Abstract
Although no longer considered therapeutically beneficial, antiretroviral treatment interruptions (TIs) still occur frequently among patients with human immunodeficiency virus (HIV) infection for a variety of reasons. TIs typically result in viral rebound and worsening immunosuppression, which in turn are risk factors for neurocognitive decline and dementia. We sought to determine the extent of neurocognitive risk with TIs and subsequent reintroduction of highly active antiretroviral therapy (HAART) by using a comprehensive, sensitive neuropsychological assessment and by concurrently determining changes in plasma and cerebrospinal fluid (CSF) viral load and CD4 counts. Prospective, serial, clinical evaluations including neuropsychological (NP) testing and measurement of plasma HIV RNA and CD4 count and mood state were performed on HIV-1–infected individuals (N=11) at three time points: (1) prior to a TI, while on HAART; (2) after TIs averaging 6 months; and (3) after reinitiating HAART therapy. During TI, plasma HIV RNA increased and CD4 counts declined significantly, but NP performance did not change. Following reinitiation of HAART, viral loads fell below pre-TI levels, and CD4 counts rose. Improved viral suppression and immune restoration with reinitiation of HAART resulted in significant improvement in neurocognitive performance. No changes on comprehensive questionnaires of mood state were observed in relation to TI. NP performance and mood state remained stable during TIs despite worsened viral loads and CD4 counts. Because “practice effects” are generally greatest between the first and second NP testing sessions, improvement at the third, post-TI time point was unlikely to be accounted for by practice. TIs of up to 6 months appear to be neurocognitively and psychiatrically safe for most patients.
Keywords: HIV, viral load, treatment interruption, HAART, neuropsychological assessment
Introduction
Treatment of human immunodeficiency virus (HIV) infection with highly active antiretroviral therapy (HAART) restores immune function, thereby reducing opportunistic disease and acquired immunodeficiency syndrome (AIDS)-related mortality. Nevertheless, the costs, side effects, and problems with access to antiretroviral medications often lead to treatment interruption (TI), which has become sufficiently common that recommendations on TI implementation have been recently formulated (Taylor et al, 2007). Although the landmark STRATEGIES FOR MANAGEMENT OF ANTI-RETROVIRAL THERAPY (SMART) study clearly demonstrated the superiority of continuous antiretroviral therapy to CD4-guided treatment interruption (El-Sadr et al, 2006), other recent studies indicate that TIs remain common. For example, in a study of 8300 subjects, 19.3% of subjects initially on stable HAART-interrupted treatment for a median duration of 189 days (Touloumi et al, 2006).
Although TIs remain common, no prospective studies have comprehensively assessed their neuropsychological (NP) and psychiatric impact. One small study showed no loss of motor speed during TI (Price and Deeks, 2004). However, the NP battery was limited and thus might have overlooked clinically significant deterioration. There is considerable evidence supporting the notion that ongoing viral replication may worsen neurocognitive function in HIV (Ellis et al, 1997, 2002). The purpose of this study was to evaluate whether TI and the ensuing rebound of viral replication and loss of CD4 cells adversely affected cognitive functions and mood. Additionally, we examined the same individuals after reinitiating antiretroviral therapy.
Results
Baseline demographic and select subject clinical characteristics are shown in Table 1. Subjects were treated with their pre-TI HAART regimens for a median of 10 months (IQR: 2–28; 95% CI: 1–80) at the time of pre-TI NP testing. TIs lasted a median of 6 months (IQR: 3–12; 95% CI: 3–14) and TI NP testing occurred a median of 3 months (IQR: 2–6; 95% CI: 1–10) after cessation of therapy. Post-TI, three subjects restarted their previous antiretroviral regimen, whereas eight subjects initiated a new HAART regimen. Post-TI NP testing occurred a median of 6 months (IQR: 5–12; 95% CI: 4–17) after TI NP testing, at which time subjects had resumed HAART for a median of 3 months (IQR: 2–6; 95% CI: 1–11). The median number of months between pre-TI and post-TI testing was 12 (IQR: 11–25; 95% CI: 6–37). Table 2 contains information regarding HAART regimens pre- and post-TI.
Table 1.
