Abstract
Ginsenoside compound K (C-K) is attracting a lot of interest because of its biological and pharmaceutical activities, including hepatoprotective, antitumor, anti-wrinkling, and anti-skin aging activities. C-K has been used as the principal ingredient in skin care products. For the effective application of ginseng extracts to the manufacture of cosmetics, the PPD-type ginsenosides in ginseng extracts should be converted to C-K by enzymatic conversion. For increased yield of C-K from the protopanaxadiol (PPD)-type ginsenosides in red-ginseng extract (RGE), the α-l-arabinofuranoside-hydrolyzing α-l-arabinofuranosidase from Caldicellulosiruptor saccharolyticus (CS-abf) was used along with the β-d-glucopyranoside/α-l-arabinopyranoside-hydrolyzing β-glycosidase from Sulfolobus solfataricus (SS-bgly) because SS-bgly showed very low hydrolytic activity on the α-l-arabinofuranoside linkage in ginsenosides. The optimal reaction conditions for C-K production were as follows: pH 6.0, 80°C, 2 U/mL SS-bgly, 3 U/mL CS-abf, and 7.5 g/L PPD-type ginsenosides in RGE. Under these optimized conditions, SS-bgly supplemented with CS-abf produced 4.2 g/L C-K from 7.5 g/L PPD-type ginsenosides in 12 h without other ginsenosides, with a molar yield of 100% and a productivity of 348 mg/L/h. To the best of our knowledge, this is the highest concentration and productivity of C-K from ginseng extract ever published in literature.
Introduction
Ginseng (Panax ginseng C. A. Meyer), one of the most valuable herbs, has been used in traditional medicine in Asian countries for over 2000 years [1]. The active components of ginseng are ginsenosides, which possess diverse biological and pharmaceutical activities, including anti-fatigue [2], anti-allergic [3], anti-oxidant [4], anti-inflammatory [5], anti-cancer [6], and anti-skin aging [7] activities. Ginsenosides are classified into three groups on the basis of the types of the aglycone structures, namely, oleanane, protopanaxadiol (PPD), and protopanaxatriol (PPT). Oleanane, containing oleanolic acid aglycone, exists in only one ginsenoside, that is, Ro. PPD- and PPT-type ginsenosides exist in glycosylated compounds consisting of a non-sugar component with PPD or PPT aglycone and a sugar component with 1−4 glycoside molecules [8]. Glycosylated major ginsenosides (Rb1, Rb2, Rc, Rd, Rg1, and Re) constitute more than 80% of total ginsenosides in wild ginseng [9]. Deglycosylated minor ginsenosides exhibit higher biological activity than major glycosylated ginsenosides because of their smaller size, higher bioavailability, and better permeability across the cell membrane [10]. Commonly, wild ginseng is processed into white ginseng and red ginseng to improve its preservation and efficacy. White ginseng is produced by sun drying, while red ginseng is heated by steaming and then dried. The ginsenoside content in red ginseng differs from that in white ginseng because of the heating process. Red ginseng contains higher content of PPD-type ginsenosides in total ginsenosides than PPT-type ginsenosides, whereas white ginseng contains higher content of PPT-type ginsenosides than PPD-type ginsenosides. The concentration of PPD-type ginsenosides in red ginseng is higher than that in white ginseng. Because red ginseng exerts stronger activities than white ginseng toward anti-skin aging [7], skin damage protection [11], and burn wound healing [12], it has also been used in the production of cosmetics. Red ginseng is fermented using Lactobacillus species to enhance the anti-aging potential by [13–15]. Fermented red ginseng increases the content of ginsenosides such as Rg3, Rh1, F2, Rg2, and compound K (C-K) [15].
The minor ginsenoside C-K is one of the most biologically and pharmaceutically active PPD-type ginsenosides [16]. C-K has attracted attention in recent years because of hepatoprotective activity [17], inhibition of tumor invasion [18], and induction of tumor cell apoptosis [19]. C-K is also effective against wrinkling and skin damage [20, 21]. Therefore, C-K has been used as the principal ingredient of skin care products. Because C-K is not present in ginseng, the production of C-K from major PPD-type ginsenosides have been intensively tried by biotransformations, including cell conversion [22–24], fermentation [25], and enzymatic conversion [26–28]. Of these methods, enzymatic conversion showed the highest selectivity, yield, and productivity for C-K production. For the industrial production of C-K, ginseng extract should be used as the substrate, instead of purified ginsenosides. However, most studies on C-K production have used purified ginsenosides; only some have used ginseng extracts [29–33]. For the effective application of ginseng extracts to the manufacture of cosmetics, PPD-type ginsenosides in ginseng extracts should be converted to C-K by enzymatic conversion because C-K is an effective agent for anti-wrinkling and anti-skin aging.
In this study, the PPD-type ginsenosides in red ginseng extract (RGE) were completely converted to C-K by the enzymatic reaction involving the β-d-glucopyranoside/α-l-arabinopyranoside-hydrolyzing β-glucosidase from Sulfolobus solfataricus (SS-bgly) supplemented with the α-l-arabinofuranoside-hydrolyzing α-l-arabinofuranosidase from Caldicellulosiruptor saccharolyticus (CS-abf).
Materials and Methods
Materials
The ginsenoside standards Rb1, Rb2, Rc, Rd, F2, compound Mc (C-Mc), and C-K were purchased from BTGin (Daejon, Korea). Red ginseng was purchased from a local ginseng market (Geumsan, Korea). To prepare RGE, 100 g of dry red ginseng powder was added to 1 L of methanol/water mixture (4:1, v/v) and left at 80°C for 1 h. After cooling, the mixture was filtered through a 0.45-μm filter, the filtrate was evaporated to remove methanol, and the residue was dissolved in 1 L of distilled water. The dissolved solution was adsorbed onto a Diaion HP-20 resin, which was further rinsed with distilled water to elute the free sugars. The ginsenosides attached to the resin were successively eluted with methanol, the eluant was evaporated to remove methanol, and the residue was dissolved in the same volume of distilled water as the original loading volume. Sugar-free RGE was used because the Maillard reactions between free sugars and the enzyme occurred at temperatures above 70°C.
Cloning and gene expression
S. solfataricus DSM 1617 and C. saccharolyticus DSM 8903, Escherichia coli ER2566, and the plasmids pET-24a and pTrc99A were used as the sources of β-glycosidase and α-l-arabinofuranosidase genes, host cells, and expression vectors, respectively. The genomic DNA from S. solfataricus and C. saccharolyticus was extracted using the genomic DNA extraction kit (Qiagen, Hilden, Germany). The genes encoding SS-bgly and CS-abf were amplified from each genomic DNA by polymerase chain reaction (PCR) using Pfu DNA polymerase (Solgent, Daejeon, Korea). The sequences of the oligonucleotide primers used for gene cloning were based on the DNA sequences of SS-bgly (GenBank accession number, WP 009992676) and CS-abf (GenBank accession number, YP 001180344). Forward (5'-GCGTCTGCATATGTACTCATTTCCAAATAGC-3') and reverse (5'-GCGAATGGATCCTTAGTGCCTTAATGGCTTTAC-3') primers were designed to introduce the NdeΙ and BamHΙ restriction sites (underlines), respectively, for SS-bgly gene insertion, and forward (5'-TTTGGATCCATGAAAAAAGCAAAAGTCATCTA-3') and reverse (5'-TTTCTGCAGTTAATTTTCTCTCTTCTTCAATCTG-3') primers were designed to introduce the BamHΙ and PstΙ restriction sites (underlined), respectively, for CS-abf gene insertion. Each amplified DNA fragment obtained by PCR was purified and digested with the corresponding restriction endonuclease [34, 35]. The digested DNA fragments of SS-bgly and CS-abf were extracted using a gel extraction kit (Qiagen, Hilden, Germany), and then inserted into the pET-24a vector and pTrc99A vectors, respectively, and digested with the same restriction enzymes. E. coli ER2566 strain was transformed with the ligation mixture and plated on Luria-Bertani (LB) agar containing 50 μg/mL of ampicillin. Ampicillin resistant colony was selected, and plasmid DNA from the transformant was isolated using a plasmid purification kit (Promega, Madison, WI). DNA sequencing was performed at the Macrogen facility (Seoul, Korea). The expression of SS-bgly and CS-abf genes was evaluated by both sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and enzyme activity assay.
