Abstract
Plant responses to heavy metal contamination may depend on the presence of arbuscular mycorrhizal fungi (AMF). Elsholtzia splendens is an indicator species for the presence of copper (Cu) mines because both its flowering phenology and reproduction are tolerant to heavy metals. To test whether effects of Cu on the flowering phenology and reproduction of E. splendens depend on the presence of AMF, we conducted a factorial experiment with two Cu treatments (with or without Cu addition) crossed with two AMF treatments (with or without AMF inoculation). Without AMF, Cu addition significantly delayed the onset dates, ending dates and peak dates of flowering and decreased flowering duration. However, AMF inoculation reversed the effects of Cu stress, with recovered flowering onset and ending dates and increased the flowering duration. Cu addition significantly decreased inflorescence width and number, inflorescence biomass, vegetative biomass and total seed number, but significantly increased 1000-seed weight. AMF inoculation significantly increased vegetative biomass. Two-way ANOVA results showed that the interactive effects between Cu addition and AMF inoculation were significant on the inflorescence number, vegetative biomass and total seed number. These results indicate that AMF can alleviate the Cu stress on the flowering phenology and reproduction of E. splendens.
Introduction
Copper (Cu) is a common trace element that occurs naturally in soil, and plays an essential role in plant growth [1]. Cu is important for the synthesis of enzymes and proteins that are used by plants for various metabolic processes [1]. However, high Cu concentration is highly phytotoxic and can inhibit the photosynthetic and metabolic pathways in plants, thus resulting in decreased biomass and delayed flowering and fruiting [2–4].
Arbuscular mycorrhizal fungi (AMF) are commonly associated with roots of plants, forming symbionts [5]. It is well documented that AMF can help plants adapt to heavy metal stress such as Cu [5–7]. Recently, AMF was used as a Cu toxicity alleviator to enhance metal tolerance in plants, thereby ensuring their survival in Cu-contaminated soils [7–9]. The alleviating effects of AMF are reported to reduce the metal uptake, restricte the translocation of metals to the shoots, improve the soil structure and the nutritional status of plants, and biosorpt the metal sequestration onto the cell wall [8–12]. To date, however, few studies have focused on the interactive effect of Cu and AMF on plant flowering phenology and reproduction.
Flowering phenology is critical to the survival and reproduction of plants [13–15], and reproductive output is important for the long-term persistence of the plant species [16]. Both flowering phenology and reproduction are very sensitive to heavy metals [17]. For instance, soil contamination by heavy metals delayed flowering phenology of Hieracium pilosella [17,18] and reduced sexual reproduction of plants [19]. On the other hand, AMF can increase reproductive output and also change flowering phenology [19,20]. We thus hypothesized that AMF will attenuate the negative effects of Cu stress on flowering phenology and reproduction of plants.
Elsholtzia splendens is an annual herb that belongs to the Labiatae family. E. splendens is an indicator of Cu mines and is widely distributed on Cu mining wastes and Cu-contaminated soils along the middle and the lower reaches of the Yangtze River, China [21,22]. This species is Cu tolerant and thus has been used as an Cu-accumulation plant [23]. Previous studies have focused on biochemical and physiological responses of E. splendens to Cu stress, the ability of E. splendens to accumulate metal, and the chemical forms of Cu that exist in E. splendens [23,24]. Roots of E. splendens were found to form symbiont with AMF [25], and AMF plays an important role in the absorption and accumulation of heavy metals in E. splendens [26]. Here, we conducted a pot experiment to test the potential interactive effects of Cu and AMF on flowering phenology and reproductive allocation of E. splendens. Specially, we aimed to answer the question of how Cu and AMF interact to affect the flowering phenology and reproductive allocation of E. spelendens. These results could serve as a basic reference for the selection of Cu-tolerant or Cu-resistant plants used for phytoremediation of Cu-contaminated soils.
