Abstract
Ruminant animals (domesticated or wild) emit methane (CH4) through enteric fermentation in their digestive tract and from decomposition of manure during storage. These processes are the major sources of greenhouse gas (GHG) emissions from animal production systems. Techniques for measuring enteric CH4 vary from direct measurements (respiration chambers, which are highly accurate, but with limited applicability) to various indirect methods (sniffers, laser technology, which are practical, but with variable accuracy). The sulfur hexafluoride (SF6) tracer gas method is commonly used to measure enteric CH4 production by animal scientists and more recently, application of an Automated Head-Chamber System (AHCS) (GreenFeed, C-Lock, Inc., Rapid City, SD), which is the focus of this experiment, has been growing. AHCS is an automated system to monitor CH4 and carbon dioxide (CO2) mass fluxes from the breath of ruminant animals. In a typical AHCS operation, small quantities of baiting feed are dispensed to individual animals to lure them to AHCS multiple times daily. As the animal visits AHCS, a fan system pulls air past the animal’s muzzle into an intake manifold, and through an air collection pipe where continuous airflow rates are measured. A sub-sample of air is pumped out of the pipe into non-dispersive infra-red sensors for continuous measurement of CH4 and CO2 concentrations. Field comparisons of AHCS to respiration chambers or SF6 have demonstrated that AHCS produces repeatable and accurate CH4 emission results, provided that animal visits to AHCS are sufficient so emission estimates are representative of the diurnal rhythm of rumen gas production. Here, we demonstrate the use of AHCS to measure CO2 and CH4 fluxes from dairy cows given a control diet or a diet supplemented with technical-grade cashew nut shell liquid.
Keywords: Environmental Sciences, Issue 103, greenhouse gas, methane, measurement technique, rumen, Greenfeed, cattle
Introduction
Animal production represents a significant source of greenhouse gas (GHG) emissions worldwide, generating CH4 and nitrous oxide either directly (e.g., from enteric fermentation and manure management) or indirectly (e.g., from feed-production activities and conversion of forest into pasture or cropland). Estimates for livestock contribution to the global GHG emission vary from about 71 to 18%2, depending on the boundaries of the analysis and methods used. In the United States, livestock represented 3.1% of the total GHG emissions in 20093.
Enteric CH4 is the largest contributor to GHG emissions from livestock. Therefore, animal scientists have focused their research on discovering mitigation technologies for reducing enteric CH4 production from ruminants. In many cases, results are of questionable scientific value due to inadequate experimental design or measurement techniques1. Thus, the accuracy and precision of the measurement techniques are critically important components of GHG mitigation research. A large body of literature has been published on this topic in recent years4-7. There are several established approaches for measuring enteric CH4 production in ruminants, including respiration chambers (highly accurate but with limited applicability), tracer gases (sulfur hexafluoride; SF6), and head-chambers. Although respiration chambers are considered the “gold standard” for measuring rumen gas emissions, their major disadvantage is that the number of animals on trial is usually limited due to the limited number of chambers available at a particular research facility. The most practical and widely used techniques for measuring enteric CH4 production are the SF6 tracer gas method and more recently, an Automated Head-Chamber System (AHCS, GreenFeed) that can monitor CH4 and CO2 mass fluxes from the breath and eructation gas of ruminants8. Both the SF6 technique and AHCS enable emissions to be analyzed on a large number of animals in free grazing conditions or in free- and tie-stall barns. The SF6 technique utilizes SF6 as a tracer gas, which is continuously released from a permeation tube inserted in the rumen of the animal, collection of a sample of the exhaled gases, and analysis of the gas for SF6:CH4 ratio. AHCS is an automated, head-chamber type system that is also based on the use of a tracer gas (propane). Compared with the respiration chamber method, where animals are confined under abnormal feeding and behavior conditions, and with the SF6 tracer method, which requires special analytical skills and equipment (for gas collection and SF6 analysis) plus extensive animal handling, AHCS is non-intrusive and is less expensive to acquire and operate. Major shortcomings of AHCS include unrepresentative sampling (in applications, such as grazing systems, where the animals have to voluntarily visit the unit) and the use of bait feed, which could represent up to 5% of the animal’s dry matter intake during a gas measurement event. Recent comparative experiments have concluded that AHCS produces emission rates comparable to those estimated using respiration chambers or the SF6 technique9,10.
