Abstract
Repeated blood collection is one of the most common techniques performed on laboratory animals. However, a non-lethal protocol for blood collection from zebrafish has not been established. The previous methods for blood collection from zebrafish are lethal, such as lateral incision, decapitation and tail ablation. Thus we have developed a novel “repeated” blood collection method, and present here a detailed protocol outlining this procedure. This method is minimally invasive and results in a very low mortality rate (2.3%) for zebrafish, thus enabling repeated blood sampling from the same individual. The maximum volume of blood sampling is dependent on body weight of the fish. The volume for repeated blood sampling at intervals should be ≤0.4% of body weight every week or ≤1% every 2 weeks, which were evaluated by measurements of blood hemoglobin. Additionally, hemoglobin, fasting blood glucose, plasma triacylglycerol (TG) and total cholesterol levels in male and female adult zebrafish were measured. We also applied this method to investigate the dysregulation of glucose metabolism in diet-induced obesity. This blood collection method will allow many applications, including glucose and lipid metabolism and hematological studies, which will increase the use of zebrafish as a human disease model organism.
Keywords: Basic Protocol, Issue 102, Repeated blood collection, adult zebrafish, dorsal aorta, hemoglobin, fasting blood glucose, plasma triacylglycerol, total cholesterol, animal model
Introduction
Zebrafish are gaining increasing popularity as a valuable model of human diseases because their organs and genetics are similar to those of humans1,2. In the field of developmental biology, many studies have demonstrated that zebrafish and human show marked similarity in hematopoiesis3, hemostasis4,5, and myelopoiesis6. Adult zebrafish are also used for studying immunological7, neurodegenerative8 and obesity-related diseases9 because this model organism shares common pathways with those disrupted in human diseases. For obesity and obesity-related diseases (diabetes, hepatic steatosis and nonalcoholic steatohepatitis and atherosclerosis), zebrafish blood glucose and lipids levels have been thoroughly investigated in several transgenic and diet-induced obesity models10-13.
Repeated blood sampling from individual animals will reduce animal use and decrease interindividual differences. However, repeated sample collection is technically difficult in small animals such as zebrafish because of their relatively small blood volume and the lack of easily accessible vessels. Several methods for one-time blood collection from zebrafish have been developed, although these methods have their own drawbacks, including lethality, associated tissue damage and limited blood volume. For example, 1–5 µl blood can be harvested from a lateral incision of approximately 0.3 cm in length in the region of the dorsal aorta5. Decapitation with scissors by cutting through the pectoral girdle can collect 5–10 µl blood10. Another convenient blood sampling method is tail ablation14. Cardiac puncture is one potential alternative method for repeated blood collection from the same fish, but the very small amount obtained (approximately 50 nl) with this procedure limits the number of analyses that can be performed11. Accordingly, a new protocol is needed to enable repeated non-lethal blood sampling, which would be a critical advance necessary for this organism to be a standard model organism for human diseases. This technique would allow for testing pharmacological response, discovery of molecular biomarkers for diagnosis, determination of prognosis, and the monitoring of various diseases, such as metabolic diseases, degenerative diseases and several types of malignancies.
We therefore developed a minimally invasive method for obtaining blood from zebrafish serially15. Here we demonstrate the procedure visually and provide a detailed protocol for this technique. Using this method, the normal value based on various parameters, including hemoglobin, fasting blood glucose, and lipids in the blood of healthy adult zebrafish were evaluated. Additionally, we also evaluated whether this method is suitable for studies that require serial samples by monitoring the temporal changes in blood glucose levels during overfeeding experiments.
Protocol
All animal procedures were approved by the Ethics Committee of Mie University, and were performed according to Japanese animal welfare regulation ‘Act on Welfare and Management of Animals’ (Ministry of Environment of Japan) and complied with international guidelines.
1. Preparation of the Needle
NOTE: All experiments were performed under anesthesia, and all efforts were made to minimize suffering. For euthanasia, fish were immersed in an ice–water bath (5 parts ice/1 part water at ≤4 °C) for ≥20 min.
Prepare the glass microcapillary needles by pulling a 1.0-mm-outer-diameter glass capillary with a needle puller (Figure 1A).