Baseline subject demographic and select clinical characteristics.
| Baseline characteristics | |
|---|---|
| Age | 35 (32–42) |
| No. (%) male | 9 (82) |
| No. (%) Caucasian | 5 (45) |
| Education (years) | 12 (12–15) |
| Duration of HIV infection (years) | 9 (4–12) |
| CD4 nadir (cells/μl) | 200 (23–371) |
| % CDC classification of AIDS | 45 |
| % neuropsychologically impaired | 27 |
| Beck Depression Inventory score | 5 (1–15) |
| POMS (z-score) | 1.0 (−0.1–1.6) |
Note. Values are median (interquartile range) unless otherwise specified.
Table 2.
Characteristics of study subjects’ antiretroviral (ARV) drug regimens pre- and post-TI.
| Pre-TI | Post-TI | |
|---|---|---|
| Number of ARVs in regimen (median [range]) |
3 [3–5] | 3 [3–7] |
| % containing PIs | 73 | 36 |
| % containing NRTIS* | 100 | 100 |
| % containing NNRTIs | 27 | 73 |
| % reporting at least 95% adherence to HAART† | 82 | 91 |
NRTIs include NARTIs.
Adherence information not available for one subject.
PI = protease inhibitors; NRTIs = nucleoside reverse transcriptase inhibitors; NNRTIs = non-nucleoside reverse transcriptase inhibitors; NARTIs = nucleotide analogue reverse transcriptase inhibitors.
Detailed results of analyses are presented in Table 3. Primary findings are outlined below by assessment interval. Changes in plasma and cerebrospinal fluid (CSF) HIV RNA and GDS scores at the three serial evaluations are depicted in Figure 1.
Table 3.
Clinical and laboratory characteristics at serial time points.
| Pre-TI | TI | Post-TI | |
|---|---|---|---|
| Global Deficit Score | .31 (0.12–0.76) | .31 (0.18–0.71) | .18 (0.00–0.41)a |
| Beck Depression Inventory | 5 (1–15) | 5 (1–13) | 4 (1–19) |
| Profile of Mood States (z-score)† | 1.1 (−0.1–1.6) | 0.7 (−0.2–1.7) | 1.0 (−0.2–1.6) |
| Plasma HIV RNA (log copies/ml) | 2.60 (2.60–4.02) | 4.80 (4.22–5.45)a | 2.60 (2.60–4.74)b |
| Virologically suppressed*, no. (%) | 6 (55%) | 0 (0%) | 7 (64%) |
| CD4+ T lymphocytes (cells/μl) | 363 (86–717) | 247 (16–395)a | 310 (27–546) |
Note. Values are median (IQR), unless otherwise specified.
Data not available for one subject.
Plasma HIV RNA <400 copies/ml.
Significantly different from pre-TI (p < .01).
Significantly different from TI (p < .01).
Figure 1.
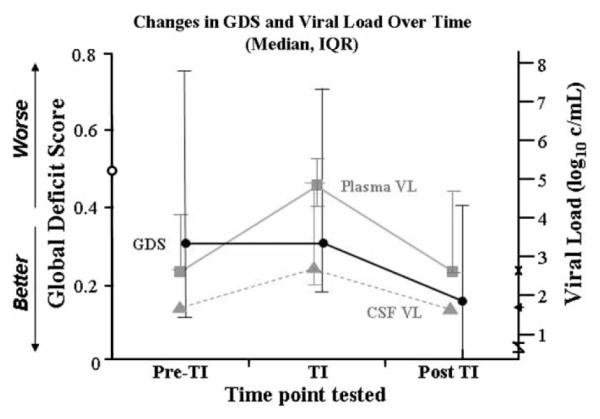
GDS scores evaluated at three serial time points compared to plasma and CSF viral loads (copies/ml). GDS scores >0.5 are considered cognitively impaired (indicated by 0 on left x-axis) Limits of viral load detection are shown on the right x-axis. x = plasma (2.6 log10 copies/ml)j ← = CSF (1.7log10 c/mL). No error bars are shown at pre-TI and post-TI time points for CSF because viral load is undetectable.
