Abstract
Gram-negative bacilli of the genus Aeromonas are primarily inhabitants of the aquatic environment. Humans acquire this organism from a wide range of food and water sources as well as during aquatic recreational activities. In the present study, the diversity and distribution of Aeromonas species from freshwater lakes in Malaysia was investigated using glycerophospholipid-cholesterol acyltransferase (GCAT) and RNA polymerase sigma-factor (rpoD) genes for speciation. A total of 122 possible Aeromonas strains were isolated and confirmed to genus level using the API20E system. The clonality of the isolates was investigated using ERIC-PCR and 20 duplicate isolates were excluded from the study. The specific GCAT-PCR identified all isolates as belonging to the genus Aeromonas, in agreement with the biochemical identification. A phylogenetic tree was constructed using the rpoD gene sequence and all 102 isolates were identified as: A. veronii 43%, A. jandaei 37%, A. hydrophila 6%, A. caviae 4%, A. salmonicida 2%, A. media 2%, A. allosaccharophila 1%, A. dhakensis 1% and Aeromonas spp. 4%. Twelve virulence genes were present in the following proportions—exu 96%, ser 93%, aer 87%, fla 83%, enolase 70%, ela 62%, act 54%, aexT 33%, lip 16%, dam 16%, alt 8% and ast 4%, and at least 2 of these genes were present in all 102 strains. The ascV, aexU and hlyA genes were not detected among the isolates. A. hydrophila was the main species containing virulence genes alt and ast either present alone or in combination. It is possible that different mechanisms may be used by each genospecies to demonstrate virulence. In summary, with the use of GCAT and rpoD genes, unambiguous identification of Aeromonas species is possible and provides valuable data on the phylogenetic diversity of the organism.
Introduction
Aeromonas organisms are oxidase-positive, polar flagellated, non-sporulating facultative anaerobic rods [1]. These Gram-negative aeromonads are essentially ubiquitous in the microbial biosphere and found in almost every environmental niche, including aquatic habitats, fish, foods, domesticated pets, birds and soil. The aquatic environment is the natural habitat of aeromonads and they can be isolated from rivers, lakes, ponds, groundwater, surface water and chlorinated water. They are well known as causative agents of disease in fish, prawns, shrimps, oysters and other seafood [2]. A. salmonicida cause furunculosis and septicemia that result in huge economical loss in the fishing industry [3,4]. Disease may also be caused by the mesophilic A. hydrophila which has been linked to several epidemic outbreaks in the fishing industry [2,5]. In humans, aeromonads have been reported to be responsible for both gastrointestinal and extraintestinal infections particularly in immunocompromised patients [2,6,7]. Humans acquire aeromonads from a wide range of food and water [2]. Recreational activities such as boating, skiing, fishing and diving pose risks leading to infections [4,8].
The taxonomy of Aeromonas is in transition and presently this genus consists of 30 species [9]. Identification of Aeromonas to the species level can be difficult due to its complex phenotypic and genotypic heterogeneity [10]. The use of molecular approaches has led to a more refined identification that has revealed a number of discrepancies in the biochemical identification of this organism [11]. A molecular identification for Aeromonas species using GCAT and rpoD genes was reported by Puthucheary et al. [12]. GCAT is a highly conserved lipase gene present in practically all Aeromonas strains and a specific PCR probe was designed by Chacón et al. [13] that avoids confusion with other genera, such as Vibrio and Plesiomonas. The rpoD gene, a housekeeping gene, was reported to be an excellent tool for identification and for inferring the taxonomy of the genus Aeromonas [11]. Enterobacterial repetitive intergenic consensus (ERIC) sequences are short repetitive sequences in genomes of bacteria and the ERIC-PCR approach has been widely used for genomic fingerprinting of a broad range of bacterial species [14]. This method allows both phylogenetic inference and clonal differentiation of bacterial strains.
The production of virulence factors is essential for bacteria to establish infections and in Aeromonas a number of virulence genes have been described [15–18]. Pore-forming aerolysins aer and enterotoxins act, alt and ast are virulence determinants associated with gastroenteritis and diarrheal syndromes [19–21]. Other virulence factors described are extracellular lipases lip, lipH3, pla and plc that alter the host plasma membranes [22]. Type 3 secretion system (T3SS) effectors, AexT and AexU with the ability to cause host cell death have also been characterised [23,24]. Virulence in Aeromonas is a complex process and the detection of virulence factors is necessary in determining the potential pathogenicity and subsequent possible targets for vaccines. Hence the objectives of this study were (a) to isolate aeromonads from their natural aquatic habitat, (b) to investigate the clonality of the isolates using ERIC-PCR, (c) to identify and speciate these isolates using the GCAT and rpoD genes and (d) to screen for 15 virulence determinants.
Materials and Methods
Sample collection and bacterial isolation
Surface water samples were collected from 5 fresh water multi-purpose recreational lakes in Selangor, i.e., Tasik Aman (lake 1) (N 03.10261 °, E 101.62477 °), Tasik Taman Jaya (lake 2) (N 3.10418 °, E 101.64929 °), Tasik Varsiti Universiti Malaya (lake 3) (N 3.11941 °, E 101.65808 °) and two unnamed lakes in Rawang (lake 4 and lake 5) (N 03.36606 °, E 101.63717 ° and N 03.36752 °, E 101.63043 °). All samples were kept at 4°C and analysed within 30 hours of collection.
