Abstract
Objective
To prospectively evaluate the association of maternal self-esteem measured when their offspring were toddlers with the subsequent development of attention-deficit-hyperactivity-disorder (ADHD)-like behavior in their school-age offspring and the potential modifying effects of prenatal lead exposure.
Study design
We evaluated a subsample of 192 mother-child pairs from a long-running birth-cohort project that enrolled mothers in Mexico from 1994 to 2011. Prenatal lead exposure was assessed using cord blood lead and maternal bone lead around delivery (tibia and patella lead, measured by K-x-ray-fluorescence). When children were 2 years old, maternal self-esteem was measured using the Coopersmith-Self-esteem-Inventory. When children were 7-to-15 years old, children's blood lead levels and ADHD symptoms were assessed, and Conners’ Parental-Rating-Scales-Revised (CPRS-R) and Behavior-Rating-Inventory-of-Executive-Function-Parent Form (BRIEF-P) were used as measures of ADHD-like behavior.
Results
Adjusting for family economic status, marital status, maternal education and age, child's age and sex, and children's current blood lead levels, increased maternal self-esteem was associated with reduced child inattention behavior. Compared with those among high prenatal lead exposure (P25-P100), this association was stronger among low prenatal lead exposure groups (P1-P25, p-values for the interaction effects between prenatal lead exposure and maternal self-esteem levels < 0.10). Each 1-point increase in maternal self-esteem scores was associated with 0.6-to-1.3-point decrease in CPRS-R and BRIEF-P T-scores among groups with low cord blood lead and patella lead (P1-P25).
Conclusions
Children experiencing high maternal self-esteem during toddlerhood were less likely to develop inattention behavior at school-age. Prenatal lead exposure may play a role in attenuating this protective effect.
Keywords: Maternal self-esteem, prenatal exposure, lead, child, inattention
Compared with single exposure studies, mixed exposures of neurotoxic chemicals and social context may better reflect real world exposure scenarios, and therefore, are of particular importance.1-3 The potential effects of the interaction between the neurotoxic chemicals and social environment on children's neurodevelopment has been previously suggested in animal studies,4 but few studies have focused on such interaction effects in human populations.
Attention deficit hyperactivity disorder (ADHD) affects around 5% of school-age children,5 with the potential to persist into adulthood. A growing body of evidence suggests that early-life lead exposure, even at very low levels of exposure (i.e. children's blood lead levels below 5 μg/dL), is a potential contributor to ADHD.2, 6-8
Maternal self-esteem is used to reflect the overall emotional evaluation of a mother's own worth. Self-esteem and stress are closely related because high self-esteem may buffer stressors and low self-esteem may cause a higher level of perceived stress.9 Maternal low self-esteem and maternal stress may share a similar mechanism of developmental neurotoxicity.10,11 Although study results are equivocal, associations between maternal stress and ADHD in children have been shown in clinical studies as well as epidemiologic studies that controlled for potential confounders.12-14 However, the exact association between maternal self-esteem and her child's ADHD-like behavior is still unknown.
The epidemiologic research has shown that, compared with low self-esteem, higher maternal self-esteem attenuates the negative effects of lead on children's cognitive performances.11 Previous studies also showed that maternal lead exposure and stress exposure may interact such that impacts of either risk could change in the presence of the other.1,2,4
Therefore, we utilized data from a long-running birth cohort project to examine prospectively the effects of maternal self-esteem on school-age-children's ADHD-like behavior and the potential modifying effects of prenatal lead exposure.
Methods
Subjects were recruited from three birth cohorts in Mexico city that composed the Early Life Exposures in Mexico to Environmental Toxicants (ELEMENT) project during: 1994–1997 (cohort 1), 1997–2000 (cohort 2), and 2001–2005 (cohort 3). Pregnant women with low to medium income were enrolled from 3 maternity hospitals. Cohort 1 was enrolled at the time of delivery, and cohort 2 and 3 were during pregnancy. The mother-offspring pairs were excluded if the mother or child had pathological factors affecting child development.15-18 All the 3 cohorts used identical exclusion criteria.15-18
Maternal bone lead levels were measured in vivo within one month of delivery. Of 1756 mother-offspring pairs who completed a baseline assessment during recruitment (with at least one measurement of prenatal lead exposure including maternal bone lead or cord blood lead), 349 pairs completed an assessment of maternal self-esteem when the offspring were 2 years old. The mother-child pairs were re-interviewed to assess childhood ADHD symptoms and children's current blood lead levels when the children were 7-15 years old. Of the 349 pairs, 192 (55.0%) completed the 7-15-year follow-up assessment. The most common reason for the subject's lost to follow-up was lack of time to undergo the assessment. The 192 mother-child pairs were not statistically different in demographic characteristics, maternal self-esteem, cord blood lead and maternal bone lead from the 157 pairs who were not followed-up (data not shown).
