Abstract
Mice emit ultrasonic vocalizations (USVs) during a variety of conditions, such as pup isolation and adult social interactions. These USVs differ with age, sex, condition, and genetic background of the emitting animal. Although many studies have characterized these differences, whether receiver mice can discriminate among objectively different USVs and show preferences for particular sound traits remains to be elucidated. To determine whether mice can discriminate between different characteristics of USVs, a playback experiment was developed recently, in which preference responses of mice to two different USVs could be evaluated in the form of a place preference.
First, USVs from mice were recorded. Then, the recorded USVs were edited, trimmed accordingly, and exported as stereophonic sound files. Next, the USV amplitudes generated by the two ultrasound emitters used in the experiment were adjusted to the same sound pressure level. Nanocrystalline silicon thermo-acoustic emitters were used to play the USVs back. Finally, to investigate the preference of subject mice to selected USVs, pairs of two differing USV signals were played back simultaneously in a two-choice test box. By repeatedly entering a defined zone near an ultrasound emitter and searching the wire mesh in front of the emitter, the mouse reveals its preference for one sound over another. This model allows comparing the attractiveness of the various features of mouse USVs, in various contexts.
Keywords: Behavior, Issue 103, Ultrasonic vocalization, Mate choice, sexual selection, social recognition, pup isolation call, BALB/c mice, C57BL/6 mice, ultrasound emitter
Introduction
Many animals use vocalizations for intraspecific communication. In the mouse Mus musculus, one important type of communication signal is ultrasonic vocalizations (USVs), which have frequencies higher than 20 kHz. USVs emitted by mice are considered a component of social recognition in male-female1-4, female-female1, 5, and male-male1, 6 interactions. USVs are also emitted by pups when they are isolated from their mother, which increases her pup-retrieving behavior, and therefore pup survival 7. Although many reports have analyzed and categorized mouse USVs8,9, the behavioral responses and neural mechanisms of the receiving animal have been less documented10, 11. The latter is necessary to clarify the biological significance of the various characteristics of USVs. To reveal these mechanisms, the playback experiment is an efficient method. Recent playback studies have revealed that female mice are attracted to USVs12, and that they prefer USVs from males that are different from their parents13, 14.
This article explains the playback test used to evaluate USV preference in mice. A two-choice test box was developed in which two different USVs can be played back simultaneously in two compartments of a test enclosure, as shown in Figure 1. This type of test box prevents sound contamination by dividing the test area in three sub-chambers, using lead walls. The ultrasound emitters are located outside each room. In the wall between the rooms and ultrasound emitters are holes covered with wire mesh. Mice can move freely in the three rooms, and show a “searching the mesh” behavior, as if to respond to USVs played back by the ultrasound emitters. In this test, mice stay for periods of different duration close to one sound emitter or the other. These parameters can be logged to obtain a sensitive measure of sound preference.
To play the USVs back, nanocrystalline silicon thermo-acoustic emitters (i.e., “nc-Si emitter”) were used as in previous studies15-17. These devices are composed of a thin-film heater electrode, a nano-porous silicon layer, and a single-crystalline silicon wafer. The digital sound file is converted to an analog signal and then passed through the heater electrode. The device converts the resulting voltage-dependent thermal signals into significant sound pressure with low distortion. This device is unique in that, unlike common sound generators that depend on mechanical vibrations, it can reproduce sound without the need of a diaphragm. The emitter exhibits a flat sound pressure level at frequencies from 20 to 160 kHz (Figure 2), and can reproduce digitally recorded murine USVs very accurately in terms of duration, frequency, and sound pressure level15, 18, 19.
In a representative experiment shown in Figure 3, C57BL/6 (B6) females were allowed to choose between BALB/c (BALB) male USVs and background noise. In addition, Figure 4 shows the choice of B6 and BALB females between simultaneous USV playbacks from a BALB and a B6 male, as reported in a previous study14. The characteristics of male USVs differ between B6 and BALB strains20. As shown by these results, the attractiveness of USVs can be assessed with the present protocol, in which sounds are recorded from a live individual, acoustically analyzed, and played back to other individuals.