The samples were pre-filtered to remove residue and subsequently filtered through a 0.45 μm nitrocellulose membrane (Sartorius, Germany) using a vacuum system. The membranes were then suspended in broth and plated onto m-Aeromonas selective media (Biolife, Italia Srl) supplemented with ampicillin (10 mg/l) [25]. Yellow colonies on the agar plates due to dextrin fermentation, after 18–24 hours of incubation at 30°C were presumed to be Aeromonas species and tested with oxidase reagent (bioMérieux, France), checked for growth on MacConkey agar and 6.5% (w/v) NaCl-Luria Bertani (LB) broth. Oxidase-positive colonies growing on MacConkey agar but not in 6.5% NaCl-LB broth were further confirmed to genus level by the API 20E system (bioMérieux, France), then grown in LB broth, cryopreserved in 20% (v/v) glycerol at -80°C and maintained in LB agar and broth as working cultures.
Bacterial DNA extraction
Genomic DNA extraction was carried out using GeneAll® Exgene™ Cell SV DNA isolation kit (GeneAll Technology, Korea). Overnight cultures were pelleted, lysed using 20 mg/ml Proteinase K, passed through a spin column and washed with buffers and the purified DNA was subjected to concentration and standardisation for subsequent molecular analysis.
Molecular identification
ERIC-PCR analysis
All isolates were subjected to ERIC-PCR fingerprinting using primers and PCR conditions as described previously [26]. The amplification products were electrophoresed in 1.5% (W/V) agarose gels containing ethidium bromide at 56V for 6 hours in Tris-borate-EDTA buffer. Gene Ruler 100 bp DNA Ladder Plus (Fermentas) was used as a molecular size reference. The electrophoresed gels were visualised using a UV light transilluminator. The digitised profiles were analysed by BioNumerics software, version 7.5 (Applied Maths, Belgium). Similarity between the fingerprints was calculated with the band-matching Dice coefficient. Cluster analysis was performed using the unweighted pair-group method with average linkages (UPGMA). Representative samples from each cluster were amplified using the rpoD gene and sent for sequencing for verification.
Genus identification
The GCAT gene was amplified using primer pair as reported previously [13]. Presence of this gene (237 bp) was visualised on 1.5% agarose gel stained with ethidium bromide.
Species identification
Strains with the GCAT gene were further subjected to rpoD gene sequencing whereby the 816 bp gene region was amplified by a touch-down PCR using a degenerate primer pair as described by Yamamoto et al. [27]. The amplified product of the rpoD gene was resolved on a 2% agarose gel, excised and subjected to purification using QIAquick Gel Extraction kit (Qiagen, Germany). The gel was dissolved completely to release the PCR product which was then passed through a spin column and washed with buffers for purification, then sent for sequencing using specific primers as reported by Yamamoto et al. [27]. The resulting DNA sequences were then compared with the GenBank database using Basic Local Alignment Search Tool (BLAST).
Phylogenetic analysis
Phylogenetic data analysis using a partial nucleotide sequence (670 bp) of the rpoD gene was performed, the sequences (GenBank accession numbers: KT187565 to KT187686) were aligned using the ClustalW program and pairwise sequence identity matrices were calculated using the Bioedit software version 7.0.9 [28]. A phylogenetic tree was constructed based on neighbour-joining method via the MEGA 6 program with bootstrapping for 1000 replicates [29]. The genetic distances were also computed using Kimura's two-parameter model. The rpoD gene sequences of type strains representing all the known Aeromonas species were obtained from National Centre for Biotechnology Information (NCBI) database and used as reference gene sequences in the phylogenetic tree construction (Table 1). Vibrio parahaemolyticus ATCC 43996 (JQ015347.1) was included as an outgroup.
Table 1. Reference gene sequences used in the phylogenetic tree construction.
| Species | Accession no. | |
|---|---|---|
| 1 | A. allosaccharophila CECT 4199 | HQ442825 |
| 2 | A. australiensis strain 266 | FN773335 |
| 3 | A. bestiarum CECT 4227 | HQ442854 |
| 4 | A. bivalvium CECT 7113 | HQ442817 |
| 5 | A. carvenicola CECT 7862 | HQ442864 |
| 6 | A. caviae CECT 838 | HQ442790 |
| 7 | A. dhakensis CECT 7289 | HQ442798 |
| 8 | A. diversa CECT 4254 | HQ442805 |
| 9 | A. encheleia CECT 4342 | HQ442778 |
| 10 | A. eucrenophila CECT 4224 | HQ442770 |
| 11 | A. fluvialis strain 717 | FJ603453 |
| 12 | A. hydrophila CECT 839 | HQ442791 |
| 13 | A. jandaei CECT 4228 | HQ442840 |
| 14 | A. media CECT 4232 | HQ442785 |
| 15 | A. molluscorum CECT 5864 | HQ442812 |
| 16 | A. piscicola CECT 7443 | HQ442859 |
| 17 | A. popoffii CECT 5176 | HQ442853 |
| 18 | A. rivuli DSM 22539 | FJ969433 |
| 19 | A. salmonicida CECT 894 | HQ442843 |
| 20 | A. sanarellii strain A2-67 | FJ807275 |
| 21 | A. schubertii CECT 4240 | HQ442809 |
| 22 | A. simiae CIP 107798 | HQ442811 |
| 23 | A. sobria CECT 4245 | HQ442867 |
| 24 | A. taiwanensis strain A2-50 | FJ807271 |
| 25 | A. tecta CECT 7082 | HQ442762 |
| 26 | A. trota CECT 4255 | HQ442822 |
| 27 | A. veronii CECT 4257 | HQ442833 |
Screening of virulence genes
Isolates identified as Aeromonas species by rpoD gene analysis were subjected to direct PCR to detect the existence of the following 15 known virulence genes encoding for, DNAse (exu), heat-labile cytotonic enterotoxin (alt), serine protease (ser), aerolysin/hemolysin (aer), cytotoxic enterotoxin (act), heat-stable cytotonic enterotoxin (ast), lipase (lip), flagellin (fla), elastase (ela), ADP-ribosyltransferase toxins (aexT and aexU), DNA adenine methyltransferase (dam), enolase (enolase), T3SS membrane component (ascV) and hemolysin (hlyA). Screening was performed using the PCR primers reported previously (Table 2) at annealing temperatures from 55 to 68°C. Two-tailed Fisher’s exact test was carried out to determine the presence of combination of virulence genes. The purified PCR products of the genes, detected by QIAquick gel extraction kit (Qiagen, Germany) were sent for sequencing for validation.