The stand-alone AHCS system is constructed around a robust automatic feeder that is easily transportable by hand or can be mounted to a trailer equipped with solar panels (or other power sources) for autonomous field operation and long distance travel. The system includes an animal Radio-Frequency Identification system (RFID), a baiting system, an air handling and measurement system, a gas tracer system, electronics and communication system, and a data handling system (Figure 1). More details can be found in the original patent documentation11.
The example AHCS operation protocol described below is for lactating dairy cows housed in a tie-stall barn. The procedure is applicable to other categories of cattle (non-lactating dairy cows, heifers, or beef cattle) housed in similar facilities. The objective of this experiment is to demonstrate the principles and operation of AHCS for the measurement of CH4 and CO2 emissions from ruminant animals.
Protocol
Animals involved in the experiment described in representative results were cared for according to the guidelines of the Pennsylvania State University Animal Care and Use Committee. The committee reviewed and approved the experiment and all procedures carried out in the study. Details, such as animal and diet composition information and experimental design, can be found in the full publication of this experiment12.
Note: For a list of equipment and supplies needed to conduct the experiment, see Materials Table.
1. Experimental Design
Obtain Institutional Animal Care and Use Committee approval for the experiment. This is a non-invasive procedure that causes no pain to the animal and is classified as USDA Category C (Slight or momentary pain or distress or no pain or distress). Anesthesia is not necessary.
Select intact (i.e., non-cannulated) cows for the experiment based on lactation stage, age, and milk production. Do not use cannulated cows with AHCS because of potential leakage of rumen gas through the cannula. A device designed to alleviate this problem is currently being tested but results are not reported here.
If a crossover design (i.e., Latin square, for example) is utilized, use 8 to 12 cows, depending on number of treatments, in a replicated design balanced for residual effects. If, for example, 4 treatments are tested, 8 cows will yield a replicated 4 × 4 design trial, etc. The minimum recommended duration of these types of experiments is 21 to 28 days, with the first 14 to 21 days for adaptation to treatment and 7 days for data collection.
If a randomized block design is utilized, use 12 to 15 cows per treatment. Include a 2-week covariate period before data collection begins. The recommended duration of these experiments is from 8 to 12 weeks, with the first 2 weeks being for adaptation to treatment.
Equip each experimental animal with an ISO 11784 or 11785-compatible RFID identification tag.
2. Training of the Animals to Use AHCS
Before the experiment begins, move AHCS into the facility where the cows are located. Place the unit within eyesight of the cows. Leave the unit there for at least 2 days.
Prepare a bait feed that the animals like. Different feeds may be tried to entice cows, although a mixture of 70% ground corn, 27% dry molasses, and 3% soybean oil (as-is weight basis) has been successfully used in the laboratory. Avoid sticky and dusty feeds that may clog the air filter and the feed delivery system of AHCS. It is preferred that the feed is pelleted.
Give a small amount (a handful) of feed to all animals by placing it on top of the feed they are used to, in order for them to become familiar with the bait feed.
Very slowly move AHCS to about 1.5 m from the animal.
Place about 1 kg of bait feed into a bucket and allow the animal to smell and taste the bait feed. Gradually move the bucket towards the feeding trough of the AHCS unit, forcing the animal to stretch and reach towards the AHCS feeding trough.
Dump some of the bait feed from the bucket into the AHCS feeding trough and slowly move the AHCS unit towards the cow. If at any point of training, the cow becomes apprehensive or scared, move the unit away from her and try again at another time or day.
Over the course of several days, repeat the training until the animals are accustomed and excited by the AHCS unit, (i.e., the bait feed). If an animal cannot get used to AHCS, replace with another animal before the experiment begins and train the new animal following the above procedure.
3. Calibration of AHCS
Note: The concentration range of the CO2 sensor is 0 to 5%; the range for the CH4 sensor is 0 to 2%. The detection lower limits are 20 ppm for CH4 and 50 ppm for CO2. There are no concerns about high background levels of CH4 and CO2 because the detection limits are far greater than safe high background levels of these gases in animal facilities.
For maximum accuracy, perform this calibration procedure 5 times at the beginning and 3 times at the end of each gas measurement experiment.