Materials and Methods
Experimental design
Plants were propagated in potting soil that was a mixture of peat soil, sand and vermiculite, at a volume ratio of 6:3:1. The soil mixture was autoclaved at 121°C for 2 h to eliminate native AMF propagules and other soil biota [27]. The autoclaved soil mixture had a pH of 5.73 ± 0.04, with an organic matter content of 20.16 ± 0.26 g·kg-1, total nitrogen content of 14.61 ± 0.53 mg·kg-1, available phosphorus content of 17.86 ± 0.49 mg·kg-1, and available potassium content of 56.67 ± 0.16 mg·kg-1 soil.
On December 20, 2012, E. splendens seeds were collected from plants grown in clean soil (without Cu contamination) on an abandoned field in Tainan village (31°30.632’N, 114°32.620’E; altitude 118 m), Hong’an County, Hubei Province, China, and stored at low humidity at room temperature. No specific permissions were required for collecting the seeds.
On December 21, 2012, soil from a depth of 0 to 20 cm was collected from Cu mine tailings located on Chimashan Mountain (29°59.776’N, 115°05.856’E; altitude 138 m), Yangxin County, Hubei Province, China. The soil type was sandy, and the soil texture was sandy clay. The vegetation on the tailings was dominated by E. Splendens with some pioneer plant species, including Cynodon dactylon, Xanthium sibiricum, Artemisia capillaries, Silene fortunei and Commelina communis. The soil was air-dried and passed through a 2-mm sieve to remove plant residues, large stones and soil fauna, and was then stored at -20°C for further use as the source of soil microbes.
On May 1, 2013, pots (19 cm in inner diameter and 15 cm deep) were sterilized using 75% ethanol and then filled with 1.7 kg sterilized soil mixture. Pots were placed on the ground in a greenhouse under natural light and ambient temperature. The pot locations within the greenhouse were randomized. Four treatments, coded as -Cu-AMF (no AMF and no Cu), +Cu (Cu addition), +AMF (AMF inoculation) and +Cu+AMF (Cu addition and AMF inoculation), were included in the experiment. A total of 60 pots, with 15 replicates for each treatment, were used. On May 5, 2013, 50 ml liquid with a concentration of 34 mg/ml CuSO4·5H2O was applied to each pot in the +Cu and +Cu+AMF treatments. On May 6, 2013, the soil collected from Cu mine tailings was incubated for 48 hours at room temperature. A total of 85 g soil was extracted with 50 ml Milli-Q water (Millipore Cooperation, Bedford, MA, USA); this extract was then applied to each pot in the -Cu+AMF and +Cu+AMF treatments. For the -Cu-AMF treatment and the +Cu-AMF treatment, 50 ml extraction water was filtered through 2 mm, 1 mm, 0.5 mm, 0.1 mm, 0.075 mm and 11 μm Whatman filter paper, and the filtrate was applied to the appropriate pots.
On May 5, 2013, E. splendens seeds were surface-sterilized with 0.5% NaClO, washed several times with sterilized distilled water and then sowed in trays containing the autoclaved soil mixture; seeds maintained in a greenhouse at Taizhou University (121°17’E, 28°87’N), Linhai City, Zhejiang Province, China for germination. On June 5, 2013, 12-cm-tall seedlings were transplanted into experimental pots with one seedling per pot. Pots were watered with tap water as necessary, and their weight was monitored to ensure that the soil moisture content was consistent.
Measurement
E. splendens has a compound spike verticillaster inflorescence. The phenology of individual E. splendens was monitored by determining the inflorescence level every two days from October 10 to November 10, 2013 [28]. The onset of flowering for each individual was recorded as the date on which each spikelet had at least one open floret. The date when 25% individuals were flowering were recorded as the onset date of the treatment. The peak flowering date was recorded as the date on which 50% of the spikelets had more than one opened floret. The end of flowering was recorded as the date on which 95% of the spikelets had more than one open floret. The duration was the difference between the first date on which an open floret was observed and the date on which the last floret opened across all spikelets.
