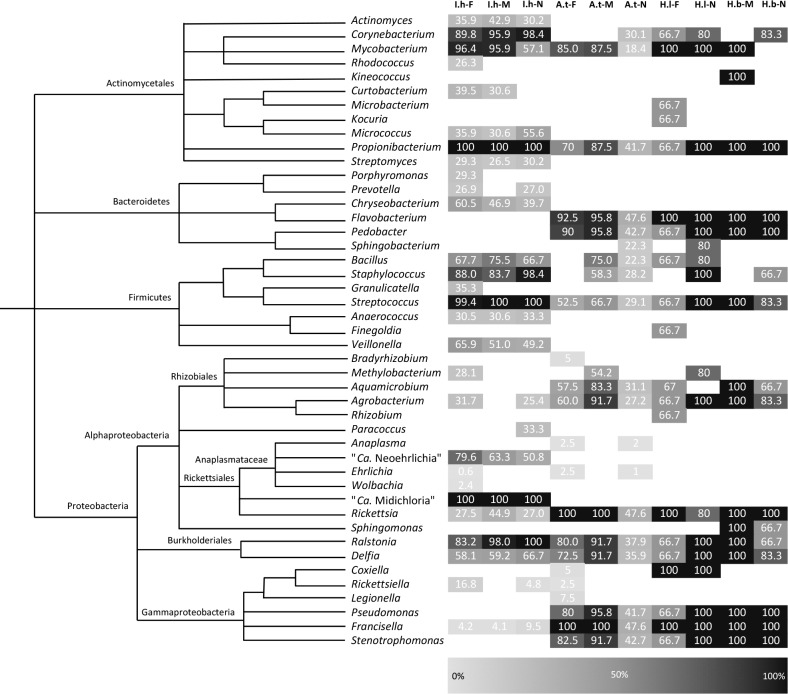
The most prevalent organisms identified in I. holocyclus, A. triguttatum, H. bancrofti, and H. longicornis ticks were environmental or commensal bacteria that included 34 genera within Actinomycetales, Bacteriodetes, Firmicutes, Rhizobiales, Burkholderiales, and Gammaproteobacteria. The genera Propionibacterium, Staphylococcus, and Streptococcus, which live as commensals on mammalian skin were identified in all tick species (Fig 2). Other environmental genera identified, such as Bacillus, Agrobacterium, Corynebacterium, Delftia, Flavobacterium, Methylobacterium, Mycobacterium, Pseudomonas, Ralstonia, and Stenotrophomones are considered as either ubiquitous in the environment, or associated with soil and moist leaf litter environments in which ticks spend a large proportion of their life cycle (Fig 2). No Borrelia sp. sequences were identified in any of the 460 ticks.
Fig 2. Cladogram and heat map showing the prevalence of bacterial genera in tick species and life stages.
I.h, A.t, H.l, and H.b indicate I. holocyclus, A. triguttatum, H. longicornis, and H. bancrofti tick species, respectively. Female, Male and Nymph life stages are indicated by F, M, and N, respectively. The level of shading corresponds to the prevalence of the genera in the tick species and life stage. Blank shading indicates that bacterial genera were not detected in that tick species life stage.
Bacterial endosymbionts in human-biting ticks
Proposed bacterial endosymbionts were highly prevalent in all ticks studied, with each tick species having one or two predominant endosymbiont species and one to three less prevalent endosymbiotic associations. As anticipated from a previous study [7], the Ixodes tick endosymbiont “Ca. Midichloria mitochondrii” (16,519 unique sequences) was found in all I. holocyclus ticks, however, as expected (due to the use of a blocking primer during PCR) [7], 16S sequences from this abundant bacterium only comprised 4–17% of sequences per sample. In addition, Wolbachia, Francisella, and Rickettsiella spp. were also identified in 1.4%, 5.4%, and 11.1% of I. holocyclus ticks, respectively (Fig 2). Bacteria of the genus Rickettsia (7,069 unique sequences) were also identified in 27.5% of females, 44.9% of males, and 27% of nymph I. holocyclus ticks, with a total prevalence 30.5% in this tick species. Unfortunately Rickettsia 16S reads were unable to be given species designation due to high sequence homology (> 99%) between many Rickettsia species at the 16S locus analysed.
All A. triguttatum, H. bancrofti, and H. longicornis ticks studied were dual-infected with Francisella (12,990 unique sequences) and Rickettsia spp. (7,069 unique sequences) (Fig 2). Francisella and Rickettsia spp. sequences from these ticks were highly abundant in the NGS results, comprised between 12%-98% and 2%-88% of sequences per sample, respectively. Francisella sequences from all ticks were more than 98% similar to known endosymbiotic Francisella spp. from A. maculatum (GenBank: AY375407) and Dermacentor spp. (GenBank: AY375403, AY375401, JX101605) ticks from the northern hemisphere, and less than 94% similar to the infectious human pathogen Francisella tularensis (GenBank: NR074666), which has never been reported in Australia. In addition to endosymbiotic Francisella and Rickettsia spp., all H. bancrofti ticks also harboured a Coxiella sp., presumed to be an endosymbiont, as did 5% of A. triguttatum females. Coxiella sp. sequences were highly abundant in H. bancrofti ticks, comprising 23%-92% of sequences per sample. These Coxiella sp. sequences were more than 99% similar to Coxiella sp. endosymbionts reported previously from H. lagrangei and H. longicornis from Thailand and Korea (GenBank: KC170756, AY342036), respectively, but less than 94% similar to the infectious pathogen C. burnetii (GenBank: HG825990).