Ethics approval was received from the Institutional Review Boards of the Harvard School of Public Health, National Institute of Public Health of Mexico, University of Michigan, University of Toronto, Mount Sinai School of Medicine and attending hospitals. Women and children old enough signed informed consent letters before enrollment.
The assessment of prenatal and current lead exposure
Maternal tibia and patella lead were measured in vivo using a Cd-109 K-shell X-ray fluorescence instrument (ABIOMED, Danvers, MA, USA).18 Cord blood lead levels were measured using an atomic absorption spectrometry instrument (AAS, model 3000, PerkinElmer, USA). Children's current blood lead levels were measured using inductively coupled plasma mass spectrometry (ICP-MS, Elan 6100, PerkinElmer, USA).18 No blood lead levels were below the limit of detection.
The assessment of maternal self-esteem
The Coopersmith Self-esteem Inventory (Spanish version, adult short form), with 25-items and good reliability and validity, was used to measure maternal self-esteem levels. The self-esteem total scores ranged from 1 to 25, and higher total scores indicated higher maternal self-esteem levels.11
Other covariate data including maternal age at enrollment, family economic status, years of maternal and paternal education, marital status, the child's sex and age, was collected by questionnaire. Because the low maternal education was associated with maternal attention and hyperactivity problems, maternal education was adjusted to partially control the heritable impacts of maternal attention and hyperactivity levels on her child's ADHD-like behavior.
The assessment of the school-age-child's ADHD-like behavior
The validated Spanish versions of Conners’ Parental Rating Scale-Revised (CPRS-R) and Behavior Rating Inventory of Executive Function-Parental (BRIEF-P) Form were used for the assessment.
The 27-question CPRS-R was designed to obtain the parents’ reports on children's behavioral problems in children at 3-17 years old with a good test-retest reliability and internal consistency.19 We specially focused on 4 scales including ADHD Index (P-ADHD), DSM-IV ADHD-Inattention (P-DSMI), DSM-IV ADHD-Hyperactivity/Impulsivity (P-DSMHI) and DSM-IV ADHD-combined indexes (P-DSMT). The P-ADHD was associated with the risk for ADHD. P-DSMI, P-DSMHI and P-DSMT were correspondent with the DSM-IV diagnostic criteria for inattentive, hyperactivity-impulsive and combined subtypes of ADHD, respectively.17,19 The BRIEF-P Form, a reliable parent-report inventory, was used to assess behavioral regulation and metacognition in children at 5-18 years old.20 BRIEF-P contained 8 clinical scales. We focused on 2 summary scales (behavioral regulation index (P-BRI) and metacognition index (P-MI)) and another scale reflecting overall functioning (P-GEC).20,21 T-scores of CPRS-R and BRIEF-P scales were associated with the risk of ADHD. A child with the T-score of 40-60 was considered average (typical levels of concern), and higher T-scores indicated increasing severity of behavioral problems.19.20
Statistical analyses
Simple linear regression was performed to quantify unadjusted associations between T-scores of CPRS-R and BRIEF-P scales, and the independent variables of interest (maternal self-esteem, blood or bone lead, as well as other covariates). Using multivariable linear regression, we modeled the adjusted relationship between maternal self-esteem and T-scores. The child's current blood lead level was further adjusted to focus on the effects of prenatal lead exposure. The relationship between maternal self-esteem and lead and other demographic factors were explored using multivariable linear regression.
