Abstract
Decellularization and recellularization of parenchymal organs may enable the generation of functional organs in vitro, and several protocols for rodent liver decellularization have already been published. We aimed to improve the decellularization process by construction of a proprietary perfusion device enabling selective perfusion via the portal vein and/or the hepatic artery. Furthermore, we sought to perform perfusion under oscillating surrounding pressure conditions to improve the homogeneity of decellularization. The homogeneity of perfusion decellularization has been an underestimated factor to date. During decellularization, areas within the organ that are poorly perfused may still contain cells, whereas the extracellular matrix (ECM) in well-perfused areas may already be affected by alkaline detergents. Oscillating pressure changes can mimic the intraabdominal pressure changes that occur during respiration to optimize microperfusion inside the liver. In the study presented here, decellularized rat liver matrices were analyzed by histological staining, DNA content analysis and corrosion casting. Perfusion via the hepatic artery showed more homogenous results than portal venous perfusion did. The application of oscillating pressure conditions improved the effectiveness of perfusion decellularization. Livers perfused via the hepatic artery and under oscillating pressure conditions showed the best results. The presented techniques for liver harvesting, cannulation and perfusion using our proprietary device enable sophisticated perfusion set-ups to improve decellularization and recellularization experiments in rat livers.
Keywords: Bioengineering, Issue 102, Rat Liver Decellularization, Rat Liver Perfusion Device, Oscillating Pressure Conditions, Rat Liver Engineering, Arterial Rat Liver Perfusion, Portal Venous Rat Liver Perfusion
Introduction
Decellularization and recellularization may enable the generation of functional, transplantable organs in vitro 1. By removing cells and antigenic material (e.g., DNA, alpha-Gal epitopes) from an organ, the non- or less-immunogenic extracellular matrix (ECM) can be obtained. This matrix conserves the three-dimensional microanatomy of an organ and can serve as the ideal biomatrix for repopulation with cells of a different, possibly xenogeneic origin 2. Thus, a decellularized rat liver matrix could be repopulated with human liver cells. This humanized micro-liver could serve as an ex vivo model for research on diseases (e.g., inborn metabolic diseases, viral diseases or malignancies) or for preclinical pharmaceutical testing 3.
Several different protocols for rat liver perfusion decellularization have already been published 4-13. In all protocols, decellularization was achieved by perfusion of alkaline ionic or non-ionic detergents via the cannulated portal vein. To the best of our knowledge, we were the first group to report rat liver decellularization by selective perfusion via the portal vein and/or the rat hepatic artery 14. Enabling the selective perfusion of the different vascular systems in the liver may enable better decellularization results and, furthermore, may play an important role in cellular repopulation.
In the study detailed here, livers were perfused in a custom-made proprietary perfusion device, enabling perfusion under oscillating pressure conditions. These pressure conditions mimic the physiologic respiratory-dependent perfusion of the liver: in situ, the liver hangs under the copula of the diaphragm, whose movement during respiration has a direct impact on liver perfusion. Inspiration specifically leads to lowering of the diaphragm and squeezing of the liver, optimizing hepato-venous outflow, whereas expiration leads to elevation of the liver and lowering of the intraabdominal pressure to optimize portal-venous inflow 15.
Our aim was to evaluate whether oscillating pressure conditions have an impact on the homogeneity of rat liver perfusion decellularization by mimicking intraabdominal conditions ex vivo. The homogeneity of the decellularization process may be an underestimated factor in perfusion decellularization. All known agents used for liver decellularization cause alterations to the ECM. Cells in poorly perfused areas remain within the ECM, whereas other areas are already completely decellularized. To dissolve the remaining cells, the perfusion duration or pressure must be elevated, causing more alterations to the well-perfused areas. Thus, detergents for decellularization should be distributed homogenously within the organ.
Protocol
Animals were kept at the Facility for Experimental Medicine (FEM, Charité, Berlin, Germany), and all experimental protocols were reviewed and approved by the State Office of Health and Local Affairs (LAGeSo, Berlin, Germany; Reg. No. O 0365/11).
1. Liver Harvesting
- Pre-surgical Preparation
- Use a cork plate for fixation. Put a medical drape on the plate. Using four needles, fix an inhalation mask on the cork plate for intraoperative inhalation narcosis.