On the peak flowering date, the inflorescence size, including length and width, was measured using Vernier calipers with 0.01 mm precision. When all the seeds matured, the plants were harvested. Plants were separated into vegetative structures, seeds and inflorescences. The numbers of flowers and seeds were recorded, and the weight of 1000 seeds was calculated. The biomass of vegetative structures, seeds and inflorescences were measured.
Fine roots were sampled and the success of AMF inoculation was verified by the mycorrhizal colonization rate. Fine roots were cut into 1-cm-long segments and fixed using formalin acetic alcohol fixation solution. Root samples were cleaned with 10% KOH solution at 90°C for 40 min, acidified in 2% HCl for 5 min, stained with 0.01% acid fuchsin [29], and observed by microscopy. A root segment was considered to be mycorrhizal when arbuscules, vesicles, or intercellular hyphal coils could be clearly identified. The mycorrhizal colonization rate was calculated using the following formula: AMF colonization rate (%) = 100 × root length infected/root length observed [30].
Statistical analysis
Data are presented as the mean ± 1 standard deviation. A two-way analysis of variance (ANOVA) was used to test the effect of AMF (inoculated vs. uninoculated) and Cu (added vs. not added) on the growth of E. splendens, with Cu and AMF as fixed factors. A least significant difference (LSD) test was employed to compare the treatment effects (-Cu-AMF, +Cu-AMF, -Cu+AMF, +Cu+AMF) at the 0.05 level of significance. All analyses were performed using the SPSS 17.0 software package for Windows. All figures were created in SigmaPlot 11.0.
Results
AMF colonization
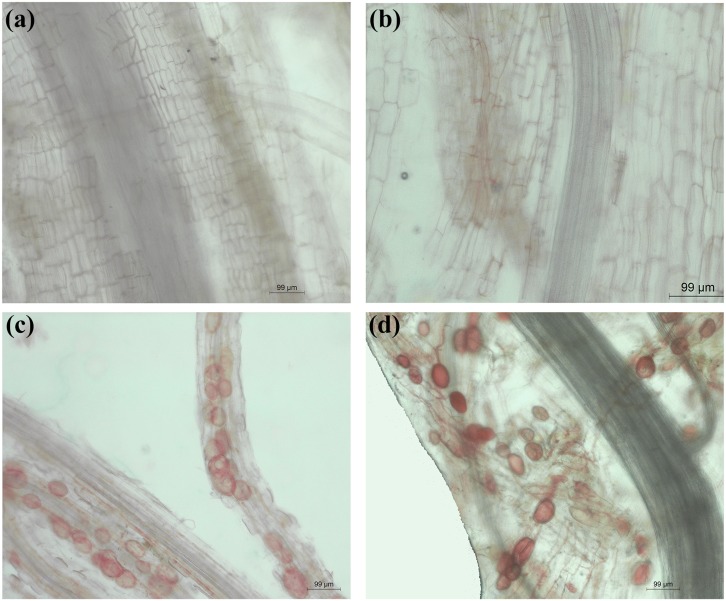
The AMF colonization rate for the -Cu+AMF and +Cu+AMF treatments was 42.5% and 52.8%, respectively, and the rate for the -Cu-AMF and +Cu-AMF treatments was 0 (Fig 1). These findings indicated that the inoculation with AMF was successful for both +AMF and +Cu+AMF treatments.
Fig 1. Microscopy of the fine roots of E. splendens under the four treatments.
(a) -Cu-AMF treatment (no AMF and no Cu), (b) +Cu-AMF treatment (Cu addition), (c) -Cu+AMF treatment (AMF inoculation), (d) +Cu+AMF treatment (Cu addition and AMF inoculation).
Effects of Cu and AMF on flowering phenology
Cu addition delayed the onset and end dates of flowering by up to 18 and 11 days, respectively, whereas AMF inoculation advanced the onset and end dates, regardless of whether Cu was added or not (Table 1). Cu addition delayed the peak flowering date, whereas AMF inoculation alone had no effect on the peak flowering date but advanced that of E. splendens under Cu stress (Table 1). Cu addition decreased the duration of flowering by 14.4 days, whereas AMF inoculation increased flowering by 1.4 days in the absence of Cu and by 6.5 days in the presence of Cu (Table 1).