Cut the tips of the needles obliquely using fine scissors (Figure 1B). The ideal tip diameter should be approximately 100 – 200 µm (Figure 1C). If the tip diameter is too narrow, blood will not enter the needle.
Dissolve heparin in saline to a concentration of 5 mg/ml.
Place a precut needle in the nosepiece end of an aspirator tube assembly and hold the mouthpiece in the mouth, or connect the needle to a bulb dispenser. Immerse the needle tip into the heparin solution, and heparinize the needle by suction and blowing through the solution (Figure 1D and 1E). Note: The aspirator tube assembly has been used for zebrafish sperm cryopreservation16. Long rubber tube can stop any blood flying into mouth. Setting a filter in the midway of the tube can avoid the hazards.
Store the heparinized needles in a 10-cm Petri dish and air-dry for at least 1 hr. A large number of needles can be prepared in advance.
2. Anesthesia
Prepare the anesthetic solution in a small plastic case by mixing 200 ml of fish water with 100 µl of 2-phenoxyethanol (2-PE). Final concentration of the anesthetics is 500 ppm. CAUTION! 2-PE has a rapid effect.
Remove desired number of fish (AB strain) from the circulation system.
Use a net to transfer the fish into the anesthetics for 1 – 2 min (Figure 1F). Observe the fish gradually swim, spread the pectoral fins horizontally, gasp, and have rapid operculum movements within 1 min.
As the time goes on, observe the fish lay on the bottom of the case and finally stop swimming. The surgical plane of anesthesia will reach when the fish stops to gasp and the operculum movements are slow. At this point, fish is ready for blood collection.
Using a skimmer lift the anesthetized fish from the 2-PE and gently place it on a paper towel soaked with the anesthetics ( Figure 1G). Cover the fish’s head with soft tissue paper also soaked with 2-PE solution to prevent eye dryness and use another dry soft tissue paper to gently dry off the body surface.
3. Blood Collection
Place a heparinized needle in the nosepiece end of the aspirator tube assembly (or the bulb dispenser) and hold the mouthpiece end of the aspirator tube assembly in mouth.
Grasp the nosepiece end and the needle together, and carefully remove the interfering scales with the needle tip. Insert the needle at a 30 – 45° angle into the blood collection site. Avoid puncture of the gastrointestinal tract (Figure 1H). In case of using bulb dispenser, press the bulb with thumb and middle finger, and block the hole at the tip of the bulb with first finger, then insert the needle as described above. Note: The site for blood collection is along the body axis and posterior to the anus in the region of the dorsal aorta. The dorsal aorta (DA) and the posterior cardinal vein (PCV) are just ventral to the spine (Figure 2).
Begin to suck the mouthpiece end of the aspiration tube assembly when the needle is felt touching the spine. If blood does not rise, move the needle tip subtly by hand to encourage blood flow. Note that once blood is rising into the needle, immediately stop shaking and suck gently (Figure 1I). In case of using bulb dispenser, release the pressure of bulb to aspirate the blood.
Observe the blood will slowly rise into the needle in a pulsatile manner without suction, which is likely because of the arterial blood pressure. Thus, it is not necessary to suck if the needle correctly penetrates the artery.
Stop suction after the appropriate volume of blood is collected. Remove the needle from the fish and press the puncture site using soft tissue paper to stop any bleeding. Observe the bleeding stop after approximately 10 – 20 sec of finger pressure (Figure 1J).
After the bleeding is stopped, immediately transfer the fish back to a clean warm–water (~ 28 °C) tank. Help the fish to recover by gently swirling water towards the gills until it begins to swim. Note: Keep up to 5 postsampling fish in a 2 L tank and connect the tank to the circulation system to oxygenate the fish. Addition of antibiotics to the fish water is not necessary. Maintain the fish with normal housing and feeding.
Expel the blood from the needle onto a clean area of a piece of parafilm (Figure 1K).
- Measure blood glucose using any commercial handheld glucometer (Figure 1L). Note: The glucometer uses a glucose dehydrogenase–flavin adenine dinucleotide electrode and requires a sample volume of 0.6 µl.