Novel Anaplasmataceae species identified in human-biting ticks
The genus “Ca. Neoehrlichia” (11,493 unique sequences) was identified in all I. holocyclus life stages studied, with a prevalence of 76.6%, 63.3%, and 50.8% in females, males, and nymphs, respectively, and a total prevalence of 88.9%. “Candidatus Neoehrlichia” sequences formed two distinct clusters, herein putatively named species A and B, which were 6–7% dissimilar from each other (S1 Table). The closest known relative to putative “Ca. Neoehrlichia” species A and B was “Ca. N. mikurensis” (94.6–94.9% similarity) (GenBank: AB196304) from Japan. Putative species A and B sequence were also highly similar to “Ca. N. lotoris” (95.9–96.3% similarity) (GenBank: EF633744), although the sequence query coverage was only 90.8%. Putative “Ca. Neoehrlichia” species A was most common, being found in 68.8% of “Ca. Neoehrlichia”-positive I. holocyclus ticks, compared to species B (31.2%). All sequences from both “Ca. Neoehrlichia” putative species A and B were more than 99% similar to “Ca. Neoehrlichia” spp. 16S sequences recently obtained by NGS from I. holocyclus ticks from NSW, Australia [7], with species A and B most similar to “Ca. Neoehrlichia” sp. isolates PI808 (GenBank: KT203915), and PI800 (GenBank: KT203914), respectively. Among all of the “Ca. Neoehrlichia”-positive I. holocyclus ticks, there were no cases of co-infection with both putative species A and B.
Interestingly, “Ca. Neoehrlichia” sequences were not detected in any A. triguttatum or Haemaphysalis ticks; however, two other Anaplasmataceae species were identified in A. triguttatum ticks and a single I. holocyclus female. Novel Anaplasma sp. sequences (284 unique sequences) were identified in three A. triguttatum ticks (1.8%), including one female (2.5%), and two nymphs (2%). These Anaplasma sp. sequences were most similar (98%) to an uncultured Anaplasma sp. (GenBank: JN862824) from southeast China, and the closest recognised species (97%) was A. bovis (GenBank: KJ659040). All three A. triguttatum ticks infected with this novel Anaplasma sp. originated from Yanchep National Park, Western Australia. Novel Ehrlichia sp. sequences (206 unique sequences) were also identified in two (1.2%) A. triguttatum ticks including one nymph, one female, and one I. holocyclus female (0.6%). These novel Ehrlichia sp. sequences were most similar (97%) to E. ruminantium (GenBank: DQ482921, CR925677), and another unresolved Ehrlichia sp. from H. longicornis ticks from Japan (GenBank: AY309970, HQ697588). The two A. triguttatum ticks infected with this novel Ehrlichia sp. both originated from Bullsbrook, Western Australia and the I. holocyclus tick originated from Pimpama, Queensland.
Anaplasmataceae, Borrelia, and Rickettsia-specific PCR
All 460 I. holocyclus, A. triguttatum, and Haemaphysalis ticks were negative for Borrelia spp. by nested PCR, confirming the 16S community profiling results. The spotted fever and typhus group-specific qPCR did not amplify any Rickettsia from I. holocyclus ticks. However, all Rickettsia-positive A. triguttatum and Haemaphysalis ticks (by NGS) were amplified with this qPCR assay, indicating the Rickettsia spp. in these ticks are within, or closely related to spotted fever and typhus group Rickettsia species. The Anaplasmataceae-specific PCR assay returned 37 positive I. holocyclus ticks (12.9%), including 19 females (11.4%), eight males (16.3%), and 10 nymphs (15.9%), and five positive A. triguttatum ticks (3%), including two females (5%), and three nymphs (2.9%). No Haemaphysalis ticks were positive for Anaplasmataceae species.
Anaplasmataceae Phylogenetic Analysis
Bayesian phylogenetic reconstruction of 1,265 bp 16S Anaplasmataceae sequences revealed that 36 (12.9% of all I. holocyclus) of the 37 positive I. holocyclus samples grouped with high confidence within the genus “Ca. Neoehrlichia”. Furthermore, the 16S sequences from these ticks clustered into two distinct groups, one containing identical sequences from 25 I. holocyclus ticks (9%) comprising putative “Ca. Neoehrlichia” species A (GenBank: KT803957), and the other containing identical sequences from 11 I. holocyclus ticks (4%), comprising putative “Ca. Neoehrlichia” species B (GenBank: KT803958) (Fig 3). Sequence from putative “Ca. Neoehrlichia” species A and B shared 96.2% similarity (S2 Table). The two known members of the genus, “Ca. N. lotoris” (GenBank: EF633744) and “Ca. N. mikurensis” (GenBank: AB074460, AB084582), were 98.1–98.6% similar at the 16S loci, however, putative “Ca. Neoehrlichia” species A and B were only 95.7–96.2%, and 97.3–98.4% similar to these species, respectively (S2 Table).