- Prearrangement of the Portal Venous Cannula
- Connect a peripheral venous catheter (G 20) to a three-way stopcock. The venous catheter should be cut obliquely, with a length of approx. 1.5 cm.
- De-air the system using a 10 ml syringe filled with Ringer solution. It is very important to carefully de-air because gas embolism may negatively affect the perfusion decellularization.
- Prearrangement of the Arterial Cannula
- Use a butterfly cannula and cut the needle to a length of 5 mm. Fit a 5 cm tube (ID 0.58 mm; OD 0.96 mm) to the 5 mm needle and fix it with superglue. Insert a 2 cm-long tube (ID 0.28 mm; OD 0.61 mm) into the bigger tube and fix it with superglue.
- Slip another tube over this construction as an abutment and fix it with superglue. De-air the arterial cannula with a 5 ml syringe filled with Ringer solution.
- Anesthesia and Analgesia
- Induce narcosis of the rat in the anesthesia induction chamber by 3.5% isoflurane in oxygen at a flow rate of 1.5 L/min.
- To ensure safe analgesia during the operation, inject metamizol (100 mg/kg body mass) and low-dose ketamine/medetomidine (10 mg/0.1 mg per kg body mass) intraperitoneally.
- Supply 1.5% isoflurane in 1 L/min oxygen via the inhalation mask for subsequent maintenance of surgical anesthesia.
- Preoperative Arrangements
- Shave the abdomen liberally with an electric shearing machine. Moisten the shaved incision site on the abdomen with 70% ethanol for disinfection.
- Place the animal in the supine position on the prepared plate, insert the head into the inhalation mask and fix the limbs with medical tape and pins. Remove excess ethanol and fur with a gauze compress.
- Anesthesia Assessment
- Assess the anesthesia depth by watching the respiratory rate. When the anesthesia seems deep enough (40-60 breaths/min), check whether analgesia is sufficient by trying to trigger the toe pinch reflex using surgical forceps to pinch the skin between the toes of both hind limbs.
- Abdominal Approach
- Make a median incision into the caudal abdominal wall above the bladder. Cut from this point to both lateral costal arches in a V-shaped manner. Take care not to cut into the diaphragm. Pull the cut abdominal wall over the chest.
- Fix the xiphoid with an overholt clamp, pull it cranially and fix it with a rubber band. Use wet cotton swabs to evacuate the intestine. Wrap the intestine in a wet gauze bandage. Furthermore, cover all abdominal margins with wet bandages.
- Liver Preparation
- Dissect the falciform ligament. Use a wet gauze bandage to hold the medial and left liver lobes cranially under the dome of the diaphragm. Expose the hepatoduodenal ligament and the upper omental liver lobe.
- Use two microforceps and spring scissors to carefully dissect the ligament attached to the upper omental lobe until the lobe is mobile. Move the lobe using wet cotton swabs under the gauze compress,holding the medial and left lobes in place.
- After mobilization of the upper omental liver lobe, move the stomach to the left. Fix the stomach with a clamp and dissect the minor omentum with microforceps and the spring scissors.
- Mobilize the lower omental liver lobe until it is mobile and subsequently move the lobe back into its cavity. Use a Backhaus clamp to retain the duodenum. Move the duodenum to the left to stretch and expose the hepatoduodenal ligament. Circumscribe the common bile duct in the stretched hepatoduodenal ligament.
- Dissect the bile duct from the adipose and pancreatic tissue. Cut the bile duct 1.5 cm from the bile duct bifurcation. Dissect the portal vein from the surrounding adipose tissue. Expose the gastroduodenal vein.
- Ligate the gastroduodenal vein twice with silk 6-0 and divide the vein between the 2 ligatures. Dissect the celiac trunk and the common hepatic artery from the surrounding tissue until all arterial rami are revealed.
- Liberate the gastroduodenal artery from the surrounding tissue. Tie the gastroduodenal artery twice with silk 6-0, with the ties distant from each other. Dissect the gastroduodenal artery between the 2 ligatures.
- Liberate the splenic artery from the surrounding tissue. Tie the splenic artery twice with silk 6-0, with the ties distant from each other. Dissect the splenic artery between the 2 ligatures.
- Liberate the left gastric artery from the surrounding tissue. Tie the left gastric artery twice with silk 6-0, with the ties distant from each other. Dissect the artery between the 2 ligatures.
- Circumscribe the inferior caval vein between the right kidney and the right lateral liver lobe. Dissect the vein from the retroperitoneal space. Expose the aorta by following the celiac trunk.