- Insert a test strip completely into the meter and directly touch the blood drop. Observe the blood draw into the test strip automatically and obtain the blood glucose result 5 sec on the display area. Record the result and discard the test strip. Use a new test strip for each measurement.
(optional step) Take accurate quantities of blood by a pipetting and transfer the blood to a microcentrifuge tube for further analyses (hemoglobin, triacylglycerol (TG), total cholesterol, etc.). If necessary, dilute the whole blood from one fish with saline. Centrifuge the blood samples for 3 min at 680 x g at RT and harvest the plasma. Transfer the plasma into a new tube. At this point, it is ready to be used in biochemical analyses.
Representative Results
This blood collection method causes minimal injury to zebrafish (a <1 mm puncture; Figure 1J) and yields a very low mortality rate of 2.3%. We examined the maximum volume of blood that could be collected from a single fish and evaluated the relationship to its body weight (Figure 3). We found that the maximum blood volume collected was linearly correlated with body weight (R = 0.813). The largest volume of blood collected from an individual fish (body weight = 1.071 g) was 25 µl, and the smallest volume was 1.3 µl from a fish weighing 0.115 g. This suggests that the maximum volume of blood collected depends on the body weight of the zebrafish.
Biochemical analysis of hemoglobin, blood glucose, TG and total cholesterol were performed after blood collection (Table 1). Male and female healthy adult zebrafish (4–6 months old) were fasted for 18 hr before blood collection. The biochemical analysis revealed that the normal value of hemoglobin (male 9.91 ± 0.49 g/dl and female 10.02 ± 0.48 g/dl) and TG (male 417 ± 45 mg/dl and female 404 ± 35 mg/dl) did not differ significantly between the two groups. However, the fasting blood glucose and total cholesterol levels of the male group (44 ± 3 mg/dl and 365 ± 18 mg/dl, respectively) were significantly lower (p <0.05) than the female group (69 ± 3mg/dl and 511 ± 52 mg/dl, respectively).
While the minimal trauma to zebrafish using the present method enables repeated blood sampling from the same individual, the effects of repeated blood drawing have not been evaluated. We investigated these effects using measurements of blood hemoglobin level (Figure 4). As we have shown in a previous publication15, adult male fish weighing approximately 0.5 g each were assigned to four groups. Repeated blood sampling (2 µl each time) of the same individual fish once daily for 7 days (for a total of seven blood samples) resulted in significant decrease (p <0.01) in hemoglobin levels from 10.82 ± 0.78 g/dl to 2.38 ± 0.8 g/dl. Removal of 2 µl of blood every 2 days or a single collection of 5 µl per week also yielded a significant decrease (p <0.05) in hemoglobin levels. In addition, one week after a single collection of a 2 µl blood sample, hemoglobin levels were slightly below normal (from 8.11 ± 1.15 g/dl to 7.15 ± 1.17 g/dl). The hemoglobin levels had no effects after a single collection of a 2 or 5 µl blood sample for a 2-week recovery period. Thus, we concluded that repeated collection of 2 µl of blood (0.4% of body weight) per week or 2–5 µl (0.4–1% of body weight) per 2 weeks from individual fish can avoid blood loss anemia.
We further applied this method to the study of glucose metabolism. The changes in blood glucose levels of each individual in the normal diet group (once daily feeding) and overfeeding group (five daily feedings) were monitored over a 5-week period. Normal diet-fed zebrafish (Fish A, B, C) exhibited stable blood sugar levels all the time, while the overfed zebrafish (Fish D, E, F) experienced high blood glucose levels as early as week 1, and maintained this hyperglycemia condition throughout the 5-week study period (Figure 5).
 Figure 1: Procedure for Blood Collection From Adult Zebrafish. (A) Glass needles prepared using a needle puller. (B) Cutting the tip of the needle obliquely using a fine scissors. (C) A precut needle with a tip diameter of approximately 135 µm. Scale bar = 1 mm. (D) Blood collection devices: an aspirator tube assembly (left) and a bulb dispenser (right). Arrows indicate the nosepiece to hold the microcapillary needle. The arrowhead shows the mouthpiece of the aspirator tube assembly. The needle is positioned in the end of the nosepiece before sample collection. (E) Heparinizing the needle. (F) An anesthetized fish. (G) Place the fish on a paper towel soaked with the anesthetics. (H) Insert the needle at a 30–45° angle into the blood collection site. (I) Blood rising into the needle. (J) Bleeding has stopped and a <650 µm puncture is circled and shown in a high magnification. (K) Expelling the blood from the needle onto a piece of parafilm. (L) Measurement of blood glucose using a glucose meter.