Table 2. Primers selected to detect virulence genes.
| Gene | Primer sequence (5’ to 3’), F/R | Size (bp) | Reference |
|---|---|---|---|
| exu |
(A/G)GACATGCACAACCTCTTCC/
GCTTGGTATTGCC(C/T)TGCAA(C/G) |
323 | [30] |
| ser |
ACGGAGTGCGTTCTTCCTACTCCAG/
CCGTTCATCACACCGTTGTAGTCG |
211 | [31] |
| aer |
CCTATGGCCTGAGCGAGAAG/
CCAGTTCCAGTCCCACCACT |
431 | [15] |
| fla |
TCCAACCGTYTGACCTC/
GMYTGGTTGCGRATGGT |
608 | [18] |
| act |
AGAAGGTGACCACCAAGAACA/
AACTGACATCGGCCTTGAACTC |
232 | [32] |
| ela |
ACACGGTCAAGGAGATCAAC/
CGCTGGTGTTGGCCAGCAGG |
513 | [18] |
| aexT |
CGTGGCCATCAAAGAGTGG/
GCAGCTGGCTCATCGCCTC |
425 | [33] |
| lip |
ATCTTCTCCGACTGGTTCGG/
CCGTGCCAGGACTGGGTCTT |
382 | [18] |
| alt |
AAAGCGTCTGACAGCGAAGT/
AGCGCATAGGCGTTCTCTT |
320 | [34] |
| ast |
ATCGTCAGCGACAGCTTCTT/
CTCATCCCTTGGCTTGTTGT |
504 | [34] |
| dam |
ATGAAAAAAACACGCGCTTTTTTAAAATGG/
TCAGCCGAGTGGCGCCAGTTCGGCGTCG |
873 | [16] |
| enolase |
ATGTCCAAGATCGTTAAAGTGAT/
TTAAGCCTGGTTCTTCACTTCTT |
1302 | [35] |
| ascV |
ATGAAGCCCGCTTCGCCTATCAA/
TCACAGGCAGACCCTTCCCAGC |
2166 | [36] |
| aexU |
ATGCAGATTCAAACACATACCAGCGGC/
TTACAGATAGTCAGCCCCGACACCGAT |
1539 | [23] |
| hlyA |
ATGAGTTTTGCCGATAGTTTATTTTTCCTGA/
TTACGATTCCTGAGCGGGCTTGTCGGCCGGCGTG |
1320 | [37] |
Results and Discussion
A total of 122 probable aeromonad strains were isolated from the 5 lakes in Selangor and identified as Aeromonas spp. using the API20E system, i.e. 27 from lake 1, 21 from lake 2, 15 from lake 3, 18 from lake 4 and 21 from lake 5.
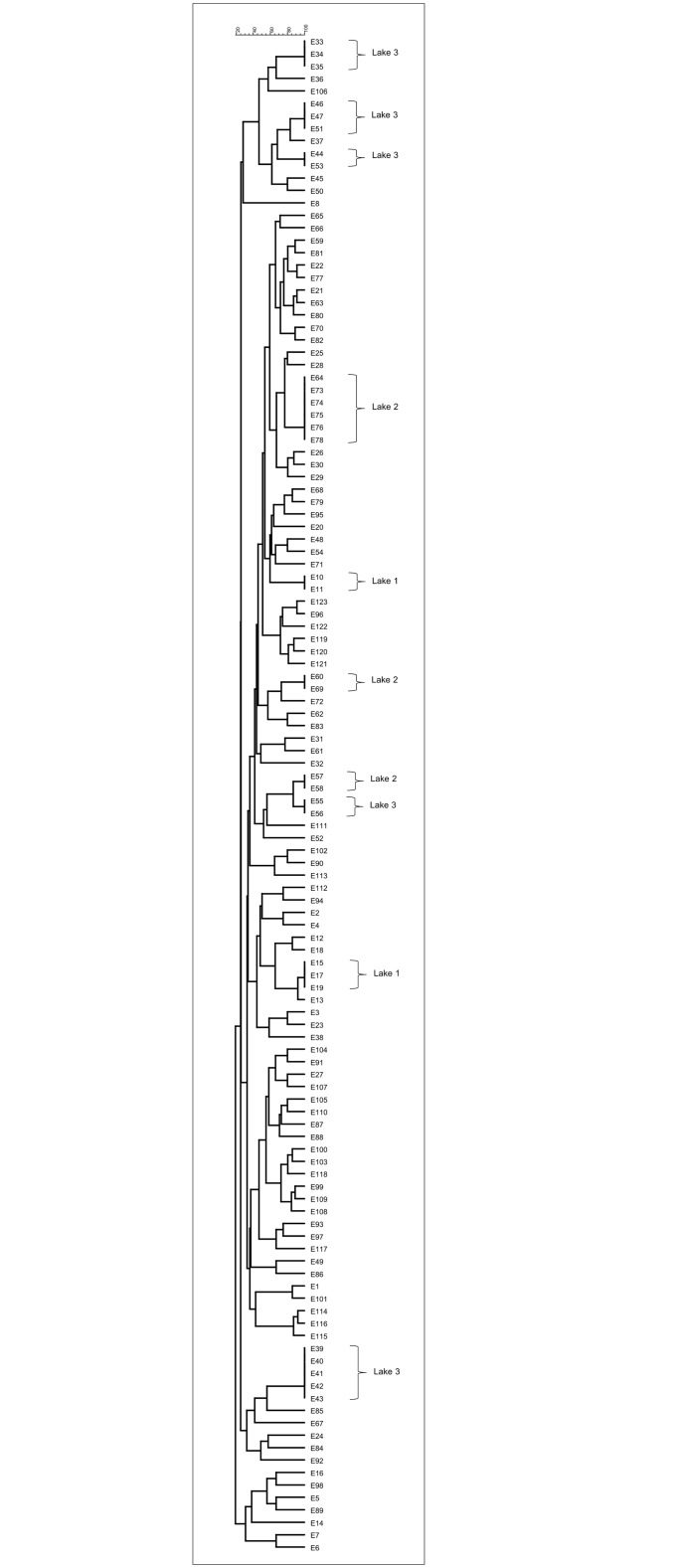
ERIC fingerprinting revealed 10 clusters consisting of 30 isolates at the 100% similarity level (Fig 1). The strains within each cluster appeared to have identical fingerprints and were considered as belonging to the same clone. The verification test demonstrated identical rpoD gene sequences for the representative isolates of the same cluster thus confirming the clonality. Replicate isolates were excluded from the study and 102 samples were subjected to further analyses.
