Abstract
For most people, adolescence is synonymous with emotional turmoil and it has been shown that early difficulties with emotion regulation can lead to persistent problems for some people. This suggests that intervention during development might reduce long-term negative consequences for those individuals. Recent research has highlighted the suitability of real-time fMRI-based neurofeedback (NF) in training emotion regulation (ER) networks in adults. However, its usefulness in directly influencing plasticity in the maturing ER networks remains unclear. Here, we used NF to teach a group of 17 7–16 year-olds to up-regulate the bilateral insula, a key ER region. We found that all participants learned to increase activation during the up-regulation trials in comparison to the down-regulation trials. Importantly, a subsequent Granger causality analysis of Granger information flow within the wider ER network found that during up-regulation trials, bottom-up driven Granger information flow increased from the amygdala to the bilateral insula and from the left insula to the mid-cingulate cortex, supplementary motor area and the inferior parietal lobe. This was reversed during the down-regulation trials, where we observed an increase in top-down driven Granger information flow to the bilateral insula from mid-cingulate cortex, pre-central gyrus and inferior parietal lobule. This suggests that: 1) NF training had a differential effect on up-regulation vs down-regulation network connections, and that 2) our training was not only superficially concentrated on surface effects but also relevant with regards to the underlying neurocognitive bases. Together these findings highlight the feasibility of using NF in children and adolescents and its possible use for shaping key social cognitive networks during development.
Highlights
-
•
Brain-based emotion regulation networks develop continuously throughout adolescence.
-
•
These networks can strengthen in response to fMRI neurofeedback (NF).
-
•
Here we used NF to increase insula response in a group of 7–17 year-olds.
-
•
Our NF training also had a strengthening effect on the emotion regulation network.
-
•
Future research could begin to explore the use of NF for facilitating clinical change.
Introduction
For most people, adolescence is synonymous with emotional turmoil (Guyer et al., 2012, Moor et al., 2010, Sebastian et al., 2011), which goes along with an increased risk for developing psychiatric disorders (Kessler et al., 2005, Paus et al., 2008). Yet the current scientific evidence suggests that emotional reactivity per se does not change much in the transition from childhood to adulthood (McRae et al., 2012). Rather, most research up to date has shown that the observed change in emotional behaviour is due to continuous developmental improvement in the control and regulation of emotional responses (McRae et al., 2012, Silvers et al., 2012). The improvements in emotion control abilities are only part of a general programme of development in that they go along with substantial cognitive and physiological maturation (Blakemore, 2008, Burnett et al., 2011). At the brain level, the on-going development is reflected in both grey and white matter changes (Giedd et al., 1999, Harris et al., 2011, Lebel and Beaulieu, 2011, Petanjek et al., 2011, Tamnes et al., 2013), as well as increased functional connectivity in default and resting state brain networks (Fair et al., 2007, Fair et al., 2008). All these changes affect not only the brain structure, but also the functional responsiveness and processing abilities of the developing brain. It has been suggested that the timing of this transformational process, which coincides with a period of significant social cognitive change, could help explain the increased risk for developing certain mental disorders (Haller et al., in pressa, Haller et al., in pressb, Keshavan et al., 2014, Paus et al., 2008).
With regard to emotion regulation, a handful of developmental functional magnetic resonance imaging (fMRI) studies have consistently found changes in subcortical emotion regulation regions, such as the amygdala (Scherf et al., 2012, Scherf et al., 2013) as well as anterior and lateral functional subdivisions of the prefrontal cortex (PFC) in response to emotional stimuli across childhood and adolescence (Guyer et al., 2012, Moor et al., 2010, Sebastian et al., 2011). These have been interpreted as improved recruitment of prefrontal regions in order to effectively down-regulate subcortical arousal (Nelson et al., 2005). Further support for this interpretation comes from data showing that functional regulatory connections between PFC and subcortical regions continue to mature throughout childhood and adolescence (Crone, 2014, Hare et al., 2008, Perlman and Pelphrey, 2011, Pitskel et al., 2011). For example, a recent study by Gee et al. (2013) reported a shift towards negative connectivity in the amygdala–medial PFC network (with decreasing amygdala responsivity corresponding to an increase in medial PFC activity) during the viewing of negative faces from the age of 10 years onwards. We note however that a mere focus on maturational changes in subcortical emotion processing regions, such as the amygdala (Scherf et al., 2013) and prefrontal cortex regions neglects substantial concurrent changes in social cognitive processes and peer interactions, all of which are likely to shape emotion processing to a similar extent (Blakemore and Mills, 2014, Crone and Dahl, 2012, Pfeifer and Allen, 2012).
In view of the prolonged developmental trajectories of the neurocognitive bases of emotion regulation abilities, it seems plausible that neuro-behavioural plasticity – and hence the window for successful interventions – is also extended (Cohen Kadosh et al., 2013, Thompson-Schill et al., 2009). For example, one could imagine that while emotion regulation networks are being set-up, they may also be more amenable to interventions that aim to shape both cognitive processing strategies as well as functional responsiveness in the emerging brain regions (Cohen Kadosh et al., 2013, Gogtay et al., 2004, Tamnes et al., 2013, Thompson-Schill et al., 2009). One such intervention approach is real-time fMRI-based neurofeedback (NF). NF is a newly emerging technique that utilises the latest developments of real-time data processing and pattern analysis in order to train participants in the self-modulation of neural networks. It has been suggested that fMRI-based NF could be used to help influence brain responses at crucial developmental junctures (Cohen Kadosh et al., 2013, Haller et al., in pressa, Haller et al., in pressb, Platt et al., 2013). Specifically, it could be used as a tool to explore response plasticity in the developing cortical networks for emotion regulation and, most importantly, to help shape these networks in the most optimal way (Cohen Kadosh et al., 2013).
In fMRI-based NF studies, participants are presented with real-time brain activation in specific regions of interest (for example through a visually-presented thermometer) and they learn to reliably regulate their online brain response with high spatial precision (deCharms, 2007, deCharms et al., 2005, Johnston et al., 2010, Weiskopf et al., 2004a, Weiskopf et al., 2004b). NF has proven particularly useful for up- or down-regulating the brain regions involved in healthy adults' emotional responses (Johnston et al., 2010, Johnston et al., 2011, Paret et al., 2014, Zotev et al., 2011, Zotev et al., 2013). In addition, it has been used to change brain responses in clinical populations, such as participants with schizophrenia (Ruiz et al., 2013) depression (Linden et al., 2012, Young et al., 2014, Yuan et al., 2014) or Parkinson's (Subramanian et al., 2011). One particular advantage of fMRI-based (compared to EEG-based) NF lies in its high spatial resolution, which can be used to directly target and train brain networks rather than single regions. For example, in two recent studies by Zotev et al., 2011, Zotev et al., 2013, where healthy adults learned to successfully up-regulate their left amygdala, they also observed significant increases in functional connectivity between different regions of the amygdala network comprising the right medial frontal polar cortex, the bilateral dorsomedial prefrontal cortex, the left anterior cingulate cortex, and bilateral superior frontal gyri. This is important, as it shows that NF does not only affect brain responses within a specific brain region (i.e. the left amygdala), but also the processing flow within a larger network of regions.
NF may be particularly useful in targeting brain regions that are undergoing maturational change — and which may be more responsive to external interventions. Moreover, the network-based effect is important from a developmental perspective, as it would allow us to time interventive approaches to coincide with a period of substantial brain and cognitive development such as adolescence. In addition, it seems likely that any changes to neurocognitive circuitry will have knock-on effect on behaviour that is stronger and more persistent than at other developmental stages (Cohen Kadosh et al., 2013). However, up until now, all NF-based research on emotion regulation networks has been conducted with healthy (Caria et al., 2010, Johnston et al., 2010, Johnston et al., 2011, Paret et al., 2014, Zotev et al., 2011) or clinical (Linden et al., 2012, Ruiz et al., 2013, Young et al., 2014, Yuan et al., 2014) adult populations. The current study aimed to establish the feasibility of using NF in children and adolescents. Our main aim was to teach children and adolescents to gain control over the insula region in a simple NF up-regulation task in comparison to a rest condition. In the up-regulation condition, participants were given NF information with an instruction to keep activation levels high using a specific strategy. In the rest condition, participants also received NF information and an instruction to keep the signal low but with no specific strategy. We therefore subsequently refer to this rest condition as the down-regulation condition. We chose the right insula region, as it is a key region in the emotion regulation network (Kohn et al., 2014, Wager and Feldman-Barrett, 2004). It is also functionally well connected with the amygdala and PFC regions, which are all relevant for improving emotion regulation abilities during development (Gee et al., 2013, Pitskel et al., 2011). In addition, previous studies have shown that the insula responds reliably to modulation interventions (Pitskel et al., 2011), and particularly NF-based ones where the NF-intervention does have a more wide-spread effect on the emotion regulation network (Ruiz et al., 2013). A second aim of the study was therefore to assess the wider effect of NF training on the developing emotion regulation network, and particularly on changes in bottom-up and top-down Granger information flow between the different brain regions for the two task conditions (up-regulation vs down-regulation).