Figure 1: Procedure for Blood Collection From Adult Zebrafish. (A) Glass needles prepared using a needle puller. (B) Cutting the tip of the needle obliquely using a fine scissors. (C) A precut needle with a tip diameter of approximately 135 µm. Scale bar = 1 mm. (D) Blood collection devices: an aspirator tube assembly (left) and a bulb dispenser (right). Arrows indicate the nosepiece to hold the microcapillary needle. The arrowhead shows the mouthpiece of the aspirator tube assembly. The needle is positioned in the end of the nosepiece before sample collection. (E) Heparinizing the needle. (F) An anesthetized fish. (G) Place the fish on a paper towel soaked with the anesthetics. (H) Insert the needle at a 30–45° angle into the blood collection site. (I) Blood rising into the needle. (J) Bleeding has stopped and a <650 µm puncture is circled and shown in a high magnification. (K) Expelling the blood from the needle onto a piece of parafilm. (L) Measurement of blood glucose using a glucose meter.
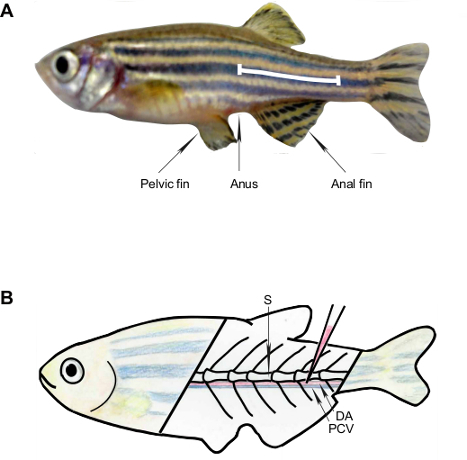 Figure 2: Schematic Representation of the Anatomic Landmarks for Blood Collection from the Adult Zebrafish. (A) The white line shows the puncture site for blood collection, which is along the body axis and posterior to the anus in the region of the dorsal aorta. (B) The primary vessels are the dorsal aorta and posterior cardinal vein; these are located ventral to the spine. S, spine; DA, dorsal aorta; PCV, posterior cardinal vein.
Figure 2: Schematic Representation of the Anatomic Landmarks for Blood Collection from the Adult Zebrafish. (A) The white line shows the puncture site for blood collection, which is along the body axis and posterior to the anus in the region of the dorsal aorta. (B) The primary vessels are the dorsal aorta and posterior cardinal vein; these are located ventral to the spine. S, spine; DA, dorsal aorta; PCV, posterior cardinal vein.
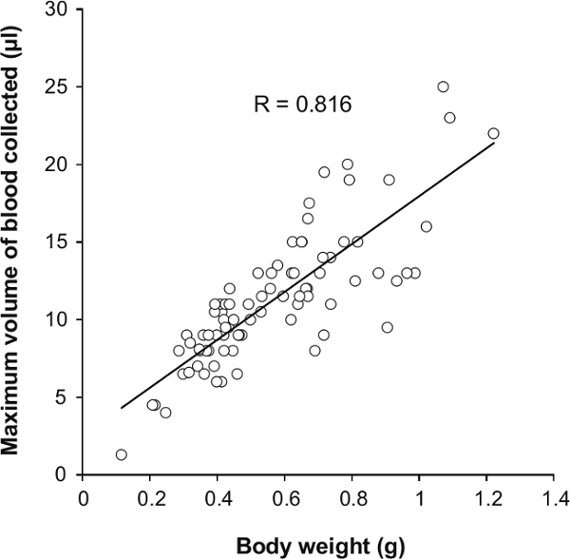 Figure 3: The Relationship of Maximum Volume of Blood Sampling and Body Weight. A total of 83 zebrafish (2–6 months old, 42 male and 41 female) underwent maximal blood collection.