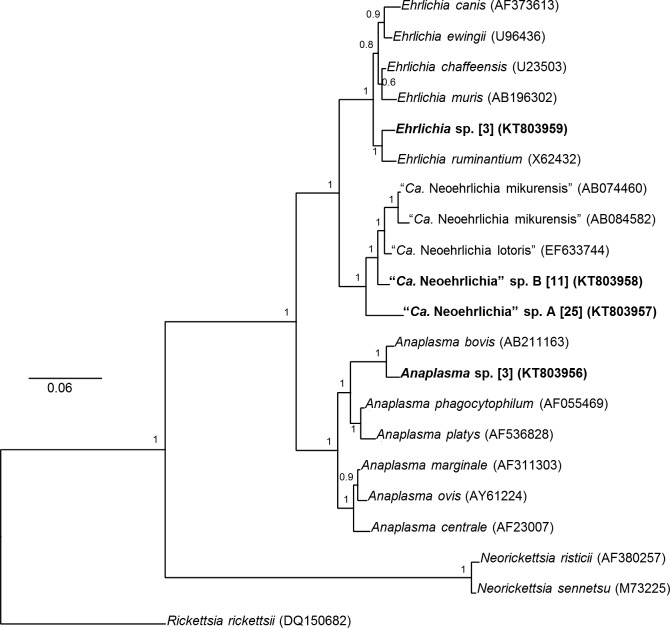
Fig 3. Bayesian phylogenetic analysis of 1,265 bp novel Anaplasmataceae 16S rRNA sequences from I. holocyclus and A. triguttatum.
Bayesian posterior probabilities are displayed at each node. Bold type indicates sequences from this study. Rounded parentheses indicate GenBank accession numbers, and square parentheses indicate the number of ticks from which identical sequences were obtained.
The level of divergence at the 16S loci both between putative “Ca. Neoehrlichia” species A and B, and between these and known “Ca. Neoehrlichia” spp., confirms the clustering pattern observed in the NGS data, and described previously [7]. All I. holocyclus ticks positive here for novel “Ca. Neoehrlichia” spp. were also positive for “Ca. Neoehrlichia” spp. by NGS, although the prevalence of “Ca. Neoehrlichia” spp. was significantly lower as determined by nested PCR (12.9%) than by NGS (88.9%).
Three identical novel Anaplasma sp. 16S sequences (GenBank: KT803956) from A. triguttatum ticks (1.8%), including one female and two nymphs, clustered with high confidence, but were distinct (98.7% similarity) from A. bovis (GenBank: AB211163) (Fig 3, S2 Table). In addition, a further three identical novel Ehrlichia sp. sequences (GenBank: KT803959) from two A. triguttatum ticks (1.2%), including one female (2.5%) and one nymph (0.97%), and one I. holocyclus female (0.6%) clustered with high confidence, but was distinct (98.3% similarity) from E. ruminantium (GenBank: X62432) (Fig 3, S2 Table). The level of divergence between these novel Anaplasma sp. and Ehrlichia sp. 16S sequences, and their closest relatives, is within the range of divergence among all Anaplasma species (94.7–99.4%) and Ehrlichia species (97.3–98.9%) (S2 Table). All ticks positive by nested PCR for novel Anaplasma sp. and Ehrlichia sp. were also positive for these taxa through NGS.
Discussion
This study follows a preliminary investigation of the bacterial microbiome associated with I. holocyclus in a localised region of NSW, with the aim of investigating a collection of human-biting ticks over a greater geographical range, including areas of Sydney, NSW, where numerous patients have been diagnosed with a Lyme disease-like illness. In Australia, approximately eight species of hard ticks, and one species of soft tick (Ornithodorus capensis) are known to bite humans [8, 17, 33]. Consistent with previously published and anecdotal reports, the Australian paralysis tick (I. holocyclus) and the ornate kangaroo tick (A. triguttatum) were most frequently associated with attachment and engorgement on the skin of people in this study [8]. The introduced ‘bush’ tick, H. longicornis, normally a parasite of cattle, and the native wallaby tick (H. bancrofti) are also well known to bite people in Australia [8]. Curiously, we did not receive any specimens of the brown dog tick (R. sanguineus), the common marsupial tick (I. tasmani) or the southern paralysis tick (I. cornuatus) for analysis in this study, all of which have previously been associated with human tick bites in Australia.
Although the external cuticle of all ticks was decontaminated with ethanol and 10% hypochlorite solution prior to molecular analyses, a range of common environmental and commensal bacteria were still prevalent among all ticks surveyed. This is most likely due to remnant bacterial DNA that survived the decontamination process, perhaps in bacterial plaques that may have accumulated in less accessible places such as between leg joints or underneath the tick’s palps. In future studies careful dissection of the tick’s main internal tissues (midgut, salivary gland, and gonads) may prove useful in distinguishing the microbiome of the internal tissues from environmental bacteria on the tick’s external surfaces. Because all ticks surveyed were collected while actively feeding on humans it must be acknowledged that some bacteria in tick samples, such as Staphylococcus spp. and Propionibacterium spp., may have been from the blood and skin of the human hosts. However, most bacteria identified in the present study have been associated previously with ticks as members of genera that contain either known tick-borne pathogens, or arthropod endosymbionts.
Consistent with previous analysis [7], endosymbiotic “Ca. M. mitochondrii”, Wolbachia, Francisella, and Rickettsia spp. were identified in I. holocyclus ticks. All A. triguttatum, H. bancrofti, and H. longicornis ticks studied were dual-infected with endosymbiotic Francisella and Rickettsia spp., which comprised a large proportion of NGS sequencing output for these samples. Although Francisella endosymbionts have been described from northern hemisphere Amblyomma and Dermacentor ticks [34–36], and previously in I. holocyclus [7], this is the first description of Francisella spp. in a native Australian Amblyomma or Haemaphysalis tick. Species-specific blocking primers have been shown to be effective at inhibiting specific endosymbiont 16S sequences in I. holocyclus and I. ricinus [7], allowing the detection of less abundant bacterial taxa. It is probable that the use of Francisella and Rickettsia-specific blocking primers during 16S bacterial profiling of A. triguttatum and Haemaphysalis spp. ticks may similarly reveal more information about the less abundant bacterial taxa associated with these ticks.