Methods
Participants
Nineteen children and adolescents (average age = 11.6 years, SD = 2.5, range 7–16 years, 8 females) were recruited from the local Cardiff community via word-of-mouth. We specifically chose to recruit across a large age-range to establish the feasibility of this research approach for children and adolescents. All participants had normal or corrected-to-normal vision and reported no history of neurological or psychological illness (as determined via self-report). Informed consent was obtained from the primary caregiver and informed assent was obtained from the child/adolescent prior to testing. Participants received an Amazon voucher (£20) for participating in the experiment. The study was approved by the local ethics committee (School of Psychology, Cardiff University).
Experimental task and stimuli
Localiser task
We use a modified version of the Overlap task (Bindemann et al., 2005, Cohen Kadosh et al., 2014) to localise the target region for the subsequent NF runs (Fig. 1). The Overlap task consists of a stimulus set of 9 colour photographs of female faces (3 women × 3 emotional expressions (fearful, happy, and neutral)) that were selected from the NimStim set.1 All pictures were cropped to show the face in frontal view and to exclude the neck and haircut of the person. For the face + target stimuli, a fixation cross was superimposed onto the face between the two eyes, and two black peripheral lines were presented on each side of the face. In total, 36 different stimuli (3 women × 3 expressions × target right or left of the face × green/red fixation cross (go/no-go trials)) were created. Note that we used only female faces in the current study in order to keep any task-irrelevant stimulus variation at a minimum. This approach was chosen, as it has been shown that facial identity serves a reference frame for interpreting emotional expressions (Cohen Kadosh, 2011, Ganel and Goshen-Gottstein, 2004) and that sex changes influence identity processing (Ganel and Goshen-Gottstein, 2002).
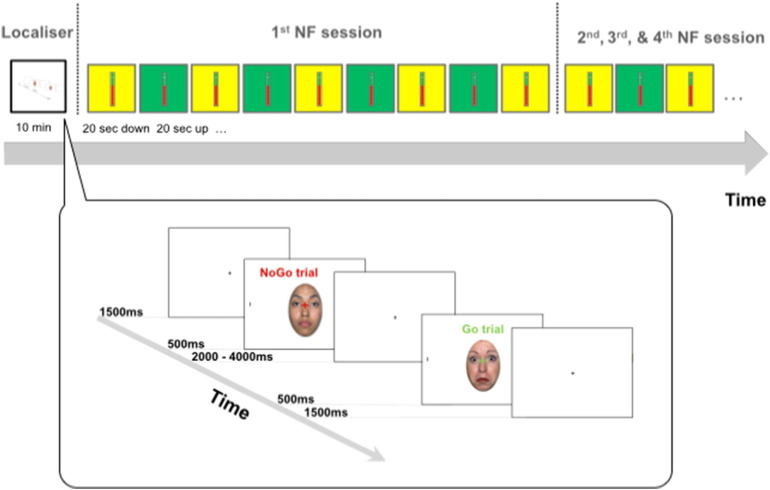
Fig. 1.
Top: Experimental procedure. During the neurofeedback runs (4 in each of the 4 sessions), participants alternated between 20 s periods of down-regulation and 20 s periods where they had to up-regulate activity in the target area. The level of activation was fed back in real time (updated for each TR of 2 s) through the thermometer display. Bottom: Two sample trials in the localiser task. A fixation cross was replaced by an emotional face + fixation cross flanked by two bars. A red fixation cross indicated a NoGo trial, where no action was required. A green fixation cross indicated a Go trial, where participants had to disengage from the face as quickly as possible in order to detect the horizontal target bar.
Procedure
We used a 3 Tesla 3T GE (General Electric) HDx MR system to acquire MRI and fMRI data at the Cardiff University Brain Research Imaging Centre. Each participant first underwent a localiser scan, which was followed by four NF runs, using a single shot echo-planar imaging sequence (TR = 2 s, TE = 35 ms, 30 slices, 3 mm slice thickness, inplane resolution 2 mm × 2 mm). Following the functional scans, a T1-weighted structural image (1 mm3 resolution) was acquired for co-registration and display of the functional data. Two participants, one female and one male did not continue on to participate in the NF runs and 1 male completed only 2 NF runs. Immediately following the scanning session, participants were asked to complete the Moods and Feelings questionnaire (Angold et al., 1995) and the Cognitive Emotion Regulation Questionnaire (CERQ) (Garniefski et al., 2001).
Localiser task
Each trial began with a central black fixation cross on a white background, being presented for 1500 ms. The fixation cross was then replaced for 500 ms by the face + target stimulus, with a red or green fixation cross super-imposed onto a face flanked by two peripheral black lines. The colour of the fixation cross indicated whether the trial was a go trial (green colour) or a no-go trial (red colour). During the go trials, the participant's task was to indicate which of the two lines on either side of the face was presented horizontally. Participants were instructed to indicate the location of the target stimulus via a button press on a response box, with the right button corresponding to a target on the right side of the face and the left button corresponding to a target on the left side of the face. During no-go trials, participants were instructed not to respond and to wait for the next trial to begin. The face + target stimulus was followed by a white screen with black fixation cross, which was displayed for 2000–4000 ms, or until a response was registered (Fig. 1 bottom). Each session began with 12 practice trials (6 go trials, 6 no-go trials), with each emotional expression being shown 4 times. The practice was followed by 4 blocks of 36 trials with a ratio of 2:1 go (24) to no-go (12) trials, with each facial expression (fearful/neutral/happy) being shown an equal number of times in the trials. Additionally, we created three pseudo-randomised variations of the task to ensure that each emotional expression and trial type varied systematically throughout the blocks.
Neurofeedback task
The localiser task was followed by four NF runs. We used TurboBrainvoyager (BrainInnovations, Maastricht, Netherlands) for the online analysis during the NF runs. Each run consisted of 5 20 second down-regulation blocks and 4 20 second up-regulation blocks (Fig. 1 top). Each participant's target area (right anterior insula) was identified based on an average effect contrast across all conditions (2 trial types × 3 emotional expressions) in the preceding localiser task. The participant's task was to increase activity in the insula region during the regulation blocks and to keep activation low during the down-regulation blocks. For the up-regulation runs the thermometer was superimposed on a green background and participants were instructed to ‘think happy thoughts’ (to induce activation), i.e. to try and think of something that would ‘make them feel happy’. During the down-regulation runs, the thermometer was superimposed on a yellow background and they were told ‘to relax’, almost like ‘turning off a car engine’, and to keep the thermometer low. During the runs, a continuous signal from the target area (updated every TR and thus every 2 s) was displayed using the picture of a thermometer whose dial indicated the amplitude of the fMRI signal in the target area (Fig. 1 top). We note that the thermometer provided feedback on real-time brain responses in both conditions. Changes in the amplitude were indicated as the percent of signal change, calculated using the current signal intensity value and comparing it with the average value determined from the down-regulation period immediately preceding each up-regulation block. The scaling of the thermometer was in steps of 0.05%, with a maximum value of 0.5%. This range was chosen based on previous, successful NF studies in both healthy and clinical populations (e.g., Linden et al., 2012). A change of background colour every 20 s indicated to participants whether their task was to up-regulate (green background) or to down-regulate activation (yellow background).
The online GLM was computed with one predictor for the regulate state, convolved with a haemodynamic reference function. The top one-third (defined by the t value for the contrast between the regulate predictor and baseline) of the voxels from the target region (the right insula for all participants) was used to compute the feedback signal. Participants were also instructed to keep head movement to a minimum and fixate the middle of the display during both, the localiser and the NF in order to avoid eye movements.
