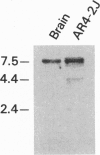
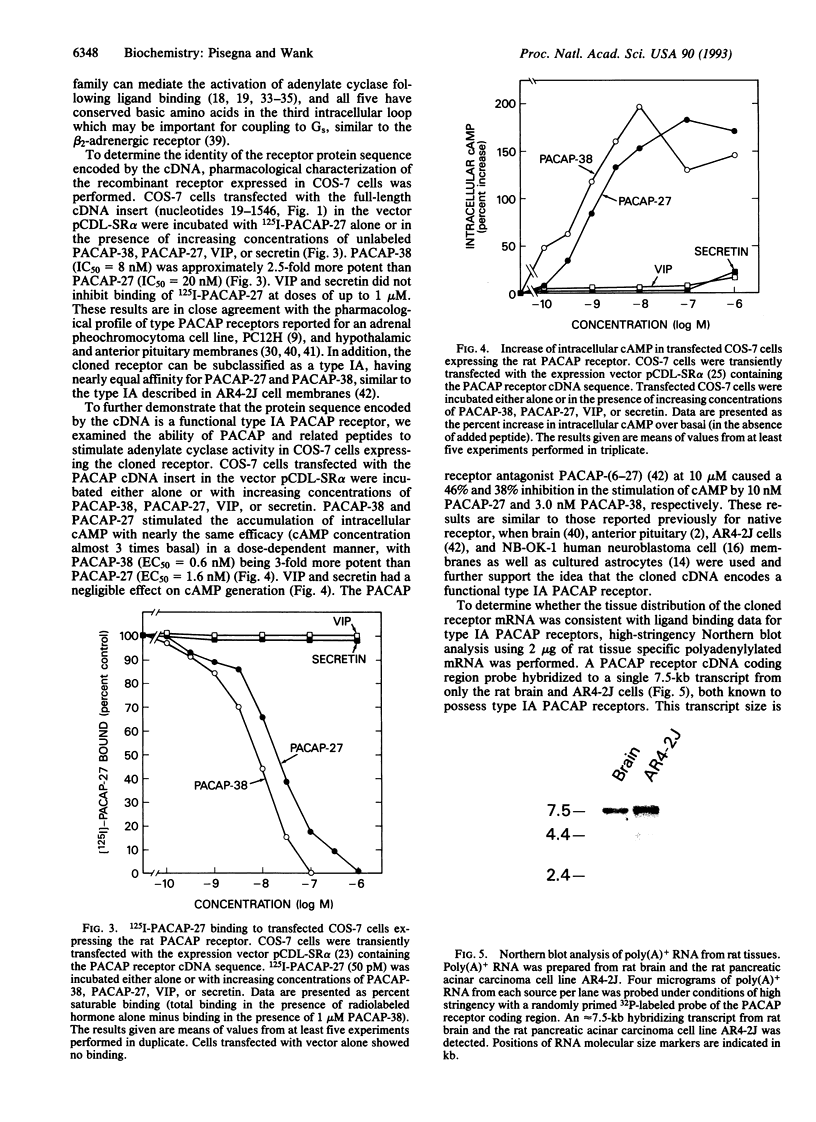
Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP), a neuropeptide belonging to the vasoactive intestinal peptide (VIP)/secretion/glucagon family of peptides, interacts with a distinct high-affinity receptor (type I receptor) on a number of tissues. These PACAP type I receptors have a high affinity for PACAP and a low affinity for VIP and are present in the hypothalamus and anterior pituitary, where they regulate the release of adrenocorticotropin, luteinizing hormone, growth hormone, and prolactin, and in the adrenal medulla, where they regulate the release of epinephrine. Type I PACAP receptors are also present in high concentrations in testicular germ cells, where they may regulate spermatogenesis, and some transformed cell lines, such as the rat pancreatic acinar carcinoma cell AR4-2J. Here we report the molecular cloning and functional expression of the PACAP type I receptor isolated from an AR4-2J cell cDNA library by cross-hybridization screening with a rat VIP receptor cDNA. The cDNA sequence encodes a unique 495-amino acid protein with seven transmembrane domains characteristic of guanine nucleotide-binding regulatory protein-coupled receptors. A high degree of sequence homology with the VIP, secretin, glucagon-like peptide 1, parathyroid, and calcitonin receptors suggests its membership in this subfamily of Gs-coupled receptors. Results of binding studies and stimulation of cellular cAMP accumulation in COS-7 cells transfected with this cDNA are characteristic of a PACAP type I receptor. Cloning of the PACAP type I receptor will enhance our understanding of its distribution, structure, and functional properties and ultimately increase our understanding of its physiological role.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Samra A. B., Jüppner H., Force T., Freeman M. W., Kong X. F., Schipani E., Urena P., Richards J., Bonventre J. V., Potts J. T., Jr Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2732–2736. doi: 10.1073/pnas.89.7.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura A., Somogyvári-Vigh A., Miyata A., Mizuno K., Coy D. H., Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991 Nov;129(5):2787–2789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- Buscail L., Gourlet P., Cauvin A., De Neef P., Gossen D., Arimura A., Miyata A., Coy D. H., Robberecht P., Christophe J. Presence of highly selective receptors for PACAP (pituitary adenylate cyclase activating peptide) in membranes from the rat pancreatic acinar cell line AR 4-2J. FEBS Lett. 1990 Mar 12;262(1):77–81. doi: 10.1016/0014-5793(90)80158-f. [DOI] [PubMed] [Google Scholar]
- Cauvin A., Buscail L., Gourlet P., De Neef P., Gossen D., Arimura A., Miyata A., Coy D. H., Robberecht P., Christophe J. The novel VIP-like hypothalamic polypeptide PACAP interacts with high affinity receptors in the human neuroblastoma cell line NB-OK. Peptides. 1990 Jul-Aug;11(4):773–777. doi: 10.1016/0196-9781(90)90194-a. [DOI] [PubMed] [Google Scholar]