Table 1. Phenology data for E. splendens in the four experimental treatments.
| -Cu-AMF | +Cu-AMF | -Cu+AMF | +Cu+AMF | |
|---|---|---|---|---|
| Onset | Oct. 6 | Oct. 24 | Oct. 4 | Oct. 20 |
| Duration | 36.6±2.0a | 22.2±2.6b | 38.0±2.5c | 28.7±2.4d |
| Peak flowering date | Oct. 23±2.3b | Nov. 8±2.7a | Oct. 23±1.4b | Nov. 6±1.7a |
| End date | Nov.15 | Nov. 26 | Nov.14 | Nov. 22 |
| Amplitude (flower/plant/day) | 3.38±2.75a | 4.44±1.97a | 3.09±2.27a | 4.57±3.67a |
Note: Values are given as the mean ± standard deviation (n = 15). Different small letters in the same line indicate the significant difference at P< 0.05. -Cu-AMF indicates no AMF and no Cu, +Cu-AMF indicates Cu addition, -Cu+AMF indicates AMF inoculation, +Cu+AMF indicates Cu addition and AMF inoculation.
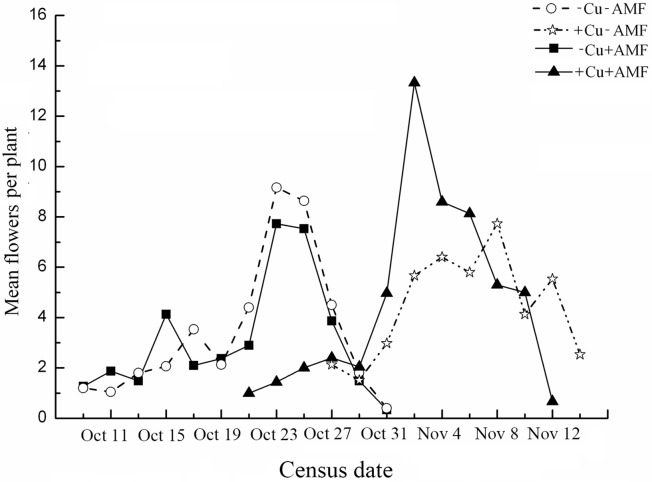
The flowering process of E. splendens in the four treatments showed a single peak process (Fig 2). AMF inoculation decreased the mean flowering amplitude, whereas Cu addition increased the amplitude, regardless of AMF inoculation (Table 1 and Fig 2).
Fig 2. Mean flowering amplitudes for E. splendens under different treatments.
Census intervals were 2 days. Amplitude shown is the mean number of flowers per day. -Cu-AMF indicates no AMF and no Cu, +Cu-AMF indicates Cu addition, -Cu+AMF indicates AMF inoculation, +Cu+AMF indicates Cu addition and AMF inoculation.
Effects of Cu and AMF on E. splendens reproduction
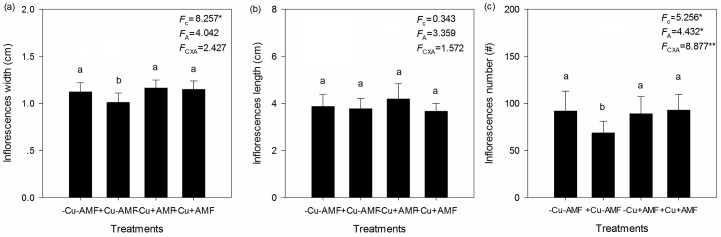
Cu addition significantly decreased inflorescence width and number, but had no effect on inflorescence length (Fig 3). AMF inoculation and the interaction between Cu addition and AMF inoculation had no effect on inflorescence length or width, but did significantly affect inflorescence number (Fig 3).
Fig 3. Effects of Cu addition and AMF inoculation on inflorescence length (a), width (b) and number (c).