The very high prevalence of Rickettsia spp. in A. triguttatum and Haemaphysalis ticks in this study suggest these Rickettsia spp. are likely endosymbiotic, and either advantageous or benign to the fitness of these tick species. The fact that these species were amplified with a qPCR assay designed to amplify only spotted fever and typhus group Rickettsia species and not the ancestral R. bellii species group [28], suggests these likely bacteria are more closely related to the spotted fever and typhus group than the R. bellii species group [37]. However, the spotted fever and typhus group qPCR did not amplify Rickettsia spp. found in I. holocyclus ticks, suggesting that these species are more closely related to the ancestral R. bellii group, which are typically endosymbionts of arthropods [37]. Further studies should include species-specific PCR and Sanger sequencing of a more informative marker gene to resolve the phylogenetic identity of Rickettsia spp. endosymbionts in Australian ticks, and to determine the prevalence of pathogenic Rickettsia spp. in Australia.
The absence of Borrelia sp. in the ticks studied here is somewhat unexpected considering the recent description of a single relapsing fever Borrelia sp. isolate found in a recent survey of I. holocyclus ticks using the same NGS method as in the present study. In that case the Borrelia-infected I. holocyclus tick was removed from an echidna, which is not a typical host for I. holocyclus. Surveying the microbial communities of ticks that share a close association with echidnas, such as Bothriocroton concolor and B. hydrosauri, may reveal more Australian Borrelia sp. isolates.
Based on the phylogenetic inference of 1,265 bp 16S sequences, the novel “Ca. Neoehrlichia”, Ehrlichia, and Anaplasma detected in the present study appear to be putative species, as the levels of divergence between their sequences and those of their closest relatives, is within the range of accepted species separation at the 16S rRNA gene loci [38–41]. However, formal descriptions of them as new species will require analysis at multiple loci such as the citrate synthase gene (gltA), RNA polymerase sub-unit β (rpoB) and heat shock operon (groESL), or whole genomes [27, 42–46].
The overall prevalence of novel “Ca. Neoehrlichia” species A and B across all I. holocyclus life stages was 88.9% by NGS but only 12.9% by nested PCR. There are several reasons that may explain this discrepancy; firstly the nested PCR amplified a large fragment (the primary amplicon was approximately 1.4 kb and the secondary amplicon was approximately 1.3 kb), which is known to reduce the efficiency of PCR [47]. For NGS, the amplicon size was much smaller (250–320 bp) and would therefore be expected to amplify with much greater efficiency [47, 48]. Secondly, NGS allows the detection of low abundant sequences, and mixed sequences that would not be detected with Sanger sequencing [48]. Further studies should include use a “Ca. Neoehrlichia”-specific droplet digital PCR quantitation assay targeting small amplicon sizes, as this will allow for more accurate quantitation [49, 50] and determination of the true prevalence of novel “Ca. Neoehrlichia” species in I. holocyclus.
All recognised members of the genera Anaplasma, Ehrlichia, and “Ca. Neoehrlichia” are obligate intracellular tick-borne mammalian pathogens that typically infect haematopoietic (mammalian) or endothelial (mammalian and tick) cells [25, 51–53]. There has been no confirmed transovarial transmission of Anaplasma, Ehrlichia, or “Ca. Neoehrlichia” species in vector-ticks or mammals, and therefore their persistence is attributed predominantly to infected mammalian reservoir populations [51–53]. Throughout Europe, Asia, and North America several Anaplasmataceae species are pathogens of veterinary significance (such as E. canis and E. ruminantium) and important emerging human pathogens, such as E. chaffeensis, E. ewingii, A. phagocytophilum, and “Ca. N. mikurensis”
“Ca. Neoehrlichia” is a recently described genus that currently comprises two species, “Ca. N. lotoris”, and “Ca. N. mikurensis” [27, 45]. Of these “Ca. N. mikurensis” is now recognised an emerging tick-borne zoonosis vectored by several tick species (I. ricinus, I. ovatus, and I. persulcatus), and is one of the most prevalent tick-borne infections in wildlife and ticks throughout Europe and Asia [27, 35, 54–68]. Clinical reports of human infections are steadily increasing, due in part to increased awareness and testing [53]. Infection with “Ca. N. mikurensis” (neoehrlichiosis) is typically severe, with a wide variety of non-specific symptoms reported [69–75]. In Europe, neoehrlichiosis usually manifests in immunocompromised patients, however in China, there are increasing reports of this infection in immunocompetent people, and asymptomatic infections in humans have also been reported [76, 77]. In contrast, “Ca. N. lotoris” is a tick-borne pathogen of racoons (Procyon lotor), and to date there are no reports of human infection [45, 78]. In the northern hemisphere treatment of patients suffering neoehrlichiosis with doxycycline (1 x 200 mg/day) for 3–6 weeks has been shown to be effective [71, 75, 79, 80], and may have implication if human or animals infections are found to occur in Australia.