FMRI analyses
Data were analysed using SPM8 (Wellcome Department of Imaging Neuroscience; http://www.fil.ion.ucl.ac.uk/spm). The pre-processing analysis was identical for the localiser and NF runs. First, a slice-scan time correction was applied to all runs. Then, EPI volumes were spatially realigned to correct for movement artifacts, normalised to the Montreal Neurological Institute (MNI) standard space (Ashburner and Friston, 2003a, Ashburner and Friston, 2003b) and smoothed using an 8-mm Gaussian kernel. We note that in order to maintain high levels of comparability across the entire participant age range (7–17 years), we chose to use the same template for spatial normalisation in all subjects. Further, there is also good evidence that brain scans from participants aged 5 years and onwards can be reliably mapped onto adult space (Kang et al., 2003).
For the localiser run, a general linear model was computed with 6 regressors, one for each condition in the design (2 trial types × 3 emotional expressions). In addition, a covariate was included with the mean accuracy rates for each participant (collapsed across emotional expressions, as the main effect of expression or the interaction between trial type × expression was not significant) to prevent the possibility of proficiency-dependent differences affecting the fMRI results. We note that participants across the age range achieved good accuracy levels (accuracy rates (mean/standard deviation): fear: 83%/15%; happy trials: 85%/13%; neutral: 78%/19%). To account for (linear) residual movement artifacts, the model also included 6 further regressors representing the rigid-body parameters estimated during realignment (note that none of the participants included in this data set exhibited greater than 3-mm deviation in the centre of mass in any direction). Voxelwise parameter estimates for these regressors were obtained by restricted maximum-likelihood estimation using a temporal high-pass filter (cutoff = 128 s) to remove low frequency drifts, and modelling temporal autocorrelation across scans with an AR(1) process. Images of these parameter estimates comprised the data for a second GLM that treated participants as the only random effect. This GLM included the 6 conditions of interest, using a single pooled error estimate, whose nonsphericity was estimated using restricted maximum-likelihood estimation as described in Friston et al. (2002). Note that apart from the region of interest (ROI) analyses, the results from the localiser task will not be reported here.
For the NF runs, each block was modelled as an epoch of 20 s and convolved with a canonical hemodynamic response function. Voxel-wise parameter estimates for these regressors were obtained by restricted maximum likelihood estimation (ReML), using a temporal high-pass filter (cut-off 128 s) to remove low-frequency drifts, and modelling temporal autocorrelation across scans with an Auto-regression (1) process. Finally, to obtain the areas for the ROI analyses and the subsequent Granger causality analyses, eight 10-mm ROIs were localised based on group local maxima for an average effect contrast in the localiser task, as well as two 10-mm ROIs based at the peak voxel coordinates in the bilateral insula in the individual, using the same contrast. This independent analysis approach was chosen to avoid the issue of double dipping (Kriegeskorte et al., 2009, Vul et al., 2009). For the NF ROI analyses, we first extracted the BOLD time series in the bilateral insula in each participant individually (mean and standard deviation of the coordinates are for peak voxel location, x, y, and z, in MNI space): left insula (lINS): − 37(6), 9(7), 4(3); right insula (rINS): 39(4), 8(8), 1(4). These insula clusters correspond to the individual NF target areas. In addition, for the Granger causality analysis, we extracted the time-series of BOLD activations in 8 core emotion regulation network regions. The selection of these 8 regions was based on a recent meta-analysis of 23 studies (Kohn et al., 2014), which used fMRI or PET to investigate cognitive emotion regulation in adults, as well as a recent fMRI-based-NF study on emotion regulation in patients with schizophrenia (Ruiz et al., 2013), who were taught to gain control over the bilateral insula regions. The following ROIs were selected for the Granger Causality Analysis: left amygdala (lAMY): − 21, − 3, − 7; lINS: − 39, 14, 3; rINS: 36, 15, 3; left mid-cingulate cortex (MCC): − 6, 18, 39; left middle frontal gyrus (lMFG): − 38, 34, 27; left medial frontal gyrus/supplementary motor area (lSMA): − 2, 16, 50; left intra-parietal lobule (lIPL): − 60, − 48, 35; left precentral gyrus (lPreG): − 48, 2, 32. (See also Table S4 and Fig. S2 for a whole-brain analysis of the NF runs).
Granger causality analysis
Following the ROI of our NF target regions, we conducted a Granger causality analysis (GCA) to assess the extended effect of NF-induced changes on the extended emotion regulation network. GCA is a widely used research approach that allows us to investigate how changes in brain activation over time in different brain regions relate to each other (Palaniyappan et al., 2013, Wen et al., 2012, Hamilton et al., 2011, Ge et al., 2012, Guo et al., 2008, Luo et al., 2011, Luo et al., 2013a, Luo et al., 2013b). Crucially, GCA also can provide insights into the directional Granger information flow between brain regions, also known as effective connectivity, which is currently impossible to explore experimentally (Park and Friston, 2013). For the current fMRI study, we adopted a previously successful GCA analysis approach (Wen et al., 2013), which included several important pre-processing steps (Smith et al., 2012), such as outlier removal, baseline correction and an analysis of the percent signal change within blocks (see also supplementary Tables S2–3).
However, the assumption that the time series models during the up-regulation blocks in different sessions would stay the same is likely to be an oversimplification, as fluctuations in the model coefficients are almost as certain as the physiological oscillations in the BOLD signal. As discussed in detail previously (Luo et al., 2013b), assuming a static model to a time-varying casual structure usually leads to misleading estimation of Granger causality. We proposed and demonstrated the reliability of an averaged Granger causality (avGC), which was a new framework to tackle the time-varying causal structure (Luo et al., 2013b). Basically, the Granger causality for a pair of brain regions was estimated at each up-regulation/down-regulation block, and then the avGC was established by averaging the estimated Granger causality during up-regulation/down-regulation blocks across different sessions. Here, we used the avGC to measure the directed Granger information flow between brain regions.
To detect significant differences in the directed Granger information flow as a function of the two task conditions (up-regulation vs down-regulation), the avGC during up-regulation/down-regulation were tested against the null hypothesis of non-causality by the distribution of sum of many independent F statistics (Luo et al., 2013b). The results that survived the false discovery rate correction (FDR, p < 0.05) were reported for the different conditions at the group level. After the Granger information flow (i.e., the avGC) estimated at each direction between brain regions for each subject during down-regulation and during up-regulation separately, the paired t test was applied to compare the avGC during up-regulation with that during down-regulation at each The resulting p-values were FDR (p < 0.05) corrected for multiple comparisons.
Last, in order to understand the functional meaning of the directed Granger information flow detected by the avGC, we computed Pearson's correlation coefficients across subjects between the two experimental conditions (defined by contrast map given by SPM8) in the bilateral insula with age and sex in each subject as covariates.
Results
Successful insular cortex self-regulation during NF
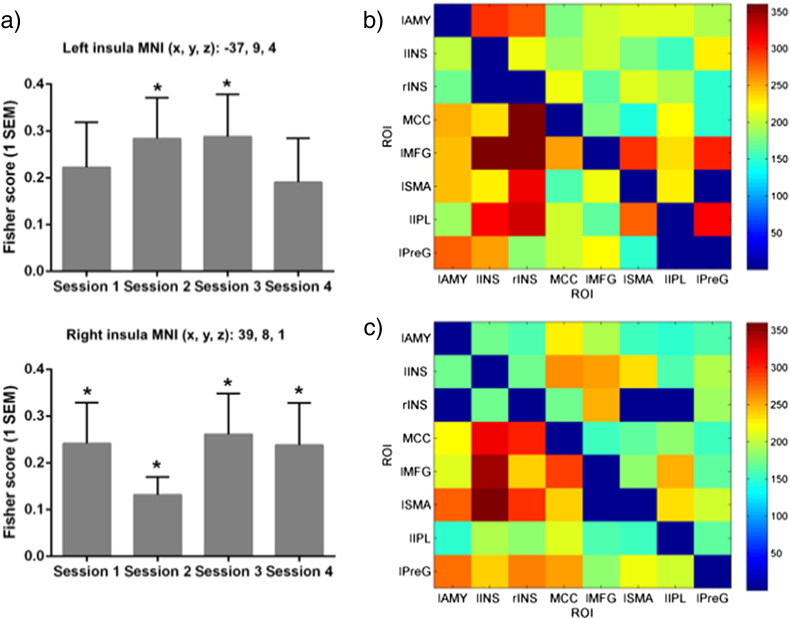
To assess the effect of the NF training on the BOLD signal increase in the left insula and right insula in each NF session, we computed a Fisher score (FS) (Ruiz et al., 2013), which measures the discriminability between BOLD signals of two conditions (in this case “down-regulation” and “up-regulation” blocks). A FS-based analysis allows us to take into account both, the variance and the mean BOLD signal change between two conditions, rather than just the mean difference between the two conditions, as is done conventionally (Ruiz et al., 2013). The FS is defined as the ratio of the square of the difference between the mean BOLD values in each time-series to the sum of the variance in the time-series. In order to assess whether the NF training was successful, we conducted one-sample t-tests on the Fisher scores to assess whether the up-regulation was statistically significant for all sessions in the bilateral insula. After applying Bonferroni correction for multiple comparisons, we found that participants were able to up-regulate the left insula in session 2 [(t(15) = 3.0, p = .01, bootstrapped CI 95%[.100, .426]) and 3 (t(15) = 3.1, p = .008, bootstrapped CI 95%[.113, .420]), but not in the first and last session where effects were trending only [session 1: (t(15) = 2.29, p = .044, bootstrapped CI%[.042, .328]); session 4: (t(15) = 2.0, p = .066, bootstrapped CI%[.030, .322])]. For the right insula however the picture looked different, with successful up-regulation in all four sessions [session 1: (t(15) = 2.7, p = .016, bootstrapped CI 95%[.087, .400]); session 2: (t(15) = 3.0, p = .009, bootstrapped CI 95%[.051, .192]); session 3: (t(15) = 2.93, p = .010, bootstrapped CI 95%[.103, .417]); session 4: (t(15) = 2.53, p = .023, bootstrapped CI 95%[.077, .443])]. (Fig. 2a, see also supplementary Fig. S1 for individual regulation success). We also conducted a one-factorial ANOVA with session as the within-subject factor (4 levels) to assess whether up-regulation differed between the four sessions to look at possible learning effects. We found that up-regulation did not differ significantly across the four sessions in both the left insula [(F(3, 45) = .427, p = .725) and the right insula (F(3, 45) = .474, p = .688)].