Using multiple regression models, separate models with interaction terms between each prenatal lead exposure variable (maternal tibia lead, patella lead or cord blood lead) and maternal self-esteem were estimated to assess the modifying effects of prenatal lead exposure on the association between maternal self-esteem and her child's behavior. Prenatal lead levels (continuous variables) were categorized to simplify the interpretation of the interaction. We estimated the association separately within each quartile of prenatal lead exposure. We found that for each of the 3 highest quartiles of cord blood lead or patella lead, maternal self-esteem was usually non-significantly related to the T-scores, but in the lowest quartile, maternal self-esteem was significantly negatively related to the T-scores. Therefore, we combined the 3 highest quartiles as the high lead group. The associations between maternal self-esteem and the T-scores among children with the lowest quartile (P1-P25) and among children with the highest three quartiles (P25-P100) are presented. Data analysis was performed using SAS (version 9.2, SAS Institute Inc., USA). The significance level was set at 0.05 (two sided), and p-values < 0.10 were used to identify interaction effects. The adjusted associations between maternal self-esteem and her child's behavior stratified by prenatal lead exposure were also shown via Spline smoothing plots using Empower(R) (version 2.13.9, X&Y solutions, Inc., USA) and R (version 2.15.3, Robert Gentleman and Ross Ihaka, New Zealand).
Results
The characteristics of the 192 mother-child pairs are presented in Table I. All the mothers were Mexican. The mean T-scores were all within normal ranges (40-60, typical levels of concern). The cord blood lead levels (geometric mean: 5.3 μg/dL) were slightly above the intervention limit for blood lead in pregnant women (5.0 μg/dL) currently recommended by U.S. CDC. 22 However, the children's current blood lead levels were much lower (geometric mean: 2.8 μg/dL) than the current recommendations by US CDC on children's blood lead (5 μg/dL).23 The Spearman correlation coefficients between patella and tibia lead, between patella and cord blood lead, and between tibia and cord blood lead were 0.4 (p=0.000), 0.4 (p=0.000) and 0.2 (p=0.085), respectively. Spearman correlation between any of prenatal lead exposure variables and the child's current blood lead was not significant (p > 0.05).
Table I.
Characteristics of the study participants (192 mother-child pairs)
| Variables | N (%) | Mean ± SD or Median (P25, P75) |
|---|---|---|
| Maternal Characteristics during index pregnancy | ||
| Maternal age (years) | 192 | 25.9±5.2 |
| Maternal education (years) | 190 | 10.3±2.8 |
| Family economic level | 187 | 8.3±3.7 |
| Marital status/ Single: Married | 62 (32.3): 130 (67.7) | |
| Patella lead (μg/g) | 155 | 12.6 (3.2, 21.7) |
| Tibia lead (μg/g) | 128 | 10.2 (4.1, 16.0) |
| Maternal self-esteem (children at 2 years) | 192 | 17.1±4.8 |
| Children's characteristics at birth and at 7-15-year follow-up | ||
| Sex/ Boy: Girl | 109 (56.8): 83 (43.2) | |
| Birth weight | 192 | 3.2±0.4 |
| Birth order | 192 | 2.3±1.3 |
| Cord blood lead (μg/dL) | 119 | 5.5 (3.5, 8.1) |
| Age (yrs) | 192 | 11.1±3.4 |
| Current blood lead (μg/dL) | 148 | 2.7 (2.0, 4.0) |
| P-ADHD | 192 | 52.5±8.5 |
| P-DSMI | 192 | 51.8±8.4 |
| P-DSMHI | 192 | 55.7±9.5 |
| P-DSMT | 192 | 54.0±8.6 |
| P- BRI | 192 | 54.2±10.9 |
| P-MI | 192 | 54.6±10.9 |
| P-GEC | 192 | 54.9±10.9 |
Prenatal lead exposure levels and maternal self-esteem scores were not significantly correlated. After adjusting for maternal age, marital status, child sex and birth orders, more maternal educational years or higher family economic levels were significantly associated with higher maternal self-esteem scores (p <0.05, data not shown).
In unadjusted models, one point increase in maternal self-esteem scores was associated with 0.4-to-0.8 point decrease in T-scores in the 7 scales (p-values <0.05, data not shown). After adjusting for the potential confounders, one point increase in maternal self-esteem scores was associated with 0.4-to-0.7 point decrease in T-scores (Table II).
Table II.