Fig 1. Dendrogram showing ERIC fingerprints of the 122 strains of Aeromonas isolates using Dice similarity coefficient and UPGMA cluster method.
The genus-specific GCAT gene present in the 102 isolates, confirmed that all the environmental isolates belonged to the genus Aeromonas. Hence, the biochemical identification system API20E is in agreement with the GCAT-PCR in identifying Aeromonas to the genus level. But the API20E has limitations in accurately identifying Aeromonas species without additional biochemical, morphological and physiological tests, thus the necessity of molecular methods for a higher resolution identification of Aeromonas species.
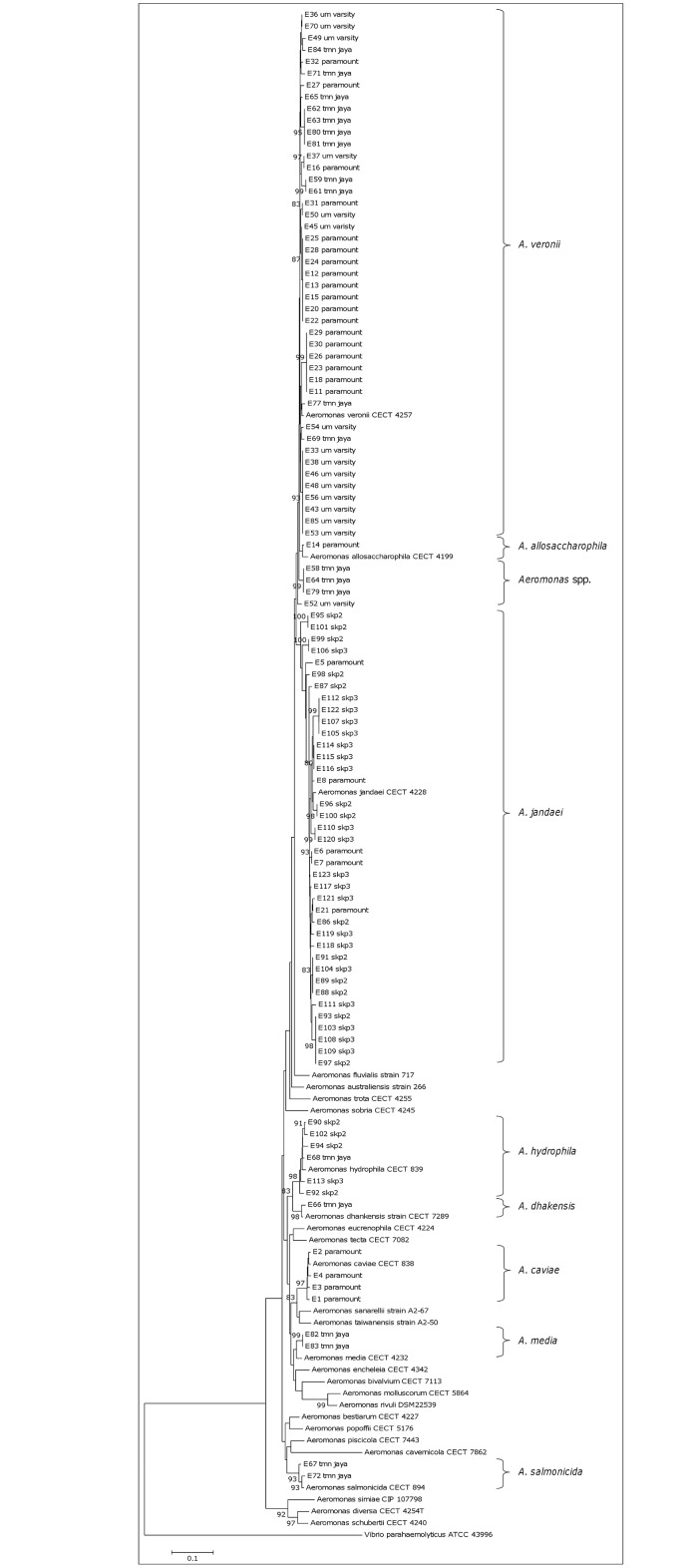
Based on the constructed phylogenetic tree using the partial rpoD sequence, 98 isolates showed clustering with the reference strains of respective species and were identified as: A. veronii—44, A. jandaei—38, A. hydrophila—6, A. caviae—4, A. salmonicida—2, A. media—2, A. allosaccharophila—1 and A. dhakensis—1 (Fig 2). Four isolates were not grouped into any cluster with known species strain. They formed clusters branching close to A. allosaccharophila and A. veronii, suggesting that these isolates need further investigation using more than one target gene or other approaches. The 4 isolates were considered as “Aeromonas spp.” in this study. The pairwise sequence identity matrix using the rpoD gene revealed that intraspecies similarities for the isolates were 96.2–100% for A. veronii, 95.2–100% for A. jandaei, 97.3–98.6% for A. hydrophila, 97.1–99.4% for A. caviae, 98.0% for A. salmonicida and 100% for A. media and 96.7–100% for Aeromonas spp.