Fig. 2.
Results from the fMRI-based neurofeedback training: a). Fisher score (+ 1 standard error of the mean (SEM)) indicating the group BOLD-signal change in the left (top) and right (bottom) insula in the up-regulation vs the down-regulation blocks in the 4 neurofeedback sessions. Stars indicated a significant up-regulation effect vs down-regulation. b–c). Granger causality analysis of the directed Granger information flow in the emotion regulation network insula during the up-regulation condition (b) and the down-regulation condition (c). Abbreviations: lAMY = amygdala; lINS = left insula; rINS = right insula; IPL = left inferior parietal lobule; MCC = mid cingulate cortex; lMFG = left middle frontal gyrus; MNI = Montreal Neurological Institute template; lPreG = left precentral sulcus; lSMA = left supplementary motor area.
Immediately following the scanning session, we debriefed participants on their subjective strategies and experiences with the NF training. We found that on a scale from one (easy) to four (difficult), participants found the task on average fairly easy to fairly difficult (average score: 2.75, SD: 0.80). Participants were then asked in greater detail about their approach to generating happy thoughts by ticking one or several out of 4 possible answers. We found that 12 subjects thought of things that happened in the past, 11 subjects thought of things they would like to happen in the future, 2 tried not thinking about something that had been making them unhappy, and 6 thought about someone.
Last, we found that neither the participant's age, the perceived task difficulty, the Mood & Feelings correlated with up-regulation success in the bilateral insula [participant's age × left insula: (rs(16) = .190, p = .480, CI 95% [− .363, .649]); × right insula: (rs(16) = − .263, p = .326, CI 95% [− .837, .337]) (see also Fig. S3); perceived task difficulty × left insula: (rs(16) = .003, p = .991, CI 95% [− .459, .496]); × right insula: (rs(16) = .122, p = .652, CI 95% [− .417, .602]); Mood & Feelings score × left insula: (rs(16) = .296, p = .267, CI 95% [− .251, .710]); × right insula: (rs(16) = .118, p = .663, CI 95% [− .558, .476])]. This was different for the correlation of average left insula up-regulation × CERQ (trend-level) where (rs(14) = .552, p = .044, CI 95% [− .011, .887), however the CERQ × right insula up-regulation was not significant: (rs(14) = − .367, p = .197, CI 95% [− .802, .255], see also Fig. S4).
GCA reveals differential NF effect on information flow within the emotion regulation networks
We then used GCA to assess the effect of the NF training in the two task conditions on information in- and out-flow in the 8 emotion regulation network regions. For the up-regulation condition, we found a significant information in-flow from the lAMY, MCC, lMFG, lIPL, lSMA, lPreG to the bilateral insula (see Fig. 2b/c for all directed Granger information flow during up-regulation vs down-regulation). In contrast, during the down-regulation blocks, no bottom-up in-flow from the lAMY to rINS, and no significant in-flow from lIPL to rINS. To detect the significant change in Granger information flow during regulating, we statistically compared the Granger information flow at each direction between up-regulation and down-regulation conditions by paired t test, and the differences in Granger information flows at two directions, lAMY➔rINS (t = 3.97, p = 0.0011) and lPreG➔lMFG (t = 4.51, p = 0.0004), survived the multiple comparison correction (FDR, p < 0.05). The Granger information flows increased at both directions during up-regulation compared with down-regulation.
NF-dependent changes information flow to and from the bilateral insula
We then assessed whether the in- and out-flow of information in the bilateral INS regions correlated with the INS percent signal change activation (Figs. 3a,b, Table 1).
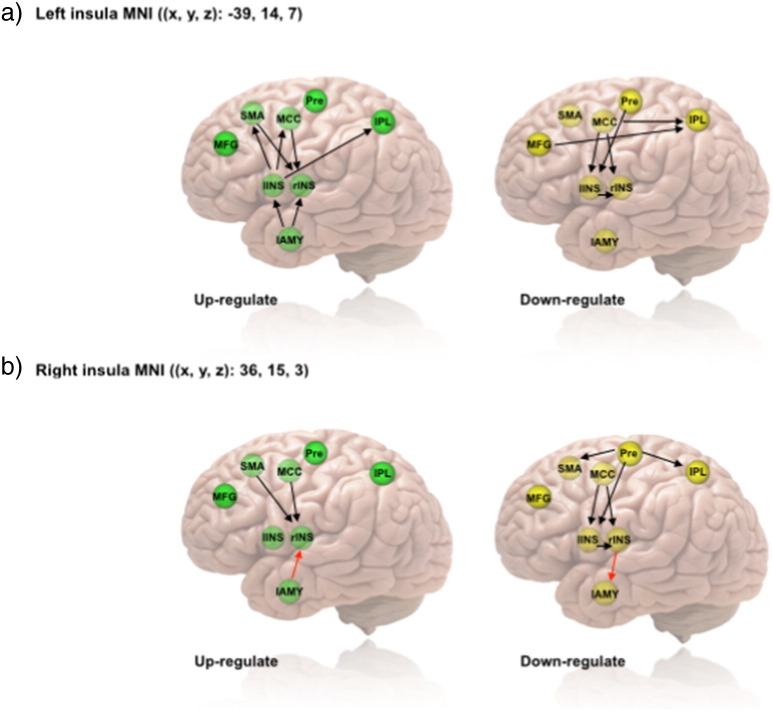
Fig. 3.
Granger causality analysis of the effective connectivity in the emotion regulation network as a function of percent signal change in the bilateral insula during the up-regulation condition a) and the down-regulation condition b). All arrows indicate significant correlations, whereas the red arrows indicate significant differences in amygdala–insula regulation (see Fig. 4) Abbreviations: lAMY = amygdala; lINS = left insula; rINS = right insula; IPL = left inferior parietal lobule; MCC = mid cingulate cortex; MFG = left middle frontal gyrus; Pre = left precentral sulcus; SMA = left supplementary motor area.
Table 1.
Comparison of correlations between directed Granger information flow and brain activity at insula for the two conditions. Only significant (p < 0.05, uncorrected) correlation were listed between the brain activities at bilateral insula and the directed Granger information flow in different directions among the brain regions of interest (i.e., ).