-Cu-AMF indicates no AMF and no Cu, +Cu-AMF indicates Cu addition, -Cu+AMF indicates AMF inoculation, +Cu+AMF indicates Cu addition and AMF inoculation.
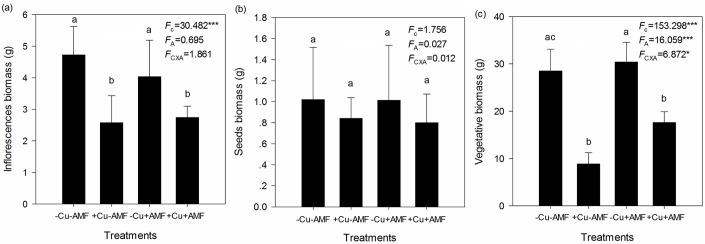
Cu addition significantly decreased inflorescence and vegetative biomass, but had no effect on seed biomass (Fig 4). AMF inoculation significantly increased vegetative biomass, but had no effect on inflorescence or seed biomass (Fig 4). Significant interactive effects between Cu addition and AMF inoculation were found on vegetative biomass, but not on inflorescence or seed biomass (Fig 4).
Fig 4. Effects of Cu addition and AMF inoculation on the biomass of flowers (a), seeds (b), and vegetative tissues (c).
-Cu-AMF indicates no AMF and no Cu, +Cu-AMF indicates Cu addition, -Cu+AMF indicates AMF inoculation, +Cu+AMF indicates Cu addition and AMF inoculation.
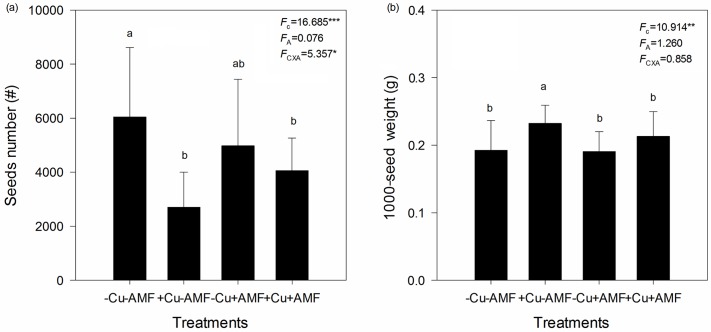
Cu addition significantly decreased total seed number, but significantly increased 1000-seed weight (Fig 5). AMF inoculation had no effect on total seed number or 1000-seed weight (Fig 5). A significant interactive effect between Cu addition and AMF inoculation was found on total seed number, but not on 1000-seed weight (Fig 5).
Fig 5. Effects of Cu addition and AMF inoculation on the seeds number (a) and 1000-seed weight (b).
-Cu-AMF indicates no AMF and no Cu, +Cu-AMF indicates Cu addition, -Cu+AMF indicates AMF inoculation, +Cu+AMF indicates Cu addition and AMF inoculation.
Discussion
Flowering phenology has important demographic consequences for plants [15] and was very sensitive to heavy metals [17]. In this study, Cu addition delayed the onset and the end dates of E. splendens by up to 18 and 11 days, and also delayed the peak flowering date. The similar effect of Cu addition on flowering phenology was found on Kummerowia stipulacea [31]. A longer flowering period could benefit plants with subsequent increases in reproductive output if pollinators are present [15]. In this study, Cu addition decreased the duration of flowering of E. splendens by 14.4 days, which could result in fewer pollinators and then decreased reproductive outputs.