- Dissect the retroperitoneal adipose tissue. Inject 1,000 I.E. heparin in 1 ml saline into the inferior vena cava. Retract the cannula and close the incision with a cotton pad. Wait 1-2 min for the systemic effect of heparin.
- Circumscribe the aorta with forceps. Dissect the aorta and exsanguinate the rat. Furthermore, dissect the inferior vena cava distally from the kidney.
- Portal Venous Cannulation (Use the cannula prepared in section 1.2)
- Make a fish-mouth incision in the distal portal vein. Open the incision with forceps and cannulate the portal vein. Flush the liver with 20 ml Ringer solution until the liver decolorizes and fix the cannula with ligatures (silk 6-0).
- Arterial Cannulation (Use the cannula prepared in section 1.3)
- Dissect the aorta above the celiac trunk. Liberate the aorta segment from the retroperitoneal space. Cut the aorta segment longitudinally to generate an aortic patch. Insert the self-built cannula into a static holder.
- Use 2 forceps to slip the aortic patch over the cannula. Ligate the hepatic artery with silk 6-0 over the cannula and fix the patch at the abutment.
- Liver Explantation
- Open the diaphragm, make a circular incision and dissect the liver from the surrounding structures.
- Storage
- Store the liver in a sterile 500 ml tank filled with 350 ml Ringer solution until use.
2. Detergent Solutions
- 10% Triton X-100 stock solution
- Fill a 1,000 ml bottle with 900 ml aqua dest. and add 100 ml Triton X-100 (99,9%). Use a stirrer to dissolve the Triton X-100. Fill the bottle with aqua dest. until a total volume of 1,000 ml is reached.
- 1% Triton X-100
- Add 100 ml of the stock solution (10%) to a 1,000 ml bottle. Add 900 ml aqua dest. and shake the bottle twice. Filter the 1% Triton X-100 solution through a sterile filter.
- 10% SDS Stock Solution
- Fill a 1,000 ml bottle with 900 ml aqua dest. and add 66.99 g SDS (99.9%, density 0.67). Use a stirrer to dissolve the SDS. Fill the bottle with aqua dest. until a total volume of 1,000 ml is reached.
- 1% SDS
- Add 100 ml of the stock solution (10%) to a 1,000 ml bottle. Add 900 ml aqua dest. and shake the bottle twice. Filter the 1% SDS solution through a sterile filter.
3. Decellularization
Set-up of Perfusion Device
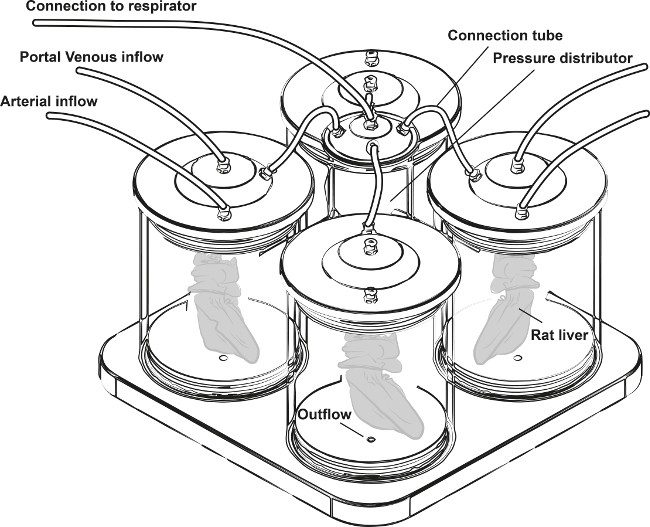
Figure 1:Scheme of the Perfusion Device for Selective Arterial and Portal Venous Perfusion of Four Rat Livers under Oscillating Pressure Conditions. (modified from Struecker et al. 14)
Link the drain to a three-way stopcock and a Heidelberger extension to regulate the fill level and fill the reactor with 500 ml 0,9% NaCl.