Use the following gases (see Materials Table): 0.15% CH4 and 1% CO2 (grade certified master class, ± 2% accuracy) for span gas and 100% N2 (99.999% pure) for zero gas.
Fill a sample bag with 2 L of zero gas, and another bag with 2 L of span gas mixture. Be sure to use fully deflated bag. Fill the bags with new gas on the day of calibration.
Replace bags after 1 year of use or less, if necessary.
Take the gas standards to the place where the gas measurement experiment takes place. If the animals and measurement take place in an enclosed facility (i.e., a dairy barn), turn the barn fans ON during the entire calibration process. This is necessary to minimize the effect of methane concentrations in the background air.
Turn AHCS ON and let it warm before the calibration for at least 30 min. Remove the stopper from the calibration tube located inside the front panel. Make sure there is no water in the calibration tube. Remove water, if necessary. The water will destroy the gas concentration sensors.
Connect the N2 sample bag (zero gas) to the calibration tube. Unscrew the plastic valve on the sample bag counter-clockwise 1 full turn to allow flow.
Turn the “RUN-CALIBRATE” knob located on the instrument control panel of AHCS to “CALIBRATE”. This will begin pumping the sample out of the bag. Once the flow starts, press and hold the “CALIBRATE” button for 10 sec then release it.
Wait for the sample bag to deflate to about 10% of its capacity. Do not completely empty the bag, it may damage the sensor. Once the bag is at about 10% of its capacity, turn the “RUN-CALIBRATE” knob back to “RUN”.
Close the sample bag and disconnect it from the calibration tube. Wait 2 min and then, connect the span gas mixture bag to the calibration tube.
Repeat step 3.7.
Once the flow starts, press and hold the “CALIBRATE” button for 3 sec and then release it.
Repeat step 3.10.
Close the sample bag and disconnect it from the calibration tube. Replace the stopper into the calibration tube. Note: After calibration is completed, “factor” values will appear in the data tab on the web page. The coefficient of variation of the factors should be less than 3% and 1% for CH4 and CO2, respectively. If not within this range, repeat calibration.
4. CO2 Recovery Test
Perform the CO2 recovery test at least once (3 releases = 1 cylinder of CO2) before each gas measurement experiment. In continuous applications, perform the recovery test once per month.
Ensure that the CO2 recovery test valve is OFF (the valve is perpendicular to the outlet nozzle). Attach a new CO2 cylinder to the release system and turn the valve clockwise until tight.
Level and zero the mass scale. Place the entire release system on the scale to ensure it is accurate. Test to ensure CO2 is flowing: open the ON/OFF valve and quickly close it again while listening for CO2 exiting the nozzle. There should be a “whishing” sound when the CO2 is flowing.
Attach the CO2 cylinder holder to the feeding trough. From now on, do not let animals get near/breathe into the feeder. People should also not breathe into the feeder.
Weigh the CO2 cylinder with the release system. Record this mass as initial mass. Record the current local time at the start of every CO2 release.
Place the CO2 cylinder and release system into the CO2 cylinder holder (feeding trough) and direct the nozzle into the manifold. Do not breathe into the feeder.
Turn on the ON/OFF valve to release CO2 and record start time of release. Back several feet away from the feeder and wait 3 min. After 3 min, turn off the ON/OFF valve and record stop time of release.
Remove the CO2 cylinder plus release system from the feeder. Do not unscrew the CO2 cylinder from the release system. Hold the CO2 cylinder in a tub of warm water (37 to 43 °C). Place only the cylinder in water, not the whole release system.
Once the CO2 cylinder is warmed up, remove it from the water and use a drying cloth to wipe all the water from it. Weigh the CO2 cylinder with release system and record this as final mass.
Wait at least 3 min before the next release. During the 3 min wait time, do not allow any animal or person near the unit. A 90 g CO2 cylinder will provide about 3 releases so use multiple cylinders if there are more than 3 releases. When a cylinder is empty, weigh the empty cylinder and continue with a new cylinder, as described above.
Repeat steps 4.3 through 4.9 at least 3 times, marking the new start time, stop time, initial mass, and final mass of each release.
After the final release, wait at least 3 min before allowing animals to access the unit. When finished, unscrew the CO2 cylinder from the release system.
5. Gas Measurement
Note: Prior to gas measurement, a recent (within a week) calibration of AHCS is required. See steps 3, Calibration of AHCS and 4, CO2 recovery test. Make sure the animal’s RFID tag is in place.