Culture conditions
S. solfataricus and C. saccharolyticus were grown at 70°C under anaerobic conditions in the presence of 100% N2 gas for 5 days on Sulfolobus medium (DSM Media Formulation No. 88) and Caldicellulosiruptor medium (DSM Media Formulation No. 640), respectively. The recombinant E. coli was cultivated with shaking at 200 rpm in a 2-L flask containing 500 mL of LB medium at 37°C with 20 μg/mL of kanamycin. When the optical density of the bacteria at 600 nm reached 0.8, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 0.1 mM to induce enzyme expression, and the culture was incubated at 16°C with shaking at 150 rpm for 16 h.
Enzyme purification
The induced E. coli cells were harvested from the culture broth by centrifugation at 6,000g for 30 min at 4°C, washed twice with 0.85% NaCl, and resuspended in 50 mM citrate/phosphate buffer (pH 6.0) containing 0.1 mM phenylmethylsulfonyl fluoride as the protease inhibitor. The resuspended cells were disrupted by sonication using Sonic Dismembrator (Model 100, Fisher Scientific, Pittsburgh, PA, USA) on ice for 10 min. The unbroken cells and cell debris were removed by centrifugation at 13,000×g for 20 min at 4°C, and the supernatant obtained was used as the crude extract. The crude extract was subsequently heated at 75°C for 10 min, and the suspension was centrifuged at 13,000×g for 20 min to remove insoluble denatured proteins. The supernatant obtained was used as the soluble enzyme.
Enzyme reactions
One unit (U) of SS-bgly or CS-abf activity was defined as the amount of enzyme required to liberate 1 nmol of C-K or ginsenoside Rd as a product from ginsenoside Rd or Rc as a substrate, respectively. Unless otherwise stated, the reactions were performed at 80°C in 50 mM citrate/phosphate buffer (pH 6.0) containing 1.25 g/L PPD-type ginsenosides in RGE for 2 h.
Optimization of the supplementation ratio of SS-bgly and CS-abf
The effect of the supplementation ratio of SS-bgly and CS-abf on the conversion of ginsenoside Rc to C-K was investigated by the enzymatic reactions of SS-bgly supplemented with CS-abf at the ratios of 0:2 to 2:0 U/mL, at a total concentration of 2 U/mL. The reactions were performed at 80°C in 50 mM citrate/phosphate buffer (pH 6.0) containing 0.4 g/L ginsenoside Rc and two enzymes, at a total concentration of 2 U/mL, for 1 h. The time-course reactions for the conversion of PPD-type ginsenosides in RGE to C-K were performed at 80°C in 50 mM citrate/phosphate buffer (pH 6.0) containing 1.25 g/L PPD-type ginsenosides in RGE, 0.4 U/mL SS-bgly, and 1.6 U/mL CS-abf in 20 h. The effect of the supplementation ratio of CS-abf to SS-bgly on the production of C-Mc from PPD-type ginsenosides in RGE was conducted by supplementing CS-abf, ranging from 0.0 to 1.6 U/mL, with 0.4 U/mL SS-bgly. The reactions were performed with 1.25 g/L PPD-type ginsenosides in RGE for 20 h.
Effects of pH and temperature on C-K production
The effects of pH and temperature on the production of C-K from PPD-type ginsenosides in RGE were investigated by varying the pH from 4.5 to 7.5, and the temperature from 65 to 95°C, respectively. The reactions were performed in 50 mM citrate/phosphate buffer containing 0.4 U/mL SS-bgly, 0.6 U/mL CS-abf, and 1.25 g/L PPD-type ginsenosides in RGE for 2 h. The effect of temperature on the stability of SS-bgly supplemented with CS-abf was monitored as a function of incubation time by maintaining the solution of the enzymes at five different temperatures (70, 75, 80, 85, and 90°C) in 50 mM citrate/phosphate buffer (pH 6.0). Samples were withdrawn at time intervals and assayed.
Effects of the concentrations of enzymes and substrate on C-K production
The optimal concentration of the two enzymes required for C-K production was determined by varying the concentrations of SS-bgly and CS-abf at the ratio of 1:1.5, ranging from 0.4 and 0.6 U/mL to 4 and 6 U/mL at a constant substrate concentration of 7.5 g/L PPD-type ginsenosides in RGE. To determine the optimal substrate concentration, the concentration of PPD-type ginsenosides was varied from 1.25 to 10 g/L at constant enzyme concentrations of 2 U/mL SS-bgly and 3 U/mL CS-abf. The reactions were performed in 50 mM citrate/phosphate buffer (pH 6.0) at 80°C for 2 h.
Production of C-K from PPD-type ginsenosides in RGE
The time-course reactions for the conversion of PPD-type ginsenosides in RGE to C-K were performed at 80°C in 50 mM citrate/phosphate buffer (pH 6.0) containing 7.5 g/L PPD-type ginsenosides in 17.4% (w/v) RGE and 2 U/mL SS-bgly or 2 U/mL SS-bgly supplemented with 3 U/mL CS-abf for 12 h.
HPLC analysis
A reaction solution containing digoxin as an internal standard was extracted with an equal volume of n-butanol. The n-butanol fraction was then evaporated until dry, and methanol was added. Ginsenosides were assayed using an HPLC system (1100, Agilent, Santa Clara, CA, USA) equipped with a UV detector at a detection wavelength of 203 nm and a C18 column (YMC, Kyoto, Japan). The column was eluted at 37°C with a linear gradient of acetonitrile/water from 20:80 to 80:20 (v/v) for 80 min at a flow rate of 1 mL/min.
TLC analysis
Three microliters of the ginsenoside standards and the reaction products of ginsenoside Rc were spotted onto a TLC plate (silica gel 60 F254, Merck, Darmstadt, Germany). The compounds on the plate were developed using a solvent mixture of chloroform, methanol, and water (40:20:10, v/v/v). The spots on the plate were detected by spraying 10% sulfuric acid, followed by incubation at 110°C in a dry oven for 5 min.
Results and Discussion
Conversion of major PPD-type ginsenosides to C-K by SS-bgly supplemented with CS-abf
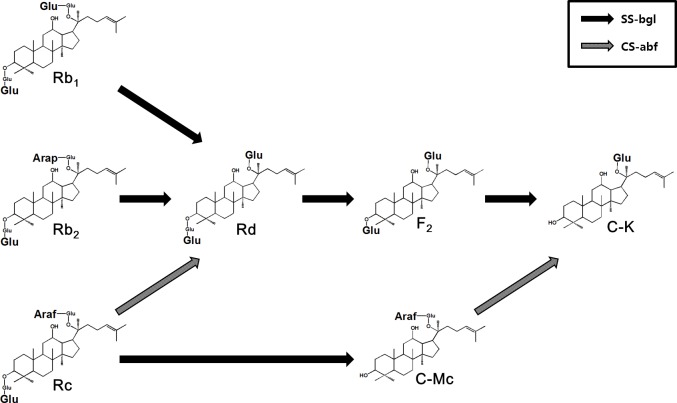
SS-bgly is a useful enzyme for C-K production because the enzyme simultaneously hydrolyzes the outer β-d-glucopyranoside or α-l-arabinopyranoside linked to C-20, and the inner and outer β-d-glucopyranosides linked to C-3 in PPD-type ginsenosides [32]. However, the enzyme exhibits very low hydrolytic activity for α-l-arabinofuranoside in PPD-type ginsenosides, indicating that SS-bgly does not convert ginsenosides Rc and C-Mc to Rd and C-K, respectively. Thus, CS-abf, which showed hydrolytic activity for α-l-arabinofuranoside [36], was supplemented to SS-bgly for the complete conversion of all PPD-type ginsenosides to C-K (Fig 1).