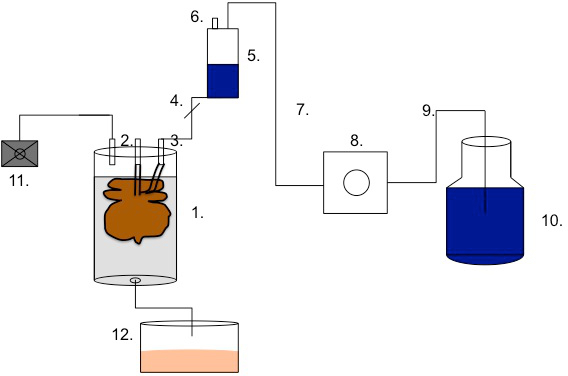
Figure 2: Scheme of the perfusion set-up. Link the perfusion chamber (1,400 cm3)(1) to the distal end (4) of the bubble trap (5) via the portal venous access (2) or via the arterial access (3). The bubble trap (5) should be equipped with an obturation (4) at the distal end and a vent (6). Connect the bubble trap (5) to a Heidelberger extension (7). Link the Heidelberger extension to the pump segment (8). Furthermore, connect the pump segment (8) to another Heidelberger extension (9). Insert the last Heidelberger extension (9) into the bottle of detergent solution (10). Connect the respirator (11) to the perfusion chamber (or to the pressure distributor in the case of multiple perfusions). To drain the reactor fluids, connect the outflow to the waste bottle (12).
Insert the pump segment into the pump. Start the pump to fill the cycle with PBS, close the distal end of the bubble trap and open the vent. When the bottom of the bubble is securely covered with PBS (2-3 cm), close the vent and open the distal end of the bubble trap.
- Liver Installation
- Link the three-way stopcock of the portal vein cannula to the related reactor access. Connect the arterial cannula to the arterial access. Be careful to ensure that no air enters the systems.
- Application of Oscillating Pressure Conditions
- Use a respirator with a frequency of 15 bpm and an amplitude of 26 mbar (min. 4 mbar, max. 30 mbar). Connect the respirator to the pressure distribution chamber and connect the chamber to the reactor. Start pressure application.
- Decellularization Protocol
- Perform perfusion steps with a flow rate of 5 ml/min. Start the decellularization with perfusion of the liver with 1% Triton X-100 for 90 min. Allow waste effluence via the outflow of the perfusion device to keep the fluid level inside the perfusion chamber constant and above the perfused liver. After 90 min, change the detergent to 1% SDS.
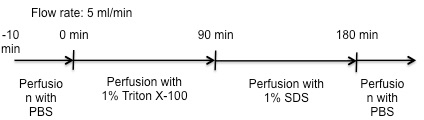

Figure 3: Perfusion Protocol for All Experimental Groups.
Representative Results
The homogeneity and thus the effectiveness of different decellularization protocols were evaluated by macroscopic observation, histological analysis, and analysis of the remaining DNA content within decellularized liver matrices. Furthermore, corrosion casting was performed to visualize the intact microanatomy of livers after decellularization.
Macroscopy
During decellularization, livers become lucent, indicating the removal of cellular content. Livers perfused via the portal vein showed inhomogeneous elucidation (and thus decellularization), with macroscopically visible remaining cells during and after decellularization (white arrows). Livers perfused via the hepatic artery showed a more homogenous decellularization course and no visible remaining cells. If livers were perfused with the application of oscillating pressure conditions, no remaining cells were visible, irrespective of the perfusion route.
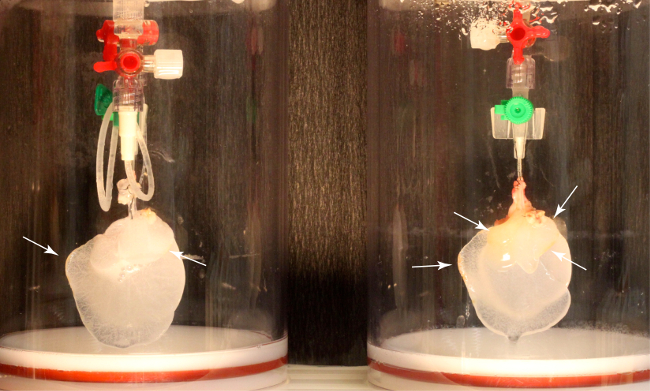 Figure 4: Macroscopic Results of Rat Liver Decellularization without Oscillating Pressure Conditions. Left: Rat liver decellularized via the hepatic artery. The liver appears lucent, without macroscopically visible remaining cells. Right: Liver perfused via the portal vein. The liver appears inhomogeneously decellularized, with macroscopically visible cells
Figure 4: Macroscopic Results of Rat Liver Decellularization without Oscillating Pressure Conditions. Left: Rat liver decellularized via the hepatic artery. The liver appears lucent, without macroscopically visible remaining cells. Right: Liver perfused via the portal vein. The liver appears inhomogeneously decellularized, with macroscopically visible cells
 Figure 5: Macroscopic results of rat liver decellularization under oscillating pressure conditions. Left: Rat liver decellularized via the hepatic artery. Right: Liver perfused via the portal vein. Both livers appear lucent, without visible remaining cells.