Figure 3: The Relationship of Maximum Volume of Blood Sampling and Body Weight. A total of 83 zebrafish (2–6 months old, 42 male and 41 female) underwent maximal blood collection.
 Figure 4: Changes in Hemoglobin Levels Over a 1-week Period with Repeated Blood Sampling.A 2 µl sample of blood was collected from the same individual fish daily, once in 2 days, once weekly or 5 µl once weekly (n = 5). The hemoglobin levels of each group before blood collection (day 0, white bar) and after repeated blood sampling (day 7, gray bar) are shown. Values are means ± standard error of the mean (SEM). *p <0.05, **p <0.01 vs. day 0. Adapted from ref.15.
Figure 4: Changes in Hemoglobin Levels Over a 1-week Period with Repeated Blood Sampling.A 2 µl sample of blood was collected from the same individual fish daily, once in 2 days, once weekly or 5 µl once weekly (n = 5). The hemoglobin levels of each group before blood collection (day 0, white bar) and after repeated blood sampling (day 7, gray bar) are shown. Values are means ± standard error of the mean (SEM). *p <0.05, **p <0.01 vs. day 0. Adapted from ref.15.
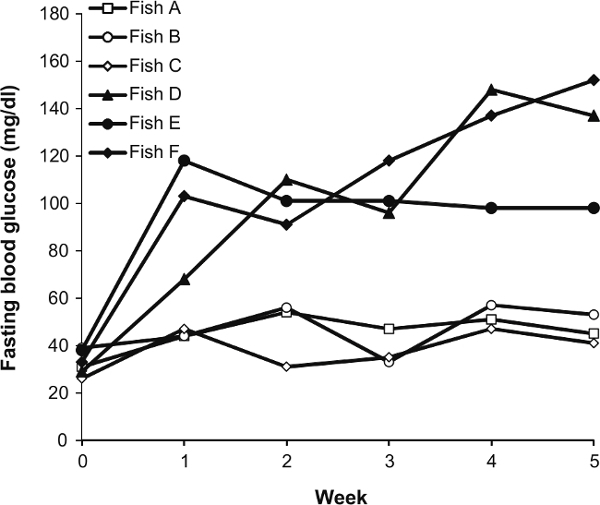 Figure 5: Changes in the Fasting Blood Glucose Concentrations of Six Individual Male Fish over a 5-week Period. Fish A, B and C were the normal diet group. Fish D, E and F were the overfed group.
Figure 5: Changes in the Fasting Blood Glucose Concentrations of Six Individual Male Fish over a 5-week Period. Fish A, B and C were the normal diet group. Fish D, E and F were the overfed group.
 Table 1:
Hemoglobin, Blood Glucose, TG and Total Cholesterol Levels for Male and Female Zebrafish 4
–
6 Months of Age.
Table 1:
Hemoglobin, Blood Glucose, TG and Total Cholesterol Levels for Male and Female Zebrafish 4
–
6 Months of Age.
Discussion
We present here a detailed protocol for serially obtaining blood from adult zebrafish. This method is straightforward to carry out and we use it in lab on a daily basis. This blood collection method is based on inserting a glass capillary needle into the zebrafish’s dorsal aorta. During this procedure, it is critical to be careful to not ablate the spine because it is the criterion for searching for the dorsal aorta. Reducing the spine injury will improve the survival rate. Although this technique is simple and easy to master, there are best practices that can ensure high success and survival rates. A skillful researcher will take 1–2 min to carry out the blood collection procedure (Protocol 2.3 to 3.6, which is the time that the fish is out of water). To save time in large-scale experiments, a double-team approach is recommended to perform blood collection. For example, one researcher could perform the blood sampling, while a second could handle the fish, perform the anesthesia and analyze the collected blood (measuring blood glucose, recording, or moving the blood to microcentrifuge tube, etc.).