Protocol
All procedures were approved by the Ethics Committee of Azabu University. All experiments were carried out in a soundproof chamber.
1. Animal Preparation
- Males for Recording
- Obtain sexually mature male mice experienced in mating.
- Female Subjects
- Obtain virgin female mice that have been housed with 2 to 5 littermates per cage (usually 8 - 12 weeks old).
- Obtain vaginal smears daily to determine the phase of the estrous cycle before the test, according to McLean21. Care should be taken to minimize the vaginal stimulation to avoid pseudopregnancy.
- Stain the smears with Giemsa, and determine phase of the estrous cycle based on the presence or absence of leukocytes, cornified epithelial cells, and nucleated epithelial cells, according to Nelson22.
2. Devices (Figure 1)
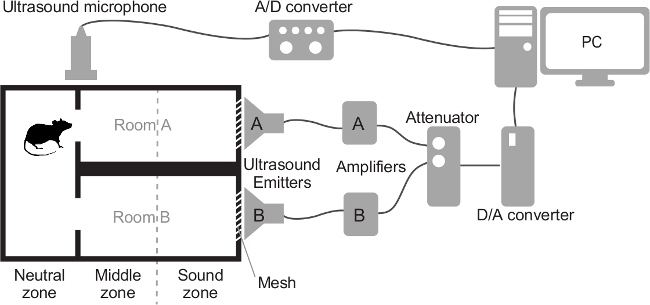 Figure 1. Schematic of the Two-choice Test Box and Devices. Mice can access rooms A and B through the small gates located between them and the neutral zone. The two-choice box and two ultrasound emitters are placed on the floor of a soundproof chamber. The microphone is suspended inside the soundproof chamber. Abbreviation: PC, personal computer. Please click here to view a larger version of this figure.
Figure 1. Schematic of the Two-choice Test Box and Devices. Mice can access rooms A and B through the small gates located between them and the neutral zone. The two-choice box and two ultrasound emitters are placed on the floor of a soundproof chamber. The microphone is suspended inside the soundproof chamber. Abbreviation: PC, personal computer. Please click here to view a larger version of this figure.
- For Recording
- Connect an ultrasonic condenser microphone to a PC using a USB A/D converter.
- Set the gain level of the A/D converter to “7”.
- Record acoustic data in 16-bit format files with a sampling rate of 400 kHz using audio capture software.
- For Playback
- Connect a D/A converter to the PC using a USB interface.
- Connect two amplifiers to the D/A converter through an attenuator. (The latter device serves to prevent overload.)
- Connect an ultrasound emitter to each amplifier.
3. USV Recording
Place a male mouse in a small- (17 x 10 x 11 cm) or medium-sized (21 x 14 x 13 cm) cage with a 6 cm-diameter hole in the sidewall and the hole covered with a 0.5 cm wire mesh. When using a medium-sized cage, construct a partition across the center of the cage to reduce the area.
Do not use bedding in the cage to avoid rustling noises that could contaminate the recordings.
Place a microphone beside the mesh.
Begin ultrasonic vocalization monitoring with the recording setup shown in step 2.1.
Place a sexually mature female mouse in either metestrus or diestrus in the cage with the male mouse.
Record USVs for 3 – 5 min.
4. Test Box
Build the test box using acrylic board (Figure 1). Note: The test box (35 x 20, and x 20 cm high) is divided in three compartments: a neutral zone (15 x 20 cm), and rooms A and B (20 x 10 cm, each). Rooms A and B each have a 4 cm-diameter hole that is covered with a 0.5cm wire mesh. The holes and mesh are located at each end of rooms A and B. Make gates (5 x 5 cm each) between the neutral zone and rooms A and B.
To prevent sound leakage insulate each ultrasound emitter, except for the side facing the mesh, using rubber plates, sealing them to the box around the perimeter of each mesh.
Place lead seals on both sides of the wall between rooms A and B for sound insulation.