Pre-TI to TI
Global Deficit Scores (GDSs) did not change significantly from pre-TI to TI testing (p = .72). Profile of Mood States (POMS) and Beck Depression Inventory (BDI) total scores also did not change significantly from pre-TI to TI testing (p = .74 and p = .63). Plasma HIV RNA levels increased significantly from pre-TI (median = 2.60 log copies/ml; IQR = 2.60–4.02; 95% CI: 2.60–6.15) to TI (median = 4.80 log copies/ml; IQR = 4.22–5.45; 95% CI: 3.95–6.00) time points (z = 2.76, p = .006). Plasma viral load increased in all subjects excluding one, and 9 of 11 experienced an increase of ≥1 log. A Spearman’s rho analysis indicated that increase in plasma HIV RNA levels was not correlated with length of time since cessation of HAART (p = .43), suggesting that all patients had rebounded to their viral load set point at the time of the TI assessment. CD4 counts decreased significantly from pre-TI (median = 363 cells/μl; IQR = 86–717; 95% CI: 40–1214) to TI (median = 247 cells/μl; IQR = 16–395; 95% CI: 0–814; z = −2. 67, p = .008). Median decrease in CD4 count was not significantly correlated with length of time since cessation of HAART (p = .44).
TI to Post-TI
GDSs showed a statistical trend towards improvement from TI (.31) to post-TI (.18) assessments (p = .04). A Spearman’s rho analysis indicated variable delays from HAART resumption to post-TI NP testing were not associated with GDS improvement (p = .47). POMS and BDI total scores did not change significantly from TI to post-TI testing (p = .22 and p = .16). Plasma viral load decreased significantly from TI (median = 4.80 log copies/ml; IQR = 4.22–5.45; 95% CI: 3.95–6.00) to post-TI study visits (median = 2.60 log copies/ml; IQR = 2.60–4.74; 95% CI: 2.60–5.89; z = −2.85, p = .004). Decline in HIV RNA TI to post-TI was not significantly associated with length of time on post-TI HAART regimen (p = .08). There was a trend toward increase in CD4 count from TI to post-TI visits (p = .04), but duration of HAART was not significantly related to CD4 change (p = .40).
Pre-TI to Post-TI
GDSs improved significantly from pre-TI (median = 0.31; IQR = 0.12–0.76; 95% CI: 0.07–2.21) to post-TI assessments (median = 0.18; IQR = 0.00–0.41; 95% CI: 0.00–2.00; z = −2.81, p = .005). The median improvement in GDS was .13 (IQR = .07–.22; 95% CI: 0.00–0.35). POMS and BDI scores did not change significantly (p = .37 and p = .21). Plasma HIV RNA levels did not change significantly (p = .75), although a somewhat larger proportion of subjects achieved virologic suppression on their new HAART regimens. CD4 counts did not change significantly between the two assessments (p = .48). Three subjects (27%) showed a pre- to post-TI increase in CD4 count, whereas the rest did not reach pre-TI CD4 counts. GDS improvement was not related to the length of the interval between evaluations (p = .88) or to the magnitude of change in viral load (p = .36) or CD4 count (p = .31).
Discussion
Contrary to expectations, we found that overall cognitive performance and mood remained stable in 11 individuals undergoing TIs averaging 6 months, despite adverse changes in viral load and CD4 count. Our data are consistent with those of Price and Deeks (2004), and expand on their findings with a comprehensive neuropsychological evaluation and a more methodologically rigorous design that includes follow-up assessment post-TI. These findings support that time-limited TI can be undertaken safely from a neurocognitive and psychiatric standpoint.
Several well-established observations imply that TI might lead to neurocognitive decline. First, elevated plasma and CSF HIV RNA levels, reflecting high levels of ongoing viral replication, are associated with a higher risk of cognitive impairment in HIV infection (Childs et al, 1999; Ellis et al, 2002; Marcotte et al, 2003). Second, reduction of viral load following HAART initiation is associated with improvements in neurocognitive performance and reduced risk of incident impairment (Price et al, 1999; Richardson et al, 2002; Tozzi et al, 1999). Given that TI leads to marked increases in viral load in most patients, TI may deleteriously affect cognitive performance. This prospective study utilized comprehensive neuropsychological (NP) and mood assessments, reducing the likelihood that clinically significant cognitive or psychiatric declines were overlooked.