Using this method, we demonstrated that the maximum blood sample volume that could be collected from individual fish is approximately 2% of body weight regardless of sex15, suggesting that the total circulating blood volume of zebrafish is greater than 2% of body weight. Previous studies demonstrated that teleost fish (Osteichthyes spp. and Salmogairdnerigairdneri) possess a total blood volume ranging between 1.8–3.8% of body weight17,18, thus we predict the total circulating blood volume of zebrafish to be 2–3.8% of body weight in zebrafish. For a single blood collection, we strongly recommend that blood sampling should be performed from 3 months old or >0.3 g body weight zebrafish to obtain ≥5 µl of blood. It is noteworthy that approximately half of the zebrafish survived even though 2% of body weight of their blood was removed, which revealed that zebrafish may cope well with blood loss.
The most significant advantage of this method is that it enables repeated blood sampling from the same individual. We determined the optimal volume and frequency of blood sampling by measuring the changes in hemoglobin levels (Figure 4). We recommend that the volume and interval for repeated blood sampling be ≤0.4% of body weight per week and ≤1% of body weight per 2 weeks to avoid blood loss anemia and hemorrhagic death. This conclusion is consistent with the practice guidelines for repeated blood sampling of rodent model animals19,20.
If the experiment allows the sacrifice of zebrafish, the needle can be inserted into a position along the body axis, posterior to the gill in the region of the dorsal aorta as a troubleshooting or an alternative method. This site is near the heart and aorta is relatively big, which can make the blood collection procedure easier.
Zebrafish have been successfully used in modeling the interrelated conditions of metabolic syndromes, including diabetes21,22, obesity23,24, fatty liver disease25 and atherosclerosis26. We further applied this technique to observe glucose metabolism in diet-induced obesity (Figure 5). Similar to mammals, zebrafish also developed abnormal glycolipid metabolism when fed a high-fat diet. The changes in fasting blood glucose of each individual showed individual differences in the response to overfeeding similar to that in humans15,27. This result indicates that an investigation of the temporal changes in blood biochemical parameters of individual fish will provide a good opportunity to determine the individual differences in metabolic disorders such as obesity and type 2 diabetes mellitus, thus further confirming its value as an animal model for human disease.
Overall, we developed a novel method for repeated blood collection from adult zebrafish. This method is essential for zebrafish research requiring repeated blood samples, such as studies of toxicokinetics, pharmacokinetics, and hematology. Furthermore, this repeated blood sampling method can also be applied to other small aquarium fish in biomedical research, for example, medaka (Oryzias latipes) or Xiphophorus (Xiphophorushelleri).
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number 25860294 and 25590073. We would like to thank Ms. Yui Namie for the hand-drawn illustration, and Mr. Koshi Kataoka and Ms. Sayuri Ichikawa for assistance with the zebrafish maintenance.
References
- Lieschke G, Currie P. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8(5):353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Penberthy WT, Shafizadeh E, Lin S. The zebrafish as a model for human disease. Front Biosci. 2002;7:d1439–d1453. doi: 10.2741/penber. [DOI] [PubMed] [Google Scholar]
- Stachura DL, Traver D. Cellular dissection of zebrafish hematopoiesis. Methods Cell Biol. 2011;101:75–110. doi: 10.1016/B978-0-12-387036-0.00004-9. [DOI] [PubMed] [Google Scholar]
- Jagadeeswaran P, Sheehan JP. Analysis of blood coagulation in the zebrafish. Blood Cells Mol Dis. 1999;25(3-4):239–249. doi: 10.1006/bcmd.1999.0249. [DOI] [PubMed] [Google Scholar]
- Jagadeeswaran P, Sheehan JP, Craig FE, Troyer D. Identification and characterization of zebrafish thrombocytes. Br J Haematol. 1999;107(4):731–738. doi: 10.1046/j.1365-2141.1999.01763.x. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Oates AC, Crowhurst MO, Ward AC, Layton JE. Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult. Blood. 2001;98(10):3087–3096. doi: 10.1182/blood.v98.10.3087. [DOI] [PubMed] [Google Scholar]