Power up AHCS and leave ON for 30 min to warm up before making any measurements.
Position AHCS so that airflow from barn fans is allowed to enter into the feeding trough. Wait 2 min. Press the Feed Delivery button and hold for 3 sec to deliver approximately 50 g of feed. Visually validate that feed has been delivered into the feed trough.
Roll AHCS in front of a cow. Record the time in experiment notebook. The unit will read the animal’s RFID tag.
Deliver feed 5 additional times over a 5 min sampling period, spacing them to attempt to keep the animal’s head continuously in the feeding trough. If extra feed is required (to keep the animal’s head into the feeding trough), make note of it in the experiment notebook.
Note: Feed/pellets are usually delivered once every 50 sec for a total of 6 drops (300 g/measurement event) to obtain individual measurement periods of 5 min. The AHCS is equipped with infrared sensors to continuously monitor the distance of the animal’s head relative to the air intake manifold. These data are subsequently used to identify periods when the animal’s head was not in position and these measurement data are discarded.
Once the 5 min sampling period has expired, pull AHCS away from the animal and position unit so that airflow from barn fans is allowed to enter into the feeding trough. Wait 2 min to flush air through the system and to collect background air composition data.
Repeat steps 5.2 through 5.5 for additional animals.
Repeat sampling 8 times during a 24 hr feeding cycle, staggered in time over a 3-day period. The following sampling schedule has been successfully used: 0900, 1500, and 2100 hr (sampling day 1), 0300, 1200, and 1700 hr (sampling day 2), and 0000, and 0500 hr (sampling day 3). This sampling schedule will deliver 8 samples per animal and per sampling period. The sampling times may vary depending on feeding and milking times. Note: When gas sampling is completed, retrieve emission data from the web page.
Representative Results
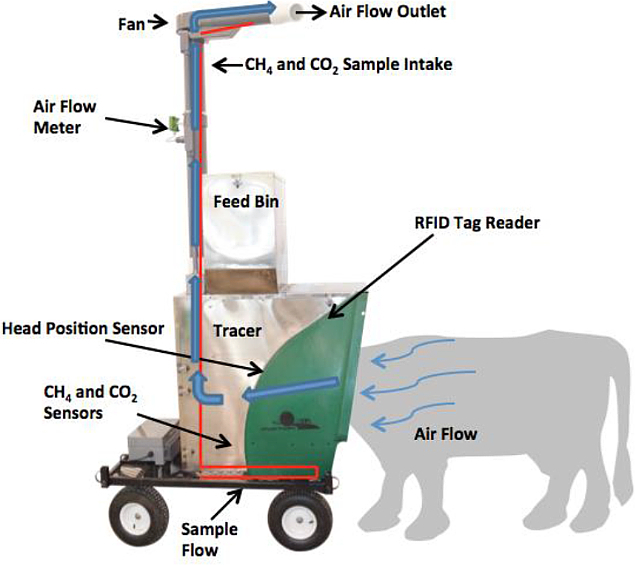
Figure 1: Components of the Automated Head-Chamber System (AHCS, GreenFeed) for measuring CH4 production in ruminant animals.
Methane production in the rumen is a substrate-dependent microbiological process7. Production of CH4 and CO2 increases after the animal is fed and decreases thereafter. Figure 2 demonstrates the increase in CH4 production from a dairy cow fed ad libitum at around 0600 hr (unpublished data by A.N. Hristov, Pennsylvania State University).
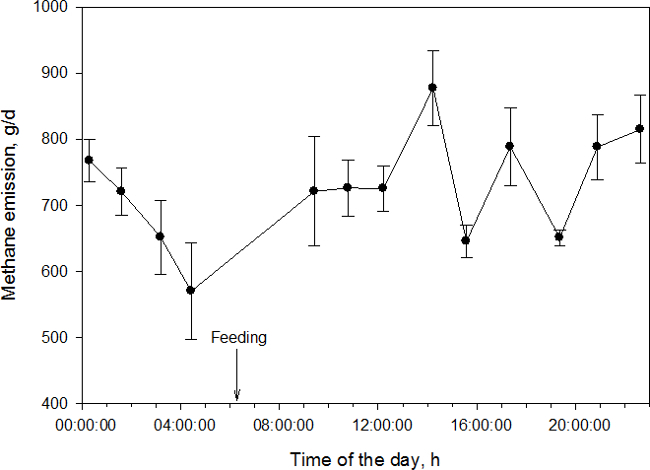 Figure 2: Diurnal CH4 emissions from a dairy cow fed once daily measured using AHCS (error bars represent SE; data courtesy of A. N. Hristov, Pennsylvania State University).
