Abstract
During joint actions, people typically adjust their own actions according to the ongoing actions of the partner, which implies that the interaction modulates the behavior of both participants. However, the neural substrates of such mutual adaptation are still poorly understood. Here, we set out to identify the kinematics-related brain activity of leaders and followers performing hand actions.
Sixteen participants as 8 pairs performed continuous, repetitive right-hand opening and closing actions with ~3-s cycles in a leader–follower task. Subjects played each role for 5 min. Magnetoencephalographic (MEG) brain signals were recorded simultaneously from both partners with a dual-MEG setup, and hand kinematics was monitored with accelerometers. Modulation index, a cross-frequency coupling measure, was computed between the hand acceleration and the MEG signals in the alpha (7–13 Hz) and beta (13–25 Hz) bands.
Regardless of the participants' role, the strongest alpha and beta modulations occurred bilaterally in the sensorimotor cortices. In the occipital region, beta modulation was stronger in followers than leaders; these oscillations originated, according to beamformer source reconstructions, in early visual cortices. Despite differences in the modulation indices, alpha and beta power did not differ between the conditions.
Our results indicate that the beta modulation in the early visual cortices depends on the subject's role as a follower or leader in a joint hand-action task. This finding could reflect the different strategies employed by leaders and followers in integrating kinematics-related visual information to control their own actions.
Keywords: Social interaction, Hyperscanning, MEG, Hand kinematics, Modulation index, Sensorimotor integration
Highlights
-
•
Pairs of subjects performed hand movements as a leader and follower in a dual-MEG setup.
-
•
Alpha and beta powers did not differ between followers and leaders.
-
•
Alpha and beta modulation indices were strongest at bilateral sensorimotor cortices.
-
•
Beta modulation was stronger in leaders than followers in the early visual cortex.
-
•
The role might influence the integration of kinematics-related visual information to control one's own movements.
Introduction
Our daily social life is filled with joint actions, during which we adjust our movements according to the ongoing actions of others to fit the demands of various tasks. Behavioral studies have shown that interacting individuals dynamically adapt their motor behaviors during joint action (Konvalinka et al., 2010, Noy et al., 2011). Such a smooth adaptation likely relies on the individual action–perception loops (Hari and Kujala, 2009) that make it possible to both perform appropriate actions and to represent the actions of others. However, the neural basis of such between-individuals mutual adaptation is still unclear.
In studies of social cognition, increasing attention is currently being paid to interacting individuals (Babiloni and Astolfi, 2014, Dumas et al., 2010, Dumas et al., 2011, Dumas et al., 2012, Schilbach et al., 2013, Sebanz et al., 2006). This trend is in part due to recent developments in dual-scanning (or hyperscanning) methods using fMRI (Montague et al., 2002, Scholkmann et al., 2013), fNIRS (Cui et al., 2012, Scholkmann et al., 2013), EEG (Babiloni et al., 2006), and MEG (Baess et al., 2012, Hirata et al., 2014, Zhdanov et al., 2015) to record brain activity of two or more persons at the same time to facilitate studying the neural substrates of social interaction (for reviews see, Babiloni and Astolfi, 2014 and Koike et al., 2015).
Hyperscanning studies have provided insight into the modulation of band-specific EEG power within brains of interacting subjects in different social contexts (Konvalinka et al., 2014, Naeem et al., 2012). However, these studies have not linked the brain activity to movement kinematics that, in addition to the context within which the movement occurs, provides important cues for interpreting the actions of others (Grafton and Hamilton, 2007).
A number of brain-imaging studies have demonstrated that limb-kinematics parameters, such as velocity and acceleration, are coherent with MEG brain signals during both executed and observed actions (Bourguignon et al., 2011, Bourguignon et al., 2012, Bourguignon et al., 2013, Piitulainen et al., 2013). The coherence peaks at the movement frequency and its harmonics, and the coherent brain signals mainly arise from the contralateral primary sensorimotor cortex (Bourguignon et al., 2011, Bourguignon et al., 2012, Jerbi et al., 2007, Piitulainen et al., 2013). Qualitatively similar but weaker coherence has been detected in the primary sensorimotor cortex during action observation between the observer's MEG and the acting subject's hand kinematics (Bourguignon et al., 2013). Moreover, during action observation, the amplitude of the beta-band (about 20 Hz) sensorimotor rhythm co-varies with kinematics parameters (Press et al., 2011). Coupling between limb kinematics and brain activity thus seems a useful measure to study the neural underpinnings of one's own movements.
The present study aimed to clarify how social interaction modulates movement parameters and the brain activity related to hand kinematics. For this purpose, we adopted a joint hand-movement task in which one subject of a dyad either followed or led the movements of their partner. The brain signals of the two participants were recorded simultaneously using two accurately synchronized MEG systems located 5 km apart (Zhdanov et al., 2015), and the hand movements were monitored at the same time using accelerometers. Phase–amplitude modulation index (MI; Tort et al., 2008), a cross-frequency coupling measure, was used to quantify the coupling between the phase of the acceleration signal and the amplitude of MEG signals. We focused our analysis on the alpha (7–13 Hz) and beta (13–25 Hz) frequencies, especially in the sensorimotor cortices, where their role in both action execution and action observation is clearly established (Caetano et al., 2007, Hari et al., 1998).
Methods
Subjects
Nine gender-matched pairs of subjects (altogether 6 females and 12 males; mean ± SD age 27.1 ± 5.6 years, range 21–43 years) participated in the experiment. All subjects were right-handed by self-report, had no motor disorders, and had normal or corrected-to-normal vision. Before participation, each subject signed a written informed consent form. The study had a prior approval by the Ethics Committee of the Hospital District of Helsinki and Uusimaa. The experiment was conducted in accordance with the Declaration of Helsinki.
Behavioral task
During the experiment, Subject A (SA) of a dyad was seated inside a three-layer magnetically shielded room (Imedco AG, Hägendorf, Switzerland) in the MEG Core of Aalto NeuroImaging in Espoo and Subject B (SB) in another three-layer magnetically shielded room (Euroshield/ETS Lindgren Oy, Eura, Finland) at the BioMag laboratory in Helsinki, 5 km apart. Subjects' upper bodies were visible to the other partner as a video image on a screen about 1 m in front of them (Fig. 1A). The end-to-end lag of the video transmission was about 100 ms due to the delays in the camera, Internet connection and projector (Zhdanov et al., 2015). The movement task used in the present study was, however, robust with respect to the lag, since no interactive adaptation for the movement was necessary.