5. Preparing for Sound Playback
- Creation of Playback Sounds
- Capture a 20 sec segment of USV from the recorded file using digital audio editing software.
- Select and capture background noise (0.35 sec) from the 20 sec USV segment file. Create a 20 sec background noise file by repeating the noise segment.
- To play back the two sound files simultaneously, export them as a single stereo sound file, with one file for the “left ear” and the other file for the “right ear.”
- Filter the sound files using as high-pass filter with a cutoff of 40 kHz.
- Reduce noise using the noise-reduction tool in post-processing software. Select and capture a segment of the file that contains no USVs as the noise-reduction profile, and run noise reduction with level 40.
- Calibration of Playback Sounds
- Ensure that the space between ultrasound emitters and microphone is 10 cm.
- To calibrate sound pressure levels from the ultrasound emitters, perform ultrasonic monitoring using the configuration described in Step 2.1. Measure the sound pressure level by the microphone in decibels (dB).
- Using the attenuator and amplifiers, adjust the volume of the USVs generated by the ultrasound emitters to the same sound pressure level as the male USVs recorded in Step 3.
- When using the two ultrasound emitters and a sound file composed of USVs and background noise, confirm that the two ultrasound emitters exhibit the same sound pressure level using a calibration sound (e.g., a 75-kHz pure tone) before performing the behavioral test.
- When reproducing a sound file composed of two streams of USVs, adjust the two generated sound pressure levels to the same level, before testing.
6. Two-choice Test
Place the ultrasound emitters behind the meshes of the test box.
Close the gates to rooms A and B with an acrylic board, and habituate the female subject to the neutral zone for 30 min. Cover the box with acrylic board to avoid that the mouse can escape.
Start video recording using a CCD camera mounted above the box. The camera (not shown in Figure 1) covers the area of all three compartments.
Remove the gate and cover-board and allow the female to explore freely the test box, including the sound zones.
Once the female has investigated both meshes, and has returned to the neutral zone, start playing a 20 sec sound file repeatedly for 5 or 10 min.
Conduct behavioral monitoring for 5 or 10 min.
To reduce undesired olfactory cues deposited from the previous subject, clean the test cages between the tests with 70% ethanol.
Switch the place of ultrasound emitters A and B randomly to avoid inherent side bias effect towards rooms A or B.
7. Statistical Analyses
Using behavioral event-scoring software, analyze the following parameters: total number of entries in each room; total duration of stay in each middle zone; total duration of stay in each sound zone; and total duration of search of each mesh in front of ultrasound emitter. Figure 1 illustrates what is meant by “middle zone” and “sound zone.” Note: In cases where the mouse stays on the dividing line between the two zones (Figure 1, dashed line) decide the location by the direction of the head. When the head faces the mesh side, it is scored as a stay in the sound zone.
For each behavioral parameter, compare responses for rooms A and B using a Wilcoxon signed-rank test, or paired t-tests with a significance level of 0.05.
Representative Results
The USVs recorded from one BALB-male (161 syllables per 20 sec), as well as background noise were used as playback sounds in the representative experiment shown in Figure 3. In this experiment, 7 female B6 mice were used at 9 weeks of age. To determine the best duration of testing of female response to playback sounds, the behavioral parameters were analyzed separately for the first and last five min of the total 10 min test time.
First, there was no significant difference in total duration of stay in rooms A or B during investigation time before playback (Figure 3A), demonstrating that there was no side bias.The results also showed that there was no difference between the background-noise side and the male-USVs side in the number of room entries, analyzing the first 5 min, the last 5 min, and total testing time (Figure 3B). Figure 3C shows cumulative seconds of duration of stay in each room during playback (i.e., total duration of stay in the middle zone, and in the sound zone). The subjects spent significantly longer time in the BALB male-USV side than in the background-noise side when analyzing the first 5 min (p = 0.043), or the total testing time (p = 0.043). There were no significant differences in these parameters when analyzing by last 5 min. There were no significant differences between the background-noise side and the male-USVs side in the middle zone (Figure 3D). However, B6 females spent significantly longer time in the sound zone and searching the mesh in the BALB male-USV side, compared to the background-noise side, when analyzing the first 5 min (Sound zone, p = 0.018, Figure 3E; Mesh, p = 0.018, Figure 3F) or total testing time (Sound zone, p = 0.028, Figure 3E; Mesh, p = 0.043, Figure 3F). The results clearly demonstrate that a B6 female approaches more the reproduced male-USVs than the background noise, particularly in the first 5 min.
