Abstract
Schizophrenia neurocognitive domain profiles are predominantly based on paper-and-pencil batteries. This study presents the first schizophrenia domain profile based on the Computerized Multiphasic Interactive Neurocognitive System (CMINDS®). Neurocognitive domain z-scores were computed from computerized neuropsychological tests, similar to those in the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB), administered to 175 patients with schizophrenia and 169 demographically similar healthy volunteers. The schizophrenia domain profile order by effect size was Speed of Processing (d=−1.14), Attention/Vigilance (d=−1.04), Working Memory (d=−1.03), Verbal Learning (d=−1.02), Visual Learning (d=−0.91), and Reasoning/Problem Solving (d=−0.67). There were no significant group by sex interactions, but overall women, compared to men, showed advantages on Attention/Vigilance, Verbal Learning, and Visual Learning compared to Reasoning/Problem Solving on which men showed an advantage over women. The CMINDS can readily be employed in the assessment of cognitive deficits in neuropsychiatric disorders; particularly in large-scale studies that may benefit most from electronic data capture.
Keywords: psychosis, neuropsychology, MATRICS, MCCB, speed of processing, memory, sex
1. Introduction
Patients with schizophrenia show significant cognitive deficits (Dickinson et al., 2007; Heinrichs and Zakzanis, 1998; Schaefer et al., 2013). These deficits are present in first-episode patients (Mesholam-Gately et al., 2009), non-ill relatives (Dickinson et al., 2007), and individuals at clinical (De Herdt et al., 2013; Giuliano et al., 2012) or genetic risk (Agnew-Blais and Seidman, 2013) for psychosis, suggesting that they are associated with disease liability and not merely a consequence of the disease or its treatment. Cognitive deficits among patients with schizophrenia have been associated with poorer functioning (Green et al., 2000; Green et al., 2004a) and hence may provide important treatment targets. This study assesses the schizophrenia neurocognitive domain profile based on the Computerized Multiphasic Interactive Neurocognitive System (CMINDS®; www.neurocomp.com), to determine its usability in large-scale studies of neuropsychiatric illness.
Neuropsychological test performance across cognitive domains (e.g., attention, working memory, verbal learning, etc.) is often presented as a cognitive domain profile (Saykin et al., 1991; Saykin et al., 1994). These profiles are created by normalizing scores using control means and standard deviations and grouping test scores to allow for visualization of putatively ‘differential’ deficits between cognitive domains. Many studies use different tests that have dissimilar discriminating power (Chapman and Chapman, 1973) within each of these cognitive domains, making comparisons of profiles across studies difficult. Meta-analyses on neuropsychological deficits in schizophrenia handle this issue by computing effect sizes based on individual test scores rather than on cognitive domain scores, which may vary in composition across studies (Dickinson et al., 2007; Schaefer et al., 2013).
The issue of variability in the composition of test batteries is of particular relevance with regard to the assessment and comparison of putative pro-cognitive treatments (Green et al., 2004b). To advance the development of such treatments, the National Institutes of Mental Health (NIMH) funded the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative to develop a consensus cognitive battery for use in schizophrenia clinical trials (Kern et al., 2008; Nuechterlein et al., 2008). The MATRICS initiative (1) selected tasks based on reliability, repeatability, sensitivity to site effects, practicality, tolerability, and relationship to functional outcome, (2) put forward the MATRICS Consensus Cognitive Battery (MCCB) comprised of 6 cognitive domains –Speed of Processing, Attention/Vigilance, Working Memory, Verbal Learning, Visual Learning, and Reasoning/Problem Solving– and one domain of Social Cognition (Nuechterlein et al., 2008) and (3) has co-normed the MCCB (Kern et al., 2008). Training on the battery is provided via Neurcog Trials, Inc. (http://www.neurocogtrials.com). The MCCB has norms for English and Spanish versions in the United States, and co-norming and standardization of the battery are taking place in several other countries (Jedrasik-Styla et al., 2012; Mohn et al., 2012; Rapisarda et al., 2013; Rodriguez-Jimenez et al., 2011). In addition, a number of studies have now reported cognitive domain profiles based on the MCCB in adult patients with schizophrenia (August et al., 2011; Freedman et al., 2008; Javitt et al., 2012; Keefe et al., 2011; Kern et al., 2011; Marx et al., 2009; Pietrzak et al., 2009; Rajji et al., 2013; Shamsi et al., 2011; Silverstein et al., 2010), early-onset schizophrenia-spectrum disorders (Holmen et al., 2009), non-ill siblings (Nam et al., 2009), adolescents with psychotic symptoms (Kelleher et al., 2012), and youth at clinical high-risk for psychosis (De Herdt et al., 2013).