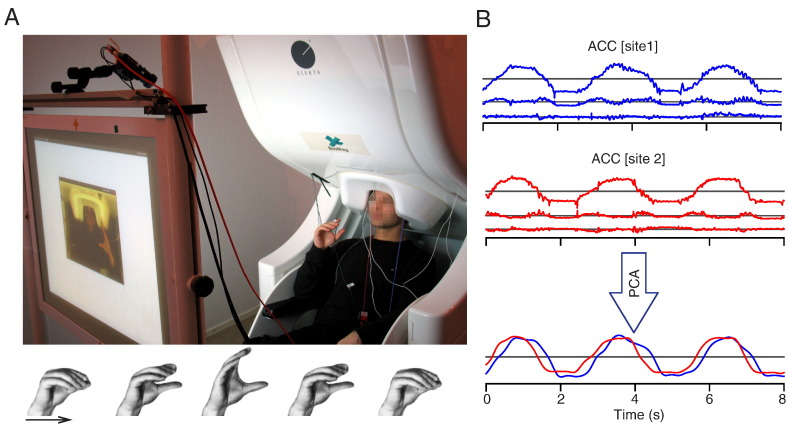
Fig. 1.
Experimental setup and summary of acceleration (ACC) signal analyses. (A) Experimental setup at one laboratory (upper panel, reproduced from Zhdanov et al., 2015) and one cycle of hand movement (lower panel). (B) Sample of ACC signals from a representative pair of subjects. ACC signals were band-pass filtered between 0.05 and 195 Hz and notched at 50, 100 and 150 Hz, followed by principal component analysis (PCA). The first component of ACC signals was further low-pass filtered at 3 Hz.
Subjects were asked to keep their right hand on the right side of the body, at the height of their face, and to perform ~0.5 Hz repetitive flexion–extensions of the right-hand fingers with fingers touching the thumb at the most-flexed phase of the movement (see Fig. 1A). Hand movements were performed in three 5-min sessions: (1) SA leading and SB following, (2) SA following and SB leading, (3) SA and SB synchronizing their movements with no specified leader or follower. The behavioral data of the synchronous condition (3) have been presented in a previous publication that describes the dual-MEG setup (Zhdanov et al., 2015); here we did not analyze data from this condition since the movements were almost sinusoidal and thus quite different from those in the follow–lead conditions. The order of the sessions was randomized and counterbalanced across the dyads. We will refer to the condition as FOLLOW when a subject is following and LEAD when he/she is leading.
Recordings
Brain signals were recorded simultaneously from both subjects using two similar 306-channel whole-scalp MEG devices: Elekta Neuromag® (Elekta Oy, Helsinki, Finland) at MEG Core of Aalto and Vectorview™ at BioMag (Elekta Oy, Helsinki, Finland); see Baess et al. (2012) and Zhdanov et al. (2015) for the full details of the dual-MEG setup. Both devices have 102 sensor elements, each comprising one magnetometer and two orthogonal planar gradiometers. Subjects' hand movements were monitored with a 3-axis accelerometer (ACC; ADXL335 iMEMS Accelerometer, Analog Devices, Inc., Norwood, MA, USA) attached to the index finger. The MEG and ACC data were band-pass filtered at 0.01–330 Hz and digitized at 1 kHz.
Because one follower–leader data block was not recorded for one of the pairs due to an operator error, we excluded this pair from further analysis. ACC and MEG data of SA and SB were synchronized with an accuracy of ~3 ms, as previously described (Baess et al., 2012). From the 5-min recordings, we analyzed 4 min of data; the first 20 s were excluded to give the subjects time to adapt to the task and the last 40 s of data were discarded to avoid possible fatigue at the end of the recording.
Analysis of ACC data
Figure 1B indicates preprocessing of ACC data. The ACC time series from the three orthogonal channels were band-pass filtered between 0.05 and 195 Hz using a finite-impulse-response (FIR) filter implemented in FieldTrip Matlab toolbox (Oostenveld et al., 2011). The FIR filter was used for the rest of the analysis unless stated otherwise. The power-line noise was removed with notch filters centered at 50, 100, and 150 Hz (bandwidths 10 Hz). Principal component analysis (PCA) was then applied to the pre-processed ACC time series.
Since our subjects performed the movements mainly in the up–down direction, the first principal component of the ACC signals accounted for as much as 82.3% ± 11.6% (mean ± SD) of the total variance. Accordingly, this first component was retained for the MI analyses and multiplied by ±1 to match the polarities between the two subjects of each dyad. Since the movements were performed at frequencies well below 3 Hz, we used a 3-Hz low-pass filter to remove the sharp transients occurring when the fingers touched the thumb. Finally, the ACC signals were down-sampled to 250 Hz.
Synchronization of movements between SA and SB was evaluated using the phase locking value (PLV) (Lachaux et al., 1999), which is defined as
where N is the number of time points and ϕ1(t) (resp. ϕ2(t)) is the time series of the instantaneous phase of the ACC signal for SA (resp. SB). The phases ϕ1(t) and ϕ2(t) were extracted from the analytic signal obtained by applying Hilbert transform to the ACC signal. The PLV yields a value between 0 and 1, where 0 means no phase synchronization and 1 means perfect phase synchronization. The threshold for statistical significance of the PLV was evaluated as the 95th percentile of a distribution of 200 surrogate PLV values. These values were calculated, as described above, between the real ACC signal of SA and the surrogate ACC signals of SB, obtained by Fourier transforming the original ACC signal, randomizing the phases of Fourier coefficients, and performing an inverse Fourier transform (Faes et al., 2004).
Preprocessing of MEG data
MEG data were preprocessed off-line using signal-space separation (SSS) to suppress external interference and to correct for head movements (Taulu et al., 2004). To remove eye-blink and heartbeat artifacts, independent component analysis was applied to the preprocessed data band-pass filtered to 0.1–25 Hz (Vigário et al., 2000). Components related to artifacts were identified by visual inspection, and 0–5 components (mean ± SD 2.4 ± 1.3) were removed from the data. Artifact-free data were down-sampled to 250 Hz.
Determination of individual alpha frequencies
Individual frequencies of the alpha rhythm were identified based on a global MEG spectral power (computed based on all gradiometer sensors). To do so, power spectral density at each gradiometer sensor was estimated with Welch's method (window length 1024 samples, overlap 512 samples, hamming window), based on the artifact-free data from all conditions. The global spectrum was then computed by averaging across all gradiometers. Given that individual spectral peaks were clearly within the 7–13-Hz frequency bands (see Results), we selected that frequency range for further analyses of the alpha band.