This two-choice test is useful to compare the characteristics of USVs. In a previous study, this test was used to determine USV preference of B6 and BALB female mice for USVs from males of these strains11. When using a playback combination of USVs from a B6 (133 syllables per 20 sec) and a BALB (108 syllables per 20 sec) male, female mice of each of these strains showed longer searching times for the USVs of males of the other strain than of their own one (Figure 4).
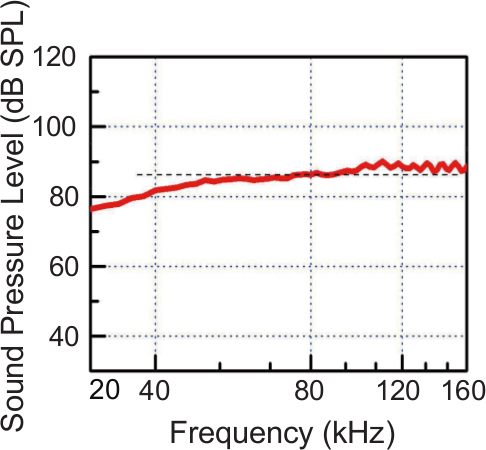 Figure 2. Measured Sound Pressure Level (SPL) Generated by the nc-Si Emitter as a Function of Frequency. An almost constant SPL of approximately 80 – 90 dB is observed between 40 and 160 kHz. AC input power is 1.3 W in this case. The emitter was placed at a distance of 20 mm from a high-frequency condenser microphone and aligned with its center. This figure has been modified from Kihara19. Please click here to view a larger version of this figure.
Figure 2. Measured Sound Pressure Level (SPL) Generated by the nc-Si Emitter as a Function of Frequency. An almost constant SPL of approximately 80 – 90 dB is observed between 40 and 160 kHz. AC input power is 1.3 W in this case. The emitter was placed at a distance of 20 mm from a high-frequency condenser microphone and aligned with its center. This figure has been modified from Kihara19. Please click here to view a larger version of this figure.
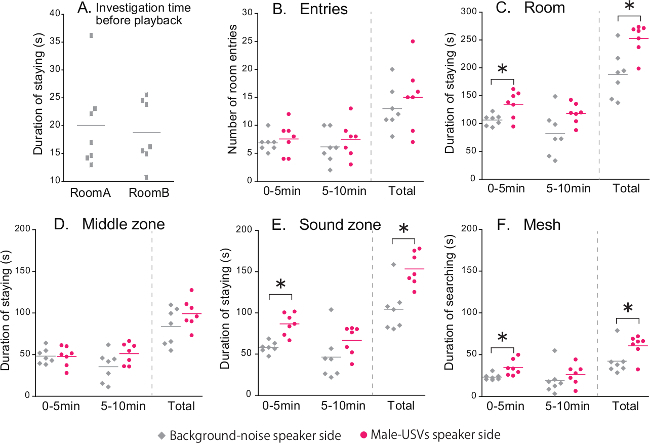 Figure 3. B6 Female Response to Background Noise and BALB Male USVs. (A) Total duration of stay in rooms A or B during investigation time before playback. (B) Number of room entries during playback. (C) Total duration of stay in the rooms. (D) Total duration of stay in the middle zone. (E) Total duration of stay in the sound zone. (F) Total duration of searching the meshes. Measurements of each individual are given in dot plots; horizontal bars in each plot indicate the mean; n = 7; * indicate significant differences (p <0.05) between background noise and male USVs for the parameters analyzed; Wilcoxon signed-rank tests. Please click here to view a larger version of this figure.