Figure 5: Macroscopic results of rat liver decellularization under oscillating pressure conditions. Left: Rat liver decellularized via the hepatic artery. Right: Liver perfused via the portal vein. Both livers appear lucent, without visible remaining cells.
Histological Evaluation
Histological evaluation of decellularized liver matrices supported the macroscopic findings. Livers perfused via the hepatic artery showed no remaining cells, irrespective of whether they were perfused under oscillating pressure conditions. Livers perfused via the portal vein showed zones of remaining cells, even if they were perfused with the application of oscillating pressure conditions. However, the application of oscillating pressure conditions reduced the remaining cell mass in livers perfused via the portal vein. The ECM of the livers was conserved, without visible differences across all applied protocols.
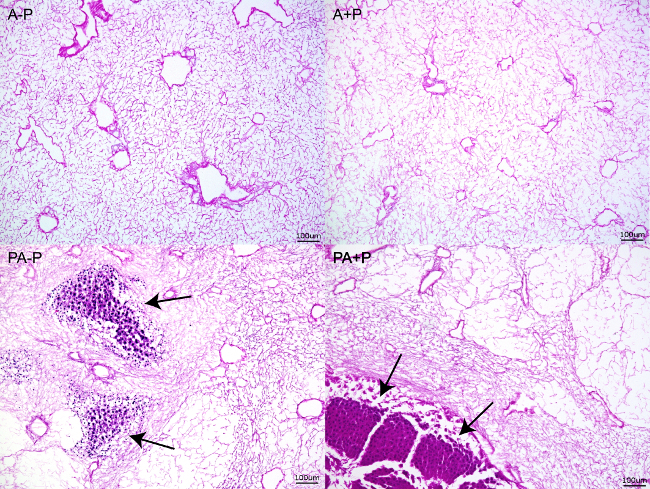
Figure 6: H/E Staining of Decellularized Liver Matrices. A-P: Decellularized liver matrix perfused via the hepatic artery without oscillating pressure conditions. A+P: Decellularized liver matrix perfused via the hepatic artery under oscillating pressure conditions. PA-P: Decellularized liver matrix perfused via the portal vein without oscillating pressure conditions. PA+P: Decellularized liver matrix perfused via the portal vein under oscillating pressure conditions. Arrows: Remaining cell clusters.
DNA content
The DNA content per dry weight of liver matrix declined in all experimental groups compared with that in native livers, although differences were only statistically significant in the groups perfused under oscillating pressure conditions (PV+P; A+P). The lowest amount of DNA per dry weight was found in livers perfused via the hepatic artery under oscillating pressure conditions.
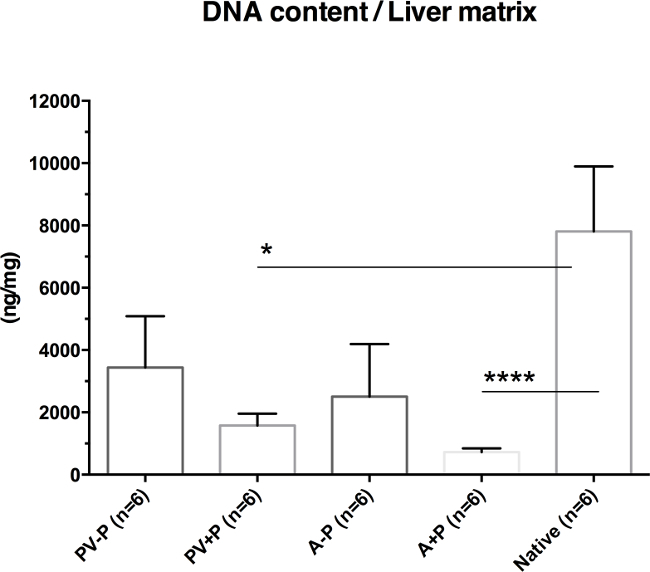
Figure 7: DNA Content Per Dry Weight of Liver Matrix (modified from Struecker et al. 14). Livers decellularized via the hepatic artery show less remaining DNA than those perfused via the portal vein do. Livers perfused under oscillating pressure conditions show less remaining DNA than those decellularized without these conditions do. Thus, livers perfused via the hepatic artery under oscillating pressure conditions show the least remaining DNA content.