Analysis of phase–amplitude coupling
The MEG data were band-pass filtered to the alpha (7–13 Hz) and beta (13–25 Hz) frequency bands. Coupling between ACC and MEG was studied with MI. First, the instantaneous amplitude of each MEG time series was obtained with a Hilbert transform of the band-pass filtered data. Next, the signals from the two planar gradiometers at each sensor location were combined into one signal by calculating their Euclidean norm at each time point. We included signals only from planar gradiometers because they are sensitive to activity right underneath the sensor. We also computed a whole-brain amplitude time series by taking the Euclidean norm of the time series from all gradiometer sensors.
A composite time series was then constructed by combining the phase time series of the ACC signal (ϕacc(t)) and the envelope of the band-pass-filtered MEG signal (Ameg(t)). The MEG power at each sensor location was calculated as the mean of the square of Ameg(t) and the whole-brain power was calculated by averaging the squared envelope time series across time and sensors.
Second, the ACC time series ϕacc(t) was binned into K = 36 bins of 10°, and the average band-limited MEG amplitude within each bin was calculated, resulting in a phase–amplitude distribution, which is denoted as < Ameg > ϕacc(i) (i = 1, 2, …, K). Then, an entropy measure H was calculated as
with pi given by
Finally, the MI was defined as the entropy H normalized by the maximum possible entropy (log(K)):
The MI was calculated separately for each gradiometer pair, frequency band, condition, and subject. We first calculated the MI between the subject's MEG and ACC signals. Then, group-level MI maps were generated by averaging MI maps across subjects.
Determination of statistical significance of the MI
The statistical significance of the MI was tested by comparing the real MI value (MIreal) with a set of 200 surrogate MI values (Canolty et al., 2006), which were calculated from real Ameg(t) and surrogate ϕacc(t). The surrogate time series ϕacc(t) was obtained from the surrogate ACC signals generated as described above. Finally, a z-score was calculated for the MIreal as z = (MIreal − μ)/δ where μ and δ are the mean and standard deviation of the surrogate MI distribution.
Statistical analysis
Comparison of the MEG power and the MI between the FOLLOW and LEAD conditions was performed for each sensor and each frequency band with a two-tailed paired t test. Data were log-transformed before the statistical analysis. Bonferroni correction was used to correct for multiple comparisons across sensors, i.e., a difference was deemed statistically significant at p < 0.05/102 = 0.00049.
Source analysis
To locate the sources of MEG signals displaying statistically significant MI, source reconstruction was performed using the widely-employed linearly constrained minimum-variance beamformer (Van Veen et al., 1997), with fixed orientation. Since we did not have the anatomical brain images for all subjects, the Montreal Neurological Institute (MNI) template brain was used for each subject in the source analysis. The MEG data and the segmented template brain were co-registered using the anatomical fiducial points and extra head-surface points. The forward solution was computed for a 1-cm uniform grid spanning the brain volume, and the contribution of volume currents taken into account using a realistically-shaped single-compartment boundary-element model. A common beamformer spatial filter was derived from the combined broadband preprocessed data of the two conditions for each subject. Then, source time series were reconstructed for each condition with the common filter. Finally, the MI at each grid point was calculated as described above, and the resulting MI maps were interpolated onto the anatomical image. A group source-level MI map was obtained by averaging across subjects within each condition.
Results
Behavioral results
All subjects succeeded in performing the task according to the experimental instructions, as confirmed by a careful review of the recorded video of the experiments. Each follower was able to follow the pace of the leader, without adding or dropping any movement cycles. The mean ± SD movement frequencies for the follower and leader were the same, on average 0.39 ± 0.17 Hz (range 0.24–0.80 Hz).
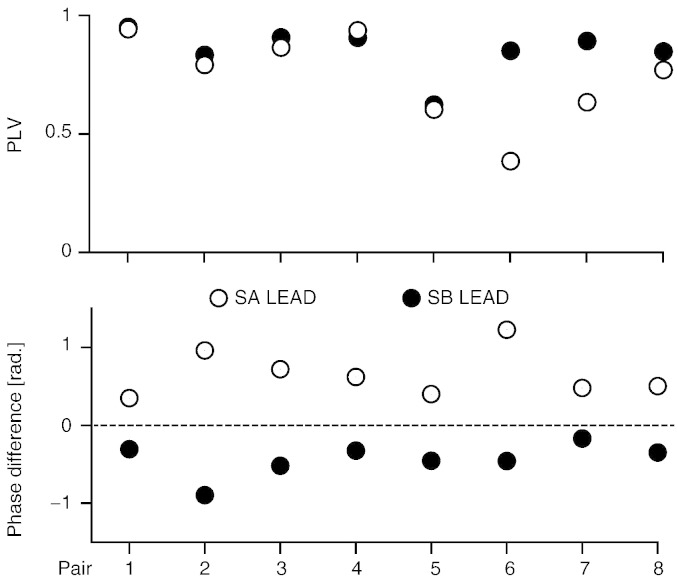
The proper behavioral performance was further validated by the strong synchronization between the ACC signals of subjects SA and SB (mean ± SD PLV 0.80 ± 0.16, all p < 0.005; upper panel in Fig. 1, Fig. 2). ACC phase differences between leaders and followers were on average 0.55 ± 0.28 rad (range 0.17–1.23 rad; lower panel in Fig. 2), confirming that all leaders were in phase lead with respect to the followers. Hence the role of leader/follower was played as instructed.
Fig. 2.
Between-subjects kinematics coupling. Phase-locking values (PLVs) and phase differences at the movement frequency between the (acceleration) ACC signals of SA and SB for each session and each pair. All pairs showed strong movement synchronization as indicated by the high PLV. The phase-difference graph demonstrates that the leader's ACC phase always preceded follower's one.
Individual alpha frequencies
The mean ± SD peak alpha frequency was 9.9 ± 1.4 Hz (range 7.1–12.2 Hz) based on the power spectral density averaged across all gradiometer sensors (thereby including both occipital and rolandic alpha). Thus, the individual spectral peaks were clearly within the 7–13 Hz frequency bands that we used for computing the modulation indices.
Power and modulation index
Table 1 summarizes the whole-brain power and MI. During the FOLLOW condition, the MI increased in both alpha and beta bands (for all subjects, p < 0.05). However, alpha and beta powers did not differ between conditions (for all subjects, p > 0.05).