Figure 3. B6 Female Response to Background Noise and BALB Male USVs. (A) Total duration of stay in rooms A or B during investigation time before playback. (B) Number of room entries during playback. (C) Total duration of stay in the rooms. (D) Total duration of stay in the middle zone. (E) Total duration of stay in the sound zone. (F) Total duration of searching the meshes. Measurements of each individual are given in dot plots; horizontal bars in each plot indicate the mean; n = 7; * indicate significant differences (p <0.05) between background noise and male USVs for the parameters analyzed; Wilcoxon signed-rank tests. Please click here to view a larger version of this figure.
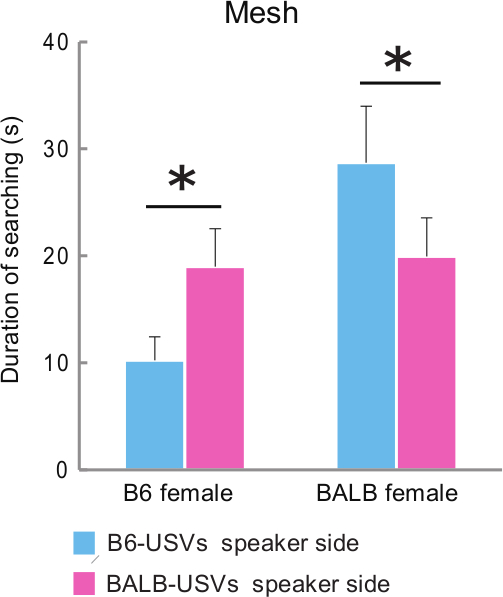 Figure 4. Female Response to USVs of Males from a Different Strain vs. the Same Strain. B6 (n = 6) and BALB (n = 10) females in diestrus exposed to male-soiled bedding before testing showed longer duration search times for the other strain USVs. Values shown are mean + standard error; * indicate significant differences (p <0.05); Wilcoxon signed-rank tests. This figure has been modified from Asaba14. Please click here to view a larger version of this figure.
Figure 4. Female Response to USVs of Males from a Different Strain vs. the Same Strain. B6 (n = 6) and BALB (n = 10) females in diestrus exposed to male-soiled bedding before testing showed longer duration search times for the other strain USVs. Values shown are mean + standard error; * indicate significant differences (p <0.05); Wilcoxon signed-rank tests. This figure has been modified from Asaba14. Please click here to view a larger version of this figure.
Discussion
Here, the results of a representative test showed that female mice can discriminate between artificial male USVs and background noise (Figure 3). The conclusion to be drawn from these results is that the discrimination signal is reflected in the duration of stay in the room and sound zone, and in the duration of searching the mesh in the first 5 min of testing, but not in the second 5 min (Figure 3C, E and F). These data indicate that mice become habituated to the playback sounds, possibly because of lack of social contact occurring near the ultrasound emitter. Therefore, the optimal testing time is 5 min.
These findings might be accounting for the fact that USVs are just “more novel sounding than noise.” However, a previous playback study showed that female mice were attracted to the sounds of male USVs, but not to novelty artificial sounds12. It is assumed that merely novel sounds are not affective to female mice.
As expected, USV preference was dependent on the phase of the estrous cycle, since social investigation towards a male is enhanced by moderate levels of estrogen23. A previous study has revealed that females in pro-estrus or estrus do not show the preference for USVs of males of a different strain observed here14. Therefore, only females in diestrus were used in the present study; in general, when using females for this test, the phase of the estrous cycle should be known.
The limitation of this test is that USVs generated by the ultrasound emitter are one-way playback sounds. This test is not appropriate to evaluate the role of USVs in complex social interactions.In addition, inbred strains of mice such as B6, can exhibit a severe hearing loss after 12 weeks of age24. Therefore, it is recommended that all mice used in this test are less than 12 week-old.