Fig 2. Phylogenetic tree of 102 Aeromonas and reference strains based on the rpoD gene sequences using neighbour-joining method with bootstrap replication of 1000.
A. veronii was the most common species– 43% followed by A. jandaei 37%, A. hydrophila 6%, A. caviae 4%, A. salmonicida 2%, A. media 2%, A. allosaccharophila 1% and A. dhakensis 1% and Aeromonas spp. 4%. We found dominance of A. veronii in environmental samples [38,39]. A. jandaei–the second common species in this study, was reported to be associated with epizootic occurrence in farmed eels [40] and possible implications for clinical infections [41]. A. salmonicida is a predominant species related to fish and fresh water samples [2]. However, only 2 strains of A. salmonicida were detected in our study and this may be due to several factors such as geographical location, type of aquatic environment selected or the temperature and pH of the water as A. salmonicida is not able to grow at temperatures around 37°C [42,43]. This variance can also be related to different genes or different regions of the same gene being targeted for the bacterial identification.
Biochemically, all Aeromonas isolates were oxidase-positive and fermented glucose and mannose, but showed negative reactions for ornithine decarboxylase, hydrogen sulfide, urease, tryptophan deaminase and rhamnose fermentation. Most A. jandaei were citrate-positive whereas only half of A. veronii were able to use citrate as the sole carbon source. Thirty-four percent of A. jandaei fermented melibiose while none of the A. veronii strains showed fermentation. The findings were in agreement with Abbott et al., [44]. However, 7 (18%) A. jandaei strains were observed to be atypically sucrose-positive.
Multiple virulence genes were present in all the 102 Aeromonas strains and all contained at least 2 of the virulence genes but none possessed only 1 virulence gene (Table 3). A single A. hydrophila isolate from the lake 4 carried a complement of 12 of the 15 virulence genes. The exu gene was the most prevalent, being present in 96% of the isolates and widely distributed in all species, except of A. media, similar to the study by Chacón et al. [15]. Other virulence genes detected were ser 93%, aer 87%, fla 83%, enolase 70%, ela 62, act 54%, aexT 33%, lip 16%, dam 16%, alt 8% and ast 4%. The sequencing results were compared to the Genbank database using BLAST. The nucleotide BLAST homology search revealed high homologies (>90%) of the virulence gene PCR products with the deposited sequences in the database.
Table 3. Presence of multiple virulence genes in 102 Aeromonas isolates.
| Species | Frequency of isolates harbouring the indicated number of virulence gene, % | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | Total | |
| (≥2) | ||||||||||||||||
| A. veronii | - | - | - | 3 | 11 | 16 | 9 | 5 | - | - | - | - | - | - | - | 44 |
| (n = 44) | 7 | 25 | 36 | 20 | 11 | 100 | ||||||||||
| A. jandaei | - | - | 2 | 2 | 6 | 21 | 6 | 1 | - | - | - | - | - | - | - | 38 |
| (n = 38) | 5 | 5 | 16 | 55 | 16 | 3 | 100 | |||||||||
| A. hydrophila | - | - | - | - | - | - | - | 1 | - | 3 | 1 | 1 | - | - | - | 6 |
| (n = 6) | 17 | 50 | 17 | 17 | 100 | |||||||||||
| A. caviae | - | - | - | - | 2 | 1 | - | 1 | - | - | - | - | - | - | - | 4 |
| (n = 4) | 50 | 25 | 25 | 100 | ||||||||||||
| A.salmonicida | - | - | - | - | - | - | - | - | - | 2 | - | - | - | - | - | 2 |
| (n = 2) | 100 | 100 | ||||||||||||||
| A. media | - | - | - | 1 | 1 | - | - | - | - | - | - | - | - | - | - | 2 |
| (n = 2) | 50 | 50 | 100 | |||||||||||||
| A. allasaccharophila | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | 1 |
| (n = 1) | 100 | 100 | ||||||||||||||
| A. dhakensis | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | 1 |
| (n = 1) | 100 | 100 | ||||||||||||||
| Aeromonas spp. | - | - | - | 2 | 1 | - | 1 | - | - | - | - | - | - | - | - | 4 |
| (n = 4) | 50 | 25 | 25 | 100 | ||||||||||||
Two-tailed Fisher’s exact test based on each virulence gene revealed statistically significant associations in A. hydrophila with alt, ast, lip, aexT and dam; A. caviae with lip; A. veronii with act and aexT and A. salmonicida with dam (Table 4). The serine protease gene was detected in all species (≥95%) with the exception of A. caviae (25%). The presence of aer gene was observed in A. veronii 94% and A. hydrophila 83%, but less frequently among A. caviae 25%, in agreement with Yousr et al. [45]. Inverse associations were observed in A. jandaei with the lip, fla and aexT genes and A. veronii with the lip, ela and enolase genes. None of the isolates demonstrated the presence of the ascV, aexU and hlyA genes. Khajanchi et al [46] reported that 30–47% of their isolates obtained from water samples were positive for the ascV, aexU and hlyA genes. This discordance can be explained by the fact that pathogenetic mechanisms of Aeromonas species may be different in different geographical locations. The discrepancies can also be related to the different dominant species of isolates in different studies. In their study, the predominant species was A. hydrophila (59.5%)—this species harboured the 3 genes. In contrast, there were only 6 strains of A. hydrophila (6%) in our study.