Table 1.
Mean ± SD whole-brain modulation index (top two rows) and log-transformed MEG power (bottom two rows; power normalized by 1 fT2 cm− 2 prior to log-transforming) in alpha and beta bands. The numbers within the brackets refer to the number of subjects with Z > 1.96. The last two columns present the result of the group-level lead vs. follow comparison.
| FOLLOW | LEAD | Cohen's dz | p value (paired t test) | |
|---|---|---|---|---|
| Modulation index | ||||
| Alpha | −3.28 ± 0.50 (15) | −3.59 ± 0.49 (12) | 0.84 | 0.0042 |
| Beta | −3.24 ± 0.52 (16) | −3.43 ± 0.49 (15) | 0.70 | 0.014 |
| MEG power, mean ± SD | ||||
| Alpha | 2.73 ± 0.23 | 2.70 ± 0.23 | 0.36 | 0.17 |
| Beta | 2.64 ± 0.15 | 2.62 ± 0.17 | 0.23 | 0.36 |
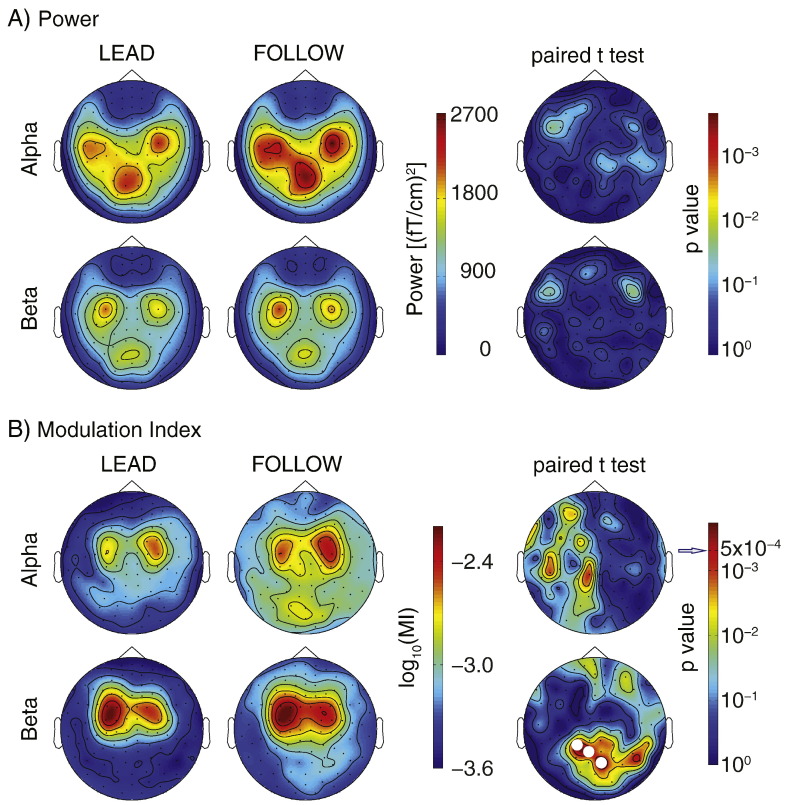
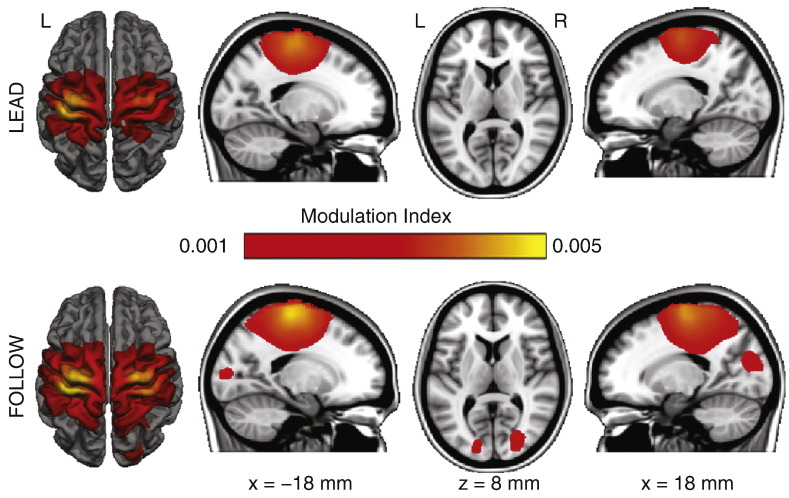
In both LEAD and FOLLOW conditions, the spatial distributions of alpha and beta power showed clear peaks at the sensors over the left and right sensorimotor cortices and the occipital region (Fig. 3A) but the conditions did not show statistically significant differences (p > 0.05, corrected). However, the ACC and MEG signals showed strong cross-frequency phase–amplitude coupling, as quantified by MI (Fig. 3B), at the sensors over both sensorimotor cortices; this effect was seen in both alpha and beta frequencies and in both FOLLOW and LEAD conditions. Source analysis showed that the strong beta MI peaked bilaterally in the primary sensorimotor and in the occipital region (Fig. 4, Table 2).
Fig. 3.
Spatial distributions and between-condition comparisons of the (A) power and (B) modulation index (MI) in the alpha and beta bands. In the FOLLOW condition, the beta MI was significantly higher than in the LEAD condition at sensors over the occipital area (indicated with white dots). The alpha and beta powers did not differ between conditions. Multiple comparisons were corrected for by using the Bonferroni method, i.e., a difference was deemed significant at p < 0.05/102 = 0.00049.
Fig. 4.
Group-level maps of beta modulation index (MI) at the source level. Maximal MI was observed in the bilateral sensorimotor cortex in both conditions. The MI also peaked in the occipital region in the FOLLOW condition, as well as in the LEAD condition but with values lower than the visualization threshold.
Table 2.