Adjusteda difference [β-coefficient, β (SE)] in T-scores of children's behavioral scales for a point increase in maternal self-esteem.
| Behavior scale | Overall effects: self-esteem coefficients | Effect modification by cord BPbb: Maternal self-esteem coefficients |
Effect modification by patella leadb: Maternal self-esteem coefficients |
||||
|---|---|---|---|---|---|---|---|
| Lowc cord BPb (≤3.5 μg/dL) | Highc cord BPb (>3.5 μg/dL) | p interact d | Lowc patella lead (≤3.2 μg/g) | Highc patella lead (>3.2 μg/g) | p interact d | ||
| P-ADHD | −0.5 (0.2)§ | −0.8 (0.3)‡ | −0.2 (0.2) | 0.082 | −1.1 (0.3)§ | −0.4 (0.2)† | 0.051 |
| P-DSMI | −0.4 (0.1)‡ | −0.7 (0.2)‡ | −0.1 (0.2) | 0.050 | −0.7 (0.3)† | −0.4 (0.2) | 0.324 |
| P-DSMHI | −0.4 (0.2)† | −0.3 (0.3) | −0.2 (0.2) | 0.722 | −0.3 (0.4) | −0.5 (0.2)† | 0.589 |
| P-DSMT | −0.4 (0.2)‡ | −0.7 (0.3)† | −0.1 (0.2) | 0.112 | −0.6 (0.3)† | −0.4 (0.2)† | 0.534 |
| P-BRI | −0.7 (0.2)§ | −0.1 (0.3)‡ | −0.4 (0.3) | 0.097 | −0.8 (0.4) | −0.7 (0.2)‡ | 0.838 |
| P-MI | −0.5 (0.2)‡ | −1.2 (0.3)§ | −0.1 (0.3) | 0.016 | −0.8 (0.5) | −0.6 (0.2)† | 0.658 |
| P-GEC | −0.7 (0.2)§ | −1.3 (0.3)§ | −0.2 (0.3) | 0.015 | −0.9 (0.5) | −0.7 (0.3)‡ | 0.688 |
Models were adjusted for economic status, marital status, maternal education and age, child's age and sex, and children's current blood lead (N=144 for overall models; N=102 and N=120 for cord blood lead and patella lead models, respectively).
p<0.05
p<0.01
p<0.001
Models to test for effect modification included, in addition to adjustment factors, cord blood (or patella) lead category (high/low), self-esteem, and the interaction between cord blood (or patella) lead and maternal self-esteem. BPb: blood lead.
Lead categories (high/low) were defined based on the 25th percentile (P25) of the lead distribution (i.e., P25=3.5 μg/dL for cord blood lead and P25=3.2 μg/g for patella lead).
pinteract is the p-value for the interaction term and indicates the difference in self-esteem coefficients comparing low vs high prenatal lead exposure.
The magnitude of maternal self-esteem associations with T-scores differed according to prenatal lead exposure. When stratified by cord blood lead, in unadjusted or adjusted models, the negative association between maternal self-esteem and T-scores was more evident among the low lead-exposure (P1-P25) groups than among the high lead-exposure (P25-P100) groups (for un-adjusted models, pinteraction values for P-ADHD, P-DSMI, P-DSMT, P-MI and P-GEC were <0.100 [data not shown]; for adjusted models, pinteraction values for P-ADHD, P-DSMI, P-MI and P-GEC were <0.100) (Table II). A similar pattern was found for P-ADHD when considering effect modification by patella lead (for un-adjusted [data not shown] or adjusted models, pinteraction values for P-ADHD were both <0.100) (Table II).
However, no significant associations were observed between maternal self-esteem and T-scores in both the lowest and highest tibia-lead groups in un-adjusted or adjusted models, and no significant tibia lead-self-esteem interaction effects on any behavior scale (data not shown), indicating the potential modification roles of cord lead or patella lead (but not tibia lead) in the association between maternal self-esteem and children's ADHD-like behavior.
In addition, the P-DSMI T-scores were more associated with maternal self-esteem than the P-DSMHI T-scores among the models adjusting for cord blood lead levels (Table II), suggesting that, in the same context of prenatal lead exposure, maternal low self-esteem may predict her child's inattention but not hyperactivity.