Because this study evaluated a relatively small sample, it cannot exclude the possibility that TI deleteriously affects a minority of HIV-infected subjects, especially those who have clinical and demographic characteristics different from those studied here. The majority of patients in this study were male, Caucasian, had moderately advanced HIV disease (AIDS-defining opportunistic illnesses, nadir CD4 < 200), and were NP normal prior to TI. Individuals with chronic, latent infection and those who were NP impaired prior to TI were less well represented in the sample. In our study, only three subjects (27%) were impaired at baseline. Patients impaired at baseline might be at higher risk for further neurocognitive decline during TI and we were unable to address this question. In addition, because no comparison group was studied (e.g., HIV-infected individuals not undergoing TI), we cannot rule out the possibility that our subjects performed more poorly or better than would have otherwise been anticipated. Studies of larger, more diverse populations in the use of comparison groups will be needed to more definitively address the neurocognitive safety of TI.
Among individuals with advanced HIV disease who are failing HAART, TI is often undertaken in an attempt to reestablish a predominant population of wild-type, antiretroviral drug–susceptible virus. The theoretical goal of TI in this setting is to improve the likelihood of a good virologic response (i.e., suppression) when HAART is reinitiated. Half of our patients were failing their current antiretroviral regimens prior to TI, however, the majority of these (4/5) experienced an increase in viral load during TI, suggesting that although subjects were not achieving complete viral suppression with ART, they were receiving some virologic benefit. Neither subjects failing ART nor those suppressed pre-TI had evidence of neurocognitive or psychiatric decline during TI or post-TI.
Lack of cognitive or affective deterioration during TIs might reflect the relatively short duration of TIs and the slow pace of pathophysiological events that lead to neurocognitive decline. Long delays in reinitiation of HIV replication did not explain this lack of progression. All our subjects showed significant viral rebound after TI, and TIs were of similar duration to, or longer than, those used in most clinical settings (Ruiz et al, 2003). Thus, if neurocognitive decline occurred frequently in patients undergoing ‘typical’ TIs, it should have been evident here.
Resumption of HAART for an average of 3 months in our subjects was associated with suppression of plasma viremia (undetectable HIV RNA) in seven subjects (64%). Because five of these subjects were suppressed prior to TI, viral loads did not change significantly from the pre- to the post-TI visit. However, reinitiation of HAART was followed by a statistically significant, albeit modest, improvement in NP performance to levels exceeding those measured before TI. Because most patients were rated as globally unimpaired at both assessments, the clinical significance of this improvement is uncertain.
Every subject maintained or improved NP performance pre- to post-TI. All participants underwent treatment interruption and nine (82%) initiated a new ARV regimen post-TI. Regimen changes may have resulted in relief from substantial drug-related side effects, allowing for a small overall improvement in functioning. In addition, the percentage of ARV regimens containing protease inhibitors (PIs) decreased dramatically pre- to post-TI, with seven subjects removing PIs from their regimens and replacing them with nucleoside (NRTIs) and/or non-nucleoside reverse transcriptase inhibitors (NNRTIs). Each class of antiretroviral drugs has a different range of permeabilities with respect to the blood-brain and blood–central nervous system (CNS) barriers. As a drug class, PIs show reduced permeability in comparison to NNRTIs or NRTIs (Letendre et al, 2000, 2001; Reddy et al, 2003). Replication of HIV in the CNS can occur independently from systemic replication, and CSF HIV RNA levels are elevated among HIV-infected individuals with neurocognitive dysfunction (Ellis et al, 1997, 2000, 2002). The replacement of PIs with ARVs conferring greater CNS penetration may have resulted in more effective treatment of HIV in the CNS, allowing for a slight improvement in NP performance. However, the small sample size of this study, variations in baseline impairment, and the contribution of other changes within regimens prohibit statistical exploration of this speculation here.
Repeated administration of the tests—i.e., practice effects—might have led to artifactual improvement. However, the pattern of changes in performance over time was incompatible with practice effects as described in previous studies. Improvements in NP performance related to practice are typically most pronounced at the second test exposure (Duff et al, 2001; Sattler, 2001), and significant improvement occurred only at the third assessment. In addition, the clinical significance of the NP improvement is uncertain as the median improvement in GDS preto post-TI was .13 (IQR = .07–.22; 95% CI: 0.00–0.35), and few subjects were significantly impaired at baseline.