Values and MNI coordinates of local maxima of beta modulation index. L left hemisphere, R right hemisphere, SD standard deviation.
| FOLLOW |
LEAD |
|||
|---|---|---|---|---|
| Region | Modulation index (mean ± SD) × 10−3 |
MNI coordinates [x y z] |
Modulation index (mean ± SD) × 10−3 |
MNI coordinates [x y z] |
| Rolandic, L | 5.1 ± 4.3 | [−32 23 67] | 5.5 ± 3.4 | [−39 −25 57] |
| Rolandic, R | 4.8 ± 5.7 | [31 −23 65] | 3.9 ± 5.5 | [31 −22 63] |
| Occipital, L | 1.1 ± 0.7 | [−16 94 7] | 0.6 ± 0.6 | [−5 −92 30] |
| Occipital, R | 1.2 ± 1.3 | [26 89 11] | 0.6 ± 0.4 | [1 −90 28] |
The occipital beta MI was higher in the FOLLOW than in the LEAD condition as revealed by between-condition comparison; the effect was statistically significant (p < 0.05, corrected) at 3 sensors in the occipital region (Fig. 3B). All 16 subjects showed significant MI in at least one of these sensors in the FOLLOW condition and 13/16 subjects in the LEAD condition (Z-score threshold 1.96, corresponding to an uncorrected p < 0.05).
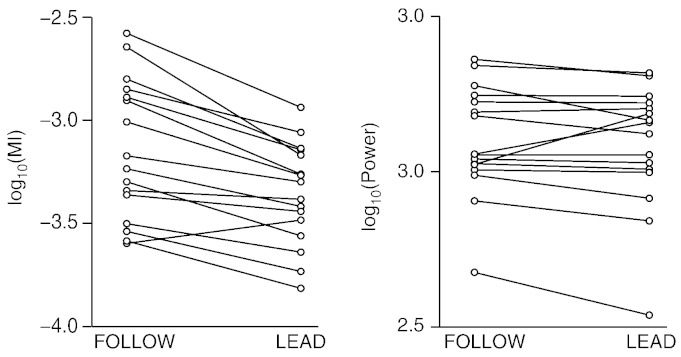
Fig. 5 shows, for each subject, the average beta MIs across these three sensors in each condition. In 15/16 subjects, the MI was higher in the FOLLOW than in the LEAD condition (mean ± SD of log10(MI) in FOLLOW −3.17 ± 0.34, in LEAD −3.40 ± 0.23; Cohen's dz = 1.44, p < 0.0001, paired t test performed on log-transformed data). Furthermore, the FOLLOW and the LEAD conditions did not differ in power (log-transformed after normalization by 1 fT2 cm− 2) in the corresponding alpha (mean ± SD in FOLLOW 3.23 ± 0.30, in LEAD 3.20 ± 0.29; dz = 0.22, p = 0.39, paired t test) and beta (FOLLOW 3.10 ± 0.18, LEAD 3.08 ± 0.20; dz = 0.24, p = 0.36).
Fig. 5.
Beta modulation index (MI) and power at the occipital sensors. The data displayed were obtained by averaging the MIs and power across the three sensors showing statistically significant group-level differences between the LEAD and FOLLOW conditions. Data from the same subjects are connected with a line, showing that all but one subject had higher occipital MI in the FOLLOW vs. LEAD condition.
To further elucidate the role of the occipital beta MI, we calculated the MI between the subjects' MEG signals and partners' ACC signals at these 3 sensors and compared it with those calculated with the subjects' MEG signals and their own ACC signals. The beta MI between subjects' MEG signals and their own ACC signals was significantly higher than that between the subjects' MEG signals and partners' ACC signals in the FOLLOW condition (mean ± SD of log10(MI) −3.25 ± 0.3, dz = 0.58, p = 0.036) but not in the LEAD condition (−3.43 ± 0.23, dz = 0.35, p = 0.19).
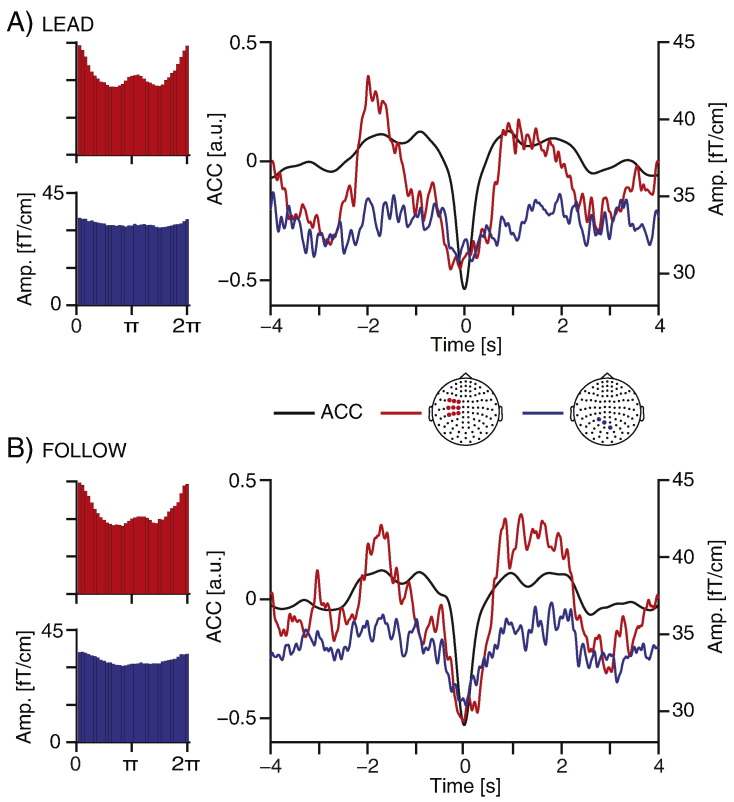
Bar plots in Fig. 6 illustrate the across-subjects averaged phase–amplitude coupling between ACC signals and beta activity. The mean phase (across subjects and conditions) of peak amplitude in the phase–amplitude distribution and its confidence interval were estimated using the CircStat Matlab toolbox (Berens, 2009). Table 3 summarizes these results. The amplitude of beta oscillations peaked at ACC phases around 0. The mean peak phase differences between the FOLLOW and LEAD conditions and that between the left rolandic area and the occipital region were not statistically significantly different from 0 rad (95% confidence interval).
Fig. 6.
Examples of beta-band modulation by the acceleration (ACC). Bar plots indicate the mean beta amplitude within each phase bin (36 in total) of the ACC in the left rolandic area (red bars) and in the occipital region (blue bars). Mean ACC (black lines) and beta amplitudes in the left rolandic area (red lines) and the occipital region (blue lines) were generated by averaging 8-s epochs (with a maximum overlap of 4 s) extracted from the ACC and beta-amplitude time series centered at the ACC signal troughs. Data are shown from representative sensors over the left rolandic area (indicated as red dots) and the occipital region (indicated as blue dots) with statistically significant condition effect on the beta modulation index. The data were separately averaged for the LEAD and FOLLOW conditions.