Figure 2: Diurnal CH4 emissions from a dairy cow fed once daily measured using AHCS (error bars represent SE; data courtesy of A. N. Hristov, Pennsylvania State University).
The error bars on this figure represent variability in CH4 emission during a sampling event (which includes multiple eructation cycles). It is apparent that in some cases (around 0400 and 0900 hr), variability was larger due to changing concentration of CH4 in exhaled gases. It is also clear that CH4 emissions increased after feeding (which was around 0600 hr in this example). The average daily CH4 emission (i.e., an average of the 13 measurement events) from this cow was 727 ± 22.9 g/days, or 26 g/kg when expressed per kg of diet dry matter intake (DMI).
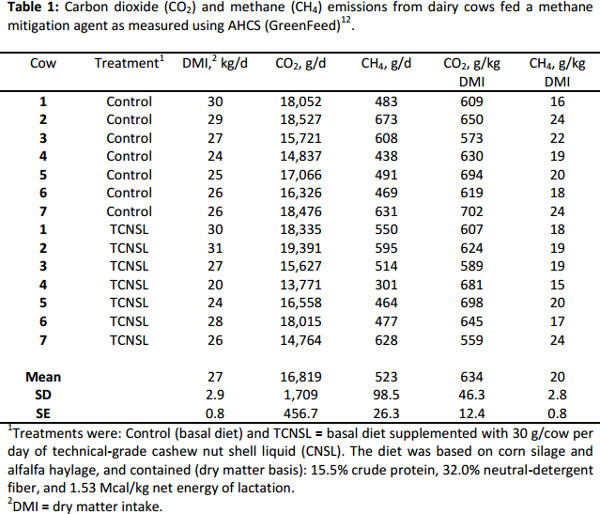
To demonstrate the range of CH4 emissions from a group of lactating dairy cows measured using AHCS, we include data from a recent crossover design trial conducted at the Pennsylvania State University that utilized technical-grade cashew nut shell liquid as a CH4 mitigation agent (Table 1). The trial was with 8 lactating Holstein dairy cows and 2 experimental periods of 21-days each. Methane data were collected during the last week of each period. Methane emission data were not collected from one cow in period 1 and data for that cow were also not used in period 2. Details of the experiment can be found in Branco et al.12. The average CO2 emission in this study was over 18,000 g/cow per day, or 634 g/kg DMI. Average CH4 emission for this group of cows was 523 g/day or 20 g/kg DMI, which is similar to the average CH4 emissions reported for a dataset of over 370 treatment means (19.1 ± 0.43 g/kg DMI)7. In the study presented in Table 1, compared with the control, technical-grade cashew nut shell liquid tended to decrease CH4 production in the rumen of the cows by about 5% (P = 0.08)12.
Discussion
The AHCS system combines elements of a dynamic enclosure technique, chamber system, and tracer technique for mass flux measurements of CH4 and CO2. Over the course of days, it collects multiple samples from each animal to define the average total daily gas mass fluxes. To identify an animal and deliver the correct amount of bait, an RFID reader is incorporated into AHCS. The RFID tag is read as the animal begins to place its head into the feeder. Once an animal is identified, AHCS determines if it is eligible to receive a bait reward at that specific time of the day (grazing or free-stall barn applications). The start and end time of each animal’s visit (determined based on the infrared sensors) is automatically recorded. The bait delivery system is used to attract animals to AHCS periodically throughout the day. Typically, the baiting feed is pelletized and may contain grass, alfalfa, grain concentrates, molasses, and vegetable oil. While an animal visits AHCS, a fan pulls air over its head (at rate of about 26 L/min), sweeping emitted CH4 and CO2 into an air intake manifold. The air flow velocity is measured continuously with a hot-film anemometer in the middle of the air collection pipe. A continuous sub-sample of air is extracted and routed into a secondary sample filter, then into two Non-Dispersive Infra-Red analyzers, one sensor for CO2 and one for CH4. AHCS also includes additional sensors for air temperature, air humidity, bait drop, system voltage, atmospheric pressure, propane flow rate, and head position. Pasture and trailer mounted versions for grazing systems include a cup anemometer (local wind speed) and wind vane (wind direction). All sensor data are stored on a local data logger and a computer, enabling AHCS to function automatically and independently. Sensor data are also stored on an internal standard USB (Universal Serial Bus) memory stick. AHCS data are normally transferred through an internet link, once per hour, to an external server where they are permanently logged. Users with internet connectivity can remotely log into AHCS and control the unit, modify baiting schedules, and review historical and real-time data as well as review and monitor AHCS function.