In summary, the results of the current study suggest that although viral load and CD4 count worsen during TIs lasting several months, no pronounced decline in NP functioning or psychiatric status are evident. In fact, improvement in clinical status with re-initiation of HAART may be accompanied by a modest “boost” in NP performance.
Methods
Subjects
Subjects were nine men and two women participating in longitudinal studies at the HIV Neurobehavioral Research Center at the University of California, San Diego, between 1999 and 2002. Study participation included multiple visits conducted over an average of 1 year. Inclusion criteria were HIV infection confirmed by measurement of HIV RNA by reverse transcriptase–polymerase chain reaction (RT-PCR) and planned TI with the intention to resume therapy in the future. Exclusion criteria included untreated central nervous system (CNS) or systemic opportunistic disease, active psychosis, or other disorder deemed likely to interfere with study participation. Sample selection is described in Figure 2 and detailed subject characteristics are provided in Table 1. Written informed consent was obtained from all participants according to a protocol approved by the institutional human subjects review panel.
Figure 2.
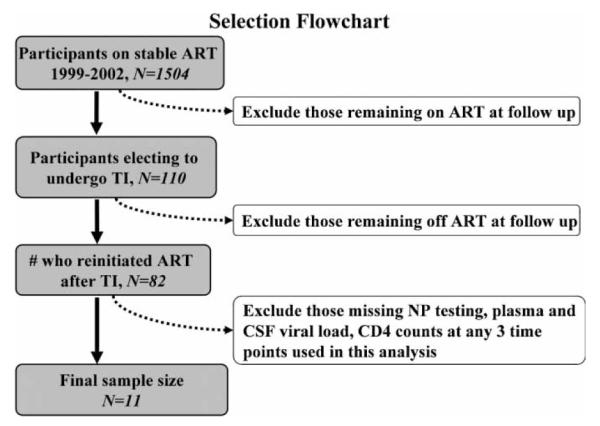
This chart explains how subjects were selected for analyses. The final sample had NP testing, plasma and CSF viral load, CD4 counts at all 3 time points.
Clinical Evaluations
Subjects were assessed at three time points: (1) pre-TI, during which each subject was on HAART; (2) TI, during which the subjects took no antiretrovirals; and (3) post-TI, during which subjects reinitiated HAART therapy. HAART was defined as an antiretroviral drug regimen containing three or more antiretroviral medications. Detailed information regarding pre- and post-TI HAART regimens are provided in Table 2. TI was defined as a planned cessation of HAART therapy. In consultation with their primary care providers, subjects decided when to interrupt and resume therapy.
At each visit, participants underwent a comprehensive neuromedical evaluation using structured clinical data forms to assess medical and medication use history, antiretroviral medications and self-reported adherence, neurological and general physical examinations, and laboratory studies including CD4 counts, routine hematology, and chemistry measurements. Participants were assessed to exclude central nervous system opportunistic disease, and to assign HIV disease stage according to the Centers for Disease Control guidelines (CDC, 1992). Adherence to antiretroviral medications was assessed in 10 of 11 subjects using the AIDS Clinical Trials Group’s brief self-report instrument (Chesney et al, 2000).
Laboratory Measures
We measured plasma HIV RNA levels by reverse transcriptase–polymerase chain reaction (RT-PCR) using the Amplicor HIV-1 Monitor Test (Roche Molecular Systems; nominal detection limit 400 copies/ml). HIV RNA values were log10-transformed prior to analysis. The assay’s nominal detection limit was used as the lower limit cut-off for HIV RNA values (2.60 log copies/ml). Virologic suppression was defined as a plasma HIV RNA level of <400 copies/ml after at least 2 months of HAART, and virologic failure was defined as a plasma HIV RNA level of >400 copies/ml while on HAART for at least 2 months. CD4+ T-lymphocyte (CD4) counts were quantified by a fluorescence-activated cell sorter.