Table 3.
Mean ± SD peak phases of the phase–beta-amplitude distributions across subjects. L left hemisphere, CI confidence interval. Phases were wrapped to (−π, π).
| FOLLOW | LEAD | FOLLOW–Lead (mean [95% CI]) |
|
|---|---|---|---|
| Rolandic, L | −0.04 ± 0.92 | −0.10 ± 0.89 | 0.1 [−0.07, 0.27] |
| Occipital | 0.23 ± 0.85 | −0.11 ± 1.05 | −0.27 [−0.95, 0.41] |
| Occipital–rolandic, L (mean [95% CI]) |
0.15 [−0.5, 0.8] | 0.37 [−0.25, 0.99] |
Furthermore, we segmented the ACC and beta-amplitude time series into 8-s epochs (with a maximum overlap of 4 s) centered at the ACC signal troughs. Line plots in Fig. 6 show the group-level ACC–beta-amplitude distributions that were obtained by first averaging within and then across subjects. The data were from representative sensors showing the maximum MI in the FOLLOW condition from the left rolandic area and for the occipital region.
Discussion
In the present study, we simultaneously measured MEG from pairs of subjects who were performing interactive repetitive right-hand movements as leaders or followers in successive runs. Strong modulations of alpha and beta amplitudes as a function of the phase of hand accelerations occurred both in the bilateral sensorimotor and occipital cortices. Phase–amplitude coupling between hand kinematics and the MEG beta activity at early visual areas was stronger in the FOLLOW than LEAD condition. In the rolandic areas, the level of the mu rhythm was dynamically modulated in the primary sensorimotor cortex, as reported by previous studies of action execution and observation (Babiloni et al., 2002, Caetano et al., 2007, Cochin et al., 1999, Hari et al., 1998, Kilner et al., 2000, Kilner et al., 2003, Press et al., 2011).
Role-specific beta modulation in the occipital cortex
The role-specific occipital beta MI (higher MI in the FOLLOW than LEAD condition), likely arising from the early visual areas, could, in principle, be related to attentional changes in the follower to both one's own and partner's movements, so as to accurately maintain the movement synchrony with the leader. However, the general effect of visual attention is to dampen the parieto-occipital alpha (~10 Hz) oscillations (Foxe et al., 1998, Klimesch, 1999) whereas no such dampening has been observed for the occipital beta (~20 Hz) power (Capilla et al., 2014, Jones et al., 2010, Van Ede et al., 2014). Our present data did not show any between-condition differences in the parieto–occipital alpha power, even when examined with a lenient statistical threshold at the site of the significant effect on the occipital beta MI. Accordingly, it is unlikely that our findings would be related to attentional effects.
A potential explanation for the role-specific occipital beta MI is that it reflects the different strategies followers and leaders employed in integrating the kinematics-related visual information to control their own movements. This interpretation is in line with functions of the dorsal visual stream, extending from early visual areas to parietal cortices, in the processing of visual information to integrate them with elaborate action plans (Goodale and Milner, 1992).
In our task, the leaders were able to perform the actions without relying on the visual information (although they were all the time seeing the follower) whereas the followers needed to accurately integrate the visual information about the leader's actions with the proprioceptive information about their own actions. This viewpoint is supported by the finding that the occipital beta MI obtained using subjects' MEG signals and their partners' ACC signals was not different from that obtained using subjects' MEG signals and own ACC signals during the leader role.
On the contrary, the followers had higher occipital beta MI calculated using their own ACC signals than when using the partners' ACC signals. However, the ACC signals between subjects in the dyad resembled each other closely, as can be seen from the high PLVs between them. Thus, the modulation of the MEG signal by one's own and partner's ACC signal could not be totally separated and we have to be cautious in interpreting this finding. In addition, as we did not have any non-interactive conditions, in which the subjects would have moved without seeing each other, further studies are needed to unravel the impact of the interactive condition itself on the beta MI.
Since we did not find any statistically significant effects of the role the subjects played on the occipital alpha MI, our results are suggestive of a functional dissociation between the occipital alpha and the occipital beta during the applied hand-movement imitation task.
Role-non-specific alpha- and beta-power modulations in rolandic cortex
Modulation of rolandic alpha and beta power has been previously reported in several interactive hand-movement tasks (Konvalinka et al., 2014, Naeem et al., 2012). In a study by Naeem et al. (2012), pairs of participants performed movements either in-phase, anti-phase, or without interaction. Anti-phase and in-phase movements were associated with significant suppression of the 10–12-Hz rolandic rhythm in comparison with a rest period, whereas such suppression was not observed for the movements without interaction (Naeem et al., 2012). Along the same line, the interactive condition was related to a stronger suppression of rolandic alpha (8–12 Hz) and beta (13–25 Hz) power compared with non-interactive condition in a synchronized finger-tapping task (Konvalinka et al., 2014). Additionally, stronger rolandic alpha suppression was associated with the role of a leader. We did not find such power differences in the alpha or beta frequencies between leaders and followers.
This discrepancy might be linked to differences in the experimental setups. Indeed, the participants of Konvalinka et al. (2014) did not see each other but instead received an auditory beat signaling the phase of the partner's tapping. More importantly, in their study, the roles of the leader and follower were not predefined but dynamically taken by the participants as the task unfolded. This task difference may be of importance since perceiving one's role as a leader vs. follower is associated with increased BOLD activity in the right inferior frontal gyrus and right inferior parietal lobule, interpreted as signs of increased cognitive control and self-processing that minimize variability of one's own performance when acting as a leader (Fairhurst et al., 2014). Our result of similar rolandic alpha and beta MIs during leader and follower roles is in line with this interpretation as it was not necessary to minimize action variability in our study since the leader and follower roles were specified at the beginning of the task instead of dynamically competed for.
Methodological considerations
The present study focused on the effects of roles in modulating the coupling between the peripheral acceleration signal and brain activity. The dual-MEG setup would also make it possible to study how social interaction modulates the interbrain functional coupling, i.e., “hyperconnectivity”. However, since the hyperconnectivity changes might reflect experimental conditions and/or synchronous changes in physiological signals, identifying the real brain signatures of social interaction remains challenging (Hari et al., 2015). Accordingly, methodological developments are needed to realize the benefit of hyperscanning in increasing our understanding of social interaction.