In an independent effort, the NIMH sponsored the development of the Computerized Multiphasic Interactive Neurocognitive System (CMINDS; www.neurocomp.com). The CMINDS includes computerized neuropsychological tasks that are structurally- and functionally similar to standard paper-and-pencil neuropsychological tasks (O'Halloran et al., 2008) and allows for immediate electronic raw data capture and automated scoring of test results. Among the tasks available in the CMINDS are tests similar to those of the MCCB, though they differ in administration, data capture, and scoring (for review see (O'Halloran et al., 2008) and Table 1S). Some tasks also differ with regard to certain task components (Kern et al., 2009; O'Halloran et al., 2008). Unfortunately, and likely in part due to ongoing development of computerized neuropsychological batteries during the development of the MCCB, few computerized tasks, with exception of the continuous performance task, were incorporated into the MCCB. Though it could be argued that electronic data capture, which eliminates the need for manual scoring and dual data entry, has at least some efficiency advantages over paper-and-pencil neuropsychological tasks. With increases in sample sizes in all areas of psychiatric research (Ripke et al., 2011; Thompson et al., 2014), efficient data capture becomes increasingly important. In addition to the CMINDS, there are the IntegNeuro computerized cognitive assessment battery which also includes some neuropsychological test similar to standard tests (Silverstein et al., 2010) as well as several more cognitive neuroscience oriented computerized test batteries (e.g., CogState (Lim et al., 2013; Pietrzak et al., 2009), STAN/JANET (Cherkil et al., 2012; Glahn et al., 2010), CDR (Wesnes et al., 2002), PENN CNP (Gur et al., 2001a; Gur et al., 2001b; Gur et al., 2012; Gur et al., 2010) and CANTAB (Fray et al., 1996; Levaux et al., 2007), all of which are employed in numerous research studies.
In this study, we report on the CMINDS cognitive domain profile of adult patients with schizophrenia (n=175) compared to demographically similar healthy volunteers (n=169) recruited into the Function Biomedical Informatics Research Network (FBIRN) Phase 3 study. The cognitive domain scores were derived from computerized tasks that are similar to those of the paper-and-pencil MCCB. We also tested for the group and sex by domain interactions on performance. Keeping in mind that some proportion of the variance in each of the domain score distributions, for patients, is affected by a schizophrenia-related generalized cognitive impairment (e.g., poor attention) as well as factors such as poor motivation, based on the first cognitive impairment profile reported on the MCCB (Kern et al., 2011), we hypothesized the following ranking of deficits across the domains, from worst to best: Speed of Processing, Working Memory, Verbal Learning, Attention/Vigilance, Visual Learning, Reasoning/Problem Solving. Based on sex differences reported on the MCCB (Kern et al., 2008), we hypothesized a male advantage on working memory and problem solving and female advantage on verbal learning. Also, given recent evidence for confounding effects of smoking status on structural brain abnormalities (Schneider et al., 2014) as well as cognitive deficits (Hagger-Johnson et al., 2013) and the unresolved issues with regard to medication effects on cognition, we examined the effects of smoking and antipsychotic medication dosing on cognitive performance. Finally, we examined the clinical correlates of global cognitive dysfunction.
2. Methods
2.1 Participants
One-hundred-and-seventy-five patients with schizophrenia (mean age±SD=39.1±11.5, 132 males) and 169 healthy volunteers (mean age±SD=37.6±11.3, 122 males) with similar mean age, sex handedness, and race distributions, from 7 sites, participated in the study (see Table 1). Patient inclusion criteria were schizophrenia diagnosis based on the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (First et al., 2002b). All patients were clinically stable outpatients whose antipsychotic medications and doses had not changed within the last two months. Current antipsychotic medication data were available for 159 of the 166 patients (antipsychotics: 132 atypical, 18 typical, and 9 both). Chlorpromazine equivalents (mean±SD=380±392) could be computed for 143 patients (www.scottwilliamwoods.com/files/Equivtext.doc). Schizophrenia patients and healthy volunteers with a history of major medical illness, drug dependence in the last 5 years (except for nicotine), current substance abuse disorder, or MRI contraindications, were excluded. Patients with significant tardive dyskinesia and healthy volunteers with a current or past history of major neurological or psychiatric illness (First et al., 2002a) or with a first-degree relative with an Axis-I psychotic disorder diagnosis were also excluded. Patient’s clinical assessments included the Positive and Negative Syndrome Scale (Kay et al., 1989), the Scale for the Assessment of Positive Symptoms (Andreasen, 1984), and the Scale for the Assessment of Negative Symptoms (Andreasen, 1983). Socioeconomic status (Hollingstead, 1975), handedness (Oldfield, 1971), basic demographics, smoking history, and premorbid IQ (Uttl, 2002) were also assessed for all subjects. The sample includes 128 paranoid, 6 disorganized, 29 undifferentiated, and 12 residual patients. Prior to data collection, experienced clinicians were jointly trained on the clinical assessment rating scales with patient interviews. The raters' assessments were compared with expert ratings. Additional training was provided when raters deviated by more than one point for each item from the expert ratings.