Fig 1. Hydrolytic pathway from ginsenosides Rb1, Rb2, and Rc to C-K via C-Mc, ginsenoside Rd, and F2 by SS-bgly supplemented with CS-abf.
SS-bgly and CS-abf were purified as soluble proteins by heat treatment of crude enzymes extracted from harvested cells. The conversion of ginsenoside Rc to C-K by the enzymatic reaction of SS-bgly supplemented with CS-abf was analyzed and confirmed by TLC using the ginsenoside standards Rc, Rd, F2, C-Mc, and C-K (S1 Fig). Ginsenosides Rd, F2 (trace), C-Mc, and C-K were obtained from ginsenoside Rc by SS-bgly supplemented with CS-abf, whereas only C-Mc was obtained by SS-bgly alone.
These results indicate that the supplementation of CS-abf to SS-bgly is an effective method for C-K production by introducing the α-l-arabinofuranoside-hydrolyzing activity, which caused the conversion of Rc and C-Mc to Rd and C-K, respectively.
Optimization of the supplementation ratio of SS-bgly and CS-abf for C-K production
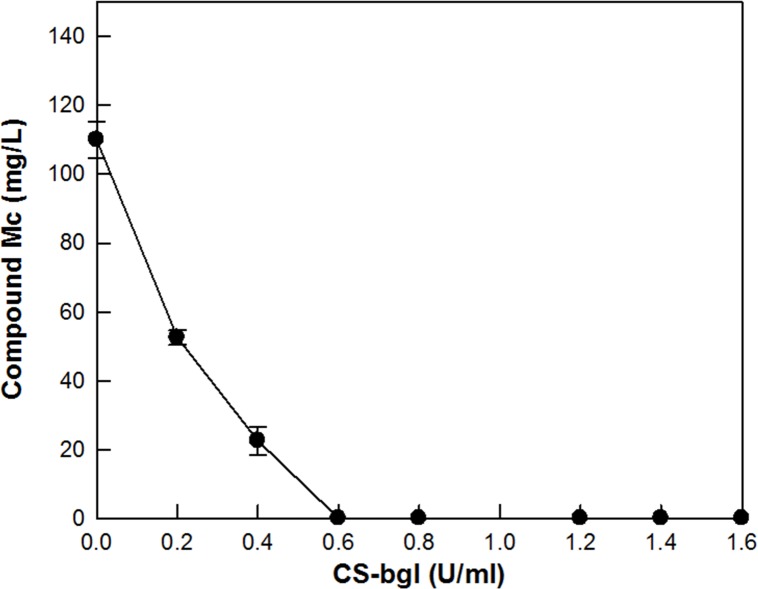
The production of C-K from ginsenoside Rc was investigated by supplementing CS-abf to SS-bgly at the ratios ranging from 0:2 to 2:0 U/mL, to make up a total concentration of 2 U/mL. The maximum production of C-K was observed at 0.4 U/mL SS-bgly and 1.6 U/mL CS-abf with a unit ratio of 1:4 (Fig 2). This ratio was used in the conversion of PPD-type ginsenosides in RGE to C-K (Fig 3). In the conversion, ginsenoside Rc and Mc disappeared after 2 h, whereas ginsenoside Rd remained at a constant concentration of approximately 100 mg/L even after 16 h. These results suggest that the concentration of CS-abf added to the reaction solution for C-K production was excess compared to that of SS-bgly. To optimize the supplementation ratio of SS-bgly and CS-abf for the production of C-K from PPD-type ginsenosides in RGE, the residual concentration of C-Mc was determined after 20 h by increasing the concentration of CS-abf from 0.0 to 1.6 U/mL, and maintaining a fixed concentration of SS-bgly at 0.4 U/mL. The residual concentration of C-Mc decreased with increasing the concentration of CS-abf, at concentrations below 0.6 U/mL CS-abf. However, C-Mc was not detected at concentrations above 0.6 U/mL CS-abf (Fig 4). Therefore, the optimal unit ratio of SS-bgly and CS-abf was 0.4 U/mL: 0.6 U/mL (1:1.5) for the production of C-K from PPD-type ginsenosides in RGE, respectively. This optimal unit ratio was used in the subsequent reactions.
Fig 2. Effect of supplementation of CS-abf to SS-bgly on the conversion of ginsenoside Rc to C-K.
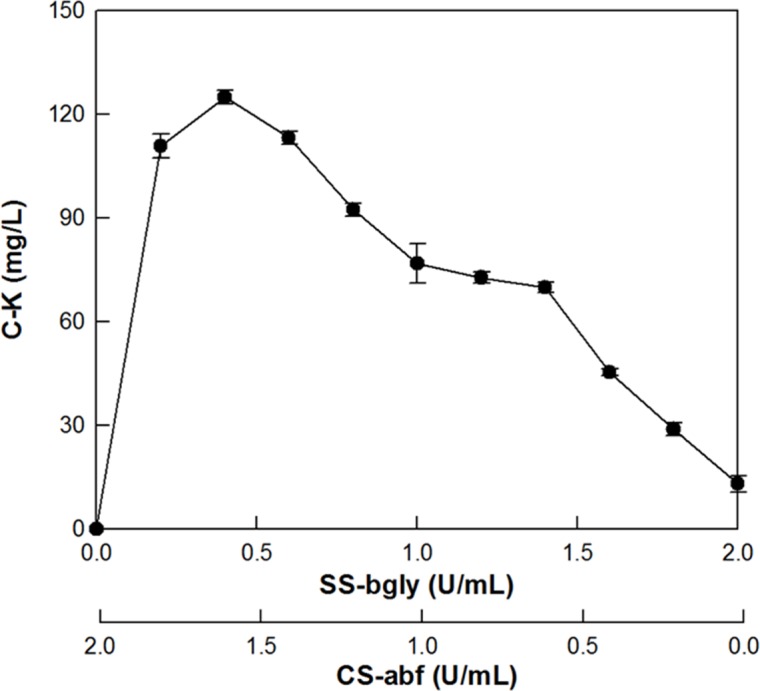
Data represent the means of three experiments and error bars represent standard deviation.
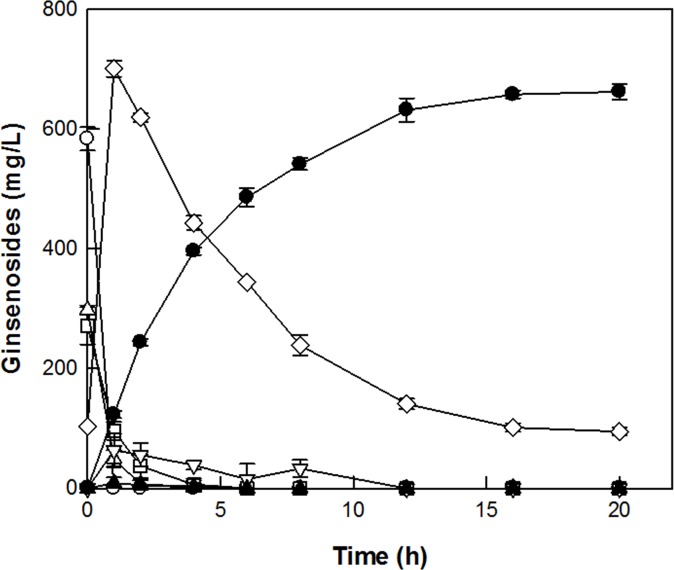
Fig 3. Production of C-K from PPD-type ginsenosides in RGE by SS-bgly supplemented with CS-abf.