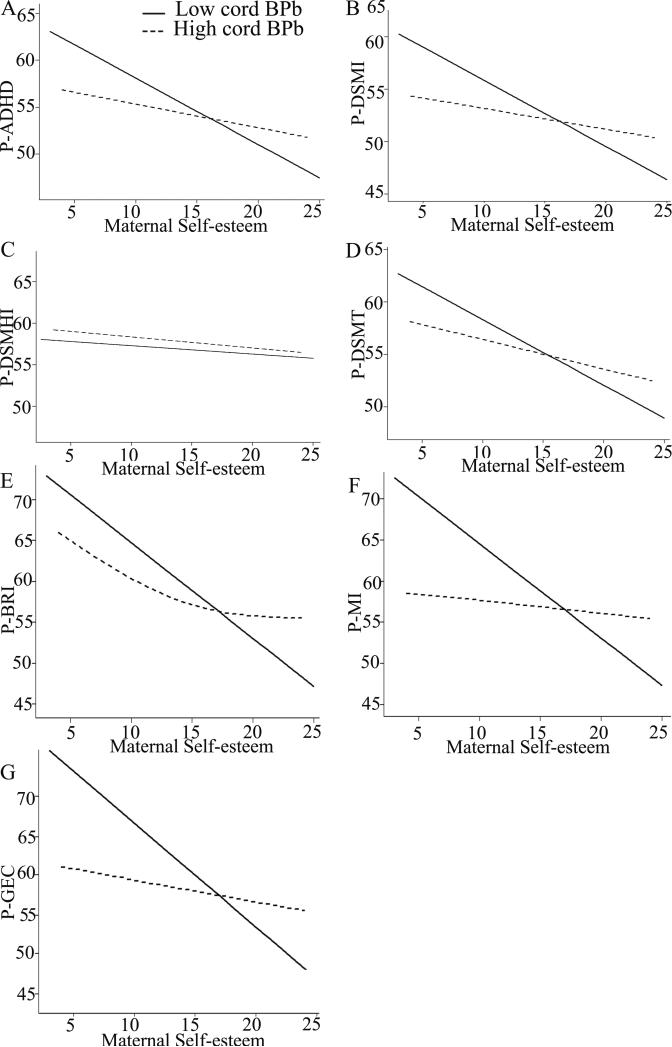
Figures 1 and 2 show adjusted associations between maternal self-esteem and behavior T-scores. Although there appears to be a slight curvilinear relationship between self-esteem and P-BRI among high cord blood lead group (Figure 1) and between self-esteem and P-DSMHI among high patella lead group (Figure 2), the curvilinear trend was modest and the association did not significantly deviate from linearity (both p > 0.05).
Figure 1.
Adjusted associations between maternal self-esteem and school-age-children's behavior T-scores when stratified by cord blood lead. P-ADHD (A), P-DSMI (B), P-DSMHI (C), P-DSMT (D), P-BRI (E), P-MI (F) and P-GEC (G). Comparison of the lowest quartile of cord blood lead (P1-P25, full line) with the highest 3 quartiles (P25-P100, dotted line). Among the low cord blood lead (P1-P25) groups, the data showed significantly inverse association between maternal self-esteem and T-scores except for the P-DSMHI scale. However, the effect was negligibly small among the high lead (P25-P100) groups. Models were adjusted for family economic status, marital status, maternal education and age, child's age and sex, and children's current blood lead levels. BPb: blood lead. N=102.
Figure 2.
Adjusted associations between maternal self-esteem and school-age-children's behavior T-scores when stratified by maternal patella lead. P-ADHD (A), P-DSMI (B), P-DSMHI (C), P-DSMT (D), P-BRI (E), P-MI (F) and P-GEC (G). Comparison of the lowest quartile of patella lead (P1-P25, full line) with the highest 3 quartiles (P25-P100, dotted line). For P-ADHD, the inverse association between maternal self-esteem and P-ADHD T-scores was more evident among the low patella lead group (P1-P25) than among the high lead group (P25-P100). Except for the P-ADHD, there was no significant interaction between patella lead and maternal self-esteem on other scales (p-interaction values > 0.10). Models were adjusted for economic status, marital status, maternal education and age, child's age and sex, and children's current blood lead levels. N=120.
Discussion
Studies on the relationship between maternal self-esteem and her child's ADHD-like behavior are very limited in number and have been cross-sectional in design. 24 Based on our results, maternal low self-esteem mainly affects the child's inattentive type of ADHD. Several potential mechanisms including behavioral, biological or both pathways may explain our results. Concerning the behavioral pathway, mothers with high self-esteem have been shown to be more likely to provide their children with more appropriate educational modes, which helps decrease the inattention behavior.25 Our results are consistent with previous studies that showed that maternal exposure to stress was associated with ADHD in the offspring.13,14,26 As for the biological pathway, maternal self-esteem may alleviate the biological response to stressful stimuli in the mother and the fetus towards more adaptive styles.10 Human studies have shown that midbrain dopaminergic system or LPHN3 gene may be involved in maternal stress-induced attention-deficit.27,28 Animal studies have shown that exposure to prenatal stress in rats leads to deficits in sustained attention, and that these impairments could be exacerbated by NMDA antagonism and mitigated by the norepinephrine uptake inhibitor.29 Prenatal stress also significantly affected the attention indexes and decreased plasma testosterone levels in male guinea pig offspring, and the replacement of plasma testosterone to control levels could reverse the attention impairment.30 These studies helped explain the findings of this study. However, further study is still needed to examine whether these mechanisms are responsible for the association of maternal self-esteem with her child's inattention behavior.