Table 1.
Sample Demographics
| Schizophrenia Patients (n=175) |
Healthy Volunteers (n=169) |
Statistic | p-value | |
|---|---|---|---|---|
| Mean Age (SD) | 39.1 (11.5) | 37.6 (11.3) | t342=1.24 | 0.22 |
| Subject Educationb (SD) | 3.4 (1.0) | 2.2 (0.9) | t342=11.46 | <0.0001 |
| Parental Educationb (SD) | 2.3 (1.9) | 2.2 (1.5) | t342=0.85 | 0.40 |
| NAART | 29.6 (12.6) | 39.9 (11.3) | t339 = −7.99 | <0.0001 |
| Age at Onset | 21.7 (7.5) | |||
| Duration of Illness | 17.5 (11.4) | |||
| PANSS positive | 15.4 (5.0) | |||
| PANSS negative | 14.6 (5.5) | |||
| PANSS general | 28.5 (7.5) | |||
| PANSS composite | 0.8 (6.4) | |||
| Sex (Male/Female) | 132/43 | 122/47 | χ21=0.46 | 0.49 |
| Handednessa (bilateral/left/right) | 3/13/159 | 2/8/159 | FET | 0.60 |
| Race | FET | 0.10 | ||
| American Indian or Alaskan Native | 4 | 3 | ||
| Asian | 21 | 14 | ||
| Black or African American | 33 | 18 | ||
| Native Hawaiian or Pacific Islander | 3 | 2 | ||
| White | 114 | 132 | ||
| Smoking Status | Χ22=68.5 | <0.0001 | ||
| Current Smoker | 81 (46%) | 14 (8%) | ||
| Ex-Smoker | 32 (18%) | 30 (18%) | ||
| Never-Smoker | 62 (36%) | 125 (74%) | ||
| Smoking - Current Pack-years | 6.7 (13.0) | 1.9 (7.2) | t341=4.2 | < 0.0001 |
| Smoking -Lifetime Pack-years | 10.7 (16.4) | 2.3 (8.7) | t340=5.9 | < 0.0001 |
FET=Fisher’s Exact Test
based on Edinburgh Handedness Inventory (Oldfield, 1971)
based on the Hollingstead Socioeconomic Status Scale (Hollingstead, 1975)
NAART = North American Adult Reading Test (Uttl, 2002)
PANSS = Positive and Negative Syndrome Scale (Kay et al., 1989)
Written informed consent, including permission to share de-identified data between the centers and with the wider research community, approved by the University of California Irvine, Los Angeles, and San Francisco, Duke University, University of North Carolina, New Mexico, Iowa, and Minnesota Institutional Review Boards, was obtained from all study participants.
2.2 CMINDS neuropsychological test battery
The Computerized Multiphasic Interactive Neurocognitive DualDisplay System (CMINDS) (http://www.neurocomp.com/Solutions/Cminds/Default.aspx?page=Page3) includes structurally- and functionally similar, computerized administration versions of standard administration version tests selected by the NIMH-sponsored CATIE (Clinical Antipsychotic Trials of Intervention Effectiveness) and MATRICS® (Measurement and Treatment Research to Improve Cognition in Schizophrenia) consortia. These computerized tests have shown high levels of agreement with the standard tests as well as high levels of test-retest reliability (O'Halloran et al., 2008). The CMINDS battery was administered in individual testing rooms by personnel trained by neurocomp and took less than 1 hour and 30 minutes to complete.