Conclusion
The present dual-MEG study sought for kinematics-related differences in brain activity of leaders and followers during a simple hand opening–closing task. Our main finding was a higher beta-band modulation index in the occipital but not in rolandic regions in followers compared with leaders, suggesting that leaders and followers employ different strategies in integrating kinematics-related visual information to control their own movements.
Acknowledgments
This work was financially supported by the European Research Council (Advanced Grant Brain2Brain #232946 to Riitta Hari), the Academy of Finland (grants #218072 and #263800), the SalWe Research Program for Mind and Body (Tekes the Finnish Funding Agency for Innovation grant 1104/10), and the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 604102 (Human Brain Project). We thank Pamela Baess, Lotta Hirvenkari, Veikko Jousmäki, Jyrki Mäkelä, Anne Mandel, Jussi Nurminen, Petteri Räisänen, Ronny Schreiber, Mia Illman¸ and Andrey Zhdanov for contributing to different phases of building and testing the Aalto MEG2MEG setup and collecting the data.
Footnotes
This study has been presented as a conference abstract at the 21st Annual Meeting of the Organization for Human Brain Mapping.
References
- Babiloni F., Astolfi L. Social neuroscience and hyperscanning techniques: past, present and future. Neurosci. Biobehav. Rev. 2014;44:76–93. doi: 10.1016/j.neubiorev.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C., Babiloni F., Carducci F., Cincotti F., Cocozza G., Del Percio C., Moretti D.V., Rossini P.M. Human cortical electroencephalography (EEG) rhythms during the observation of simple aimless movements: a high-resolution EEG study. NeuroImage. 2002;17:559–572. [PubMed] [Google Scholar]
- Babiloni F., Cincotti F., Mattia D., Mattiocco M., De Vico Fallani F., Tocci A., Bianchi L., Marciani M.G., Astolfi L. Hypermethods for EEG hyperscanning. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006;1:3666–3669. doi: 10.1109/IEMBS.2006.260754. [DOI] [PubMed] [Google Scholar]
- Baess P., Zhdanov A., Mandel A., Parkkonen L., Hirvenkari L., Mäkelä J.P., Jousmäki V., Hari R. MEG dual scanning: a procedure to study real-time auditory interaction between two persons. Front. Hum. Neurosci. 2012;6:83. doi: 10.3389/fnhum.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens P. CircStat: a MATLAB toolbox for circular statistics. J. Stat. Softw. 2009;31 [Google Scholar]
- Bourguignon M., De Tiège X., Op de Beeck M., Pirotte B., Van Bogaert P., Goldman S., Hari R., Jousmäki V. Functional motor-cortex mapping using corticokinematic coherence. NeuroImage. 2011;55:1475–1479. doi: 10.1016/j.neuroimage.2011.01.031. [DOI] [PubMed] [Google Scholar]
- Bourguignon M., Jousmäki V., Op de Beeck M., Van Bogaert P., Goldman S., De Tiège X. Neuronal network coherent with hand kinematics during fast repetitive hand movements. NeuroImage. 2012;59:1684–1691. doi: 10.1016/j.neuroimage.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Bourguignon M., De Tiège X., de Beeck M.O., Van Bogaert P., Goldman S., Jousmäki V., Hari R. Primary motor cortex and cerebellum are coupled with the kinematics of observed hand movements. NeuroImage. 2013;66:500–507. doi: 10.1016/j.neuroimage.2012.10.038. [DOI] [PubMed] [Google Scholar]
- Caetano G., Jousmäki V., Hari R. Actor's and observer's primary motor cortices stabilize similarly after seen or heard motor actions. Proc. Natl. Acad. Sci. U. S. A. 2007;104:9058–9062. doi: 10.1073/pnas.0702453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty R.T., Edwards E., Dalal S.S., Soltani M., Nagarajan S.S., Kirsch H.E., Berger M.S., Barbaro N.M., Knight R.T. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. (80.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capilla A., Schoffelen J.-M., Paterson G., Thut G., Gross J. Dissociated α-band modulations in the dorsal and ventral visual pathways in visuospatial attention and perception. Cereb. Cortex. 2014;24:550–561. doi: 10.1093/cercor/bhs343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochin S., Barthelemy C., Roux S., Martineau J. Observation and execution of movement: similarities demonstrated by quantified electroencephalography. Eur. J. Neurosci. 1999;11:1839–1842. doi: 10.1046/j.1460-9568.1999.00598.x. [DOI] [PubMed] [Google Scholar]
- Cui X., Bryant D.M., Reiss A.L. NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. NeuroImage. 2012;59:2430–2437. doi: 10.1016/j.neuroimage.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas G., Nadel J., Soussignan R., Martinerie J., Garnero L. Inter-brain synchronization during social interaction. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas G., Lachat F., Martinerie J., Nadel J., George N. From social behaviour to brain synchronization: review and perspectives in hyperscanning. IRBM. 2011;32:48–53. [Google Scholar]
- Dumas G., Martinerie J., Soussignan R., Nadel J. Does the brain know who is at the origin of what in an imitative interaction? Front. Hum. Neurosci. 2012;6:128. doi: 10.3389/fnhum.2012.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faes L., Pinna G.D., Porta A., Maestri R., Nollo G. Surrogate data analysis for assessing the significance of the coherence function. IEEE Trans. Biomed. Eng. 2004;51:1156–1166. doi: 10.1109/TBME.2004.827271. [DOI] [PubMed] [Google Scholar]
- Fairhurst M.T., Janata P., Keller P.E. Leading the follower: an fMRI investigation of dynamic cooperativity and leader–follower strategies in synchronization with an adaptive virtual partner. NeuroImage. 2014;84:688–697. doi: 10.1016/j.neuroimage.2013.09.027. [DOI] [PubMed] [Google Scholar]
- Foxe J.J., Simpson G.V., Ahlfors S.P. Parieto–occipital approximately 10 Hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport. 1998;9:3929–3933. doi: 10.1097/00001756-199812010-00030. [DOI] [PubMed] [Google Scholar]
- Goodale M.A., Milner A.D. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Grafton S.T., Hamilton A.F. de C. Evidence for a distributed hierarchy of action representation in the brain. Hum. Mov. Sci. 2007;26:590–616. doi: 10.1016/j.humov.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R., Kujala M.V. Brain basis of human social interaction: from concepts to brain imaging. Physiol. Rev. 2009;89:453–479. doi: 10.1152/physrev.00041.2007. [DOI] [PubMed] [Google Scholar]