- Iwanami N. Zebrafish as a model for understanding the evolution of the vertebrate immune system and human primary immunodeficiency. Exp Hematol. 2014;42(8):697–706. doi: 10.1016/j.exphem.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Babin PJ, Goizet C, Raldua D. Zebrafish models of human motor neuron diseases: advantages and limitations. Prog Neurobiol. 2014;118:36–58. doi: 10.1016/j.pneurobio.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Seth A, Stemple DL, Barroso I. The emerging use of zebrafish to model metabolic disease. Dis Mod Mech. 2013;6(5):1080–1088. doi: 10.1242/dmm.011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames SC, Philipson LH, Prince VE, Kinkel MD. Blood sugar measurement in zebrafish reveals dynamics of glucose homeostasis. Zebrafish. 2010;7(2):205–213. doi: 10.1089/zeb.2009.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss JB, et al. Regeneration of the pancreas in adult zebrafish. Diabetes. 2009;58(8):1844–1851. doi: 10.2337/db08-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, et al. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol. 2010;10(21) doi: 10.1186/1472-6793-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CY, et al. Overexpression of Akt1 enhances adipogenesis and leads to lipoma formation in zebrafish. PLoS One. 2012;7(5):e36474. doi: 10.1371/journal.pone.0036474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco-Santamaría YM, Korsgaard B, Madsen SS, Bjerregaard P. Bezafibrate, a lipid-lowering pharmaceutical, as a potential endocrine disruptor in male zebrafish (Danio rerio) Aquat Toxicol. 2011;105(1-2):107–118. doi: 10.1016/j.aquatox.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Zang L, Shimada Y, Nishimura Y, Tanaka T, Nishimura N. A novel, reliable method for repeated blood collection from aquarium fish. Zebrafish. 2013;10(3):425–432. doi: 10.1089/zeb.2012.0862. [DOI] [PubMed] [Google Scholar]
- Carmichael C, Westerfield M, Varga ZM. Cryopreservation and in vitro fertilization at the zebrafish international resource center. Methods Mol Biol. 2009;546:45–65. doi: 10.1007/978-1-60327-977-2_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorson TB. The partitioning of body water in Osteichthyes: phylogenetic and ecological implications in aquatic vertebrates. Biol Bull-US. 1961;120:238–254. [Google Scholar]
- Conte FP, Wagner HH, Harris TO. Measurement of blood volume in the fish (Salmo gairdneri gairdneri) Am J Physiol. 1963;205:533–540. doi: 10.1152/ajplegacy.1963.205.3.533. [DOI] [PubMed] [Google Scholar]
- Diehl KH, et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 2001;21(1):15–23. doi: 10.1002/jat.727. [DOI] [PubMed] [Google Scholar]
- Nahas K, Provost J-P, Baneux PH, Rabemampianina Y. Effects of acute blood removal via the sublingual vein on haematological and clinical parameters in Sprague-Dawley rats. Lab Anim. 2000;34(4):362–371. doi: 10.1258/002367700780387804. [DOI] [PubMed] [Google Scholar]
- Curado S, et al. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. DevDyn. 2007;236(4):1025–1035. doi: 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
- Andersson O, et al. Adenosine signaling promotes regeneration of pancreatic beta cells in vivo. Cell Metab. 2012;15(6):885–894. doi: 10.1016/j.cmet.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramitsu M, et al. Eriocitrin ameliorates diet-induced hepatic steatosis with activation of mitochondrial biogenesis. Sci Rep-UK. 2014;4:3708. doi: 10.1038/srep03708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang L, Shimada Y, Kawajiri J, Tanaka T, Nishimura N. Effects of Yuzu (Citrus junos Siebold ex Tanaka) peel on the diet-induced obesity in a zebrafish model. J Funct Foods. 2014;10:499–510. [Google Scholar]
- Schlegel A. Studying non-alcoholic fatty liver disease with zebrafish: a confluence of optics, genetics, and physiology. Cell Mol Life Sci. 2012;69(23):3953–3961. doi: 10.1007/s00018-012-1037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoletov K, et al. Vascular lipid accumulation, lipoprotein oxidation, and macrophage lipid uptake in hypercholesterolemic zebrafish. Circ Res. 2009;104(8):952–960. doi: 10.1161/CIRCRESAHA.108.189803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CD, et al. Nutrient balance and energy expenditure during ad libitum feeding of high-fat and high-carbohydrate diets in humans. Am J Clin Nutr. 1992;55(5):934–942. doi: 10.1093/ajcn/55.5.934. [DOI] [PubMed] [Google Scholar]