The CMINDS test battery employed in the FBIRN Phase 3 study included the following computerized tests (standard equivalent, Publisher): Finger Tapping (Finger Tapping Test, Reitan Neuropsychological Laboratory), the Stroop Test (Stroop Color and Word Test, Psychological Assessment Resources, Inc), the North American Adult Reading Test (North American Adult Reading Test, Public Domain), the Symbol Digit Association Test (Symbol Digit Modalities Test, Western Psychological Services), Trails A and B, (Trail Making Test Parts A and B, Reitan Neuropsychological Laboratory), the Semantic Verbal Learning Test (Hopkins Verbal Learning Test – Revised™, Psychological Assessment Resources, Inc.), the Visual Spatial Sequencing Test (WMS®-III Spatial Span, Harcourt Assessment, Inc.), the Letter Number Test (WAIS®-III Letter Number Sequencing, Harcourt Assessment, Inc.), the Maze Solving Test (WISC®-III Mazes, Harcourt Assessment, Inc.), Facial Emotion Discrimination (Facial Emotion Discrimination, Public Domain), Letter Fluency Test (Letter Fluency, Public Domain), Category Fluency Test (Category Fluency, Public Domain), the Visual Figure Learning Test (Brief Visuospatial Memory Test – Revised, Psychological Assessment Resources, Inc.), the Continuous Performance Test – Repeated Pairs (Continuous Performance Test – Identical Pairs, Biobehavioral Technologies, Inc.), and the Card Sort Test [64 trials] (Wisconsin Card Sort Test™, Psychological Assessment Resources, Inc.). For detailed CMINDS task descriptions see Supplement 1. For detailed descriptions of standard neuropsychological tasks see reference (Lezak, 1995). At each site, the test battery was administered by staff trained by CMINDS personnel. Prior to test administration each subject performed the CMINDS Perception Response Evaluation Test to determine the subject’s capability of perceiving and responding to auditory and visual stimuli and prompts.
The CMINDS-based cognitive domains, based on comparable tests to those assessed by the MCCB, were as follows: (1) Speed of Processing. This domain score was based on the mean of (a) the log-transformed, negated (worse performance is lower) elapsed time (in seconds) during Trails A, (b) the number of correct in set responses in 60 seconds on trial 1 of the Category Fluency Test – Animals, and (c) the number of correct responses during the Symbol Digit Association Test z-scores; (2) Attention/Vigilance. This domain score was based on the d-prime across blocks A-C of the Continuous Performance Test z-scores; (3) Working Memory. This domain score was based on the mean of (a) the sum of the number of correct on the Visual Spatial Sequencing Test – Forward and Backward condition, and (b) the total correct on the Letter Number Span z-scores; (4) Verbal Learning. This domain score was based on the total number of correctly recalled target words for all three trials on the Semantic Verbal Learning Test z-scores; (5) Visual Learning. This domain score was based on the square-transformed total of the Visual Figure Learning Test z-scores, and (6) Reasoning/Problem Solving. This domain score was based on the square-transformed Maze Solving Test total score z-scores. Finally, CMINDS Composite Score was created based on the average across all 6 cognitive domains.
Examination of raw score distributions indicated that the Trails A variable (elapsed time) was notably positively skewed, while Maze Solving and Visual Figure Learning test variables were negatively skewed. The distributions for the positive and negatively skewed variables were transformed using log and square transformations, respectively. Subsequently, all tests scores were normalized to the control mean and SD [normalized (z) score = (raw score – control mean raw score) ÷ control standard deviation]. Domain scores that consisted of multiple test scores (e.g, speed of processing, working memory, and the CMINDS composite score (mean of all 6 normalized domain scores) were normalized to the control mean and SD a second time to account for the averaging-associated changes in means and standard deviations (see Table 2 for absolute test score means as well as domain z-scores and their standard deviations).
Table 2.
Absolute CMINDS Test Scores and Domain Z-scores by Diagnosis and Sex
| Domain / Test (SD) | Schizophrenia Patients (n=175) |
Healthy Volunteers (n=169) |
Males (n=254) |
Females (n=90) |
|---|---|---|---|---|
| Attention/Vigilance | −1.42 (1.43) | 0 (1) | −0.81 (1.4) | −0.43 (1.4) |
| Continuous Performance Test (CPT) | 2.3 (0.9) | 3.2 (0.6) | 2.7 (0.9) | 3 (0.9) |
| Speed of Processing | −1.29 (1.09) | 0 (1) | −0.69 (1.2) | −0.56 (1.23) |
| Trails A (TA) | 36.1 (13.5) | 26.3 (9.1) | 31.6 (12.3) | 30.4 (13.2) |
| Category Fluency Test (CFT) | 20.1 (6.4) | 24.7 (6.4) | 22.2 (7) | 22.7 (6.2) |
| Symbol Digit Association Test (SDAT) | 39.3 (11.6) | 53 (11.2) | 45.6 (13.4) | 47.2 (13.1) |
| Verbal Learning | −1.28 (1.2) | 0 (1) | −0.73 (1.3) | −0.44 (1.27) |
| Semantic Verbal Learning Test (SVLT) | 22.8 (5.1) | 28.3 (4.3) | 25.2 (5.4) | 26.4 (5.4) |
| Working Memory | −1.21 (1.15) | 0 (1) | −0.61 (1.2) | −0.61 (1.21) |
| Visual Spatial Sequencing Test (VSST) | 13.9 (4.2) | 17.2 (3.2) | 15.6 (4.1) | 15.2 (4) |
| Letter Number Test (LNT) | 8.8 (2.7) | 11.7 (3) | 10.1 (3.3) | 10.6 (3.1) |
| Visual Learning | −1.06 (1.14) | 0 (1) | −0.61 (1.2) | −0.36 (1.12) |
| Visual Figure Learning Test (VFLT) | 21.6 (7.7) | 28.2 (5.6) | 24.4 (7.7) | 26 (6.9) |
| Reasoning/Problem Solving | −0.81 (1.24) | 0 (1) | −0.36 (1.2) | −0.57 (1.15) |
| Maze Solving Test (MST) | 22.8 (4.2) | 25.3 (3.2) | 24.2 (4) | 23.6 (3.6) |
| CMINDS Composite Score | −1.66 (1.34) | 0 (1) | −0.91 (1.4) | −0.66 (1.44) |
Domain Z-scores are listed in bold. Score definitions: CPT: mean d-prime across blocks A to C; TA: elapsed time in seconds; CFT: number of in set responses in 60 seconds on trial 1; SDAT: number of correct responses; SVLT: total number of correctly recalled target words for all three trials; VSST: sum of number correct on forward and backward condition; LNT: total number correct; VFLT: total number correct; MST: total number correct.