Our findings, if confirmed in larger studies, indicate the interference of prenatal lead exposure against the protective effects of maternal high self-esteem on her child's attention. Specifically, the positive effects of maternal high self-esteem on the alleviation of the child's inattention appear to be inhibited among children with high prenatal lead exposure compared with those with low prenatal lead exposure. Previous animal studies have shown that the potential mechanism for the interaction may be that lead and stress had similar competitive targets in the brain, and both acted on brain mesolimbic dopamine/glutamate systems which were associated with the attention and inhibitory response control.4,31-33 Therefore, our study suggests the potential interaction between the chemical exposure and social context on a child's ADHD-like behavior, and suggests that population-based cumulative risk assessment should be extended beyond chemicals to include psychosocial factors.
Our findings are consistent with previous studies that showed that socioeconomic status and self-esteem were positively correlated.34 This may be due to mothers with higher socioeconomic status having a higher sense of self-accomplishment, and therefore, a higher likelihood of building their self-esteem.
One of the strong points of our study is that maternal bone lead was measured as a biomarker of cumulative lead exposure over the course of pregnancy. In contrast to tibia lead, which had a half-life of decades, patella lead had a half-life of several years and had a high turnover rate during pregnancy to mobilize lead from bone into circulation.18 Therefore, it was reasonable that in our study, patella lead and cord blood lead exerted the greatest modifying impacts on children's ADHD-like behavior because these two biomarkers represent the major sources of circulating lead during pregnancy and the main sources of exposure to the fetus.18
Our study has some other advantages as well. Our data using CPRS-R corroborated our data from BRIEF-P, and this result confirmed the shared correlation between these 2 scales. We used 3 indexes including patella lead, tibia lead and cord blood lead to fully disclose the levels of prenatal lead exposure. In addition, the child's current blood lead was controlled to cancel out the confounding effect of the child's recent lead exposure. A variety of confounders were adjusted for, but the adjustment didn't greatly change the magnitudes of most estimates, suggesting that adjustment for confounders didn't substantially affect results.
In considering our study's limitations, we first note that our sample size was relatively small, which limited our statistical power. Second, our analysis was restricted to a Mexican population with a low to moderate economic level, and we cannot extrapolate our findings to the general population. Third, self-esteem was assessed using a self-reported scale that cannot be independently verified. Fourth, measurements of maternal self-esteem were limited to only a subset of the larger cohorts. Fifth, the loss of follow-up of participating subjects may have introduced bias. Finally, we didn't measure levels of prenatal lead exposure during each trimester of pregnancy, and we may have missed critical developmental windows in which the proposed interaction effects might be more prominent. Therefore, our study provided suggestive but inconclusive evidence for the effects. Confirming our results in other studies is warranted.
In conclusion, this study suggests that, independent of socioeconomic status, children of mothers with higher self-esteem during their toddlerhood tend to develop less inattention behavior at school age. However, prenatal lead exposure may lessen this association. Our findings emphasized the need to prevent prenatal lead exposure.16,18 Because childhood ADHD is a known risk for adulthood ADHD, continued follow-up of our cohort is warranted.
Acknowledgments
We thank Sirui Cao (Department of Environmental Health Sciences, University of Michigan School of Public Health) for her contribution to the data analysis. We also thank Héctor Lamadrid-Figueroa (National Institute of Public Health, Morelos, Mexico) for his contribution to the field work design and implementation.
Supported by the US National Institute of Environmental Health Sciences (NIEHS; R01ES021446, P42-ES05947, P01ES012874, RO1-ES013744, RO1 ES014930, P30-ES 00002, K23 ES000381, RO1 ES0078210), Consejo Nacional de Ciencia y Tecnología (CONACyT; 4150M9405 and CONSERVA), Department of Federal District, México, National Institute of Public Health/Ministry of Health of Mexico, National Natural Science Foundation of China (NCFS; 81373016), and Science and Technology Commission of Shanghai Municipality (STCSM; 124119a1400). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH, EPA, CONACyT, CONSERVA, NCFS or STCSM.