- Cox H. M. Pituitary adenylate cyclase activating polypeptides, PACAP-27 and PACAP-38: stimulators of electrogenic ion secretion in the rat small intestine. Br J Pharmacol. 1992 Jun;106(2):498–502. doi: 10.1111/j.1476-5381.1992.tb14363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Sigal I. S., Candelore M. R., Register R. B., Scattergood W., Rands E., Strader C. D. Structural features required for ligand binding to the beta-adrenergic receptor. EMBO J. 1987 Nov;6(11):3269–3275. doi: 10.1002/j.1460-2075.1987.tb02645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman H. G., Caron M. G., Lefkowitz R. J. A family of receptors coupled to guanine nucleotide regulatory proteins. Biochemistry. 1987 May 19;26(10):2657–2664. doi: 10.1021/bi00384a001. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Goth M. I., Lyons C. E., Canny B. J., Thorner M. O. Pituitary adenylate cyclase activating polypeptide, growth hormone (GH)-releasing peptide and GH-releasing hormone stimulate GH release through distinct pituitary receptors. Endocrinology. 1992 Feb;130(2):939–944. doi: 10.1210/endo.130.2.1346381. [DOI] [PubMed] [Google Scholar]
- Gottschall P. E., Tatsuno I., Arimura A. Hypothalamic binding sites for pituitary adenylate cyclase activating polypeptide: characterization and molecular identification. FASEB J. 1991 Feb;5(2):194–199. doi: 10.1096/fasebj.5.2.1848519. [DOI] [PubMed] [Google Scholar]
- Gottschall P. E., Tatsuno I., Miyata A., Arimura A. Characterization and distribution of binding sites for the hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide. Endocrinology. 1990 Jul;127(1):272–277. doi: 10.1210/endo-127-1-272. [DOI] [PubMed] [Google Scholar]
- Hulme E. C., Birdsall N. J., Buckley N. J. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- Ishihara T., Nakamura S., Kaziro Y., Takahashi T., Takahashi K., Nagata S. Molecular cloning and expression of a cDNA encoding the secretin receptor. EMBO J. 1991 Jul;10(7):1635–1641. doi: 10.1002/j.1460-2075.1991.tb07686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T., Shigemoto R., Mori K., Takahashi K., Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992 Apr;8(4):811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- Karnik S. S., Sakmar T. P., Chen H. B., Khorana H. G. Cysteine residues 110 and 187 are essential for the formation of correct structure in bovine rhodopsin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8459–8463. doi: 10.1073/pnas.85.22.8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch B., Lutz-Bucher B. Pituitary adenylate cyclase-activating polypeptide (PACAP) stimulates cyclic AMP formation as well as peptide output of cultured pituitary melanotrophs and AtT-20 corticotrophs. Regul Pept. 1992 Mar 5;38(1):45–53. doi: 10.1016/0167-0115(92)90071-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991 Oct 25;266(30):19867–19870. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lam H. C., Takahashi K., Ghatei M. A., Kanse S. M., Polak J. M., Bloom S. R. Binding sites of a novel neuropeptide pituitary-adenylate-cyclase-activating polypeptide in the rat brain and lung. Eur J Biochem. 1990 Nov 13;193(3):725–729. doi: 10.1111/j.1432-1033.1990.tb19392.x. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Harris T. L., Flannery M. S., Aruffo A., Kaji E. H., Gorn A., Kolakowski L. F., Jr, Lodish H. F., Goldring S. R. Expression cloning of an adenylate cyclase-coupled calcitonin receptor. Science. 1991 Nov 15;254(5034):1022–1024. doi: 10.1126/science.1658940. [DOI] [PubMed] [Google Scholar]