2.3 Statistical analyses
The group comparison of the CMINDS composite score was performed using a mixed effects model (Proc Mixed, SAS/STAT® version 9.2, SAS Institute, Inc., Cary, NC, USA), predicting the normalized composite scores with group (schizophrenia, healthy volunteer), sex, age, and site. Group comparisons of the domain scores were performed using a mixed effects model with repeated measures (MMRM). Normalized domain scores were predicted with group, domain (Speed of Processing, Attention/Vigilance, Working Memory, Verbal Learning, Visual Learning, Reasoning/Problem Solving), and the group × domain interaction. Domain was modeled as a within-subject repeated measures factor and within-subject correlations were modeled with an unstructured covariance matrix. Age, sex, and site were included as fixed effects covariates. Based on testing for group × sex, sex × domain, and group × age interactions, the sex × domain interaction was added to the model.
The significant group × domain and sex × domain interactions were followed up with 15 contrasts comparing the magnitude of the patient versus control (or female versus male) differences between each of the domains. We used the Benjamini and Hochberg (1995) (Benjamini and Hochberg, 1995) False Discovery Rate procedure (FDR<0.05) to control for Type I errors. Briefly, the pairwise comparisons were sorted (ranked) from smallest to largest pvalue and compared with the critical p-value at each rank i, defined as P(i) = (i ÷ 15) · 0.05. For all consecutive p-values for rank 1 to i, where the tests p-value < P(i) the null-hypothesis was rejected. In addition to the z-score profile, we also rank ordered the cognitive domain profile based on Cohen’s d effect size estimates. To place our findings in the context of those by others, we compared our findings to those from the most recent, and largest meta-analysis study of neuropsychological task performance (Schaefer et al., 2013) as well as published neuropsychological profiles based on the MCCB (August et al., 2011; Freedman et al., 2008; Javitt et al., 2012; Keefe et al., 2011; Kern et al., 2011; Marx et al., 2009; Pietrzak et al., 2009; Rajji et al., 2013; Shamsi et al., 2011; Silverstein et al., 2010).
Possible confounding influences of smoking status on cognitive performance deficits (Hagger-Johnson et al., 2013) were assessed by including dummy-coded smoking status (current smoker, ex-smoker, never-smoker), current pack-year, or lifetime pack-year as covariates, respectively. We also examined the relationship between domain scores and antipsychotic medication dose (chlorpromazine equivalents). Estimate statements, which produce t-statistics, were used to determine the directionality of the relationships between continuous variables (e.g., age, medication dose) and CMINDS composite and domain scores. Finally, we examine the relationships between the CMINDS composite score and several clinical variables (age at onset, duration of illness, positive and negative symptoms). A detailed analysis of individual domain scores and clinical variables is outside the scope of this study and findings will be reported on in a separate manuscript.
3. Results
3.1 CMINDS composite score
Patients (marginal mean±SE=−1.62±0.10) had significantly lower CMINDS composite scores than controls (marginal mean±SE=0.08±0.10; t333=−12.62, p<0.0001) and the CMINDS composite score showed a significant negative association with age (t333=−6.66, p<0.0001).
3.2 CMINDS domain profile
The MMRM analysis showed significant effects of diagnosis [F(1,330)=201.10, p<0.0001], age [F(1,332)=68.46, p<0.0001], domain [F(5,339)=2.42, p<0.04], and diagnosis × domain [F(5,339)=4.51, p=0.0005] and sex × domain interactions [F(5,339)=3.26, p<0.007], but no significant effects of site, sex, or the group × sex interaction on standardized neuropsychological domain scores.