In case of failure to observe differences in response related to the playback sounds, the addition of other social cues could help make the subject more sensitive to the sounds. For instance, it is recommended that the test subject be exposed to mouse odor during the habituation time in the neutral zone. In a previous study, the preference of females for some USVs over others was potentiated when they were exposed to male-soiled bedding, or to the male pheromone ESP125 before behavioral testing. In the absence of male-odor stimuli, females did not show a preference for USVs of males of a different strain14.
This model allows comparing the various features of murine USVs. The characteristics of mouse USVs change with maturation from pup to adult26, and differ across strains1, 20, 27. In addition, the female estrous cycle influences male courtship USVs28. Moreover, the rate of male production of USVs, and the incidence of a specific syllable type show a temporal increase related to male mounting behavior28, 29. Research using models of communication disorders have demonstrated unusual repertoires of USV profiles2, 29-33. However, the question of whether the receiver mice can discriminate and show a specific response to these different USVs remains to be elucidated. The present protocol increases the usefulness of mouse USVs for understanding the context-dependence of social signal production and auditory reaction, and for uncovering the characteristics of attractive vocal communications in mice.
Disclosures
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by a Grant-in-Aid for JSPS Fellows to A.A.; by Grants-in-Aid for Scientific Research on Innovative Areas for JSPS Fellows (No. 4501 and No. 25132712) to T.K.; and by a research project grant awarded by Azabu University. Figure 2 is reprinted from Kihara, T., Harada, T., & Koshida, N. Wafer-compatible fabrication and characteristics of nanocrystalline silicon thermally induced ultrasound emitters. In: Sensors and Actuators A: Physical, volume 125, Elsevier, p. 426, (2006), with permission from Elsevier.
References
- Panksepp JB, et al. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One. 2007;2(4):e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2010;10(1):44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt K, Radyushkin K, Ehrenreich H, Fischer J. The structure and usage of female and male mouse ultrasonic vocalizations reveal only minor differences. PLoS One. 2012;7(7):e41133. doi: 10.1371/journal.pone.0041133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten S, Hoier S, Pfeifle C, Tautz D. A role for ultrasonic vocalisation in social communication and divergence of natural populations of the house mouse (Mus musculus domesticus) PLoS One. 2014;9(5):e97244. doi: 10.1371/journal.pone.0097244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato FR, Moles A. Ultrasonic vocalizations as an index of social memory in female mice. Behav. Neurosci. 2001;115(4):834–840. doi: 10.1037//0735-7044.115.4.834. [DOI] [PubMed] [Google Scholar]
- Chabout J, et al. Adult male mice emit context-specific ultrasonic vocalizations that are modulated by prior isolation or group rearing environment. PLoS One. 2012;7(1):e29401. doi: 10.1371/journal.pone.0029401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G. Infant rodent ultrasounds–a gate to the understanding of sound communication. Behav. Genet. 2005;35(1):19–29. doi: 10.1007/s10519-004-0853-8. [DOI] [PubMed] [Google Scholar]
- Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biol. 2005;3(1):e386. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J. Am. Assoc. Lab. Anim. Sci. 2007;46(1):28–34. [PubMed] [Google Scholar]
- Holfoth DP, Neilans EG, Dent ML. Discrimination of partial from whole ultrasonic vocalizations using a go/no-go task in mice. J. Acoust. Soc. Am. 2014;136(6):3401. doi: 10.1121/1.4900564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilans EG, Holfoth DP, Radziwon KE, Portfors CV, Dent ML. Discrimination of ultrasonic vocalizations by CBA/CaJ mice (Mus musculus) is related to spectrotemporal dissimilarity of vocalizations. PLoS One. 2014;9(1):e85405. doi: 10.1371/journal.pone.0085405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt K, Radyushkin K, Ehrenreich H, Fischer J. Female mice respond to male ultrasonic ‘songs’ with approach behaviour. Biol. Lett. 2009;5(5):589–592. doi: 10.1098/rsbl.2009.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musolf K, Hoffmann F, Penn DJ. Ultrasonic courtship vocalizations in wild house mice, Mus musculus musculus. Anim. Behav. 2010;79(3):757–764. [Google Scholar]