Neuropsychological Evaluation
All participants completed a comprehensive neuropsychological (NP) testing battery, which was developed to afford a brief (i.e., 2 to 3 h), but wide-ranging assessment of the NP domains affected by HIV disease. The specific domains tested were information processing speed, executive functions, working memory, verbal fluency, learning, recall, and motor coordination. The test battery consisted of (1) Hopkins Verbal Learning Test—Revised (HVLT-R trials 1 to 3 and delayed free recall) (Benedict et al, 1998); 2) Brief Visuospatial Memory Test – Revised (BVMT-R trials 1-3 and delayed free recall) (Benedict, 1997); (3) Controlled Oral Word Association Test (COWAT-FAS total correct) (Benton et al, 1994; Gladsjo et al, 1999); (4) Semantic verbal fluency—animals (total correct) (Gladsjo et al, 1999); (5) Stroop Color-Word Test (interference trial) (Golden, 1978); (6) Trail Making Test, Parts A and B (total time) (United States Army, 1994; Heaton et al, 1991); (7) Wisconsin Card Sorting Test—64 Card Version (perseverative responses) (Kongs et al, 2000); (8) Halstead Category Test (total errors) (Heaton et al, 1991; Reitan and Wolson, 1993); (9) Paced Auditory Serial Addition Test (PASAT-200 total correct) (Diehr et al, 1998; Gronwall, 1977; Gronwall and Sampson, 1974); (10) Grooved Pegboard Test (time to completion for dominant and nondominant hands) (Heaton et al, 1991; Klove, 1963); and (11) the Digit Symbol, Symbol Search, and Letter-Number Sequencing tests from the Wechsler Adult Intelligence Scale—Third Edition (WAIS-III) (The Psychological Corporation, 1997; Heaton et al, 2002). All NP tests were administered and scored by trained psychometrists who adhered to the standardized procedures outlined in the various test manuals.
For each of the NP test variables above, raw scores were converted to demographically corrected T-scores using published normative data. T-scores were transformed into deficit scores using the following conversions: ≥40T = 0; 39T–35T = 1; 34T–30T = 2; 29T–25T = 3; 24T–20T = 4; and ≤19T = 5. To obtain a measure of global NP functioning, the deficit scores from each NP test variable were averaged to derive a Global Deficit Score (GDS) for each participant. Prior research supports the construct validity of the GDS approach as an indicator of global NP functioning in persons with HIV infection (Carey et al, 2004; Heaton et al, 1995). A score of .50 or above on the GDS indicates global impairment.
To evaluate possible changes in psychiatric status, participants completed the Profile of Mood States (POMS) (McNair et al, 1992) at all three time points. The POMS is a reliable and valid comprehensive measure of affective states (e.g., anxiety, depression, confusion, fatigue) on which participants rate 65 mood-related adjectives on a scale ranging from 0 (not at all) to 4 (extremely). Raw scores were converted to z-scores using published, demographically corrected normative data (Nyenhuis et al, 1999). A z-score of >1.5 is traditionally used as a cut-point for considerable affective distress. Participants also completed the Beck Depression Inventory (BDI; Beck et al, 1996) at all three assessments. The BDI is a widely used 21-item self-report screening questionnaire on which participants indicate the severity of their experienced depressive symptoms (e.g., sadness) using a 4-point Likert-type scale. Total scores on the BDI range from 0 (no depression) to 63 (severe depression), with scores above nine typically used as an indicator of mild depression.
Statistical Analyses
Because sample sizes were relatively small, and the distribution of the clinical variables in most cases deviated significantly from normal, a series of nonparametric Wilcoxon signed-rank tests were employed in a repeated measures design to assess the direction and magnitude of changes in GDS, POMS, BDI, plasma HIV RNA levels, and CD4 counts between time points. When a significant change in one of these variables occurred, Spearman’s rho correlations were performed to determine if the change was related to variability among subjects in length of time between changes in antiretroviral status (on or off). Given the exploratory nature of the study, small sample size, and multiple comparisons, to avoid type 1 error, p values ≤.01 were considered significant.