Ginsenosides Rb1 (open circle), Rb2 (open square), Rc (open triangle), and Rd (open diamond) were converted to C-K (filled circle) via F2 (open inverted triangle) and C-Mc (filled triangle). Data represent the means of three experiments and error bars represent standard deviation.
Fig 4. Effect of supplementation of CS-abf to SS-bgly on the concentration of C-Mc in RGE.
Data represent the means of three experiments and error bars represent standard deviation.
Effects of pH and temperature on C-K production by SS-bgly supplemented with CS-abf
The maximum production of C-K by the enzymatic reaction of SS-bgly supplemented with CS-abf at the optimal unit ratio was observed at pH 6.0 and 85°C (Fig 5), and the optimal pH and temperature of only SS-bgly were also 6.0 and 85°C, respectively. The thermal inactivation of SS-bgly supplemented with CS-abf at the optimal unit ratio for the production of C-K from PPD-type ginsenosides in RGE followed first-order kinetics, with half-lives of 346, 76, 25, 15, and 1.2 h at 70, 75, 80, 85, and 90°C (S2 Fig). The half-lives of SS-bgly at 70, 75, 80, and 85°C were 700, 100, 66, and 30 h [32]; and those of CS-abf were 130, 41, 2.5, and 0.12 h [36]. Thus, the thermostability of SS-bgly supplemented with CS-abf was greater than that of CS-abf alone, but less than that of SS-bgly alone. Because of the instability of SS-bgly supplemented with CS-abf above 85°C, the reaction temperature for C-K production by SS-bgly supplemented with CS-abf was determined to be 80°C.
Fig 5. Effects of (A) pH and (B) temperature on the production of C-K from PPD-type ginsenosides in RGE by SS-bgly supplemented with CS-abf.
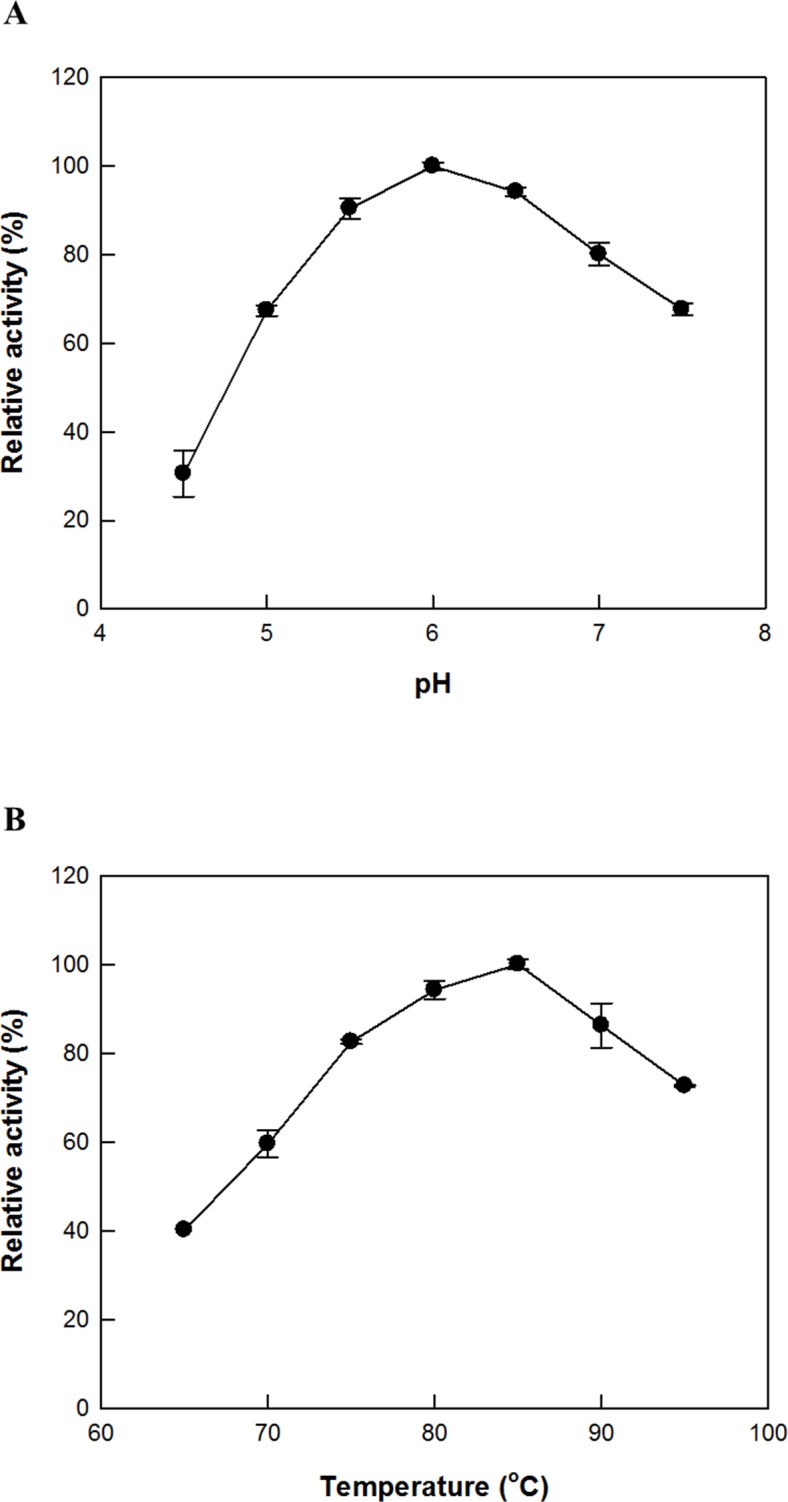
Data represent the means of three experiments and error bars represent standard deviation.
Effects of the concentrations of enzymes and substrate on C-K production by SS-bgly supplemented with CS-abf
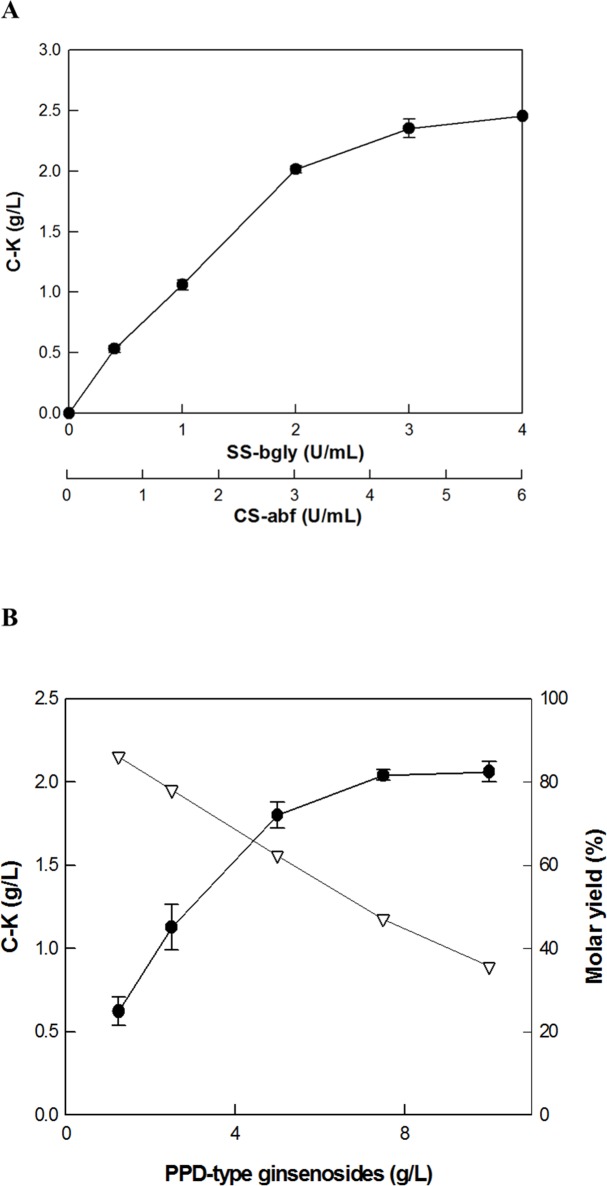
The effect of the concentration of SS-bgly supplemented with CS-abf on C-K production was investigated with 7.5 g/L PPD-type ginsenosides in RGE as a substrate by varying the concentrations of SS-bgly and CS-abf at the optimal unit ratio of 1:1.5, ranging from 0.4 and 0.6 U/mL to 4 and 6 U/mL, respectively (Fig 6A). C-K production increased with increasing enzyme concentrations, however, C-K production per enzyme concentration decreased at concentrations above 2 U/mL SS-bgly and 3 U/mL CS-abf. Thus, the optimal concentration of the enzymes required for the production of C-K from PPD-type ginsenosides in RGE was 2 U/mL SS-bgly and 3 U/mL CS-abf.