AMF inoculation has been shown to change flowering phenology [20]. The flowering date of the dominant plant species in limestone soil was advanced after the inoculation of AMF [32]. However, Sohn et al. found that AMF inoculation significantly shortened flowering time of Chrysanthemum morifolium compared with non-AMF treatments [33]. In this study, we found that AMF inoculation advanced the peak flowering date of E. splendens by 6.5 days in the presence of Cu, indicating that AMF inoculation ameliorated the flower date delay and the reduction in flowering duration caused by the addition of Cu. Although no empirical study focused on the interactive effect between Cu addition and AMF, similar alleviation effects of AMF inoculation were reported in a previous study on the negative effects of water stress on inflorescence number of Biden pilosa [32]. Ryser and Sauder mentioned that the effects of metal contamination on flowering phenology and reproduction were mostly similar to those caused by water stress, and were not associated with obvious damage to the plants [17]. The recovery of the flowering duration observed here suggests that AMF inoculation could attract more pollinators, thereby increasing the reproductive success of plants and benefit the survival of E. splendens under Cu stress. In addition, flowering phenology may be related to resource accumulation [20]. AMF infection was positively associated with the increase of phosphorus uptake, which would affect the pattern of flowering [20].
Similar effects of Cu addition and AMF inoculation were found on reproduction of E. splendens. We found that Cu addition significantly reduced both inflorescence and seed number of E. splendens, indicating that Cu addition could significantly decrease its reproductive success. Heavy metals are toxic to plants and have significant negative effects on plant reproduction [31]. Negative effects of high Cu concentrations on plant reproduction have been reported previously for many plants species, such as Hieracium pilosella [17], Poa annua, Dactylis glomerata, Senecio vulgaris, Hypochoeris radicata, and Andryala integrifolia [34]. On the other hand, we also found that AMF inoculation significantly increased reproduction, and that there was also a significant interaction effect of Cu addition and AMF inoculation on reproduction. Similar effects of AMF inoculation were also found on the number of flowers of Petunia hybrida [19], and on the number of seeds and fruits per plant of Erodium oxyrrhynchum and Plantago minuta [35]. The alleviating effects of AMF on plants under Cu stress might be due to the reduction in metal uptake or nutritional accumulation in plants induced by AMF infection [9]. The larger inflorescence and seed number of E. splendens under Cu stress induced by AMF inoculation might have contributed to the survival of plants under Cu stress, as observed for Rumex dentatus [18]. These results indicate that AMF infection could contribute to plant tolerance to Cu stress by changing flowering phenology and reproductive success during the adaptive evolution. Further studies are needed to test this hypothesis.
In conclusion, we found that Cu addition significantly delayed flowering phenology, shortened flowering duration and inhibited reproduction of E. splendens, but AMF inoculation ameliorated the flower date delay and the reduction in flowering duration caused by the addition of Cu. Consequently, AMF inoculation alleviated the negative effect of Cu addition on the reproduction output of E. splendens. The results provide basic information for developing phytoremediation strategies using E. splendens.
Acknowledgments
We are grateful to W.S. Ke and M. Guan for their help in sampling soil and seeds. We also thank the editors and reviewers for their valuable comments. This study was financially supported by Zhejiang Provincial Nature Science Foundation (LY12C03002).