The correlation was calculated by conditioning on both age and sex of each subject. In the brackets, we listed the correlation between the brain activity and the change in directed Granger information flow for the down-regulation condition to the up-regulation condition (i.e., ). Abbreviation: l = left; r = right; SMA = supplementary motor area.
| Up-regulation condition |
Down-regulation condition |
||||
|---|---|---|---|---|---|
| Left Insula | r = | p = | Left Insula | r = | p = |
| Amygdala → l Insula | 0.28 (0.27) | 0.02 (0.03) | l Insula → r Insula | 0.31 (− 0.32) | 0.01 (0.01) |
| Amygdala → r Insula | 0.34 (0.38) | 0.01 (0.01) | Mid Cingulate Cortex → l Insula | 0.32 | 0.01 |
| l Insula → Mid Cingulate Cortex | 0.27 | 0.03 | Mid Cingulate Cortex → r Insula | 0.25 | 0.05 |
| l Insula → l SMA | 0.26 | 0.04 | Mid Cingulate Cortex → Inferior parietal lobule | 0.34 | 0.01 |
| l Insula → Inferior parietal lobule | 0.26 | 0.03 | Middle frontal gyrus → Inferior parietal lobule | 0.30 | 0.02 |
| Mid Cingulate Cortex → r Insula | 0.26 (− 0.26) | 0.04 (0.04) | Precentral gyrus → l Insula | 0.32 | 0.01 |
| l SMA → r Insula | 0.31 | 0.01 | |||
| Right Insula | r = | p = | Right Insula | r = | p = |
| Amygdala → r Insula | 0.25 (0.26) | 0.05 (0.04) | r Insula → Amygdala | 0.31 | 0.01 |
| Mid Cingulate Cortex → r Insula | 0.31 | 0.01 | Mid Cingulate Cortex → l Insula | 0.32 | 0.01 |
| l SMA → r Insula | 0.28 | 0.03 | Mid Cingulate Cortex → r Insula | 0.25 | 0.05 |
| Mid Cingulate Cortex → Inferior parietal lobule | 0.34 | 0.01 | |||
| Precentral gyrus → l Insula | 0.35 | 0.01 | |||
| Precentral gyrus → SMA | 0.27 | 0.03 | |||
| Precentral gyrus → Inferior parietal lobule | 0.30 | 0.02 | |||
Right insula
A significant positive correlation (r = 0.25, p = 0.045) was found between the directed Granger information flow from the lAMY to the rINS, GClAMY → rINS(up − regulate) and the brain activity in the rINS in the up-regulation condition, but not for the down-regulation condition. Interestingly, during the down-regulation blocks, we observed a reversal in the Granger information flow from the rINS to the lAMY, GCrINS → lAMY(down − regulate), which was also positively correlated with the brain activity at rINS (r = 0.30, p = 0.015). That is, the stronger the bottom-up Granger information flow from the lAMY to the rINS, the stronger the activity in the rINS in the up-regulation condition, a finding which suggests an effective bottom-up control during these blocks. Crucially, this effect was reversed for the down-regulation condition, with a more directed Granger information flow from the rINS to the lAMY predicting stronger activity of the rINS.
The positive correlation between the brain activity of the rINS and the directed Granger information flow was observed for several other directions, including MCC➔rINS and lSMA➔rINS in the up-regulation condition, and the MCC➔rINS and lPreG➔rINS in the down-regulation condition. The bottom-up Granger information flow from lAMY to lINS was also found to increase the activity of lINS (r = 0.28, p = 0.024) during the up-regulation condition but not during the down-regulation condition.
Left insula
For the lINS, a few out-flows of lINS, including those from lINS to MCC, lSMA, and lIPL, positively correlated with the activity at lINS in the up-regulation condition, while a few in-flows, including those from MCC and lPreG to lINS were positively correlated with the activity.
NF training significantly increases amygdala–insula connectivity in the up-regulation condition
We compared the magnitudes of the directed Granger information flow for the two experimental conditions, the difference between the Granger causality during the up-regulation condition and that during the down-regulation condition at each direction.
Right insula
We found that the directed Granger information flow from the lAMY to the rINS as measured by Granger causality was significantly increased (t = 3.97, p = 0.001) for the up-regulation condition in comparison to the down-regulation condition (Fig. 4a), whereas the out-flow from bilateral insula to the other regions of interest did not differ for the two task conditions.
Fig. 4.
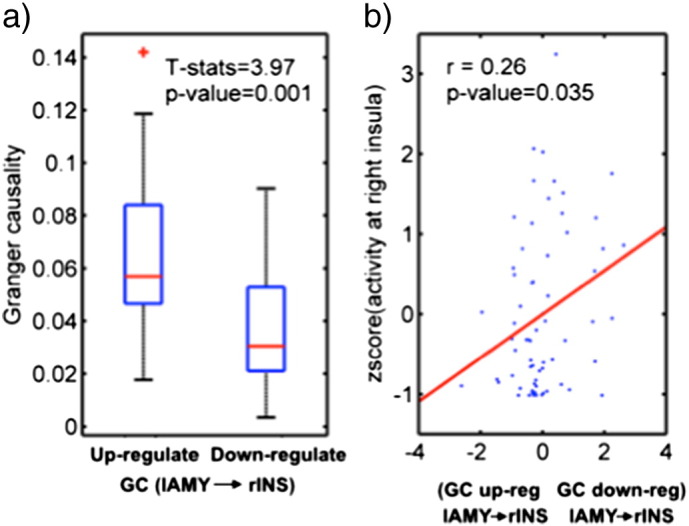
Change in bottom-up Granger information flow and its correlation with brain activity of rINS. a) Comparison of Granger causality between the average brain response in the up-regulation condition and the down-regulation condition. b) The correlation between the change in the directed information flow from lAMY to rINS and the brain activity at rINS. For each session, the brain activity was plotted against the change in the Granger causality at lAMY➔rINS. The red line is the linear fitting. See also Fig. 3b.
We then assessed the functional meaning of the change in the directed Granger information flow from the down-regulation condition to the up-regulation condition, by looking at task performance, i.e. the successful up-regulation of the insula region. To this end, we computed the partial correlation coefficient between the change in the directed Granger information flow and the brain activity at insula as a function of age and sex in each subject. The significantly change in the bottom-up Granger information flow from the lAMY to the rINS, GClAMY → rINS(up − regulate) − GClAMY → rINS(down − regulate) was found to be positively correlated (r = 0.26, p = 0.035) with the brain activity in the rINS.
Left insula
The change in the directed Granger information flow from the lAMY to the lINS from the up-regulation to the down-regulation condition was also positively correlated (r = 0.27, p = 0.028) with the brain activity in the lINS (Fig. 4b). That is, the stronger the increase in directed Granger information flow from the lINS to the rINS, the less activity was observed at lINS in the up-regulation condition. Moreover, a significant negative correlation (r = − 0.32, p = 0.009) was observed between the causality change in this direction from down-regulation to up-regulation and the brain activity of the lINS.
Discussion
The current study had two main aims: 1) to show the feasibility of using fMRI-based neurofeedback (NF) in children and adolescents and 2) to assess the differential effect of NF training on the wider emotion regulation network. Our results allowed us to fulfil both aims.
NF training enhances insula activation in children and adolescents
We found that all participants were able to up-regulate activation in the bilateral insula during the up-regulation blocks in comparison to down-regulation blocks, which supports the feasibility of using fMRI-based NF with children and adolescents. We note though that up-regulation of BOLD signal is not the same as up-regulating neuronal firing as it is a vascular measure.
The important role of the right insula in self-relevant affect has been repeatedly shown in previous research (Craig, 2003, Wager and Feldman-Barrett, 2004) and this result is particularly striking given our simple task instruction to “think happy thoughts”. The NF success in the current study opens up possibilities for new, brain-based intervention approaches, which take into account the developmental changes in the developing brain (Cohen Kadosh et al., 2013). For example, within the context of emotion regulation, NF could be used to both increase and decrease the responsiveness of age-appropriate brain networks at critical developmental stages (Paret et al., 2014). Similarly, such an approach could be useful for enhancing helpful brain network connections in at-risk populations, such as for example high socially-anxious children and adolescents (Haller et al., in pressa, Haller et al., in pressb). However in order to identify these network connections, a better understanding of the wider effects of insula regulation on the emotion regulation network is necessary.
NF training increase bottom-up driven Granger information flow in the emotion regulation network
In the present study, we also found that NF training had a differential effect on the Granger information flow within the emotion regulation network. That is the bottom-up driven Granger information flow from the amygdala to the bilateral insula and from the left insula to the MCC, SMA and the IPL during the up-regulation blocks contrasted with more top-down driven Granger information flow to the bilateral insula from MCC and PreG and IPL during the down-regulation blocks. This finding validates the effectiveness of our ‘increase’ instruction to affect brain regions beyond the NF target region. It also shows that the two task conditions had a qualitatively different effect on the brain network. We note however, that we cannot rule out at this point that participants would not have been able to achieve similar effects without the live feedback in both conditions. With regard to overall network changes, we found that by using a simple task instruction, we were able to change Granger information flow along previously established emotion regulation routes in the brain (e.g., Ruiz et al., 2013). The more widespread effect of NF training has been previously shown in both healthy adults (Rota et al., 2011, Zotev et al., 2011), as well as patients with schizophrenia (Ruiz et al., 2013), but this is, to the best of our knowledge, the first study to document NF-induced regulation effects in the developing emotion regulation network and across a wide age range. We also note that our participants did all activate the same regions of the emotion regulation network and the general NF success was not affected by age or gender, which further highlights the feasibility of this research approach. What we cannot predict however, based on the current sample of 17 participants, whether directional Granger information flow also varied as a function of age, which is a critical question for the development of future intervention approaches — especially those aimed to particular developmental junctures. Finally, as we did not obtain puberty measures for the current sample, the present study cannot assess how puberty-induced hormonal changes may have affected the results, a shortcoming, which should be addressed in future studies.