- Miyata A., Arimura A., Dahl R. R., Minamino N., Uehara A., Jiang L., Culler M. D., Coy D. H. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989 Oct 16;164(1):567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Mungan Z., Arimura A., Ertan A., Rossowski W. J., Coy D. H. Pituitary adenylate cyclase-activating polypeptide relaxes rat gastrointestinal smooth muscle. Scand J Gastroenterol. 1992 May;27(5):375–380. doi: 10.3109/00365529209000091. [DOI] [PubMed] [Google Scholar]
- Nguyen T. D., Heintz G. G., Cohn J. A. Pituitary adenylate cyclase-activating polypeptide stimulates secretion in T84 cells. Gastroenterology. 1992 Aug;103(2):539–544. doi: 10.1016/0016-5085(92)90844-o. [DOI] [PubMed] [Google Scholar]
- Ogi K., Kimura C., Onda H., Arimura A., Fujino M. Molecular cloning and characterization of cDNA for the precursor of rat pituitary adenylate cyclase activating polypeptide (PACAP). Biochem Biophys Res Commun. 1990 Dec 31;173(3):1271–1279. doi: 10.1016/s0006-291x(05)80924-6. [DOI] [PubMed] [Google Scholar]
- Ohtaki T., Watanabe T., Ishibashi Y., Kitada C., Tsuda M., Gottschall P. E., Arimura A., Fujino M. Molecular identification of receptor for pituitary adenylate cyclase activating polypeptide. Biochem Biophys Res Commun. 1990 Sep 14;171(2):838–844. doi: 10.1016/0006-291x(90)91222-e. [DOI] [PubMed] [Google Scholar]
- Osuga Y., Mitsuhashi N., Mizuno M. In vivo effect of pituitary adenylate cyclase activating polypeptide 38 (PACAP 38) on the secretion of luteinizing hormone (LH) in male rats. Endocrinol Jpn. 1992 Feb;39(1):153–156. doi: 10.1507/endocrj1954.39.153. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Abdulaev N. G., Bogachuk A. S. Two adjacent cysteine residues in the C-terminal cytoplasmic fragment of bovine rhodopsin are palmitylated. FEBS Lett. 1988 Mar 28;230(1-2):1–5. doi: 10.1016/0014-5793(88)80628-8. [DOI] [PubMed] [Google Scholar]
- Propato-Mussafiri R., Kanse S. M., Ghatei M. A., Bloom S. R. Pituitary adenylate cyclase-activating polypeptide releases 7B2, adrenocorticotrophin, growth hormone and prolactin from the mouse and rat clonal pituitary cell lines AtT-20 and GH3. J Endocrinol. 1992 Jan;132(1):107–113. doi: 10.1677/joe.0.1320107. [DOI] [PubMed] [Google Scholar]
- Propato-Mussafiri R., Kanse S. M., Ghatei M. A., Bloom S. R. Pituitary adenylate cyclase-activating polypeptide releases 7B2, adrenocorticotrophin, growth hormone and prolactin from the mouse and rat clonal pituitary cell lines AtT-20 and GH3. J Endocrinol. 1992 Jan;132(1):107–113. doi: 10.1677/joe.0.1320107. [DOI] [PubMed] [Google Scholar]