- Hari R., Forss N., Avikainen S., Kirveskari E., Salenius S., Rizzolatti G. Activation of human primary motor cortex during action observation: a neuromagnetic study. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15061–15065. doi: 10.1073/pnas.95.25.15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R., Henriksson L., Malinen S., Parkkonen L. Centrality of social interaction in human brain function. Neuron. 2015;88:181–193. doi: 10.1016/j.neuron.2015.09.022. [DOI] [PubMed] [Google Scholar]
- Hirata M., Ikeda T., Kikuchi M., Kimura T., Hiraishi H., Yoshimura Y., Asada M. Hyperscanning MEG for understanding mother–child cerebral interactions. Front. Hum. Neurosci. 2014;8:118. doi: 10.3389/fnhum.2014.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerbi K., Lachaux J.-P., N'Diaye K., Pantazis D., Leahy R.M., Garnero L., Baillet S. Coherent neural representation of hand speed in humans revealed by MEG imaging. Proc. Natl. Acad. Sci. U. S. A. 2007;104:7676–7681. doi: 10.1073/pnas.0609632104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.R., Kerr C.E., Wan Q., Pritchett D.L., Hämäläinen M., Moore C.I. Cued spatial attention drives functionally relevant modulation of the mu rhythm in primary somatosensory cortex. J. Neurosci. 2010;30:13760–13765. doi: 10.1523/JNEUROSCI.2969-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner J.M., Baker S.N., Salenius S., Hari R., Lemon R.N. Human cortical muscle coherence is directly related to specific motor parameters. J. Neurosci. 2000;20:8838–8845. doi: 10.1523/JNEUROSCI.20-23-08838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner J.M., Salenius S., Baker S.N., Jackson A., Hari R., Lemon R.N. Task-dependent modulations of cortical oscillatory activity in human subjects during a bimanual precision grip task. NeuroImage. 2003;18:67–73. doi: 10.1006/nimg.2002.1322. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Koike T., Tanabe H.C., Sadato N. Hyperscanning neuroimaging technique to reveal the “two-in-one” system in social interactions. Neurosci. Res. 2015;90:25–32. doi: 10.1016/j.neures.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Konvalinka I., Vuust P., Roepstorff A., Frith C.D. Follow you, follow me: continuous mutual prediction and adaptation in joint tapping. Q. J. Exp. Psychol. 2010;63:2220–2230. doi: 10.1080/17470218.2010.497843. [DOI] [PubMed] [Google Scholar]
- Konvalinka I., Bauer M., Stahlhut C., Hansen L.K., Roepstorff A., Frith C.D. Frontal alpha oscillations distinguish leaders from followers: multivariate decoding of mutually interacting brains. NeuroImage. 2014;94:79–88. doi: 10.1016/j.neuroimage.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Lachaux J.P., Rodriguez E., Martinerie J., Varela F.J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague P.R., Berns G.S., Cohen J.D., McClure S.M., Pagnoni G., Dhamala M., Wiest M.C., Karpov I., King R.D., Apple N., Fisher R.E. Hyperscanning: simultaneous fMRI during linked social interactions. NeuroImage. 2002;16:1159–1164. doi: 10.1006/nimg.2002.1150. [DOI] [PubMed] [Google Scholar]
- Naeem M., Prasad G., Watson D.R., Kelso J.A.S. Electrophysiological signatures of intentional social coordination in the 10–12 Hz range. NeuroImage. 2012;59:1795–1803. doi: 10.1016/j.neuroimage.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Noy L., Dekel E., Alon U. The mirror game as a paradigm for studying the dynamics of two people improvising motion together. Proc. Natl. Acad. Sci. U. S. A. 2011;108:20947–20952. doi: 10.1073/pnas.1108155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.-M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piitulainen H., Bourguignon M., De Tiège X., Hari R., Jousmäki V. Coherence between magnetoencephalography and hand-action-related acceleration, force, pressure, and electromyogram. NeuroImage. 2013;72:83–90. doi: 10.1016/j.neuroimage.2013.01.029. [DOI] [PubMed] [Google Scholar]
- Press C., Cook J., Blakemore S.-J., Kilner J. Dynamic modulation of human motor activity when observing actions. J. Neurosci. 2011;31:2792–2800. doi: 10.1523/JNEUROSCI.1595-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L., Timmermans B., Reddy V., Costall A., Bente G., Schlicht T., Vogeley K. Toward a second-person neuroscience. Behav. Brain Sci. 2013;36:393–414. doi: 10.1017/S0140525X12000660. [DOI] [PubMed] [Google Scholar]
- Scholkmann F., Holper L., Wolf U., Wolf M. A new methodical approach in neuroscience: assessing inter-personal brain coupling using functional near-infrared imaging (fNIRI) hyperscanning. Front. Hum. Neurosci. 2013;7:813. doi: 10.3389/fnhum.2013.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebanz N., Bekkering H., Knoblich G. Joint action: bodies and minds moving together. Trends Neurosci. 2006;10:70–76. doi: 10.1016/j.tics.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Taulu S., Kajola M., Simola J. Suppression of interference and artifacts by the Signal Space Separation Method. Brain Topogr. 2004;16:269–275. doi: 10.1023/b:brat.0000032864.93890.f9. [DOI] [PubMed] [Google Scholar]
- Tort A.B.L., Kramer M.A., Thorn C., Gibson D.J., Kubota Y., Graybiel A.M., Kopell N.J. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc. Natl. Acad. Sci. U. S. A. 2008;105:20517–20522. doi: 10.1073/pnas.0810524105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ede F., Szebényi S., Maris E. Attentional modulations of somatosensory alpha, beta and gamma oscillations dissociate between anticipation and stimulus processing. NeuroImage. 2014;97:134–141. doi: 10.1016/j.neuroimage.2014.04.047. [DOI] [PubMed] [Google Scholar]
- Van Veen B.D., van Drongelen W., Yuchtman M., Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 1997;44:867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Vigário R., Särelä J., Jousmäki V., Hämäläinen M., Oja E. Independent component approach to the analysis of EEG and MEG recordings. IEEE Trans. Biomed. Eng. 2000;47:589–593. doi: 10.1109/10.841330. [DOI] [PubMed] [Google Scholar]
- Zhdanov A., Nurminen J., Baess P., Hirvenkari L., Jousmäki V., Mäkelä J., Mandel A., Hari R., Parkkonen L. An Internet-based real-time audiovisual link for dual MEG recordings. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128485. [DOI] [PMC free article] [PubMed] [Google Scholar]