Fig 6. Effect of the concentration of (A) SS-bgly supplemented with CS-abf and (B) PPD-type ginsenosides in RGE.
Filled circle and open inverted triangle represent concentration of C-K and conversion yield, respectively. Data represent the means of three experiments and error bars represent standard deviation.
To investigate the effect of substrate concentration on C-K production, the concentration of PPD-type ginsenosides in RGE was varied from 1.25 to 10 g/L. The conversion yield decreased with increasing the substrate concentration (Fig 6B). However, the maximal production of C-K was exhibited above 7.5 g/L PPD-type ginsenosides. Thus, the optimal concentration of substrate was 7.5 g/L PPD-type ginsenosides in RGE.
C-K production from PPD-type ginsenosides in RGE by SS-bgly supplemented with CS-abf under optimum conditions
The optimal reaction conditions for the production of C-K from PPD-type ginsenosides in RGE by SS-bgly supplemented with CS-abf were pH 6.0, 80°C, 2 U/mL SS-bgly, 3 U/mL CS-abf, and 7.5 g/L PPD-type ginsenosides in RGE. Under these conditions, 2 U/mL SS-bgly without supplementation of CS-abf produced 3.1 g/L C-K from 7.5 g/L PPD-type ginsenosides in RGE along with other ginsenosides, 1.1 g/L C-Mc and 0.3 g/L Rd, after 12 h, with a molar yield of 75% and a productivity of 263 mg/L/h for the production of C-K from PPD-type ginsenosides (Fig 7A). To convert the remaining other ginsenosides to C-K, 3 U/mL CS-abf was supplemented to 2 U/mL SS-bgly. Under the optimized conditions, SS-bgly supplemented with CS-abf converted 7.5 g/L PPD-type ginsenosides in RGE to 4.2 g/L C-K in 12 h, with a molar yield of 100% and a productivity of 348 mg/L/h (Fig 7B). The molar yield and productivity of C-K by SS-bgly supplemented with CS-abf were 25% and 1.3-fold higher, respectively, than those by SS-bgly alone. No C-K was formed when the reaction was run under the same experimental conditions without enzymes.
Fig 7. C-K production from PPD-type ginsenosides in RGE.
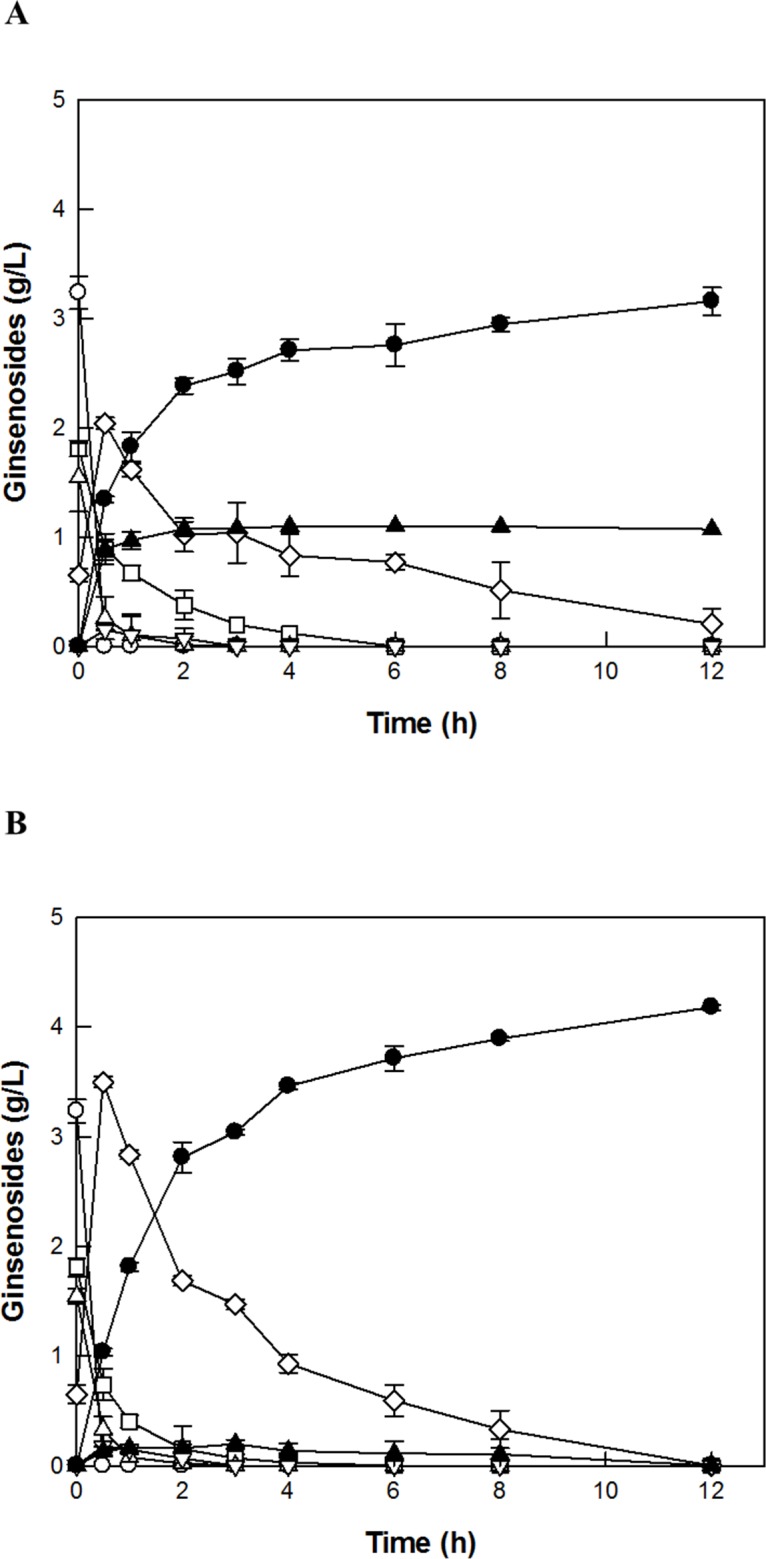
C-K (filled circle) was produced from ginsenosides Rb1 (open circle), Rb2 (open square), Rc (open triangle), and Rd (open diamond) in RGE via F2 (open inverted triangle) and C-Mc (filled triangle) by (A) SS-bgly. (B) SS-bgly supplemented with CS-abf under the optimized unit ratio of the two enzymes. Data represent the means of three experiments and error bars represent standard deviation.