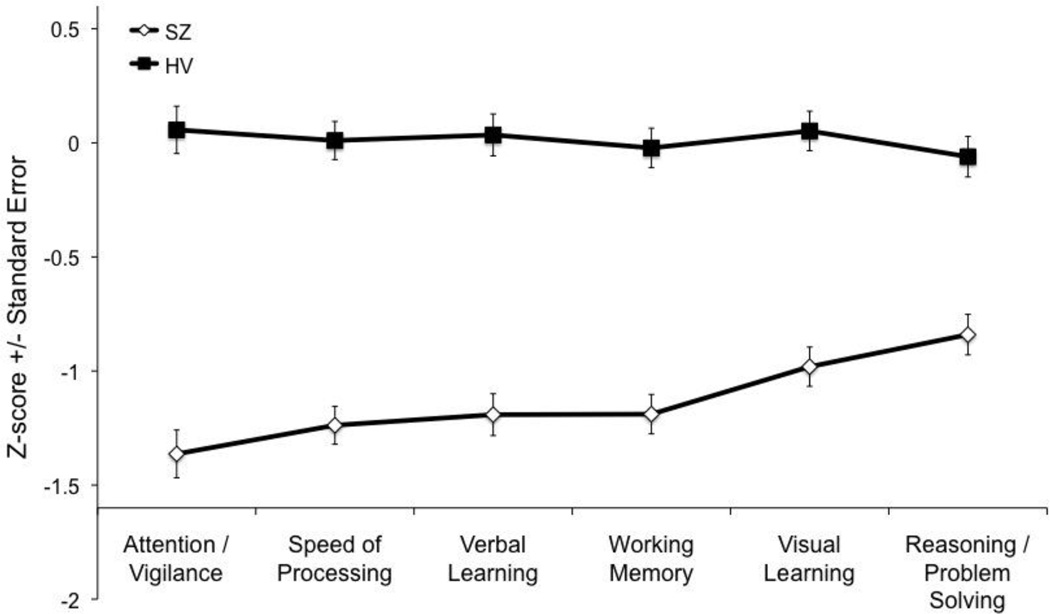
Comparisons of group (patient vs. control) contrasts between the six cognitive domains (FDR-corrected) showed that patients were significantly more impaired than controls on the Attention/Vigilance (t338=−4.26, p<0.0001), Speed of Processing (t340=−3.63, p=0.003), Working Memory (t339=−3.24, p<0.0013), and Verbal Learning domains (t339=−3.07, p=0.0023) relative to the Reasoning/Problem Solving domain, and the Attention/Vigilance domain relative to the Visual Learning domain (t338=−2.8, p<0.0054; see cognitive domain profile in Figure 1).
Figure 1.
CMINDS Schizophrenia Cognitive Domain Profile
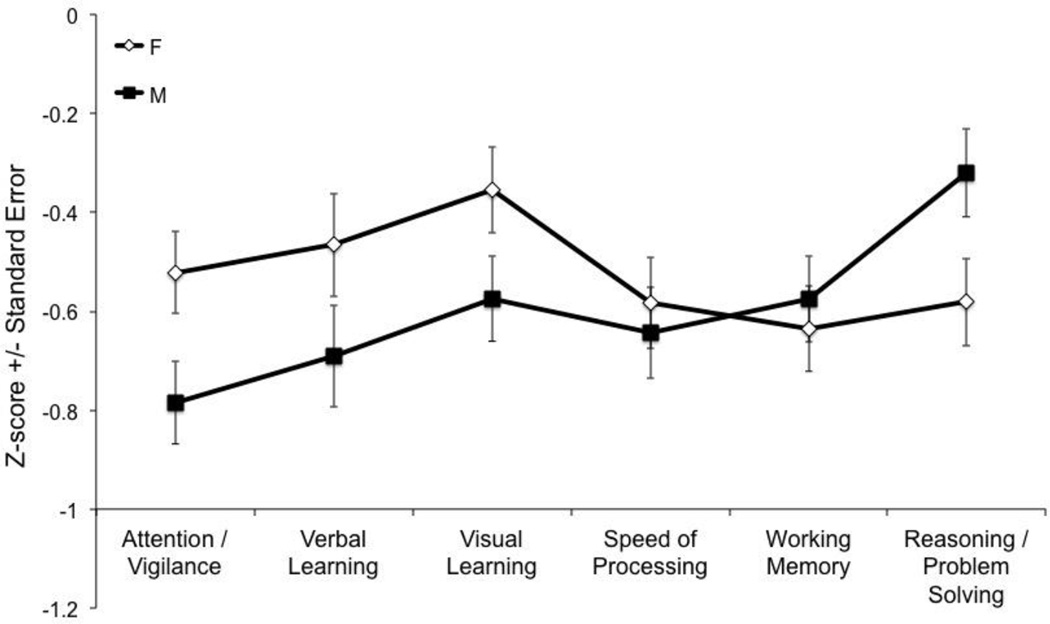
Comparisons of sex (female vs. male) contrasts between the six cognitive domains (FDR-corrected) showed that women were significantly better than men on the Attention/Vigilance (t341=3.22, p=0.0014), Verbal Learning (t339=3.06, p<0.0024), and Visual Learning (t339=2.94, p=0.0035) domains relative to Reasoning/Problem Solving domain on which men excelled compared to women (t338=2.11, p=0.04; see Figure 2).