Table 4. Distribution of single and subsets of virulence genes in 102 Aeromonas isolates.
| Single virulence gene | Frequency of isolates of the indicated species | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A. veronii (n = 44) | A. jandaei (n = 38) | A. hydrophila (n = 6) | A. caviae(n = 4) | A. salmonicida(n = 2) | A. media (n = 2) | A. allosaccharophila(n = 1) | A. dhakensis (n = 1) | Aeromonas spp. (n = 4) | Total (n = 102) | |
| exu | 42 | 38 | 6 | 4 | 2 | 0 | 1 | 1 | 4 | 98 |
| alt | 0 | 0 | 6 a | 0 | 1 | 0 | 0 | 1 | 0 | 8 |
| ser | 42 | 36 | 6 | 1 | 2 | 2 | 1 | 1 | 4 | 95 |
| aer | 41 | 35 | 5 | 1 | 2 | 0 | 0 | 1 | 4 | 89 |
| act | 41 a | 6 | 2 | 2 | 2 | 1 | 0 | 0 | 1 | 55 |
| ast | 0 | 0 | 4 a | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| lip | 0 | 2 | 5 b | 4 b | 2 | 2 | 0 | 1 | 0 | 16 |
| fla | 38 | 27 | 6 | 4 | 2 | 2 | 1 | 1 | 4 | 85 |
| ela | 11 | 37 | 6 | 4 | 2 | 2 | 0 | 1 | 0 | 63 |
| aexT | 26 a | 0 | 5 b | 0 | 1 | 0 | 1 | 0 | 1 | 34 |
| aexU | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| dam | 0 | 7 | 6 a | 0 | 2 b | 0 | 0 | 1 | 0 | 16 |
| enolase | 25 | 32 | 4 | 4 | 2 | 0 | 1 | 1 | 2 | 71 |
| ascV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| hlyA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subset of virulence gene | ||||||||||
| exu/ser/aer/fla | 36 | 26 | 5 | 1 | 2 | 0 | 0 | 1 | 4 | 71 |
| exu/ser/aer | 6 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15 |
| exu/aer/fla | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| exu/ser/fla | 2 | 1 | 0 | 0 | 0 | 0 | 1 b | 0 | 0 | 4 |
| ser/aer/fla | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| ser/fla | 1 | 0 | 0 | 0 | 0 | 2 b | 0 | 0 | 0 | 3 |
| exu/fla | 0 | 0 | 0 | 3 a | 0 | 0 | 0 | 0 | 0 | 3 |
| exu only | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
Significantly higher presence of the single/subset of virulence gene:
a p<0.0001;
b p<0.05.
Four virulence genes: exu, ser, aer and fla were the most frequently detected (>80%) and based on these 4 genes, 8 different subsets were observed and a statistically significant association was observed in A. allosaccharophila with exu/ser/fla; A. caviae with exu/fla and A. media with ser/fla (Table 4). The cytotonic enterotoxin genes alt and ast were less frequently detected (alt-7%) and (ast-3%) and however mainly present in A. hydrophila. A significant association (p<0.0001) was found between these 2 genes and A. hydrophila either singly or in combination (alt/ast), and this implies that A. hydrophila probably possess a distinct set of virulence genes vis-à-vis other Aeromonas species. The alt and ast virulence determinants are associated with diarrhea, and found to be more prevalent in children with diarrhea compared to healthy controls, probably due to the presence of enterotoxins in Aeromonas [19]. The different proportions of virulence genes present suggest that different mechanisms may be used by each subpopulation of Aeromonas to colonise and induce infections.
Conclusions
Phenotypic and genotypic diversity of Aeromonas organisms from aquatic environments were investigated in this study. The use of both the approaches are useful for the characterisation of Aeromonas, however phenotypic studies have limitations emphasising the need for molecular identification methods. Ninety-six percent of the aeromonad isolates from aquatic environments were confirmed as Aeromonas species using the GCAT and rpoD genes. The results confirm that by the use of these two genes, the definitive identification of environmental Aeromonas species is possible. A. veronii was the predominant species 43%, occurring in the freshwater lakes. Multiple virulence genes were present and different subsets of these genes existed in the various Aeromonas species, leading to the suggestion that each species has a distinct set of virulence genes.
Data Availability
All rpoD sequences were deposited in the Genbank (Accession No.: KT187565-KT187686).
Funding Statement
This study was funded by the University of Malaya Research Grant RP039A-15HTM and Postgraduate Research Grant PG082-2014B.