Abbreviations
- ADHD
attention deficit hyperactivity disorder.
- CPRS-R
Conners’ Parental Rating Scales-Revised.
- BRIEF-P
Behavior Rating Inventory of Executive Function-Parent Form.
- P-ADHD, P-DSMI, P-DSMHI and P-DSMT
ADHD index, DSM-IV ADHD Inattentive, Hyperactive-Impulsive and Combined indexes of Conners’ Parental Rating Scales-Revised.
- P-BRI, P-MI and P-GEC
behavioral regulation index, metacognition index and overall functioning index of Behavior Rating Inventory of Executive Function-Parental Form.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.Bellinger DC. Effect modification in epidemiologic studies of low-level neurotoxicant exposures and health outcomes. Neurotoxicol Teratol. 2000;22:133–40. doi: 10.1016/s0892-0362(99)00053-7. [DOI] [PubMed] [Google Scholar]
- 2.Bellinger DC. Prenatal exposures to environmental chemicals and children's neurodevelopment: an update. Saf Health Work. 2013;4:1–11. doi: 10.5491/SHAW.2013.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tong S, McMichael AJ, Baghurst PA. Interactions between environmental lead exposure and sociodemographic factors on cognitive development. Arch Environ Health. 2000;55:330–5. doi: 10.1080/00039890009604025. [DOI] [PubMed] [Google Scholar]
- 4.Virgolini MB, Rossi-George A, Lisek R, Weston DD, Thiruchelvam M, Cory-Slechta DA. CNS effects of developmental Pb exposure are enhanced by combined maternal and offspring stress. Neurotoxicology. 2008;29:812–27. doi: 10.1016/j.neuro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; Arlington (VA): 2013. [Google Scholar]
- 6.Froehlich TE, Lanphear BP, Auinger P, Hornung R, Epstein JN, Braun J, et al. Association of tobacco and lead exposures with attention-deficit/ hyperactivity disorder. Pediatrics. 2009;124:e1054–63. doi: 10.1542/peds.2009-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanphear BP, Hornung R, Ho M, Howard CR, Eberly S, Knauf K. Environmental lead exposure during early childhood. J Pediatr. 2002;140:40–7. doi: 10.1067/mpd.2002.120513. [DOI] [PubMed] [Google Scholar]
- 8.Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ Health Perspect. 2006;114:1904–9. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coopersmith S. The antecedents of self-esteem. 1st ed. W. H. Freeman & Co; San Francisco: 1967. [Google Scholar]
- 10.Creswell JD, Welch WT, Taylor SE, Sherman DK, Gruenewald TL, Mann T. Affirmation of personal values buffers neuroendocrine and psychological stress responses. Psychol Sci. 2005;16:846–51. doi: 10.1111/j.1467-9280.2005.01624.x. [DOI] [PubMed] [Google Scholar]
- 11.Surkan PJ, Schnaas L, Wright RJ, Téllez-Rojo MM, Lamadrid-Figueroa H, Hu H, et al. Maternal self-esteem, exposure to lead, and child neurodevelopment. Neurotoxicology. 2008;29:278–85. doi: 10.1016/j.neuro.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham-Bermann SA, Seng J. Violence exposure and traumatic stress symptoms as additional predictors of health problems in high-risk children. J Pediatr. 2005;146:349–54. doi: 10.1016/j.jpeds.2004.10.065. [DOI] [PubMed] [Google Scholar]
- 13.Ronald A, Pennell CE, Whitehouse AJ, Weinstock M. Prenatal maternal stress associated with ADHD and autistic traits in early childhood. Front Psychol. 2010;1:223. doi: 10.3389/fpsyg.2010.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sagiv SK, Epstein JN, Bellinger DC, Korrick SA. Pre- and postnatal risk factors for ADHD in a nonclinical pediatric population. J Atten Disord. 2013;17:47–57. doi: 10.1177/1087054711427563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu H, Téllez-Rojo MM, Bellinger D, Smith D, Ettinger AS, Lamadrid-Figueroa H, et al. Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environ Health Perspect. 2006;114:1730–5. doi: 10.1289/ehp.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ettinger AS, Lamadrid-Figueroa H, Téllez-Rojo MM, Mercado-Garcia A, Peterson KE, Schwartz J, et al. Effect of calcium supplementation on blood lead levels in pregnancy: a randomized placebo-controlled trial. Environ Health Perspect. 2009;117:26–31. doi: 10.1289/ehp.11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortenberry GZ, Meeker JD, Sánchez BN, Barr DB, Panuwet P, Bellinger D, et al. 3,5,6-trichloro-2-pyridinol (TCPY) in pregnant women from Mexico City: Distribution, temporal variability, and relationship with child attention and hyperactivity. Int J Hyg Environ Health. 