Figure 2.
CMINDS Sex Differences Cognitive Domain Profile
3.3 Ranking of cognitive domain deficits based on their effect sizes
Based on Cohen’s d effect sizes, the rank ordering of cognitive domain deficits for patients with schizophrenia compared to healthy controls was Speed of Processing (d=−1.14), Attention / Vigilance (d=−1.04), Working Memory (d=−1.03), Verbal Learning (d=−1.02), Visual Learning (d=−0.91), and Reasoning / Problem Solving (d=−0.67). Patients were significantly more impaired than controls on the Attention/Vigilance, Speed of Processing, Working Memory, and Verbal Learning domains relative to the Reasoning/Problem Solving domain, and the Attention/Vigilance domain relative to the Visual Learning domain (see section 3.2 and Figure 1).”
3.4 Smoking and medication
There were no significant effects of smoking status, current or lifetime pack-year on the CMINDS composite score or on any of the individual cognitive domain scores. Among the cognitive domain scores (including the CMINDS composite score), only Reasoning/Problem Solving was significantly negatively associated with chlorpromazine dose equivalents (t143=−0.21, p=0.01, uncorrected).
3.5 Clinical correlates
CMINDS composite scores showed significant negative correlations with PANSS Negative (r=−0.23, p<0.003), PANSS Positive (r=−0.23, p<0.003), PANSS Disorganized (item P2; r=−0.25, p<0.001), and PANSS General (r=−0.23, p<0.003) symptom severity ratings as well as duration of illness (r=−0.30, p<0.0001) but not age at onset (r=−0.15, p>0.05). The symptom correlations remained significant even after partial correlations, controlling for duration of illness, age, or age at onset, were computed. The correlation between the CMINDS composite score and duration of illness was not significant after co-varying for the effect of age (partial r=0.06, p=0.42).
4. Discussion
This is the first study to report the CMINDS schizophrenia cognitive domain profile. The ranking of CMINDS cognitive domain deficits in patients with schizophrenia based on effect sizes was Speed of Processing (d=−1.14), Attention/Vigilance (d=−1.04), Working Memory (d=−1.03), Verbal Learning (d=−1.02), Visual Learning (d=−0.91), and Reasoning/Problem Solving (d=−0.67). The cognitive domain profile based on z-scores was largely similar with the exception that the z-score for Attention/Vigilance was non-significantly lower than that of Speed of Processing. Decomposition of the significant group by domain interaction showed that patients were significantly more impaired than controls on the Attention/Vigilance, Speed of Processing, Working Memory, and Verbal Learning domains relative to the Reasoning/Problem Solving domain, and the Attention/Vigilance domain relative to the Visual Learning domain. Decomposition of the significant group by sex interaction, showed that women were significantly better than men on the Attention/Vigilance, Verbal Learning, and Visual Learning, domains relative to Reasoning/Problem Solving domain, and vice versa. Patient’s cognitive deficits were not significantly associated with smoking status, or current/lifetime pack years. Among the cognitive domains, only reasoning/problem solving showed a significant negative association with medication dose in chlorpromazine equivalents. More severe cognitive impairment was associated with higher symptom severity.
The finding of the largest deficit in speed of processing and the smallest in reasoning/problem solving in patients with schizophrenia, compared to healthy volunteers, is consistent with findings from meta-analyses (Dickinson et al., 2007; Schaefer et al., 2013) as well as many, though not all (Nam et al., 2009; Shamsi et al., 2011), studies that used the MCCB (Freedman et al., 2008; Javitt et al., 2012; Keefe et al., 2011; Kern et al., 2011; Silverstein et al., 2010). Domain profiles are traditionally based on z- or T-scores, thought it could be argued that domain profiles based on effect sizes rather than z-scores are more appropriate give that effect sizes also take into account variance in the patient sample, while z- and T-score profiles do not. The effect size for speed of processing based on the CMINDS is smaller than that observed in the MCCB battery, which is most likely due to task differences in the Digit Symbol Coding task, which in the CMINDS does not require hand-eye coordination and graphomotor speed (Kern et al., 2009) because subjects provide their answers verbally instead of in writing (see (Bachman et al., 2010) for additional analyses about the contributions of cognitive processes to digit symbol coding task performance). Importantly, despite the considerable efforts in the formation of the MCCB as well as in the creation of the CMINDS, the group by domain interaction must be interpreted with caution given the inherent difficulties in establishing tests of comparable discriminability between groups across domains (Chapman and Chapman, 1973). Some attempts to create tasks of comparable discriminability have been put forward in the cognitive sciences (e.g. (Calev, 1984)) but to our knowledge such endeavors have not been undertaken for neuropsychological tests.