The identification of four novel putative tick-borne Anaplasmataceae species in Australian human-biting ticks is of potential public health significance, especially the high prevalence of novel “Ca. Neoehrlichia” spp. in I. holocyclus ticks. Based on their phylogenetic position, as inferred here, and the disease-causing status of their close relatives, all four species are candidate human and animal pathogens, and almost certainly infective (symptomatic or asymptomatic) to Australian wildlife species. Determining whether these Ehrlichia, Anaplasma and “Ca. Neoehrlichia” species may cause disease in Australian humans, like their close relatives do overseas is of public health importance. Future studies should include the development of specific digital and qPCR assays to more accurately determine the prevalence and pathogen load in ticks, wildlife, and humans. In addition, the isolation and culture of these organisms, in pure culture or infected mammalian and tick cell culture, will significantly aid in understanding the biology and potential pathogenicity of these novel Anaplasmataceae, and the development of specific diagnostic serological test and therapeutic practices.
Supporting Information
Shading indicated > 99% similarity between sequences in putative “Ca. Neoehrlichia” spp. A and B.
(PDF)
(PDF)
Acknowledgments
The authors wish to acknowledge the assistance of Dr. John Stenos and the Australian Rickettsial Reference Laboratory for supplying R. Australia DNA, Kevin Stratford and the Pawsey Supercomputing Centre for technical assistance during data analysis, Frances Brigg and the Western Australia State Agriculture Biotechnology Centre for Sanger sequencing, and Sam Abraham for assistance with the methods.
Data Availability
All NGS 16S sequences are available from NCBI Bioproject database (PRJNA298108). Sanger sequencing results for Anaplasmataceae 16S sequences are available from the GenBank accessions cited in text.
Funding Statement
This study was part-funded by the Australian Research Council (LP13010050), www.arc.gov.au, Bayer Healthcare, www.healthcare.bayer.com, and Bayer Australia Ltd., www.bayer.com.au. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Russell RC, Doggett SL, Munro R, Ellis J, Avery D, Hunt C, et al. Lyme disease: a search for a causative agent in ticks in south-eastern Australia. Epidemiol Infect. 1994;112(2):375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wills MC, Barry RD. Detecting the cause of Lyme disease in Australia. Med J Aust. 1991;155(4):275. [DOI] [PubMed] [Google Scholar]
- 3. Mayne P, Song S, Shao R, Burke J, Wang Y, Roberts T. Evidence for Ixodes holocyclus (Acarina: Ixodidae) as a Vector for Human Lyme Borreliosis Infection in Australia. Journal of insect science (Online). 2014;14(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mayne PJ. Clinical determinants of Lyme borreliosis, babesiosis, bartonellosis, anaplasmosis, and ehrlichiosis in an Australian cohort. Int J Gen Med. 2015;8:15–26. 10.2147/IJGM.S75825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gray J, Kahl O, Lane RS, Stanek G. Lyme Borreliosis: Biology, Epidemiology, and Control: CABI; 2002. [Google Scholar]
- 6. Halperin JJ. Chronic Lyme disease: misconceptions and challenges for patient management. Infection and Drug Resistance. 2015;8:119–28. 10.2147/IDR.S66739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gofton AW, Oskam CL, Lo N, Beninati T, Wei H, McCarl V, et al. Inhibition of the endosymbiont "Candidatus Midichloria mitochondrii" during 16S rRNA gene profiling reveals potential pathogens in Ixodes ticks from Australia. Parasit Vectors. 2015;8(1):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barker SC, Walker AR. Ticks of Australia. The species that infest domestic animals and humans. Zootaxa. 2014(3816):1–144. 10.11646/zootaxa.3816.1.1 [DOI] [PubMed] [Google Scholar]
- 9. Song S, Shao R, Atwell R, Barker S, Vankan D. Phylogenetic and phylogeographic relationships in Ixodes holocyclus and Ixodes cornuatus (Acari: Ixodidae) inferred from COX1 and ITS2 sequences. Int J Parasitol. 2011;41(8):871–80. 10.1016/j.ijpara.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 10. van Nunen S. Tick-induced allergies: mammalian meat allergy, tick anaphylaxis and their significance. Asia Pacific Allergy. 2015;5(1):3–16. 10.5415/apallergy.2015.5.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Campbell RW, Domrow R. Rickettsioses in Australia: isolation of Rickettsia tsutsugamushi and R. australis from naturally infected arthropods. Trans R Soc Trop Med Hyg. 1974;68(5):397–402. [DOI] [PubMed] [Google Scholar]
- 12. Unsworth NB, Stenos J, Graves SR, Faa AG, Cox GE, Dyer JR, et al. Flinders Island spotted fever rickettsioses caused by "marmionii" strain of Rickettsia honei, Eastern Australia. Emerg Infect Dis. 2007;13(4):566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li AY, Adams PJ, Abdad MY, Fenwick SG. High prevalence of Rickettsia gravesii sp. nov. in Amblyomma triguttatum collected from feral pigs. Veterinary Microbiology. 2010;146(1–2):59–62. 10.1016/j.vetmic.2010.04.018 [DOI] [PubMed] [Google Scholar]
- 14. McDiarmid L, Petney T, Dixon B, Andrews R. Range expansion of the tick Amblyomma triguttatum triguttatum, an australian vector of Q fever. Int J Parasitol. 2000;30(7):791–3. [DOI] [PubMed] [Google Scholar]