Data Availability
All relevant data are within the paper.
Funding Statement
Support was provided by the Zhejiang Provincial National Natural Science Foundation of China (No. LY12C03002) [http://www.zjnsf.gov.cn/]. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kamali M, Pour MS, Moud AAM. Copper effects on growth parameters of hollyhock (Althaea rosea L.). J Ornamental Hortic Plants. 2012; 2: 95–101. [Google Scholar]
- 2. Bhakuni G, Dube BK, Sinha P, Chatterjee C. Copper stress affects metabolism and reproductive yield of chickpea. J Plant Nutr. 2009; 32: 703–711. [Google Scholar]
- 3. Kováčik J, Klejdus B, Hedbavný J, Bačkor M. Effect of copper and salicylic acid on phenolic metabolites and free amino acids in Scenedesmus quadricauda (Chlorophyceae). Plant Sci. 2010; 178: 307–311. [Google Scholar]
- 4. Andre CM, Larondelle Y, Evers D. Dietary antioxidants and oxidative stress from a human and plant perspective: a review. Curr Nutr Food Sci. 2010; 6: 2–12. [Google Scholar]
- 5. Ferrol N, González-Guerrero M, Valderas A, Benabdellah K, Azcón-Aguilar C. Survival strategies of arbuscular mycorrhizal fungi in Cu-polluted environments. Phytochem Rev. 2009; 8: 551–559. [Google Scholar]
- 6. Carvalho LM, Caçador I, Martins-Loução MA. Arbuscular mycorrhizal fungi enhance root cadmium and copper accumulation in the roots of the salt marsh plant Aster tripolium L. Plant Soil. 2006; 285:161–169. [Google Scholar]
- 7. Hildebrandt U, Regvar M, Bothe H. Arbuscular mycorrhiza and heavy metal tolerance. Phytochem. 2007; 68: 139–146. [DOI] [PubMed] [Google Scholar]
- 8. Meier S, Brie F, Curaqueo G, Bolan N, Cornejo P. Effects of arbuscular mycorrhizal inoculation on metallophyte and agricultural plants growing at increasing copper level. Appl Soil Ecol. 2012; 61: 280–287. [Google Scholar]
- 9. Arunakumara KKIU, Walpola BC, Yoon MH. Alleviation of phyto-toxicity of copper on agricultural plants. J Korean Soc Appl Biol Chem. 2013; 56: 505–517. [Google Scholar]
- 10. Gonzalez-Chavez M, Carrillo-Gonzalez R, Wrigth S, Nichols K. The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environ Pollut. 2004; 130: 317–323. [DOI] [PubMed] [Google Scholar]
- 11. Zafar S, Aqil F, Ahmad I. Metal tolerance and biosorption potential of filamentous fungi isolated from metal contaminated agricultural soil. Bioresour Technol. 2007; 98: 2557–2561. [DOI] [PubMed] [Google Scholar]
- 12. Cornejo P, Meiera S, Borie G, Rillig MC, Borie F. Glomalin-related soil protein in a Mediterranean ecosystem affected by a copper smelter and its contribution to Cu and Zn sequestration. Sci Total Environ. 2008; 406: 154–160. 10.1016/j.scitotenv.2008.07.045 [DOI] [PubMed] [Google Scholar]
- 13. Ollerton J, Diaz A. Evidence for stabilizing selection acting on flowering time in Arum maculatum (Araceae): the influence of phenology on adaptation. Oecologia. 1999; 119: 340–348. [DOI] [PubMed] [Google Scholar]
- 14. Torimaru T, Tomaru N. Relationships between flowering phenology, plant size, and female reproductive output in a dioecious shrub, Ilex leucoclada (Aquifoliaceae). Can J Bot. 2006; 84: 1860–1869. [Google Scholar]
- 15. Barber NA, Gorden NLS. How do belowground organisms influence plant-pollinator interactions? J Plant Ecol. 2014; 8: 1–11. [Google Scholar]
- 16. Sadras VO. Evolutionary aspects of the trade-off between seed size and number in crops. Field Crop Res. 2007; 100: 125–138. [Google Scholar]
- 17. Ryser P, Sauder WR. Effects of heavy-metal-contaminated soil on growth, phenology and biomass turnover of Hieracium piloselloides . Environ. Pollut. 2006; 140: 52–61. [DOI] [PubMed] [Google Scholar]
- 18. Huang WX, Huang Y, Ye FY, Shan S, Xion ZT. Effects of copper on phenology and reproduction in Rumex dentatus from metalliferous and non-metalliferous sites. Ecotoxicol Environ Saf. 2011; 74: 1043–1049. 10.1016/j.ecoenv.2011.01.020 [DOI] [PubMed] [Google Scholar]