Increased bottom-up Granger information flow from amygdala to insula correlates with NF success
We also found that increased bottom-up Granger information flow from the left amygdala to the right insula correlated with NF success in the up-regulation blocks. This effects runs in line with the research reported in a recent review, which highlighted the role of the left amygdala in increasing positive affect (Silvers et al., 2014). There is also evidence of amygdala–insula co-activation in humans, albeit more within the context of negatively-valenced emotions (Carlson et al., 2011, Phelps et al., 2001). Similarly, within the context of anxiety, a recent study found effective connectivity in a resting state analysis, as well as structural connectivity (Baur et al., 2013). In the current study, we did not find any evidence for top-down emotion regulation in the insula from ventromedial prefrontal cortex regions, as shown in previous studies (e.g. Hare et al., 2008, Perlman and Pelphrey, 2011, Pitskel et al., 2011). There are two possible explanations as to why this may be the case. The first has to do with the task itself and the fact that all observed effects are based on significant differences in Granger connectivity between the two conditions. Namely, our participants were not asked to control their emotions during the comparison down-regulation condition, but rather to lower insula response in these blocks. This would also reduce any top-down regulation effects during these blocks. Another possible explanation might be the considerable age range in our sample, which, given the prolonged maturational trajectory of the prefrontal cortex (Gogtay et al., 2004, Tamnes et al., 2013), is likely to have introduced considerable variance amongst our participants, which prevented us from finding a significant effect.
While the results from this study are certainly encouraging, many open questions remain concerning for example the longevity of the observed effect, and particularly the specificity and sustainability of any changes to the functional architecture of the emotion regulation network. Similarly, whereas the current design adapted training time for a sample of children and adolescents, future studies should focus on extending training time (by increasing the number of sessions), and could also include transfer runs to assess the generalisability of training effects in the brain.
In previous NF studies, some designs have also included a sham condition, where feedback is provided from a non-task-related brain region (deCharms et al., 2004). In the current study, we chose not to adopt such as design as we believe that it comes with its own, serious ethical and scientific problems particularly in the context of paediatric samples. For example, we were concerned with the ethical implications of incorrect or incoherent feedback, when our participants are trying to establish a strategy that works for them. We believe that this is particularly problematic for participants like our children and adolescents, whose emotion regulation strategies, not to mention the underlying brain networks are still very plastic and shaping up (Cohen Kadosh et al., 2013, Haller et al., in pressa, Haller et al., in pressb). For example, we would be concerned that participants might be encouraged to abandon an otherwise successful strategy, simply because the feedback does not seem to support using it. Moreover, from a developmental perspective, brain regions that are used at an earlier developmental stage would not be necessarily relevant at a later stage and it would be extremely difficult to find a sham brain region that would support a comparable function at different ages. Specifically as it has been shown that brain networks undergo considerable restructuring throughout development (Fair et al., 2009, Johnson et al., 2009, Johnson et al., 2015). Given these scientific and ethical reasons we believe that another way forward might be to provide authentic feedback during the up-regulation conditions and to compare the regulation success against a resting baseline (where feedback is still given) and to focus more on the transfer effects (e.g. behavioural emotion regulation abilities before and after training intervention, or mood assessments via questionnaires). This would still allow us to work towards establishing NF as a tool for brain-targeted interventions, while avoiding the pitfalls of interfering with a developing (or atypical) network. Another approach to establishing specificity of the NF intervention could be to target a different brain region within the same underlying brain network (such as the amygdala or the mid cingulate cortex) to compare the specificity of training for these regions both for up- vs down-regulation conditions and at rest. This would allow us to not only differentiate the directionality of the NF effect depending on task instruction, but also whether the different brain regions respond equally well. See also Arns et al. (2014) for a review of training protocols in the ERP-based NF literature.
It remains to be determined how NF relates to overt behaviour changes. Previous research in clinical populations has made some progress into this question by showing a reduction in chronic pain symptoms after 6 months (deCharms et al., 2005) or improved motor fluency in Parkinson's disease (Subramanian et al., 2011). A better understanding of the underlying mechanisms of the translational effects of NF would go a long way towards developing effective interventions during development. Finally, while our simple task instruction proved effective for the current study, we nevertheless found that in some cases this only lasted for a couple of runs, with participants failing to up-regulate in the last runs. Whether this was due to general fatigue or the lower effectiveness of our task instruction remains to be determined. Understanding these individual differences is important, if we want to enhance the effectiveness of these procedures by combining them with a more established cognitive training programme, such as attention/or cognitive bias modification (Bar-Haim, 2010, MacLeod and Holmes, 2011). Future studies are now needed to explore these individual differences in larger samples.
Conclusions
The current study provided proof-of-concept for using fMRI-based neurofeedback with children and adolescents. Within the context of an emotion regulation network, we were also able to show that NF training had a differential effect of up- and down-regulation connections within the network, suggesting that our training was not only superficially concentrated on surface effects but actually relevant with regard to the underlying neurocognitive bases of a key social cognitive ability. More research is now needed to investigate the longevity of the effects and to explore the possible combination of NF with cognitive training programmes, in particular with view of future intervention in clinical populations.
Acknowledgments
This work was supported by the European Commission FP7 Braintrain grant (602186).
The authors would like to thank Sita Deeg for help with participant recruitment and testing, and Niklas Ihssen and Isabel Habes for help with setting up the neurofeedback experiment. QL is partly supported by grants from the National Natural Sciences Foundation of China (No. 11101429, No.11471081). JF is a Royal Society Wolfson Research Merit Award holder, partially supported by the National Centre for Mathematics and Interdisciplinary Sciences (NCMIS) of the Chinese Academy of Sciences and Key Program of National Natural Science Foundation of China (No. 91230201), the National High Technology Research and Development Program of China (No. 2015AA020507), and the Key Project of Shanghai Science & Technology Innovation Plan (No. 15JC1400101).
Footnotes
Development of the NimStim Face Stimulus Set was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neuroimage.2015.09.070.
Appendix A. Supplementary data
Supplementary material.