- 15. Donohoe H, Pennington-Gray L, Omodior O. Lyme disease: Current issues, implications, and recommendations for tourism management. Tourism Management. 2015;46:408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stewart A, Glass J, Patel A, Watt G, Cripps A, Clancy R. Lyme arthritis in the Hunter Valley. Med J Aust. 1982;1(3):139 [DOI] [PubMed] [Google Scholar]
- 17. Roberts FHS. Australian ticks Commonwealth Science and Industrial Research Organisation; 1970. [Google Scholar]
- 18. Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–1. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- 19. Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–9. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Meth. 2013;10(10):996–8. [DOI] [PubMed] [Google Scholar]
- 21. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Meth. 2010;7(5):335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Applied and Environmental Microbiology. 2006;72(7):5069–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barbour AG, Maupin GO, Teltow GJ, Carter CJ, Piesman J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. The Journal of infectious diseases. 1996;173(2):403–9. [DOI] [PubMed] [Google Scholar]
- 24. Clark KL, Leydet B, Hartman S. Lyme Borreliosis in Human Patients in Florida and Georgia, USA. International Journal of Medical Sciences. 2013;10(7):915–31. 10.7150/ijms.6273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anderson BE, Dawson JE, Jones DC, Wilson KH. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29(12):2838–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paddock CD, Sumner JW, Shore GM, Bartley DC, Elie RC, McQuade JG, et al. Isolation and characterization of Ehrlichia chaffeensis strains from patients with fatal ehrlichiosis. J Clin Microbiol. 1997;35(10):2496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kawahara M, Rikihisa Y, Isogai E, Takahashi M, Misumi H, Suto C, et al. Ultrastructure and phylogenetic analysis of 'Candidatus Neoehrlichia mikurensis' in the family Anaplasmataceae, isolated from wild rats and found in Ixodes ovatus ticks. Int J Syst Evol Microbiol. 2004;54(5):1837–43. [DOI] [PubMed] [Google Scholar]
- 28. Stenos J, Graves SR, Unsworth NB. A highly sensitive and specific real-time PCR assay for the detection of spotted fever and typhus group Rickettsiae. Am J Trop Med Hyg. 2005;73(6):1083–5. [PubMed] [Google Scholar]
- 29. Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30(14):3059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular biology and evolution. 2013;30(12):2725–9. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–4. [DOI] [PubMed] [Google Scholar]
- 33. Barker SC, Walker AR, Campelo D. A list of the 70 species of Australian ticks; diagnostic guides to and species accounts of Ixodes holocyclus (paralysis tick), Ixodes cornuatus (southern paralysis tick) and Rhipicephalus australis (Australian cattle tick); and consideration of the place of Australia in the evolution of ticks with comments on four controversial ideas. Int J Parasitol. 2014;44(12):941–53. 10.1016/j.ijpara.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 34. Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol. 2003;48:307–37. [DOI] [PubMed] [Google Scholar]
- 35. Kurilshikov A, Livanova NN, Fomenko NV, Tupikin AE, Rar VA, Kabilov MR, et al. Comparative Metagenomic Profiling of Symbiotic Bacterial Communities Associated with Ixodes persulcatus, Ixodes pavlovskyi and Dermacentor reticulatus Ticks. PLoS One. 2015;10(7):e0131413 10.1371/journal.pone.0131413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wojcik-Fatla A, Zajac V, Sawczyn A, Cisak E, Sroka J, Dutkiewicz J. Occurrence of Francisella spp. in Dermacentor reticulatus and Ixodes ricinus ticks collected in eastern Poland. Ticks Tick Borne Dis. 2015;6(3):253–7. 10.1016/j.ttbdis.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 37. Stothard DR, Clark JB, Fuerst PA. Ancestral divergence of Rickettsia bellii from the spotted fever and typhus groups of Rickettsia and antiquity of the genus Rickettsia. Int J Syst Bacteriol. 1994;44(4):798–804. [DOI] [PubMed] [Google Scholar]
- 38. Clarridge JE. Impact of 16S rRNA Gene Sequence Analysis for Identification of Bacteria on Clinical Microbiology and Infectious Diseases. Clin Microbiol Rev. 2004;17(4):840–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mignard S, Flandrois JP. 16S rRNA sequencing in routine bacterial identification: a 30-month experiment. J Microbiol Methods. 2006;67(3):574–81. [DOI] [PubMed] [Google Scholar]
- 40. Janda JM, Abbott SL. 16S rRNA Gene Sequencing for Bacterial Identification in the Diagnostic Laboratory: Pluses, Perils, and Pitfalls. J Clin Microbiol. 2007;45(9):2761–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ferla MP, Thrash JC, Giovannoni SJ, Patrick WM. New rRNA Gene-Based Phylogenies of the Alphaproteobacteria Provide Perspective on Major Groups, Mitochondrial Ancestry and Phylogenetic Instability. PLoS ONE. 2013;8(12):e83383 10.1371/journal.pone.0083383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Inokuma H, Brouqui P, Drancourt M, Raoult D. Citrate Synthase Gene Sequence: a New Tool for Phylogenetic Analysis and Identification of Ehrlichia. J Clin Microbiol. 2001;39(9):3031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rar VA, Epikhina TI, Livanova NN, Panov VV, Doroshenko EK, Pukhovskaia NM, et al. Study of the heterogeneity of 16s rRNA gene and groESL operone in the dna samples of Anaplasma phagocytophilum, Ehrlichia muris, and "Candidatus Neoehrlichia mikurensis" determined in the Ixodes persulcatus ticks in the area of Urals, Siberia, and far east of Russia. Molekuliarnaia genetika, mikrobiologiia i virusologiia. 2011(2):17–23. [PubMed] [Google Scholar]