Acknowledgments
The HIV Neurobehavioral Research Center is supported by Center award 5 P30 MH62512-01 from the National Institutes of Mental Health (NIMH). Dr. R. J.Ellis is supported by R01 MH58076.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
References
- Beck A, Steer R, Brown G. BDI-II Beck Depression Inventory manual. Harcourt Brace and Company; San Antonio, TX: 1996. [Google Scholar]
- Benedict RH. Brief Visuospatial Memory Test—Revised. Psychological Assessments Resources; Odessa, TX: 1997. [Google Scholar]
- Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test—Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychologist. 1998;12:43–55. [Google Scholar]
- Benton A, Hamsher K, Sivan A. Multilingual aphasia examination. AJA Associates; Iowa City, IA: 1994. [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- CDC 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb Mort Wkly Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Childs EA, Lyles RH, Selnes OA, Chen B, Miller EN, Cohen BA, Becker JT, Mellors J, McArthur JC. Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology. 1999;52:607–613. doi: 10.1212/wnl.52.3.607. [DOI] [PubMed] [Google Scholar]
- Diehr MC, Heaton RK, Miller W, Grant I. The Paced Auditory Serial Addition Task (PASAT): norms for age, education, and ethnicity. Assessment. 1998;5:375–387. doi: 10.1177/107319119800500407. [DOI] [PubMed] [Google Scholar]
- Duff K, Westervelt HJ, McCaffrey RJ, Haase RF. Practice effects, test-retest stability, and dual baseline assessments with the California Verbal Learning Test in an HIV sample. Arch Clin Neuropsychol. 2001;16:461–476. [PubMed] [Google Scholar]
- El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, Babiker A, Burman W, Clumeck N, Cohen CJ, Cohn D, Cooper D, Darbyshire J, Emery S, Fatkenheuer G, Gazzard B, Grund B, Hoy J, Klingman K, Losso M, Markowitz N, Neuhaus J, Phillips A, Rappoport C. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Gamst AC, Capparelli E, Spector SA, Hsia K, Wolfson T, Abramson I, Grant I, McCutchan JA. Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology. 2000;54:927–936. doi: 10.1212/wnl.54.4.927. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Hsia K, Spector SA, Nelson JA, Heaton RK, Wallace MR, Abramson I, Atkinson JH, Grant I, McCutchan JA. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. Ann Neurol. 1997;42:679–688. doi: 10.1002/ana.410420503. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Moore DJ, Childers ME, Letendre S, McCutchan JA, Wolfson T, Spector SA, Hsia K, Heaton RK, Grant I. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Arch Neurol. 2002;59:923–928. doi: 10.1001/archneur.59.6.923. [DOI] [PubMed] [Google Scholar]
- Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment. 1999;6:147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- Golden C. Stroop Color and Word test. Stoelting; Chicao, IL: 1978. [Google Scholar]
- Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Motor Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Gronwall DM, Sampson H. The psychological effects of concussion. Auckland University Press; Auckland: 1974. [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ, et al. The HNRC 500—Neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead-Reitan Battery: demographic corrections, research findings, and clinical applications. Psychological Assessment Resources; Odessa, TX: 1991. [Google Scholar]
- Heaton RK, Taylor MJ, Manly JJ. Demographic effects and use of demographically corrected norms with the WAIS-III and WMS-III. In: Ledbetter M, editor. Clinical interpretation of the WAIS-III and WMS-III. Academic Press; San Diego: 2002. [Google Scholar]
- Klove H. Clinical neuropsychology. In: Forster F, editor. Medical clinics of North America. Saunders; New York: 1963. [PubMed] [Google Scholar]
- Kongs S, Thompson L, Iverson G, Heaton RK. Wisconsin Card Sorting Test—64 card computerized version. Psychological Assessment Resources; Odessa, TX: 2000. [Google Scholar]