- Asaba A, et al. Developmental social environment imprints female preference for male song in iice. PloS one. 2014;9(2):e87186. doi: 10.1371/journal.pone.0087186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A, et al. Maternal approaches to pup ultrasonic vocalizations produced by a nanocrystalline silicon thermo-acoustic emitter. Brain Res. 2007;1163:91–99. doi: 10.1016/j.brainres.2007.05.056. [DOI] [PubMed] [Google Scholar]
- Okabe S, et al. The effects of social experience and gonadal hormones on retrieving behavior of mice and their responses to pup ultrasonic vocalizations. Zoolog. Sci. 2010;27(10):790–795. doi: 10.2108/zsj.27.790. [DOI] [PubMed] [Google Scholar]
- Okabe S, et al. Pup odor and ultrasonic vocalizations synergistically stimulate maternal attention in mice. Behav. Neurosci. 2013;127(3):432–438. doi: 10.1037/a0032395. [DOI] [PubMed] [Google Scholar]
- Shinoda H, Nakajima T, Ueno K, Koshida N. Thermally induced ultrasonic emission from porous silicon. Nature. 1999;400(6747):853–855. [Google Scholar]
- Kihara T, Harada T, Koshida N. Wafer-compatible fabrication and characteristics of nanocrystalline silicon thermally induced ultrasound emitters. Sensor. Actuat. A-Phys. 2006;125(2):422–428. [Google Scholar]
- Kikusui T, et al. Cross fostering experiments suggest that mice songs are innate. PloS One. 2011;6(3):e17721. doi: 10.1371/journal.pone.0017721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SA. Performing Vaginal Lavage, Crystal Violet Staining, and Vaginal Cytological Evaluation for Mouse Estrous Cycle Staging Identification. J. Vis. Exp. 2012. p. e4389. [DOI] [PMC free article] [PubMed]
- Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol. Reprod. 1982;27(2):327–339. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]
- Tomihara K, et al. Effect of ER-beta gene disruption on estrogenic regulation of anxiety in female mice. Physiol. Behav. 2009;96(2):300–306. doi: 10.1016/j.physbeh.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR. Hearing loss associated with the modifier of deaf waddler (mdfw) locus corresponds with age-related hearing loss in 12 inbred strains of mice. Hear. Res. 2001;154(1-2):45–53. doi: 10.1016/s0378-5955(01)00215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga S, et al. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature. 2010;466(7302):118–122. doi: 10.1038/nature09142. [DOI] [PubMed] [Google Scholar]
- Grimsley JM, Monaghan JJ, Wenstrup JJ. Development of social vocalizations in mice. PLoS One. 2011;6(3):e17460. doi: 10.1371/journal.pone.0017460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H, et al. A role for strain differences in waveforms of ultrasonic vocalizations during male-female interaction. PLoS ONE. 2011;6(7) doi: 10.1371/journal.pone.0022093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hurley LM. Female presence and estrous state influence mouse ultrasonic courtship vocalizations. PLoS One. 2012;7(7):e40782. doi: 10.1371/journal.pone.0040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liang S, Burgdorf J, Wess J, Yeomans J. Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS One. 2008;3(4) doi: 10.1371/journal.pone.0001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A, Kieffer BL, D'Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304(5679):1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Hiramoto T, et al. Tbx1: identification of a 22q11.2 gene as a risk factor for autism spectrum disorder in a mouse. Hum. Mol. Genet. 2011;20(24):4775–4785. doi: 10.1093/hmg/ddr404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey E, et al. Absence of deficits in social behaviors and ultrasonic vocalizations in later generations of mice lacking neuroligin4. Genes Brain Behav. 2012. [DOI] [PMC free article] [PubMed]
- Roy S, Watkins N, Heck D. Comprehensive analysis of ultrasonic vocalizations in a mouse model of fragile X syndrome reveals limited, call type specific deficits. PLoS One. 2012;7(9):e44816. doi: 10.1371/journal.pone.0044816. [DOI] [PMC free article] [PubMed] [Google Scholar]