- 44. Sumner JW, Nicholson WL, Massung RF. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J Clin Microbiol. 1997;35(8):2087–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yabsley MJ, Murphy SM, Luttrell MP, Wilcox BR, Howerth EW, Munderloh UG. Characterization of 'Candidatus Neoehrlichia lotoris' (family Anaplasmataceae) from raccoons (Procyon lotor). Int J Syst Evol Microbiol. 2008;58(12):2794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grankvist A, Moore ERB, Stadler LS, Pekova S, Bogdan C, Geißdörfer W, et al. Multilocus sequence analysis of clinical "candidatus neoehrlichia mikurensis" strains from Europe. J Clin Microbiol. 2015;53(10):3126–32. 10.1128/JCM.00880-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mc Pherson MJ MS. PCR: The Basics from Background to Bench. UK: Taylor and Francis e-Library; 2005. [Google Scholar]
- 48. Su Z, Ning B, Fang H, Hong H, Perkins R, Tong W, et al. Next-generation sequencing and its applications in molecular diagnostics. Expert review of molecular diagnostics. 2011;11(3):333–43. 10.1586/erm.11.3 [DOI] [PubMed] [Google Scholar]
- 49. Hindson CM, Chevillet JR, Briggs HA, Gallichotte EN, Ruf IK, Hindson BJ, et al. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat Meth. 2013;10(10):1003–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pinheiro LB, Coleman VA, Hindson CM, Herrmann J, Hindson BJ, Bhat S, et al. Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Analytical chemistry. 2012;84(2):1003–11. 10.1021/ac202578x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stuen S, Granquist EG, Silaghi C. Anaplasma phagocytophilum—a widespread multi-host pathogen with highly adaptive strategies. Frontiers in Cellular and Infection Microbiology. 2013;3:31 10.3389/fcimb.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dumler JS, Bakken JS. Ehrlichial diseases of humans: emerging tick-borne infections. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 1995;20(5):1102–10. [DOI] [PubMed] [Google Scholar]
- 53. Silaghi C, Beck R, Oteo JA, Pfeffer M, Sprong H. Neoehrlichiosis: an emerging tick-borne zoonosis caused by Candidatus Neoehrlichia mikurensis. Exp Appl Acarol. 2015. [DOI] [PubMed] [Google Scholar]
- 54. Szekeres S, Claudia Coipan E, Rigó K, Majoros G, Jahfari S, Sprong H, et al. Candidatus Neoehrlichia mikurensis and Anaplasma phagocytophilum in natural rodent and tick communities in Southern Hungary. Ticks and Tick-borne Diseases. 2015;6(2):111–6. 10.1016/j.ttbdis.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 55. Földvári G, Jahfari S, Rigó K, Jablonszky M, Szekeres S, Majoros G, et al. Candidatus Neoehrlichia mikurensis and Anaplasma phagocytophilum in Urban Hedgehogs. Emerging Infectious Diseases. 2014;20(3):496–8. 10.3201/eid2003.130935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Obiegala A, Pfeffer M, Pfister K, Tiedemann T, Thiel C, Balling A, et al. Candidatus Neoehrlichia mikurensis and Anaplasma phagocytophilum: prevalences and investigations on a new transmission path in small mammals and ixodid ticks. Parasites & Vectors. 2014;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Derdáková M, Václav R, Pangrácova-Blaňárová L, Selyemová D, Koči J, Walder G, et al. Candidatus Neoehrlichia mikurensis and its co-circulation with Anaplasma phagocytophilum in Ixodes ricinus ticks across ecologically different habitats of Central Europe. Parasites and Vectors. 2014;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vayssier-Taussat M, le Rhun D, Buffet JP, Maaoui N, Galan M, Guivier E, et al. Candidatus Neoehrlichia mikurensis in bank voles, France. Emerging Infectious Diseases. 2012;18(12):2063–5. 10.3201/eid1812.120846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Silaghi C, Woll D, Mahling M, Pfister K, Pfeffer M. Candidatus Neoehrlichia mikurensis in rodents in an area with sympatric existence of the hard ticks Ixodes ricinus and Dermacentor reticulatus, Germany. Parasites and Vectors. 2012;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Richter D, Matuschka FR. "Candidatus Neoehrlichia mikurensis," Anaplasma phagocytophilum, and Lyme Disease spirochetes in questing European vector ticks and in feeding ticks removed from people. J Clin Microbiol. 2012;50(3):943–7. 10.1128/JCM.05802-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Richter D, Matuschka F-R. “Candidatus Neoehrlichia mikurensis”, Anaplasma phagocytophilum and Lyme disease spirochetes in questing European vector ticks and in feeding ticks removed from people. J Clin Microbiol. 2011:JCM.05802-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Andersson M, Zaghdoudi-Allan N, Tamba P, Stefanache M, Chitimia L. Co-infection with 'Candidatus Neoehrlichia mikurensis' and Borrelia afzelii in an Ixodes ricinus tick that has bitten a human in Romania. Ticks and Tick-borne Diseases. 2014;5(6):706–8. 10.1016/j.ttbdis.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 63. Andersson M, Bartkova S, Lindestad O, Råberg L. Co-infection with 'Candidatus Neoehrlichia mikurensis' and Borrelia afzelii in ixodes ricinus ticks in southern Sweden. Vector-Borne and Zoonotic Diseases. 2013;13(7):438–42. 10.1089/vbz.2012.1118 [DOI] [PubMed] [Google Scholar]