References
- Angold A., Costello E.J., Messer S.C., Pickles A., Winder F., Silver D. The development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. Int. J. Methods Psychiatr. Res. 1995;5:237–249. [Google Scholar]
- Arns M., Heinrich H., Strehl U. Evaluation of neurofeedback in ADHD: the long and winding road. Biol. Psychol. 2014;95:108–115. doi: 10.1016/j.biopsycho.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Rigid body transformation. In: Frakoviak R.S., Friston K.J., Frith C., Dolan R.J., Price C., Zeki S., Ashburner J., Penny W., editors. Human Brain Function. 2nd ed. Academic Press; Oxford: 2003. pp. 635–654. [Google Scholar]
- Ashburner J., Friston K.J. Spatial normalization using basis functions. In: Frakoviak R.S., Friston K.J., Frith C., Dolan R.J., Price C., Zeki S., Ashburner J., Penny W., editors. Human brain function. 2nd ed. Academic Press; Oxford: 2003. pp. 655–672. [Google Scholar]
- Bar-Haim Y. Research review: attention bias modification (ABM): a novel treatment for anxiety disorders. J. Child Psychol. Psychiatry. 2010;51(8):859–870. doi: 10.1111/j.1469-7610.2010.02251.x. (JCPP2251 [pii]) [DOI] [PubMed] [Google Scholar]
- Baur V., Hanggi J., Langer N., Jancke L. Resting-state functional and structural connectivity within an insula–amygdala route specifically index state and trait anxiety. Biol. Psychiatry. 2013;73(1):85–92. doi: 10.1016/j.biopsych.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Bindemann M., Burton A.M., Hooge I.T., Jenkins R., de Haan E.H. Faces retain attention. Psychon. Bull. Rev. 2005;12(6):1048–1053. doi: 10.3758/bf03206442. (Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16615327) [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J. The social brain in adolescence. Nat. Rev. Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J., Mills K.L. Is adolescence a sensitive period for sociocultural processing? Annu. Rev. Psychol. 2014;65:187–207. doi: 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- Burnett S., Sebastian C., Cohen Kadosh K., Blakemore S.-J. The social brain in adolescence: evidence from functional magnetic resonance imaging and behavioural studies. Neurosci. Biobehav. Rev. 2011;35(8):1654-1564. doi: 10.1016/j.neubiorev.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caria A., Sitaram R., Veit R., Begliomini C., Birbaumer N. Volitional control of anterior insula activity modulates the response to aversive stimuli. A real-time functional magnetic resonance imaging study. Biol. Psychiatry. 2010;68:425–432. doi: 10.1016/j.biopsych.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Carlson J.M., Greenberg T., Rubin D., Mujica-Parodi L.R. Feeling anxious: anticipatory amygdalo-insular response predicts the feeling of anxious anticipation. Soc. Cogn. Affect. Neurosci. 2011;6(1):74–81. doi: 10.1093/scan/nsq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K. Differing processing abilities for specific face properties in mid-childhood and adulthood. Front. Psychol. 2011;2:400. doi: 10.3389/fpsyg.2011.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K., Linden D.E.J., Lau J.Y. Plasticity during childhood and adolescence: innovative approaches to investigating neurocognitive development. Dev. Sci. 2013;16(4):574–583. doi: 10.1111/desc.12054. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh K., Heathcote L.C., Lau J.Y. Age-related changes in attentional control across adolescence: how does this impact emotion regulation capacities? Front. Psychol. 2014 doi: 10.3389/fpsyg.2014.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. (Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12965300) [DOI] [PubMed] [Google Scholar]
- Crone E.A. The role of the medial frontal cortex in the development of cognitive and social-affective performance monitoring. Psychophysiology. 2014;51(10):943–950. doi: 10.1111/psyp.12252. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- deCharms R.C. Reading and controlling human brain activation using real-time functional magnetic resonance imaging. Trends Cogn. Sci. 2007;11(11):473–481. doi: 10.1016/j.tics.2007.08.014. [DOI] [PubMed] [Google Scholar]
- deCharms R.C., Maeda F., Glover G.H., Ludlow D., Pauly J.M., Soneji D.…Mackey S.C. Control over brain activation and pain learned by using real-time functional MRI. Proc. Natl. Acad. Sci. U. S. A. 2005;102(51):18626–18631. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCharms R.C., Christoff K., Glover G.H., Pauly J.M., Whitfield S., Gabrieli J.D. Learned regulation of spatially localized brain activation using real-time fMRI. NeuroImage. 2004;21(1):436–443. doi: 10.1016/j.neuroimage.2003.08.041. (Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14741680) [DOI] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U.F., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M.…Schlaggar B.L. Development of distinct control networks through segregation and integration. Proc. Natl. Acad. Sci. U. S. A. 2007;104(33):13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Dosenbach N.U.F., Church J.A., Miezin F.M., Barch D.M.…Schlaggar B.L. The maturing architecture of the brain's default network. Proc. Natl. Acad. Sci. U. S. A. 2008;105(10):4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Power J.D., Dosenbach N.U., Church J.A., Miezin F.M.…Petersen S.E. Functional brain networks develop from a “local to distributed” organization. PLoS Comput. Biol. 2009;5(5) doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Penny W., Philips C., Kiebel S., Hinton G., Ashburner J. Classical and Bayesian inference in neuroimaging: theory. NeuroImage. 2002;16(2):465–483. doi: 10.1006/nimg.2002.1090. [DOI] [PubMed] [Google Scholar]
- Ganel T., Goshen-Gottstein Y. Perceptual integrality of sex and identity of faces: further evidence for the single route hypothesis. J. Exp. Psychol. Hum. Percept. Perform. 2002;28:854–867. [PubMed] [Google Scholar]
- Ganel T., Goshen-Gottstein Y. Effects of familiarity on the perceptual integrality of the identity and expression of faces: the parallel-route hypothesis revisited. J. Exp. Psychol. Hum. Percept. Perform. 2004;30(3):583–597. doi: 10.1037/0096-1523.30.3.583. [DOI] [PubMed] [Google Scholar]
- Garniefski N., Kraaij V., Spinhoven P. Negative life events, cognitive emotion regulation and emotional problems. Personal. Individ. Differ. 2001;30:1311–1327. [Google Scholar]
- Ge T., Feng J., Grabenhorst F., Rolls E. Componential Granger causality, and its application to identifying the source and mechanisms of the top-down biased activation that controls attention to affective vs sensory processing. NeuroImage. 2012;59(2):1846–1858. doi: 10.1016/j.neuroimage.2011.08.047. (Retrieved from citeulike-article-id:9715278) [DOI] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M.…Tottenham N. A developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. J. Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A.…Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C.…Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Wu J., Ding M., Feng J. Uncovering Interactions in the Frequency Domain. PLoS Comput. Biol. 2008;4(5) doi: 10.1371/journal.pcbi.1000087. (Retrieved from citeulike-article-id:2869965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Choate V.R., Pine D.S., Nelson E.E. Neural circuitry underlying affective response to peer feedback in adolescence. Soc. Cogn. Affect. Neurosci. 2012;7(1):81–92. doi: 10.1093/scan/nsr043. (nsr043 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S.P.W., Cohen Kadosh K., Lau J.Y.F. A developmental angle to understanding the mechanisms of biased cognition in social anxiety. Front. Hum. Neurosci. 2015 doi: 10.3389/fnhum.2013.00846. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S.P.W., Cohen Kadosh K., Scerif G., Lau J.Y.F. Social anxiety disorder: a new developmental cognitive neuroscience approach to uncover risk factors during adolescence. Dev. Cogn. Neurosci. 2015 doi: 10.1016/j.dcn.2015.02.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Chen G., Thomason M.E., Schwartz M.E., Gotlib I.H. Investigating neural primacy in Major Depressive Disorder: multivariate Granger causality analysis of resting-state fMRI time-series data. Mol. Psychiatry. 2011;16(7):763–772. doi: 10.1038/mp.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go–nogo task. Biol. Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J.J., Reynell C., Attwell D. The physiology of developmental changes in BOLD functional imaging signals. Dev. Cogn. Neurosci. 2011;1(3):199–216. doi: 10.1016/j.dcn.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.H., Grossmann T., Cohen Kadosh K. Mapping functional brain development: building a social brain through Interactive Specialization. Dev. Psychol. 2009;45:151–159. doi: 10.1037/a0014548. [DOI] [PubMed] [Google Scholar]
- Johnson M.H., Jones E.J., Gliga T. Brain adaptation and alternative developmental trajectories. Dev. Psychopathol. 2015;27(2):425–442. doi: 10.1017/S0954579415000073. [DOI] [PubMed] [Google Scholar]
- Johnston S., Boehm S., Healy D., Goebel R., Linden D. Neurofeedback: a promising tool for the self-regulation of emotion networks. NeuroImage. 2010;49:1066–1072. doi: 10.1016/j.neuroimage.2009.07.056. [DOI] [PubMed] [Google Scholar]
- Johnston S., Linden D.E.J., Healy D., Goebel R., Habes I., Boehm S.G. Upregulation of emotion areas through neurofeedback with a focus on positive mood. Cogn. Affect. Behav. Neurosci. 2011;11:44–51. doi: 10.3758/s13415-010-0010-1. [DOI] [PubMed] [Google Scholar]