Overall, experiments conducted at the Pennsylvania State University demonstrated that the AHCS system delivers reliable estimates for CH4 and CO2 emissions from dairy cows housed in tie-stall barns. The advantages of AHCS over respiration chambers is that the animal is not restricted and is in its natural environment (i.e., on pasture), or can freely move (in a free-stall barn). AHCS is also less expensive to build than a traditional respiration chamber. This relatively low cost is important, particularly for CH4 mitigation research in developing countries. Compared with the SF6 tracer method, AHCS is simpler to operate and does not require complicated and expensive analytical equipment. Perhaps the most apparent disadvantage of AHCS, compared with chambers and the SF6 methods (particularly when used in grazing or free-stall barn environments), is that the animal has to voluntarily approach the unit and therefore gas measurement events are dependent on animal visits. Within a day, these animal visits may or may not be representative of the diurnal rhythm of CH4 production. Therefore, in applications where the animal visits AHCS voluntarily, the sampling period should be long enough or repeated a sufficient number of times. The tie-stall application used at the Pennsylvania State University alleviates this problem by controlling the number and temporal distribution of gas measurements during a 24 hr feeding cycle. Sufficient sampling of eructation gas during a feeding cycle (as indicated in the above protocol) is important for representative estimation of CH4 production in the rumen of cattle. The amount of bait feed fed to the animals during measurements using AHCS has to be considered in the overall analysis (i.e., must be added to the total amount of feed consumed by the animal), so emission intensity per unit of feed DMI can be accurately estimated. Under normal feeding conditions, the bait feed represents less than 5% of the total DMI of a dairy cows and its effect on the ruminal fermentation and CH4 production is small. It is noted that AHCS (and other similar systems) does not measure CH4 production in the animal’s hindgut. Hindgut fermentation, however, contributes only around 3% of the total CH4 emissions in a ruminant animal7.
Based on experience, there are several important components of measuring enteric rumen gas production using AHCS: (1) the animal has to be accustomed to the baiting feed (and AHCS) and has to like it in order to approach and use the AHCS feeder, (2) the animal’s head has to be inserted all the way into the feeder in order to collect reliable gas emissions data, (3) the AHCS calibration procedure has to be followed strictly, (4) having sufficient time to collect background CH4 and CO2 data between sampling individual animals is important, particularly in tie- or free-stall barns, and (5) it is important that enough data are collected in a sampling cycle (covering a 24 hr period) so emission data generated by AHCS are representative of the actual diurnal CH4 or CO2 emissions by the animal.
Comparative tests with AHCS vs. established CH4 measurement techniques support the above conclusions. For example, a study with growing dairy heifers concluded that AHCS was capable of estimating CH4 emissions from livestock and emission estimates generated by AHCS were comparable to values obtained by respiration chambers9. These authors pointed out that deployment of the AHCS units and replication must be carefully considered to ensure sufficient numbers of measurements are obtained. Based on experience, 8 sampling events, staggered over a 3-day period to cover a 24 hr feeding cycle (see protocol above) are sufficient to obtain accurate measurements of gaseous emissions and relatively low variability in the data (i.e., acceptable precision). In a study with lactating dairy cows, it was concluded that CH4 emissions measured by the AHCS were similar to literature values derived from respiration chambers and between animal variability (CV of 11 to 12%; repeatability of 0.64 to 0.81) was also within the range reported for respiration chambers10. In a recently-published study with lactating cows, AHCS produced a smaller CV than the SF6 method (14.1 to 22.4% vs. 16.0 to 111% for SF6)13. In a 12-week experiment conducted at the Pennsylvania State University with 48 lactating dairy cows, in which rumen CH4 production was inhibited by 30% (P < 0.001), we concluded that AHCS and the SF6 method produced similar CH4 emission results: 319 to 481 g/cow per day (mean = 374 g/d; SEM = 15.9; CV = 13%) and 345 to 485 g/cow per day (mean = 396 g/d; SEM = 29.8; CV = 23%) for AHCS and SF6, respectively14.