- 64. Glatz M, Müllegger RR, Maurer F, Fingerle V, Achermann Y, Wilske B, et al. Detection of candidatus neoehrlichia mikurensis, borrelia burgdorferi sensu lato genospecies and anaplasma phagocytophilum in a tick population from Austria. Ticks and Tick-borne Diseases. 2014;5(2):139–44. 10.1016/j.ttbdis.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 65. Palomar AM, García-Álvarez L, Santibáñez S, Portillo A, Oteo JA. Detection of tick-borne 'Candidatus Neoehrlichia mikurensis' and Anaplasma phagocytophilum in Spain in 2013. Parasites and Vectors. 2014;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Naitou H, Kawaguchi D, Nishimura Y, Inayoshi M, Kawamori F, Masuzawa T, et al. Molecular identification of Ehrlichia species and 'Candidatus Neoehrlichia mikurensis' from ticks and wild rodents in Shizuoka and Nagano Prefectures, Japan. Microbiology and Immunology. 2006;50(1):45–51. [DOI] [PubMed] [Google Scholar]
- 67. Hodžić A, Alić A, Fuehrer HP, Harl J, Wille-Piazzai W, Duscher GG. A molecular survey of vector-borne pathogens in red foxes (Vulpes vulpes) from Bosnia and Herzegovina. Parasites and Vectors. 2015:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li H, Jiang J, Tang F, Sun Y, Li Z, Zhang W, et al. Wide distribution and genetic diversity of "candidatus neoehrlichia mikurensis" in rodents from china. Applied and Environmental Microbiology. 2013;79(3):1024–7. 10.1128/AEM.02917-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rar V, Golovljova I. Anaplasma, Ehrlichia, and "Candidatus Neoehrlichia" bacteria: pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2011;11(8):1842–61. 10.1016/j.meegid.2011.09.019 [DOI] [PubMed] [Google Scholar]
- 70. Pekova S, Vydra J, Kabickova H, Frankova S, Haugvicova R, Mazal O, et al. Candidatus Neoehrlichia mikurensis infection identified in 2 hematooncologic patients: benefit of molecular techniques for rare pathogen detection. Diagn Microbiol Infect Dis. 2011;69(3):266–70. 10.1016/j.diagmicrobio.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 71. Von Loewenich FD, Geißdörfer W, Disqué C, Matten J, Schett G, Sakka SG, et al. Detection of "Candidatus Neoehrlichia mikurensis" in two patients with severe febrile illnesses: Evidence for a European sequence variant. J Clin Microbiol. 2010;48(7):2630–5. 10.1128/JCM.00588-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Welinder-Olsson C, Kjellin E, Vaht K, Jacobsson S, Wennerås C. First case of human "Candidatus neoehrlichia mikurensis" infection in a febrile patient with chronic lymphocytic leukemia. J Clin Microbiol. 2010;48(5):1956–9. 10.1128/JCM.02423-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Grankvist A, Andersson PO, Mattsson M, Sender M, Vaht K, Höper L, et al. Infections with the tick-borne bacterium "candidatus neoehrlichia mikurensis" mimic noninfectious conditions in patients with B cell malignancies or autoimmune diseases. Clin Infect Dis. 2014;58(12):1716–22. 10.1093/cid/ciu189 [DOI] [PubMed] [Google Scholar]
- 74. Marsal J. Recurrent fever caused by Candidatus Neoehrlichia mikurensis in a rheumatoid arthritis patient treated with rituximab. Rheumatology (Oxford, England). 2015;54(2):369–71. [DOI] [PubMed] [Google Scholar]
- 75. Fehr JS, Bloemberg GV, Ritter C, Hombach M, Lüscher TF, Weber R, et al. Septicemia caused by tick-borne bacterial pathogen candidatus Neoehrlichia mikurensis. Emerging Infectious Diseases. 2010;16(7):1127–9. 10.3201/eid1607.091907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Welc-Falȩciak R, Siński E, Kowalec M, Zajkowska J, Pancewicz SA. Asymptomatic "Candidatus neoehrlichia mikurensis" infections in immunocompetent humans. J Clin Microbiol. 2014;52(8):3072–4. 10.1128/JCM.00741-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Brouqui P, Sanogo YO, Caruso G, Merola F, Raoult D. Candidatus Ehrlichia walkerii: a new Ehrlichia detected in Ixodes ricinus tick collected from asymptomatic humans in Northern Italy. Ann N Y Acad Sci. 2003;990:134–40. [DOI] [PubMed] [Google Scholar]
- 78. Yabsley MJ, Murphy SM, Luttrell MP, Wilcox BR, Ruckdeschel C. Raccoons (Procyon lotor), but not rodents, are natural and experimental hosts for an ehrlichial organism related to “Candidatus Neoehrlichia mikurensis”. Veterinary Microbiology. 2008;131(3–4):301–8. 10.1016/j.vetmic.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 79. Grankvist A, Sandelin LL, Andersson J, Fryland L, Wilhelmsson P, Lindgren PE, et al. Infections with Candidatus Neoehrlichia mikurensis and Cytokine Responses in 2 Persons Bitten by Ticks, Sweden. Emerg Infect Dis. 2015;21(8):1462–5. 10.3201/eid2108.150060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Welinder-Olsson C, Kjellin E, Vaht K, Jacobsson S, Wennerås C. First Case of Human “Candidatus Neoehrlichia mikurensis” Infection in a Febrile Patient with Chronic Lymphocytic Leukemia. J Clin Microbiol. 2010;48(5):1956–9. 10.1128/JCM.02423-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Shading indicated > 99% similarity between sequences in putative “Ca. Neoehrlichia” spp. A and B.
(PDF)
(PDF)
Data Availability Statement
All NGS 16S sequences are available from NCBI Bioproject database (PRJNA298108). Sanger sequencing results for Anaplasmataceae 16S sequences are available from the GenBank accessions cited in text.