2014;217:405–12. doi: 10.1016/j.ijheh.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang A, Hu H, Sánchez BN, Ettinger AS, Park SK, Cantonwine D, et al. Association between prenatal lead exposure and blood pressure in children. Environ Health Perspect. 2012;120:445–50. doi: 10.1289/ehp.1103736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised conners’ parent rating scale (CPRS-R): Factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:257–68. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 20.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Child Neuropsychol. 2000;6:235–8. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- 21.Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128:873–82. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Committee on Obstetric Practice Committee opinion no. 533: lead screening during pregnancy and lactation. Obstet Gynecol. 2012;120:416–20. doi: 10.1097/AOG.0b013e31826804e8. [DOI] [PubMed] [Google Scholar]
- 23.Betts KS. CDC Updates Guidelines for Children's Lead Exposure. Environ Health Perspect. 2012;120:a268. doi: 10.1289/ehp.120-a268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gamble SA, Chronis-Tuscano A, Roberts JE, Ciesla JA, Pelham WE., Jr Self-esteem reactivity among mothers of children with attention-deficit/hyperactivity disorder: the moderating role of depression history. Cognit Ther Res. 2013;37:1233–42. doi: 10.1007/s10608-013-9562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yarcheski A, Mahon NE, Yarcheski TJ, Cannella BL. A meta-analysis of predictors of positive health practices. J Nurs Scholarsh. 2004;36:102–8. doi: 10.1111/j.1547-5069.2004.04021.x. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Olsen J, Vestergaard M, Obel C. Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: a nationwide follow-up study in Denmark. Eur Child Adolesc Psychiatry. 2010;19:747–53. doi: 10.1007/s00787-010-0113-9. [DOI] [PubMed] [Google Scholar]
- 27.Baier CJ, Katunar MR, Adrover E, Pallarés ME, Antonelli MC. Gestational restraint stress and the developing dopaminergic system: an overview. Neurotox Res. 2012;22:16–32. doi: 10.1007/s12640-011-9305-4. [DOI] [PubMed] [Google Scholar]
- 28.Choudhry Z, Sengupta SM, Grizenko N, Fortier ME, Thakur GA, Bellingham J, et al. LPHN3 and attention-deficit/hyperactivity disorder: interaction with maternal stress during pregnancy. J Child Psychol Psychiatry. 2012;53:892–902. doi: 10.1111/j.1469-7610.2012.02551.x. [DOI] [PubMed] [Google Scholar]
- 29.Wilson CA, Schade R, Terry AV., Jr Variable prenatal stress results in impairments of sustained attention and inhibitory response control in a 5-choice serial reaction time task in rats. Neuroscience. 2012;218:126–37. doi: 10.1016/j.neuroscience.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapoor A, Matthews SG. Testosterone is involved in mediating the effects of prenatal stress in male guinea pig offspring. J Physiol. 2011;589:755–66. doi: 10.1113/jphysiol.2010.200543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cory-Slechta DA. Studying toxicants as single chemicals: does this strategy adequately identify neurotoxic risk? Neurotoxicology. 2005;26:491–510. doi: 10.1016/j.neuro.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Weston HI, Weston DD, Allen JL, Cory-Slechta DA. Sex-dependent impacts of low-level lead exposure and prenatal stress on impulsive choice behavior and associated biochemical and neurochemical manifestations. Neurotoxicology. 2014;44:169–83. doi: 10.1016/j.neuro.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kordas K, Ettinger AS, Bellinger DC, Schnaas L, Téllez Rojo MM, Hernández-Avila M, et al. A dopamine receptor (DRD2) but not dopamine transporter (DAT1) gene polymorphism is associated with neurocognitive development of Mexican preschool children with lead exposure. J Pediatr. 2011;159:638–43. doi: 10.1016/j.jpeds.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veselska Z, Madarasova Geckova A, Reijneveld SA, van Dijk JP. Socio-economic status and physical activity among adolescents: the mediating role of self-esteem. Public Health. 2011;125:763–8. doi: 10.1016/j.puhe.2011.09.007. [DOI] [PubMed] [Google Scholar]