Corrosion Casting
Corrosion casting of decellularized liver matrices confirmed that the microanatomy of livers (including the protein framework of the portal venous system, the arterial system and the biliary system) is conserved during decellularization.
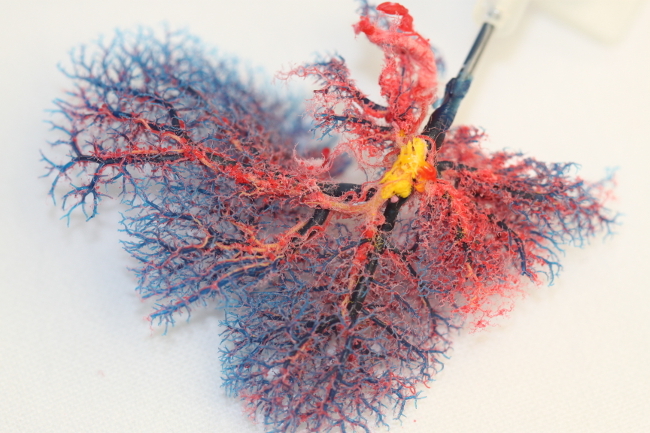 Figure 8: Photograph of a Corrosion Casting of a Decellularized Rat Liver Matrix (modified from Struecker et al. 14). Despite removal of all cells, the microanatomy of the organ, including the portal venous (blue) and arterial (red) systems, are conserved. The remaining main branches of the biliary system are casted in yellow.
Figure 8: Photograph of a Corrosion Casting of a Decellularized Rat Liver Matrix (modified from Struecker et al. 14). Despite removal of all cells, the microanatomy of the organ, including the portal venous (blue) and arterial (red) systems, are conserved. The remaining main branches of the biliary system are casted in yellow.
 Table 1: Experimental Groups for Assessing the Effect of Oscillating Pressure Conditions and the Perfusion Route on Rat Liver Decellularization.
Table 1: Experimental Groups for Assessing the Effect of Oscillating Pressure Conditions and the Perfusion Route on Rat Liver Decellularization.
Discussion
Although the presented technique for rat liver harvesting and decellularization is easily reproducible, there are certain critical steps to consider:
During preparation for liver harvesting, it is important to avoid severe bleeding because it will activate blood coagulation and may lead to blood clot formation within the liver. In our opinion, it is advantageous to incise the abdominal aorta directly before cannulation of the portal vein to avoid blood inflow via the hepatic artery during perfusion via the portal vein. Once the liver is cannulated and clearly perfused, blood clot formation is no longer an issue.
After liver harvesting and transportation to the perfusion device, the connection of livers to the perfusion device is a particularly critical step because the entry of gas bubbles into the perfusion circle must be avoided to prevent gas embolism. It is useful to prefill the three-way valve, which is connected to the liver cannula, with PBS using a syringe and a thin cannula directly before connecting the valve to the perfusion system.
After perfusion is established, whether all tube connectors, three-way valves and tubes are perfectly connected should be re-checked. If livers are perfused for longer durations (e.g., O/N), even a small error in connection can lead to air siphoning into the perfusion system, thus leading to fatal perfusion outcomes.
The presented data are limited by the fact that the optimal pressure and frequency of oscillating pressure changes for the best decellularization outcomes remain unclear. Furthermore, pressure-controlled perfusion may lead to better and more stable results than flow-controlled perfusion does. Pressure-controlled perfusion enables the application of one perfusion protocol to livers of different sizes and weights, with the same outcome, because the flow will automatically be adapted.
The hepatic artery and portal vein are significantly different in terms of size and physiologic flow rates. Thus, a constant perfusion rate (5 ml/min) will certainly result in different perfusion pressures within the two vascular systems. Similarly, a constant perfusion pressure will result in different perfusion rates. One method to compare the decellularization efficacy via the two perfusion routes would be to perform perfusion at physiologic perfusion pressures or perfusion rates via both routes. However, to make the results comparable in the current study, we chose a constant perfusion rate for both routes. Unfortunately, using our device, we were not able to measure the perfusion pressure within perfused livers.