- 19. Gaur A, Gaur A, Adholeya A. Growth and flowering in Petunia hybrida, Callistephus chinensis and Impatiens balsamina inoculated with mixed AM inoculation or chemical fertilizers in a soil of low P fertility. Sci Horic. 2000; 84: 151–162. [Google Scholar]
- 20. Shamshiri MH, Usha K, Singh B. Growth and nutrient uptake responses of Kinnow to vesicular arbuscular mycorrhizae. ISRN Agronomy. 2012; 10.5402/2012/535846 [DOI] [Google Scholar]
- 21. Tang SR, Wilke BM, Huang CY. The uptake of copper by plants dominantly growing on copper mining spoils along the Yangtze River, the People’s Republic of China. Plant Soil. 1999; 209: 225–232. [Google Scholar]
- 22. Lou LQ, Shen ZG, Li XD. The copper tolerance mechanisms of Elsholtizia haichowensis, a plant from copper-enriched soils. Environ Exp Bot. 2004; 51: 111–120. [Google Scholar]
- 23. Jiang LY, Yang XE, Chen JM. Copper tolerance and accumulation of Elsholtzia splendens Nakai in a pot environment. J Plant Nutr. 2008; 31: 1382–1392. [Google Scholar]
- 24. Liu TT, Li F, Zhang X, Zhang H, Duan DC, Shen CF, et al. Tracing intracellular localization and chemical forms of copper in Elsholtzia splendens with cluster analysis. Bio Trace Elem Res. 2014; 160: 418–426. [DOI] [PubMed] [Google Scholar]
- 25. Yang RY, Zan ST, Tang JJ, Chen X, Zhang Q. Variation in community structure of arbuscular mycorrhizal fungi associated with a Cu tolerant plant——Elsholtzia splendens . Appl Soil Ecol. 2010; 44: 191–197. [Google Scholar]
- 26. Wang FY, Lin XG, Yin R, Wu LH. Effects of arbuscular mycorrhizal inoculation on the growth of Elsholtzia splendens and Zea mays and the activities of phosphatase and urease in a multi-metal-contaminated soil under unsterilized conditions. Appl Soil Ecol. 2006; 31: 110–119. [Google Scholar]
- 27. Andrade SAL, Gratăo PL, Schiavinato MA, Silveira APD, Azevedo RA, Mazzafera P. Zn uptake, physiological response and stress attenuation in mycorrhizal jack bean growing in soil with increasing Zn concentrations. Chemosphere 2009; 75: 1363–1370. 10.1016/j.chemosphere.2009.02.008 [DOI] [PubMed] [Google Scholar]
- 28. Gutiérrez HF, Medan D, Pensiero JF. Limiting factors of reproductive success in Bromus auleticus (Poaceae). I. Flowering phenology, sexual expression, and pollen production. New Zealand J Bot. 2006; 44: 47–55. [Google Scholar]
- 29. Kormanik PP, Bryan WC, Schultz RC. Procedure and equipment for staining large number of plant roots for endomycorrhizal assay. Can J Microb. 1980; 26: 536–538. [DOI] [PubMed] [Google Scholar]
- 30. Graham JH, Syvertsen JP. Host determinants of mycorrhizal dependency of citrus rootstock seedlings. New Phytol. 1985; 101: 667–676. [Google Scholar]
- 31. Gan JH, Xiong ZT, Li JP, Chen DQ. Differential response to copper stress in the reproductive resources and allocation of metallophyte Kummerowia stipulacea . Ecotoxicol Environ Saf. 2013; 89: 204–211. 10.1016/j.ecoenv.2012.11.033 [DOI] [PubMed] [Google Scholar]
- 32.Song HX. Species diversity of AMF and its effects on eco-physiology of dominant plant species in limestone soil. Ph.D Thesis, Southwestern University China, 2007.
- 33. Sohn BK, Kim KY, Chung SJ, Kim WS, Park SM, Kang JG, et al. Effect of different timing of AMF inoculation on plant and flower quality of chrysanthemum. Sci Hortic. 2003; 98: 173–183. [Google Scholar]
- 34. Brun LA, Le Corff J, Maillet J. Effects of elevated soil copper on phenology, growth and reproduction of five ruderal plant species. Environ Pollut. 2003; 122: 361–368. [DOI] [PubMed] [Google Scholar]
- 35. Sun Y, Li XL, Feng G. Effect of arbuscular mycorrhizal colonization on ecological functional traits of ephermerals in the Gurbantonggut desert. Symbiosis. 2008; 46: 121–127. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.