The production of C-K from PPD-type ginsenosides in ginseng extracts by enzymes and cells is presented in Table 1. C-K was also synthesized from glucose by metabolically engineered yeast, however, the produced concentration of C-K was low (1.4 mg/L) [37]. β-Glycosidase from Sulfolobus acidocaldarius (SA-bgly) produced 2.0 g/L C-K from 5 g/L PPD-type ginsenosides in ginseng root extract (GRE) in 22 h, with a molar yield of 69% and a productivity of 91 mg/L/h [38], which were 6% and 2.9-fold lower than those in RGE by SS-bgly. α-l-Arabinopyranoside linked to ginsenoside Rb2 could be hydrolyzed by SS-bgly but not by SA-bgly. The content of Rc among the PPD-type ginsenosides such as Rb1, Rb2, Rc, and Rd in RGE (21.3%) was lower than that in GRE (26.9%), and the concentration of the PPD-type ginsenosides in 10% (w/v) RGE was 1.5-fold higher (Table 2), indicating that RGE is a better substrate for C-K production. Thus, SS-bgly using RGE as the substrate exhibited higher conversion yield and productivity of C-K than SA-bgly using GRE. Diverse glycosidases from Fusobacterium sp. [39], Microbacterium esteraromaticum [28], S. acidocaldarius [29], Aspergillus niger [40], Aspergillus sp. [41], Esteya vermicola [42], and Terrabacter ginsenosidimutans [43] converted reagent-grade major ginsenosides Rb1, Rb2, Rc, and Rd to C-K. However, the quantitative production of C-K from Rc was conducted by only the combined use of three-enzymes [38]. The three-enzyme system of SA-bgly, and α-l-arabinofuranosidase and β-galactosidase from C. saccharolyticus produced 2.9 g/L C-K from 5 g/L PPD-type ginsenosides in GRE in 20 h, with a molar yield of 100% and a productivity of 144 mg/L/h [38]. To the best of our knowledge, this was the highest previously reported conversion yield and productivity of C-K from ginseng extract. The concentration and productivity of C-K as obtained by the two-enzyme system of SS-bgly and CS-abf using RGE, which contained the higher content of PPD-type ginsenosides than GRE, were 1.4- and 2.4-fold higher than those obtained by the three-enzyme system using GRE, respectively. The supplementation of CS-abf to SS-bgly resulted in the complete conversion of all PPD-type ginsenosides in RGE to C-K with the highest productivity ever reported via two transformation pathways: Rb1, Rb2, and Rc → Rd → F2 → C-K and Rc → C-Mc → C-K (Fig 1). The conversion of ginsenosides Rb1, Rb2, Rc, and Rd in RGE to C-K via F2, and C-Mc was analyzed and confirmed by HPLC with the ginsenoside standards (S3 Fig).
Table 1. Production of C-K from PPD-type ginsenosides in ginseng extracts.
| Microorganism | Biocatalyst | Extract | C-K (g/L) | Molar yield (%) | Productivity (mg L−1 h−1) | Reference |
|---|---|---|---|---|---|---|
| Aspergillus niger | Pectinase | Rootlet ginseng | 2.0 | NC | 54 | [44] |
| Aspergillus niger | Cytolase PCL 5 | White ginseng extract | 2.1 | NC | 27 | [45] |
| Sulfolobus acidocaldarius, | β-Glycosidase | Ginseng root extract | 2.0 | 69 | 91 | [38] |
| Sulfolobus acidocaldarius and Caldicellulosiruptor saccharolyticus | β-Glycosidase, α-l-arabinofuranosidase, and β-galactosidase | Ginseng root extract | 2.9 | 100 | 144 | [38] |
| Saccharomyces cerevisiae | Cells | Red ginseng extract | 0.3 | NC | 3 | [46] |
| Paecilomyces bainier sp. | Cells | Notoginseng extract | 1.3 | 83 | 9 | [47] |
| Ganoderma lucidum | Cells | American ginseng root extract | 0.7 | 2 | 1 | [48] |
| Sulfolobus solfataricus | β-Glycosidase | Red ginseng extract | 3.1 | 75 | 263 | This study |
| Sulfolobus solfataricus and Caldicellulosiruptor saccharolyticus | β-Glycosidase and α-l-arabinofuranosidase | Red ginseng extract | 4.2 | 100 | 348 | This study |
NC, not calculated.
Table 2. Contents of PPD- and PPT-type ginsenosides in 10% (w/v) RGE and GRE.
| Ginsenoside | RGE | GRE | ||||
|---|---|---|---|---|---|---|
| Concentration (g/L) | Content a (%, w/w) | Content b (%, w/w) | Concentration (g/L) | Content a (%, w/w) | Content b (%, w/w) | |
| PPD-type | ||||||
| Rb1 | 1.92 | 27.4 | 44.5 | 1.39 | 21.0 | 46.8 |
| Rb2 | 1.08 | 15.4 | 25.1 | 0.49 | 7.4 | 16.5 |
| Rc | 0.92 | 13.1 | 21.3 | 0.80 | 12.1 | 26.9 |
| Rd | 0.39 | 5.6 | 9.1 | 0.29 | 4.4 | 9.8 |
| Subtotal | 4.31 | 61.6 | 100.0 | 2.97 | 44.9 | 100.0 |
| PPT-type | ||||||
| Rg1 | 0.61 | 8.7 | 1.83 | 27.6 | ||
| Rh1 | 0.12 | 1.7 | 0.06 | 0.9 | ||
| Re | 1.78 | 25.4 | 1.66 | 25.1 | ||
| Rg2 | 0.18 | 2.6 | 0.10 | 1.5 | ||
| Subtotal | 2.69 | 38.4 | 3.65 | 55.1 | ||
| Total | 7.00 | 100.0 | 6.62 | 100.0 | ||
aContents of total ginsenosides.
bContents of each type of ginsenosides.
Conclusions
SS-bgly shows hydrolytic activity on the β-d-glucopyranoside and α-l-arabinopyranoside linkage in ginsenosides without the α-l-arabinofuranoside linkage. To compensate this defect, SS-bgly was supplemented with α-l-arabinofuranoside-hydrolyzing CS-abf. This combination completely converted PPD-type ginsenosides in RGE to C-K. To the best of our knowledge, this is the highest concentration and productivity of C-K from ginseng extract ever published in literature. Our results would contribute toward improving the industrial production of C-K by biotransformation.
Supporting Information
Ginsenoside Rc was converted to the reaction products by only SS-bgly and SS-bgly supplemented with CS-abf. Lane S, ginsenoside standards; Lane 1, reaction products of ginsenoside Rc by SS-bgl alone; Lane 2, reaction products of ginsenoside Rc by SS-bgly supplemented with CS-abf.
(TIF)
The enzymes were incubated at 70°C (open square), 75°C (filled square), 80°C (open circle), 85°C (filled circle), and 90°C (filled triangle) for varying time periods. Data represent the means of three experiments and error bars represent standard deviation.
(TIF)
HPLC analysis of C-K (27.9 min) produced from ginsenosides Rb1 (9.6 min), Rb2 (11.9 min), Rc (11.4 min), and Rd (12.8 min) present in RGE via F2 (18.1 min) and compound Mc (23.4 min) by SS-bgly supplemented with CS-abf at 0, 2, and 12 h.