In conclusion, accurate, but practical techniques for measuring CH4 production in the rumen are critically important for the success of GHG mitigation efforts. AHCS is an automated gas measurement system that has been proven to deliver reliable and accurate estimates of enteric CH4 and CO2 emissions from beef and dairy cattle.
Disclosures
The authors Patrick R. Zimmerman and Scott R. Zimmerman are co-owners of C-Lock, Inc.
(Rapid City, SD), the manufacturer of GreenFeed (AHCS) described in this Article.
Acknowledgments
The authors would like to thank the staff of the Department of Animal Science’s Dairy Center for their conscientious care of the experimental cows used to generate data for this study.
References
- Hristov AN, et al. In: Mitigation of greenhouse gas emissions in livestock production – A review of technical options for non-CO2 emissions. Gerber PJ, Henderson B, Makkar PS, editors. Rome, Italy: Food and Agriculture Organization of the United Nations; 2013. (FAO Animal Production and Health Paper No. 177). [Google Scholar]
- Steinfeld H, et al. Livestock’s long shadow – Environmental issues and options. Rome, Italy: Food and Agriculture Organization of the United Nations; 2006. [Google Scholar]
- Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990-2009. Washington, DC: US Environmental Protection Agency; 2011. [Google Scholar]
- Makkar HPS, Vercoe P. Quantification of methane emission from ruminants, FAO/IAEA Publication. New York, NY: Springer Science and Business Media, Inc; 2007. p. 138. [Google Scholar]
- Williams SRO, et al. Background matters with the SF6 tracer method for estimating enteric methane emissions from dairy cows: A critical evaluation of the SF6 procedure. Anim. Feed Sci. Technol. 2011;170(3-4):265–276. [Google Scholar]
- Storm IMLD, Hellwing ALF, Nielsen NI, Madsen J. Methods for measuring and estimating methane emission from ruminants. Animals. 2012;2:160–183. doi: 10.3390/ani2020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov AN, et al. Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. J. Anim. Sci. 2013;91(9):5045–5069. doi: 10.2527/jas.2013-6583. [DOI] [PubMed] [Google Scholar]
- Zimmerman P, Zimmerman S, Utsumi S, Beede D. Development of a user-friendly online system to quantitatively measure metabolic gas fluxes from ruminants. J. Dairy Sci. 2011;94(1):760. [Google Scholar]
- Hammond KJ, et al. Methane emissions from growing dairy heifers estimated using an automated head chamber (GreenFeed) compared to respiration chambers or SF6 techniques. Adv. Anim. Biosci. 2013;4(Pt 2):391. [Google Scholar]
- Huhtanen P, Krizsan S, Cabezas Garcia EH, Hetta M, Gidlund H. Repeatability and between cow variability of enteric CH4 and total CO2 emissions. Adv. Anim. Biosci. 2013;4(Pt 2):588. [Google Scholar]
- Zimmerman P, inventor. Method and system for monitoring and reducing ruminant methane production. 2009/0288606A1. US patent. 2011
- Branco AF, et al. Effect of technical cashew nut shell liquid on rumen methane production and lactation performance of dairy cows. J. Dairy Sci. 2015;98:4030–4040. doi: 10.3168/jds.2014-9015. [DOI] [PubMed] [Google Scholar]
- Dorich CD, et al. Short communication: Use of a portable automated opencircuit gas quantification system and the sulfur hexafluoride tracer technique for measuring enteric methane emissions in Holstein cows fed ad libitum or restricted. J. Dairy Sci. 2015;98:2676–2681. doi: 10.3168/jds.2014-8348. [DOI] [PubMed] [Google Scholar]
- Hristov AN, et al. An inhibitor persistently decreased enteric methane emission from dairy cows with no negative effect on milk production. Proc Nat Acad Sci USA. 2015. [DOI] [PMC free article] [PubMed]