- Kang H.C., Burgund E.D., Lugar H.M., Petersen S.E., Schlaggar B.L. Comparison of functional activation foci in children and adults using a common stereotactic space. NeuroImage. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Keshavan M.S., Giedd J., Lau J.Y.F., Lewis D.A., Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1(7):549–558. doi: 10.1016/S2215-0366(14)00081-9. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kohn N., Eickhoff S.B., Scheller M., Laird A.R., Fox P.T., Habel U. Neural network of cognitive emotion regulation—an ALE meta-analysis and MACM analysis. NeuroImage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N., Simmons W.K., Bellgowan P.S., Baker C.I. Circular analysis in systems neuroscience: the dangers of double dipping. Nat. Neurosci. 2009;12(5):535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood to adulthood. J. Neurosci. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden D.E., Habes I., Johnston S.J., Linden S., Tatineni R., Subramanian L.…Goebel R. Real-time self-regulation of emotion networks in patients with depression. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0038115. (PONE-D-11-22815 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q., Ge T., Feng J. Granger causality with signal-dependent noise. NeuroImage. 2011;57(4):1422–1429. doi: 10.1016/j.neuroimage.2011.05.054. [DOI] [PubMed] [Google Scholar]
- Luo Q., Ge T., Grabenhorst F., Feng J., Rolls E.T. Attention-dependent modulation of cortical taste circuits revealed by Granger Causality with signal-dependent noise. PLoS Comput. Biol. 2013;9(10) doi: 10.1371/journal.pcbi.1003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q., Lu W., Cheng W., Valdes-Sosa P.A., Wen X., Ding M., Feng J. Spatio-temporal Granger causality: a new framework. NeuroImage. 2013;79:241–263. doi: 10.1016/j.neuroimage.2013.04.091. (S1053-8119(13)00450-3 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C., Holmes E.A. Cognitive bias modification for children with anxiety disorder: an intervention approach worth attending to. Am. J. Psychiatr. 2011;169(2):118–120. doi: 10.1176/appi.ajp.2011.11111682. [DOI] [PubMed] [Google Scholar]
- McRae K., Gross J.J., Weber J., Robertson E.R., Sokol-Hessner P., Ray R.D.…Ochsner K.N. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc. Cogn. Affect. Neurosci. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor B.G., van Leijenhorst L., Rombouts S.A.R.B., Crone E.A., Van der Molen M.W. Do you like me? Neural correlates of social evaluation and developmental trajectories. Soc. Neurosci. 2010;5(5-6):461–482. doi: 10.1080/17470910903526155. (Pii 927980134) [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E.B., Pine D.S. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15841674. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L., Simmonite M., White T.P., Liddle E.B., Liddle P.F. Neural primacy of the salience processing system in schizophrenia. Neuron. 2013;79(4):814–828. doi: 10.1016/j.neuron.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paret C., Kluetsch R., Ruf M., Demirakca T., Hoesterey S., Ende G., Schmahl C. Down-regulation of amygdala activation with real-time fMRI neurofeedback in a healthy female sample. Front. Behav. Neurosci. 2014;8 doi: 10.3389/fnbeh.2014.00299. (Unsp 299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.-J., Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342(6158) doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S.B., Pelphrey K.A. Developing connections for affective regulation: age-related changes in emotional brain connectivity. J. Exp. Child Psychol. 2011;108(3):607–620. doi: 10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z., Judas M., Simic G., Rasin M.R., Uylings H.B.M., Rakic P. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2011;108(32):13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Allen N.B. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends Cogn. Sci. 2012 doi: 10.1016/j.tics.2012.04.011. (S1364-6613(12)00104-0 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A., O'Connor K.J., Gatenby J.C., Gore J.C., Grillon C., Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat. Neurosci. 2001;4(4):437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Pitskel N.B., Bolling D.Z., Kaiser M.D., Crowley M.J., Pelphrey K.A. How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. Dev. Cogn. Neurosci. 2011;1(3):324–337. doi: 10.1016/j.dcn.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt B., Cohen Kadosh K., Lau J.Y. The Role of Peer Rejection in Adolescent Depression. Depress Anxiety. 2013 doi: 10.1002/da.22120. [DOI] [PubMed] [Google Scholar]
- Rota G., Handjaras G., Sitaram R., Birbaumer N., Dogil G. Reorganization of functional and effective connectivity during real-time fMRI-BCI modulation of prosody processing. Brain Lang. 2011;117(3):123–132. doi: 10.1016/j.bandl.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Ruiz S., Lee S., Soekadar S.R., Caria A., Veit R., Kircher T.…Sitaram R. Acquired self-control of insula cortex modulates emotion recognition and brain network connectivity in schizophrenia. Hum. Brain Mapp. 2013;34(1):200–212. doi: 10.1002/hbm.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf S.K., Behrmann M., Dahl R. Facing changes & changing faces in adolescence: investigating the neural basis of key developmental shifts in social-information processing. Dev. Cogn. Neurosci. 2012;2(2):199–219. doi: 10.1016/j.dcn.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K.S., Smyth J.M., Delgado M.R. The amygdala: an agent of change in adolescent neural networks. Horm. Behav. 2013;64(2):298–313. doi: 10.1016/j.yhbeh.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C.L., Tan G.C., Roiser J.P., Viding E., Dumontheil I., Blakemore S.J. Developmental influences on the neural bases of responses to social rejection: implications of social neuroscience for education. NeuroImage. 2011;57(3):686–694. doi: 10.1016/j.neuroimage.2010.09.063. [DOI] [PubMed] [Google Scholar]
- Silvers J.A., McRae K., Gabrieli J.D., Gross J.J., Remy K.A., Ochsner K.N. Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion. 2012 doi: 10.1037/a0028297. (2012-13957-001 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.A., Buhle J.T., Ochsner K.N. The neuroscience of emotion regulation: basic mechanisms and their role in development, aging and psychopathology. In: Ochsner K.N., Kosslyn S.M., editors. vol. 2. Oxford University Press; New York: 2014. pp. 53–78. (The Oxford Handbook of Cognitive Neuroscience, Vol 2: The cutting edges). [Google Scholar]
- Smith S.M., Bandettini P.A., Miller K.L., Behrens T.E., Friston K.J., David O.…Nichols T.E. The danger of systematic bias in group-level FMRI-lag-based causality estimation. NeuroImage. 2012;59(2):1228–1229. doi: 10.1016/j.neuroimage.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Subramanian L., Hindle J., Johnston S., Roberts M., Husain M., Goebel R., Linden D.E.J. Real-time fMRI neurofeedback for treatment of Parkinson's disease. J. Neurosci. 2011;31(45):16309–16317. doi: 10.1523/JNEUROSCI.3498-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C.K., Walhovd K.B., Dale A.M., Ostby Y., Grydeland H., Richardson G.…Alzheimer's Disease Neuroimaging I. Brain development and aging: overlapping and unique patterns of change. NeuroImage. 2013;68:63–74. doi: 10.1016/j.neuroimage.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill S.L., Ramscar M., Chrysikou E.G. Cognition without control: when a little frontal lobe goes a long way. Curr. Dir. Psychol. Sci. 2009;18(5):259–263. doi: 10.1111/j.1467-8721.2009.01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E., Harris C., Winkielman P., Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect. Psychol. Sci. 2009;4(3):274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Feldman-Barrett L. PsycExtra. Retrieved from; 2004. From affect to control: functional specialization of the insula in motivation and regulation. retrieved from: http://www.columbia.edu/cu/psychology/tor/ [Google Scholar]
- Weiskopf N., Mathias K., Bock S.W., Scharnowski F., Veit R., Grodd W.…Birbaumer N. Principles of a brain–computer interface (BCI) based on real-time functional magnetic resonance imaging (fMRI) IEEE Trans. Biomed. Eng. 2004;51(6):966–970. doi: 10.1109/TBME.2004.827063. [DOI] [PubMed] [Google Scholar]
- Weiskopf N., Scharnowski F., Veit R., Goebel R., Birbaumer N., Mathias K. Self-regulation of local brain activity using real-time functional magnetic resonance imaging (fMRI) J. Physiol. Paris. 2004;98:357–373. doi: 10.1016/j.jphysparis.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Wen X., Yao L., Liu Y., Ding M. Causal interactions in attention networks predict behavioral performance. J. Neurosci. 2012;32(4):1284–1292. doi: 10.1523/JNEUROSCI.2817-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X., Liu Y., Yao L., Ding M. Top-down regulation of default mode activity in spatial visual attention. J. Neurosci. 2013;33(15):6444–6453. doi: 10.1523/JNEUROSCI.4939-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K.D., Zotev V., Phillips R., Misaki M., Yuan H., Drevets W.C., Bodurka J. Real-time FMRI neurofeedback training of amygdala activity in patients with major depressive disorder. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0088785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Young K.D., Phillips R., Zotev V., Misaki M., Bodurka J. Resting-state functional connectivity modulation and sustained changes after real-time functional magnetic resonance imaging neurofeedback training in depression. Brain Connect. 2014;4(9):690–701. doi: 10.1089/brain.2014.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V., Krueger F., Phillips R., Alvarez R.P., Simmons W.K., Bellgowan P.…Bodurka J. Self-regulation of amygdala activation using real-time FMRI neurofeedback. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0024522. (PONE-D-11-06642 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V., Phillips R., Young K.D., Drevets W.C., Bodurka J. Prefrontal control of the amygdala during real-time fMRI neurofeedback training of emotion regulation. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0079184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.