Our next-generation perfusion devices will be smaller to minimize the amount of perfusion media necessary. Furthermore, pump devices will enable pressure-controlled perfusion.
The presented technique appears very useful for reproducible, homogenous rat liver decellularization. Whether the presented technique is appropriate for recellularization and organ maturation in vitro has to be further addressed. Whether oscillating pressure conditions have an impact on repopulated cells will be a subject of further experiments.
The presented technique is more sophisticated than other rat liver perfusion devices and shows several clear advantages: 1) The homogenous decellularization is reproducible, with good results when oscillating pressure conditions are applied. 2) The perfusion device is sealed and enables sterile decellularization, which is a prerequisite for matrix repopulation and long-term perfusion. 3) The presented protocol is shorter than other published protocols but is still very effective. 4) Selective perfusion of the portal vein and the hepatic artery renders selective cell repopulation via different systems possible, which may be an important tool.
The perfusion device will be applied in further decellularization experiments to refine and optimize rat liver decellularization. The effect of pressure-controlled perfusion will be experimentally evaluated and compared with flow-controlled perfusion. The addition of further elements (e.g., a “dialysis unit”, a heat exchanger, an oxygenator) for repopulation, culture and maturation experiments appears possible and reasonable to further develop the presented device.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors would like to gratefully thank Steffen Lippert, Khalid Aliyev, Korinna Jöhrens and Katharina Struecker for their help during this project.
References
- Struecker B, Raschzok N, Sauer IM. Liver support strategies: cutting-edge technologies. Nat Rev Gastroenterol Hepatol. 2014;11:166–176. doi: 10.1038/nrgastro.2013.204. [DOI] [PubMed] [Google Scholar]
- Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. Decellularized liver scaffolds effectively support the proliferation and differentiation of mouse fetal hepatic progenitors. J Biomed Mater Res A. 2013. [DOI] [PMC free article] [PubMed]
- Uygun BE, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–820. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupe T, Williams M, Brown A, Willenberg B, Petersen BE. Method for the decellularization of intact rat liver. Organogenesis. 2010;6:134–136. doi: 10.4161/org.6.2.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, et al. Construction of a portal implantable functional tissue-engineered liver using perfusion-decellularized matrix and hepatocytes in rats. Cell transplantation. 2011;20:753–766. doi: 10.3727/096368910X536572. [DOI] [PubMed] [Google Scholar]
- Baptista PM, et al. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604–617. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]
- Soto-Gutierrez A, Wertheim JA, Ott HC, Gilbert TW. Perspectives on whole-organ assembly: moving toward transplantation on demand. J Clin Invest. 2012;122:3817–3823. doi: 10.1172/JCI61974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Gutierrez A, et al. A whole-organ regenerative medicine approach for liver replacement. Tissue engineering. Part C, Methods. 2011;17:677–686. doi: 10.1089/ten.tec.2010.0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kock J, et al. Simple and quick method for whole-liver decellularization: a novel in vitro three-dimensional bioengineering tool. Archives of toxicology. 2011;85:607–612. doi: 10.1007/s00204-011-0706-1. [DOI] [PubMed] [Google Scholar]
- Park KM, Woo HM. Systemic decellularization for multi-organ scaffolds in rats. Transplantation proceedings. 2012;44:1151–1154. doi: 10.1016/j.transproceed.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Shirakigawa N, Ijima H, Takei T. Decellularized liver as a practical scaffold with a vascular network template for liver tissue engineering. J Biosci Bioeng. 2012. [DOI] [PubMed]
- Ren H, et al. Evaluation of two decellularization methods in the development of a whole-organ decellularized rat liver scaffold. Liver Int. 2013;33:448–458. doi: 10.1111/liv.12088. [DOI] [PubMed] [Google Scholar]
- Struecker B, et al. Improved rat liver decellularization by arterial perfusion under oscillating pressure conditions. J Tissue Eng Regen Med. 2014. [DOI] [PubMed]
- Struecker B, et al. Porcine Liver Decellularization Under Oscillating Pressure Conditions: A Technical Refinement to Improve the Homogeneity of the Decellularization Process. Tissue engineering. Part C, Methods. 2014. [DOI] [PubMed]