References
- 1. Nordmann P, Poirel L. Emerging carbapenemases in Gram-negative aerobes. Clin Microbiol Infect. 2002;8: 321–331. [DOI] [PubMed] [Google Scholar]
- 2. Janda JM, Abbott SL. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev. 2010;23: 35–73. 10.1128/CMR.00039-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Faisal M, Eissa AE, Elsayed EE. Isolation of Aeromonas salmonicida from sea lamprey (Petromyzon marinus) with furuncle-like lesions in Lake Ontario. J Wildl Dis. 2007;43: 618–622. 10.7589/0090-3558-43.4.618 [DOI] [PubMed] [Google Scholar]
- 4. Janda JM, Abbott SL. Evolving concepts regarding the genus Aeromonas: an expanding Panorama of species, disease presentations, and unanswered questions. Clin Infect Dis. 1998;27: 332–344. [DOI] [PubMed] [Google Scholar]
- 5. Hossain MJ, Sun D, McGarey DJ, Wrenn S, Alexander LM, Martino ME, et al. An Asian origin of virulent Aeromonas hydrophila responsible for disease epidemics in United States-farmed catfish. MBio. 2014;5: e00848–14. 10.1128/mBio.00848-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee WS, Puthucheary SD. Retrospective study of Aeromonas infection in a Malaysian urban area: a 10-year experience. Singapore Med J. 2001;42: 57–60. [PubMed] [Google Scholar]
- 7. Figueras MJ. Clinical relevance of Aeromonas sM503. Rev Med Microbiol. 2005;16: 145–153. [Google Scholar]
- 8. Bossi-Kupfer M, Genini A, Peduzzi R, Demarta A. Tracheobronchitis caused by Aeromonas veronii biovar sobria after near-drowning. J Med Microbiol. Microbiology Society; 2007;56: 1563–1564. 10.1099/jmm.0.47202-0 [DOI] [PubMed] [Google Scholar]
- 9. Beaz-Hidalgo R, Hossain MJ, Liles MR, Figueras MJ. Strategies to Avoid Wrongly Labelled Genomes Using as Example the Detected Wrong Taxonomic Affiliation for Aeromonas Genomes in the GenBank Database. PLoS One. 2015;10: e0115813 10.1371/journal.pone.0115813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lamy B, Laurent F, Verdier I, Decousser J-W, Lecaillon E, Marchandin H, et al. Accuracy of 6 commercial systems for identifying clinical Aeromonas isolates. Diagn Microbiol Infect Dis. 2010;67: 9–14. 10.1016/j.diagmicrobio.2009.12.012 [DOI] [PubMed] [Google Scholar]
- 11. Beaz-Hidalgo R, Alperi A, Buján N, Romalde JL, Figueras MJ. Comparison of phenotypical and genetic identification of Aeromonas strains isolated from diseased fish. Syst Appl Microbiol. 2010;33: 149–153. 10.1016/j.syapm.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 12. Puthucheary SD, Puah SM, Chua KH. Molecular characterisation of clinical isolates of Aeromonas species from Malaysia. PLoS One. 2012;7: e30205 10.1371/journal.pone.0030205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chacón MR, Castro-Escarpulli G, Soler L, Guarro J, Figueras MJ. A DNA probe specific for Aeromonas colonies. Diagn Microbiol Infect Dis. 2002;44: 221–225. [DOI] [PubMed] [Google Scholar]
- 14. Versalovic J, Koeuth T, Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19: 6823–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chacón MR, Figueras MJ, Castro-Escarpulli G, Soler L, Guarro J. Distribution of virulence genes in clinical and environmental isolates of Aeromonas spp. Antonie Van Leeuwenhoek. 2003;84: 269–278. 10.1023/A:1026042125243 [DOI] [PubMed] [Google Scholar]
- 16. Erova TE, Pillai L, Fadl AA, Sha J, Wang S, Galindo CL, et al. DNA adenine methyltransferase influences the virulence of Aeromonas hydrophila . Infect Immun. 2006;74: 410–424. 10.1128/IAI.74.1.410-424.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pillai L, Sha J, Erova TE, Fadl AA, Khajanchi BK, Chopra AK. Molecular and functional characterisation of a ToxR-regulated lipoprotein from a clinical isolate of Aeromonas hydrophila . Infect Immun. 2006;74: 3742–3755. 10.1128/IAI.00402-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sen K, Rodgers M. Distribution of six virulence factors in Aeromonas species isolated from US drinking water utilities: a PCR identification. J Appl Microbiol. 2004;97: 1077–1086. 10.1111/j.1365-2672.2004.02398.x [DOI] [PubMed] [Google Scholar]
- 19. Albert MJ, Ansaruzzaman M, Talukder KA, Chopra AK, Kuhn I, Rahman M, et al. Prevalence of enterotoxin genes in Aeromonas spp. isolated from children with diarrhea, healthy controls, and the environment. J Clin Microbiol. 2000;38: 3785–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chopra AK, Houston CW. Enterotoxins in Aeromonas-associated gastroenteritis. Microbes Infect. 1999;1: 1129–1137. 10.1016/S1286-4579(99)00202-6 [DOI] [PubMed] [Google Scholar]
- 21. Sha J, Kozlova E V, Chopra AK. Role of various enterotoxins in Aeromonas hydrophila-induced gastroenteritis: generation of enterotoxin gene-deficient mutants and evaluation of their enterotoxic activity. Infect Immun. 2002;70: 1924–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pemberton JM, Kidd SP, Schmidt R. Secreted enzymes of Aeromonas . FEMS Microbiol Lett. 1997;152: 1–10. 10.1111/j.1574-6968.1997.tb10401.x [DOI] [PubMed] [Google Scholar]
- 23. Sha J, Wang SF, Suarez G, Sierra JC, Fadl AA, Erova TE, et al. Further characterisation of a type III secretion system (T3SS) and of a new effector protein from a clinical isolate of Aeromonas hydrophila—part I. Microb Pathog. 2007;43: 127–146. 10.1016/j.micpath.2007.05.002 [DOI] [PubMed] [Google Scholar]