(TIF)
Data Availability
All data are all contained within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Lu JM, Yao Q, Chen C (2009) Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol 7(3): 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yoshikawa M, Morikawa T, Kashima Y, Ninomiya K, Matsuda H (2003) Structures of new dammarane-type Triterpene Saponins from the flower buds of Panax notoginseng and hepatoprotective effects of principal Ginseng Saponins. J Nat Prod 66(7): 922–7. [DOI] [PubMed] [Google Scholar]
- 3. Bae EA, Choo MK, Park EK, Park SY, Shin HY, Kim DH (2002) Metabolism of ginsenoside Rc by human intestinal bacteria and its related antiallergic activity. Biol Pharm Bull 25(6): 743–7. [DOI] [PubMed] [Google Scholar]
- 4. Cho WC, Chung WS, Lee SK, Leung AW, Cheng CH, Yue KK (2006) Ginsenoside Re of Panax ginseng possesses significant antioxidant and antihyperlipidemic efficacies in streptozotocin-induced diabetic rats. Eur J Pharmacol 550(1–3): 173–9. [DOI] [PubMed] [Google Scholar]
- 5. Wang L, Zhang Y, Chen J, Li S, Wang Y, Hu L, et al. (2011) Immunosuppressive effects of ginsenoside-Rd on skin allograft rejection in rats. J Surg Res 176(1): 267–74. 10.1016/j.jss.2011.06.038 [DOI] [PubMed] [Google Scholar]
- 6. Lee SY, Kim GT, Roh SH, Song JS, Kim HJ, Hong SS, et al. (2009) Proteome changes related to the anti-cancer activity of HT29 cells by the treatment of ginsenoside Rd. Pharmazie 64(4): 242–7. [PubMed] [Google Scholar]
- 7. Kang TH, Park HM, Kim YB, Kim H, Kim N, Do JH, et al. (2009) Effects of red ginseng extract on UVB irradiation-induced skin aging in hairless mice. J Ethnopharmacol 123(3): 446–51. 10.1016/j.jep.2009.03.022 [DOI] [PubMed] [Google Scholar]
- 8. Park CS, Yoo MH, Noh KH, Oh DK (2010) Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl Microbiol Biotechnol 87(1): 9–19. 10.1007/s00253-010-2567-6 [DOI] [PubMed] [Google Scholar]
- 9. Son JW, Kim HJ, Oh DK (2008) Ginsenoside Rd production from the major ginsenoside Rb1 by β-glucosidase from Thermus caldophilus . Biotechnol Lett 30(4): 713–6. [DOI] [PubMed] [Google Scholar]
- 10. Kim MK, Lee JW, Lee KY, Yang DC (2005) Microbial conversion of major ginsenoside Rb1 to pharmaceutically active minor ginsenoside Rd. J Microbiol 43(5): 456–62. [PubMed] [Google Scholar]
- 11. Hwang E, Sun ZW, Lee TH, Shin HS, Park SY, Lee DG, et al. (2013) Enzyme-processed Korean Red Ginseng extracts protects against skin damage induced by UVB irradiation in hairless mice. J Ginseng Res 37(4): 425–34. 10.5142/jgr.2013.37.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kimura Y, Sumiyoshi M, Kawahira K, Sakanaka M (2006) Effects of ginseng saponins isolated from Red Ginseng roots on burn wound healing in mice. Br J Pharmacol 148(6): 860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bang CS, Hong SH, Suk KT, Kim JB, Han SH, Sung H, et al. (2014) Effects of Korean Red Ginseng (Panax ginseng), urushiol (Rhus vernicifera Stokes), and probiotics (Lactobacillus rhamnosus R0011 and Lactobacillus acidophilus R0052) on the gut-liver axis of alcoholic liver disease. J Ginseng Res 38(3): 167–72. 10.1016/j.jgr.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim BG, Shin KS, Yoon TJ, Yu KW, Ra KS, Kim JM, et al. (2011) Fermentation of Korean red ginseng by Lactobacillus plantarum M-2 and its immunological activities. Appl Biochem Biotechnol 165(5–6): 1107–19. 10.1007/s12010-011-9328-6 [DOI] [PubMed] [Google Scholar]
- 15. Lee HS, Kim MR, Park Y, Park HJ, Chang UJ, Kim SY, et al. (2012) Fermenting red ginseng enhances its safety and efficacy as a novel skin care anti-aging ingredient: in vitro and animal study. J Med Food 15(11): 1015–23. 10.1089/jmf.2012.2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akao T, Kanaoka M, Kobashi K (1998) Appearance of compound K, a major metabolite of ginsenoside Rb1 by intestinal bacteria, in rat plasma after oral administration—measurement of compound K by enzyme immunoassay. Biol Pharm Bull 21(3): 245–9. [DOI] [PubMed] [Google Scholar]
- 17. Lee HU, Bae EA, Han MJ, Kim NJ, Kim DH (2005) Hepatoprotective effect of ginsenoside Rb1 and compound K on tert-butyl hydroperoxide-induced liver injury. Liver Int 25(5): 1069–73. [DOI] [PubMed] [Google Scholar]
- 18. Wakabayashi C, Murakami K, Hasegawa H, Murata J, Saiki I (1998) An intestinal bacterial metabolite of ginseng protopanaxadiol saponins has the ability to induce apoptosis in tumor cells. Biochem Biophys Res Commun 246(3): 725–30. [DOI] [PubMed] [Google Scholar]
- 19. Oh SH, Yin HQ, Lee BH (2004) Role of the Fas/Fas ligand death receptor pathway in ginseng saponin metabolite-induced apoptosis in HepG2 cells. Arch Pharm Res 27(4): 402–6. [DOI] [PubMed] [Google Scholar]
- 20. Shin DJ, Kim JE, Lim TG, Jeong EH, Park G, Kang NJ, et al. (2014) 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol suppresses UV-Induced MMP-1 expression through AMPK-mediated mTOR inhibition as a downstream of the PKA-LKB1 pathway. J Cell Biochem 115(10): 1702–11. 10.1002/jcb.24833 [DOI] [PubMed] [Google Scholar]
- 21. Lim TG, Jeon AJ, Yoon JH, Song D, Kim JE, Kwon JY, et al. (2015) 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol, a metabolite of ginsenoside Rb1, enhances the production of hyaluronic acid through the activation of ERK and Akt mediated by Src tyrosin kinase in human keratinocytes. Int J Mol Med 35(5): 1388–94. 10.3892/ijmm.2015.2121 [DOI] [PubMed] [Google Scholar]
- 22. Chi H, Ji GE (2005) Transformation of ginsenosides Rb1 and Re from Panax ginseng by food microorganisms. Biotechnol Lett 27(11): 765–71. [DOI] [PubMed] [Google Scholar]
- 23. Chi H, Kim DH, Ji GE (2005) Transformation of ginsenosides Rb2 and Rc from Panax ginseng by food microorganisms. Biol Pharm Bull 28(11): 2102–5. [DOI] [PubMed] [Google Scholar]
- 24. Fu Y, Yin Z, Wu L, Yin C (2014) Diversity of cultivable β-glycosidase-producing micro-organisms isolated from the soil of a ginseng field and their ginsenosides-hydrolysing activity. Lett Appl Microbiol 58(2): 138–44. 10.1111/lam.12166 [DOI] [PubMed] [Google Scholar]
- 25. Wu L, Jin Y, Yin C, Bai L (2012) Co-transformation of Panax major ginsenosides Rb1 and Rg1 to minor ginsenosides C-K and F1 by Cladosporium cladosporioides . J Ind Microbiol Biotechnol 39(4): 521–7. 10.1007/s10295-011-1058-9 [DOI] [PubMed] [Google Scholar]
- 26. Quan LH, Kim YJ, Li GH, Choi KT, Yang DC (2013) Microbial transformation of ginsenoside Rb1 to compound K by Lactobacillus paralimentarius . World J Microbiol Biotechnol 29(6): 1001–7. 10.1007/s11274-013-1260-1 [DOI] [PubMed] [Google Scholar]