- Raufman J. P., Malhotra R., Singh L. PACAP-38, a novel peptide from ovine hypothalamus, is a potent modulator of amylase release from dispersed acini from rat pancreas. Regul Pept. 1991 Oct 1;36(1):121–129. doi: 10.1016/0167-0115(91)90200-z. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Woussen-Colle M. C., De Neef P., Gourlet P., Buscail L., Vandermeers A., Vandermeers-Piret M. C., Christophe J. The two forms of the pituitary adenylate cyclase activating polypeptide (PACAP (1-27) and PACAP (1-38)) interact with distinct receptors on rat pancreatic AR 4-2J cell membranes. FEBS Lett. 1991 Jul 29;286(1-2):133–136. doi: 10.1016/0014-5793(91)80958-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer H., Schwarzhoff R., Creutzfeldt W., Schmidt W. E. Characterization of a guanosine-nucleotide-binding-protein-coupled receptor for pituitary adenylate-cyclase-activating polypeptide on plasma membranes from rat brain. Eur J Biochem. 1991 Dec 18;202(3):951–958. doi: 10.1111/j.1432-1033.1991.tb16455.x. [DOI] [PubMed] [Google Scholar]
- Shivers B. D., Görcs T. J., Gottschall P. E., Arimura A. Two high affinity binding sites for pituitary adenylate cyclase-activating polypeptide have different tissue distributions. Endocrinology. 1991 Jun;128(6):3055–3065. doi: 10.1210/endo-128-6-3055. [DOI] [PubMed] [Google Scholar]
- Suda K., Smith D. M., Ghatei M. A., Murphy J. K., Bloom S. R. Investigation and characterization of receptors for pituitary adenylate cyclase-activating polypeptide in human brain by radioligand binding and chemical cross-linking. J Clin Endocrinol Metab. 1991 May;72(5):958–964. doi: 10.1210/jcem-72-5-958. [DOI] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuno I., Gottschall P. E., Arimura A. Specific binding sites for pituitary adenylate cyclase activating polypeptide (PACAP) in rat cultured astrocytes: molecular identification and interaction with vasoactive intestinal peptide (VIP). Peptides. 1991 May-Jun;12(3):617–621. doi: 10.1016/0196-9781(91)90110-b. [DOI] [PubMed] [Google Scholar]
- Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddman R., Luts A., Arimura A., Sundler F. Pituitary adenylate cyclase-activating peptide (PACAP), a new vasoactive intestinal peptide (VIP)-like peptide in the respiratory tract. Cell Tissue Res. 1991 Jul;265(1):197–201. doi: 10.1007/BF00318155. [DOI] [PubMed] [Google Scholar]
- Wank S. A., Pisegna J. R., de Weerth A. Brain and gastrointestinal cholecystokinin receptor family: structure and functional expression. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8691–8695. doi: 10.1073/pnas.89.18.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Masuo Y., Matsumoto H., Suzuki N., Ohtaki T., Masuda Y., Kitada C., Tsuda M., Fujino M. Pituitary adenylate cyclase activating polypeptide provokes cultured rat chromaffin cells to secrete adrenaline. Biochem Biophys Res Commun. 1992 Jan 15;182(1):403–411. doi: 10.1016/s0006-291x(05)80159-7. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Ohtaki T., Kitada C., Tsuda M., Fujino M. Adrenal pheochromocytoma PC12h cells respond to pituitary adenylate cyclase activating polypeptide. Biochem Biophys Res Commun. 1990 Nov 30;173(1):252–258. doi: 10.1016/s0006-291x(05)81049-6. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]