- 24. Grim CJ, Kozlova E V, Sha J, Fitts EC, van Lier CJ, Kirtley ML, et al. Characterisation of Aeromonas hydrophila wound pathotypes by comparative genomic and functional analyses of virulence genes. MBio. 2013;4: e00064–13. 10.1128/mBio.00064-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Havelaar AH, Vonk M. The preparation of ampicillin dextrin agar for the enumeration of Aeromonas in water. Lett Appl Microbiol. 1988;7: 169–171. 10.1111/j.1472-765X.1988.tb01271.x [DOI] [Google Scholar]
- 26. Szczuka E, Kaznowski A. Typing of clinical and environmental Aeromonas sp. strains by random amplified polymorphic DNA PCR, repetitive extragenic palindromic PCR, and enterobacterial repetitive intergenic consensus sequence PCR. J Clin Microbiol. 2004;42: 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamamoto S, Kasai H, Arnold DL, Jackson RW, Vivian A, Harayama S. Phylogeny of the genus Pseudomonas: intrageneric structure reconstructed from the nucleotide sequences of gyrB and rpoD genes. Microbiology. 2000;146: 2385–2394. 10.1099/00221287-146-10-2385 [DOI] [PubMed] [Google Scholar]
- 28. Hall T. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41: 95–98. [Google Scholar]
- 29. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nawaz M, Khan SA, Khan AA, Sung K, Tran Q, Kerdahi K, et al. Detection and characterisation of virulence genes and integrons in Aeromonas veronii isolated from catfish. Food Microbiol. 2010;27: 327–31. 10.1016/j.fm.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 31. Nam IY, Joh K. Rapid detection of virulence factors of Aeromonas isolated from a trout farm by hexaplex-PCR. J Microbiol. 2007;45: 297–304. [PubMed] [Google Scholar]
- 32. Kingombe CI, Huys G, Tonolla M, Albert MJ, Swings J, Peduzzi R, et al. PCR detection, characterisation, and distribution of virulence genes in Aeromonas spp. Appl Environ Microbiol. 1999;65: 5293–5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vilches S, Wilhelms M, Yu HB, Leung KY, Tomás JM, Merino S. Aeromonas hydrophila AH-3 AexT is an ADP-ribosylating toxin secreted through the type III secretion system. Microb Pathog. 2008;44: 1–12. 10.1016/j.micpath.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 34. Aguilera-Arreola MG, Hernández-Rodríguez C, Zúñiga G, Figueras MJ, Castro-Escarpulli G. Aeromonas hydrophila clinical and environmental ecotypes as revealed by genetic diversity and virulence genes. FEMS Microbiol Lett. 2005;242: 231–40. 10.1016/j.femsle.2004.11.011 [DOI] [PubMed] [Google Scholar]
- 35. Sha J, Galindo CL, Pancholi V, Popov VL, Zhao Y, Houston CW, et al. Differential expression of the enolase gene under in vivo versus in vitro growth conditions of Aeromonas hydrophila . Microb Pathog. 2003;34: 195–204. 10.1016/S0882-4010(03)00028-7 [DOI] [PubMed] [Google Scholar]
- 36. Sha J, Pillai L, Fadl AA, Galindo CL, Erova TE, Chopra AK. The Type III Secretion System and Cytotoxic Enterotoxin Alter the Virulence of Aeromonas hydrophila . Infect Immun. 2005;73: 6446–6457. 10.1128/IAI.73.10.6446-6457.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Erova TE, Sha J, Horneman AJ, Borchardt MA, Khajanchi BK, Fadl AA, et al. Identification of a new hemolysin from diarrheal isolate SSU of Aeromonas hydrophila . FEMS Microbiol Lett. 2007;275: 301–311. 10.1111/j.1574-6968.2007.00895.x [DOI] [PubMed] [Google Scholar]
- 38. Hu M, Wang N, Pan ZH, Lu CP, Liu YJ. Identity and virulence properties of Aeromonas isolates from diseased fish, healthy controls and water environment in China. Lett Appl Microbiol. 2012;55: 224–233. 10.1111/j.1472-765X.2012.03281.x [DOI] [PubMed] [Google Scholar]
- 39. Sreedharan K, Philip R, Singh ISB. Characterisation and virulence potential of phenotypically diverse Aeromonas veronii isolates recovered from moribund freshwater ornamental fishes of Kerala, India. Antonie Van Leeuwenhoek. 2012;103: 53–67. 10.1007/s10482-012-9786-z [DOI] [PubMed] [Google Scholar]
- 40. Esteve C, Valera L, Gutiérrez C, Ventosa A. Taxonomic study of sucrose-positive Aeromonas jandaei-like isolates from faeces, water and eels: emendation of A. jandaei Carnahan et al. 1992. Int J Syst Evol Microbiol. Microbiology Society; 2003;53: 1411–1419. 10.1099/ijs.0.02504-0 [DOI] [PubMed] [Google Scholar]
- 41. Corry JEL, Curtis GDW, Baird RM. Handbook of culture media for food microbiology. Amsterdam: Elsevier; 2003. [Google Scholar]
- 42. Altwegg M, Steigerwalt AG, Altwegg-Bissig R, Lüthy-Hottenstein, Brenner DJ. Biochemical identification of Aeromonas genospecies isolated from humans. J Clin Microbiol. 1990;28(2): 258–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rhodes MW, Kator H. Seasonal occurrence of mesophilic Aeromonas spp. as a function of biotype and water quality in temperate freshwater lakes. Water Res. 1994;28: 2241–2251. 10.1016/0043-1354(94)90039-6 [DOI] [Google Scholar]
- 44. Abbott SL, Cheung WKW, Janda JM. The genus Aeromonas: biochemical characteristics, atypical reactions, and phenotypic identification schemes. J Clin Microbiol. 2003;41: 2348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yousr AH, Napis S, Rahmat AGR, Radu S. Detection of aerolysin and hemolysin genes in Aeromonas spp. isolated from environmental and shellfish sources by polymerase chain reaction. ASEAN food J. 2007;14: 115–122. [Google Scholar]
- 46. Khajanchi BK, Fadl AA, Borchardt MA, Berg RL, Horneman AJ, Stemper ME, et al. Distribution of virulence factors and molecular fingerprinting of Aeromonas species isolates from water and clinical samples: suggestive evidence of water-to-human transmission. Appl Environ Microbiol. 2010;76: 2313–2325. 10.1128/AEM.02535-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All rpoD sequences were deposited in the Genbank (Accession No.: KT187565-KT187686).