- 27. Yan Q, Zhou XW, Zhou W, Li XW, Feng MQ, Zhou P (2008) Purification and properties of a novel β-glucosidase, hydrolyzing ginsenoside Rb1 to CK, from Paecilomyces bainier . J Microbiol Biotechnol 18(6): 1081–9. [PubMed] [Google Scholar]
- 28. Quan LH, Min JW, Jin Y, Wang C, Kim YJ, Yang DC (2012) Enzymatic biotransformation of ginsenoside Rb1 to compound K by recombinant β-glucosidase from Microbacterium esteraromaticum . J Agric Food Chem 60(14): 3776–81. 10.1021/jf300186a [DOI] [PubMed] [Google Scholar]
- 29. Noh KH, Oh DK (2009) Production of the rare ginsenosides compound K, compound Y, and compound Mc by a thermostable β-glycosidase from Sulfolobus acidocaldarius . Biol Pharm Bull 32(11): 1830–5. [DOI] [PubMed] [Google Scholar]
- 30. Ko SR, Suzuki Y, Suzuki K, Choi KJ, Cho BG (2007) Marked production of ginsenosides Rd, F2, Rg3, and compound K by enzymatic method. Chem Pharm Bull (Tokyo) 55(10): 1522–7. [DOI] [PubMed] [Google Scholar]
- 31. Li W, Zhang M, Zheng YN, Li J, Wang YP, Wang YJ, et al. (2011) Snailase preparation of ginsenoside M1 from protopanaxadiol-type ginsenoside and their protective effects against CCl4-induced chronic hepatotoxicity in mice. Molecules 16(12): 10093–103. 10.3390/molecules161210093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Noh KH, Son JW, Kim HJ, Oh DK (2009) Ginsenoside compound K production from ginseng root extract by a thermostable β-glycosidase from Sulfolobus solfataricus . Biosci Biotechnol Biochem 73(2): 316–21. [DOI] [PubMed] [Google Scholar]
- 33. Liu C-Y, Zhou R-X, Yu H-S, Jin Y-H, Sun C-K, Zhang T-Y, et al. (2014) Preparation of minor ginsenosides C-Mc, C-Y, F2, and C-K from American ginseng PPD-ginsenoside using special ginsenosidase type-I from Aspergillus niger g.848. J Ginseng Res (0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lim YR, Yoon RY, Seo ES, Kim YS, Park CS, Oh DK (2010) Hydrolytic properties of a thermostable α-L-arabinofuranosidase from Caldicellulosiruptor saccharolyticus . J Appl Microbiol 109(4): 1188–97. 10.1111/j.1365-2672.2010.04744.x [DOI] [PubMed] [Google Scholar]
- 35. Park AR, Kim HJ, Lee JK, Oh DK (2010) Hydrolysis and transglycosylation activity of a thermostable recombinant β-glycosidase from Sulfolobus acidocaldarius . Appl Biochem Biotechnol 160(8): 2236–47. 10.1007/s12010-009-8705-x [DOI] [PubMed] [Google Scholar]
- 36. Shin KC, Lee GW, Oh DK (2013) Production of ginsenoside Rd from ginsenoside Rc by α-L-arabinofuranosidase from Caldicellulosiruptor saccharolyticus . J Microbiol Biotechnol 23(4): 483–8. [DOI] [PubMed] [Google Scholar]
- 37. Yan X, Fan Y, Wei W, Wang P, Liu Q, Wei Y, et al. (2014) Production of bioactive ginsenoside compound K in metabolically engineered yeast. Cell Res 24(6): 770–3. 10.1038/cr.2014.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shin KC, Oh HJ, Kim BJ, Oh DK (2013) Complete conversion of major protopanaxadiol ginsenosides to compound K by the combined use of α-L-arabinofuranosidase and β-galactosidase from Caldicellulosiruptor saccharolyticus and β-glucosidase from Sulfolobus acidocaldarius . J Biotechnol 167(1): 33–40. 10.1016/j.jbiotec.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 39. Park SY, Bae EA, Sung JH, Lee SK, Kim DH (2001) Purification and characterization of ginsenoside Rb1-metabolizing β-glucosidase from Fusobacterium K-60, a human intestinal anaerobic bacterium. Biosci Biotechnol Biochem 65(5): 1163–9. [DOI] [PubMed] [Google Scholar]
- 40. Liu C, Jin Y, Yu H, Sun C, Gao P, Xiao Y, et al. (2014) Biotransformation pathway and kinetics of the hydrolysis of the 3-O- and 20-O-multi-glucosides of PPD-type ginsenosides by ginsenosidase type I. Process Biochem 49(5): 813–20. [Google Scholar]
- 41. Yu H, Zhang C, Lu M, Sun F, Fu Y, Jin F (2007) Purification and characterization of new special ginsenosidase hydrolyzing multi-glycisides of protopanaxadiol ginsenosides, ginsenosidase type I. Chem Pharm Bull (Tokyo) 55(2): 231–5. [DOI] [PubMed] [Google Scholar]
- 42. Hou JG, Xue JJ, Sun MQ, Wang CY, Liu L, Zhang DL, et al. (2012) Highly selective microbial transformation of major ginsenoside Rb1 to gypenoside LXXV by Esteya vermicola CNU120806. J Appl Microbiol 113(4): 807–14. 10.1111/j.1365-2672.2012.05400.x [DOI] [PubMed] [Google Scholar]
- 43. Jin XF, Yu HS, Wang DM, Liu TQ, Liu CY, An DS, et al. (2012) Kinetics of a cloned special ginsenosidase hydrolyzing 3-O-glucoside of multi-protopanaxadiol-type ginsenosides, named ginsenosidase type III. J Microbiol Biotechnol 22(3): 343–51. [DOI] [PubMed] [Google Scholar]
- 44. Kim BH, Lee SY, Cho HJ, You SN, Kim YJ, Park YM, et al. (2006) Biotransformation of Korean Panax ginseng by Pectinex. Biol Pharm Bull 29(12): 2472–8. [DOI] [PubMed] [Google Scholar]
- 45. Kim EH, Lim S, Kim SO, Ahn SH, Choi YJ (2013) Optimization of enzymatic treatment for compound K production from white ginseng extract by response surface methodology. Biosci Biotechnol Biochem 77(5): 1138–40. [DOI] [PubMed] [Google Scholar]
- 46. Choi HJ, Kim EA, Kim DH, Shin KS (2014) The Bioconversion of Red Ginseng Ethanol Extract into Compound K by Saccharomyces cerevisiae HJ-014. Mycobiology 42(3): 256–61. 10.5941/MYCO.2014.42.3.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhou W, Yan Q, Li JY, Zhang XC, Zhou P (2008) Biotransformation of Panax notoginseng saponins into ginsenoside compound K production by Paecilomyces bainier sp. 229. J Appl Microbiol 104(3): 699–706. 10.1111/j.1365-2672.2007.03586.x [DOI] [PubMed] [Google Scholar]
- 48. Hsu BY, Lu TJ, Chen CH, Wang SJ, Hwang LS (2013) Biotransformation of ginsenoside Rd in the ginseng extraction residue by fermentation with lingzhi (Ganoderma lucidum). Food Chemistry 141(4): 4186–93. 10.1016/j.foodchem.2013.06.134 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ginsenoside Rc was converted to the reaction products by only SS-bgly and SS-bgly supplemented with CS-abf. Lane S, ginsenoside standards; Lane 1, reaction products of ginsenoside Rc by SS-bgl alone; Lane 2, reaction products of ginsenoside Rc by SS-bgly supplemented with CS-abf.
(TIF)
The enzymes were incubated at 70°C (open square), 75°C (filled square), 80°C (open circle), 85°C (filled circle), and 90°C (filled triangle) for varying time periods. Data represent the means of three experiments and error bars represent standard deviation.
(TIF)
HPLC analysis of C-K (27.9 min) produced from ginsenosides Rb1 (9.6 min), Rb2 (11.9 min), Rc (11.4 min), and Rd (12.8 min) present in RGE via F2 (18.1 min) and compound Mc (23.4 min) by SS-bgly supplemented with CS-abf at 0, 2, and 12 h.
(TIF)
Data Availability Statement
All data are all contained within the paper and its Supporting Information files.