- Letendre S, Capparelli EV, Ellis RJ, McCutchan JA. Indinavir population pharmacokinetics in plasma and cerebrospinal fluid. The HIV Neurobehavioral Research Center Group. Antimicrob Agents Chemother. 2000;44:2173–2175. doi: 10.1128/aac.44.8.2173-2175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S, Ellis R, Grant I, McCutchan A, the HNRC Group The CSF concentration (conc)/IC50 ratio: a predictor of CNS antiretroviral (ARV) efficacy. Eighth Conference on Retroviruses and Opportunistic Infections; Chicago. Feb 5–8, 2001; 2001. Abstract K1004p. [Google Scholar]
- Marcotte TD, Deutsch R, McCutchan JA, Moore DJ, Letendre S, Ellis RJ, Wallace MR, Heaton RK, Grant I. Prediction of incident neurocognitive impairment by plasma HIV RNA and CD4 levels early after HIV seroconversion. Arch Neurol. 2003;60:1406–1412. doi: 10.1001/archneur.60.10.1406. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. Profile of Mood States (POMS) manual. Educational and Industrial Testing Services; San Diego: 1992. [Google Scholar]
- Nyenhuis DL, Yamamoto C, Luchetta T, Terrien A, Parmentier A. Adult and geriatric normative data and validation of the profile of mood states. J Clin Psychol. 1999;55:79–86. doi: 10.1002/(sici)1097-4679(199901)55:1<79::aid-jclp8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Price RW, Deeks SG. Antiretroviral drug treatment interruption in human immunodeficiency virus-infected adults: clinical and pathogenetic implications for the central nervous system. J NeuroVirol. 2004;10(Suppl 1):44–51. doi: 10.1080/753312752. [DOI] [PubMed] [Google Scholar]
- Price RW, Yiannoutsos CT, Clifford DB, Zaborski L, Tselis A, Sidtis JJ, Cohen B, Hall CD, Erice A, Henry K. Neurological outcomes in late HIV infection: adverse impact of neurological impairment on survival and protective effect of antiviral therapy. AIDS Clinical Trial Group and Neurological AIDS Research Consortium study team. AIDS. 1999;13:1677–1685. doi: 10.1097/00002030-199909100-00011. [DOI] [PubMed] [Google Scholar]
- Reddy YS, Kashuba A, Gerber J, Miller V. Roundtable report: importance of antiretroviral drug concentrations in sanctuary sites and viral reservoirs. AIDS Res Hum Retroviruses. 2003;19:167–176. doi: 10.1089/088922203763315669. [DOI] [PubMed] [Google Scholar]
- Reitan R, Wolson D. The Halstead-Reitan Neuropsychological Test Battery. 2nd ed Neuropsychology Press; Tuscon, AZ: 1993. [Google Scholar]
- Richardson JL, Martin EM, Jimenez N, Danley K, Cohen M, Carson VL, Sinclair B, Racenstein JM, Reed RA, Levine AM. Neuropsychological functioning in a cohort of HIV infected women: importance of antiretroviral therapy. J Int Neuropsychol Soc. 2002;8:781–793. doi: 10.1017/s1355617702860064. [DOI] [PubMed] [Google Scholar]
- Ruiz L, Ribera E, Bonjoch A, Romeu J, Martinez-Picado J, Paredes R, Diaz M, Marfil S, Negredo E, Garcia-Prado J, Tural C, Sirera G, Clotet B. Role of structured treatment interruption before a 5-drug salvage antiretroviral regimen: the Retrogene Study. J Infect Dis. 2003;188:977–985. doi: 10.1086/378411. [DOI] [PubMed] [Google Scholar]
- Sattler JM. Assessment of children: cognitive applications. 4th ed Jerome M. Sattler; La Mesa, CA: 2001. [Google Scholar]
- Taylor S, Boffito M, Khoo S, Smit E, Back D. Stopping antiretroviral therapy. AIDS. 2007;21:1673–1682. doi: 10.1097/QAD.0b013e3281c61394. [DOI] [PubMed] [Google Scholar]
- The Psychological Corporation, editor. Wechsler Adult Intelligence Scale—Third Edition (WAIS-III) The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Touloumi G, Pantazis N, Antoniou A, Stirnadel HA, Walker SA, Porter K. Highly active antiretroviral therapy interruption: predictors and virological and immunologic consequences. J Acquir Immune Defic Syndr. 2006;42:554–561. doi: 10.1097/01.qai.0000230321.85911.db. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Galgani SP, Narciso P, Ferri F, Sebastiani G, Amato CC, Affricano C, Pigorini F, Pau F, De Felici A, Benedetto A. Positive and sustained effects of highly active antiretroviral therapy on HIV-1-associated neurocognitive impairment. AIDS. 1999;13:1889–1897. doi: 10.1097/00002030-199910010-00011. [DOI] [PubMed] [Google Scholar]
- United States Army . Army Individual Test Battery—manual of directions and scoring. War Department, Adjutant General’s Office; Washington, DC: 1994. [Google Scholar]