We did not find significant group by sex interaction effects on cognitive domain scores and the presence of differential sex effects on cognition in schizophrenia remains controversial (Krysta et al., 2013). We did find that women performed better than men on Attention/Vigilance, Verbal Learning, and Visual Learning compared to Reasoning/Problem solving on which men performed better than women. Better performance among women than men on verbal learning and worse performance on reasoning problem solving are consistent with findings from the MCCB battery (Kern et al., 2008; Mohn et al., 2012; Rodriguez-Jimenez et al., 2011), though findings for attention/vigilance are mixed, with two studies showing better performance in men than women (Mohn et al., 2012; Rodriguez-Jimenez et al., 2011) and one showing no difference between the sexes (Kern et al., 2008). Small sex differences in performance on some neuropsychological tasks, including male advantage for some spatial tasks (e.g., block design) and female advantage for short term memory, verbal fluency, and verbal memory (Gur and Gur, 2002) are well-documented, though it has long been recognized that a simple spatial versus verbal domain accounting of sex differences is insufficient (Kolb and Whishaw, 1990). The neural underpinnings of sex differences in cognitive performance are being investigated using state-of-the art neuroimaging tools (Satterthwaite et al., 2014). A recent resting state functional magnetic resonance imaging study found that men showed stronger between-module connectivity while women showed stronger within-module connectivity and that subjects’ pattern of connectivity, i.e., the relative strength of between- versus within-module connectivity, was associated with a more masculine or feminine cognition profile, even when examined within the sexes (Satterthwaite et al., 2014).
With regard to possible confounding effects of smoking, consistent with some (Van Haren et al., 2013) but not all structural imaging studies (Schneider et al., 2014), we found no confounding effect of smoking status, current pack-years, of lifetime pack-years on neurocognitive domain deficits. With regard to confounding effects of antipsychotic medications, we only found that medication dose (in chlorpromazine equivalents) predicted worse reasoning/problem solving among patients. This effect did not survive a multiple comparison corrected threshold and must therefore be treated with caution until replicated by other studies. We found significant correlations between the CMINDS composite score and negative, positive, and general symptom severity. These findings suggest that general cognitive impairment as measured by the CMINDS composite score is associated with severity of illness. The cognitive domains contributing to these significant correlations require detailed further analyses that are outside the scope of the current manuscript. While we observed a significant correlation between the CMINDS composite score and duration of illness, this correlation did not remain significant after co-varying for age. Given that we also did not observe a significant group by age interaction effect on cognitive performance, we cannot conclude that cognitive performance among patients decreases with duration of illness or is differentially influenced by age in patients with schizophrenia compared with controls.
Silverstein and colleagues (2010) have reported an increased interest in computerized neuropsychological batteries for use in clinical trails in their report on the comparison between the IntegNeuro computerized neuropsychological test battery and the MCCB (Silverstein et al., 2010). The main goal of the MATRICS initiative was to achieve a standardized battery to test pro-cognitive agents and clearly the further development of computerized neurocognitive battery efforts should not get in the way of this important objective. Even so, given the higher efficiency of data collection, e.g., no need for manual scoring and dual data entry, the field as a whole may want to consider the use of computerized batteries. With the ever increasing sample sizes of studies aimed at making discoveries on disease etiology (Ripke et al., 2011; Thompson et al., 2014) and treatment (Keefe et al., 2011; Rajji et al., 2013), we believe that computerized batteries will have and important role to play in future cognitive assessments in neuropsychiatric disorders.
Strengths of the study are the large sample of 175 patients with schizophrenia and 169 demographically similar controls, and the use of a mature computerized test battery, the CMINDS, with tasks similar to those in the MCCB. Several weaknesses must also be noted. First, the study only included the CMINDS such that a direct comparison to the MCCB profile was not possible. A second weakness is that the CMINDS does not include tests directly comparable to the one used to measure the domain of social cognition in the MCCB. A third weakness is that the computerized digit symbol coding task differs from the paper and pencil task in that the former does not require hand-eye coordination/graphomotor integration –though, this alternative version could have advantages when assessing patients with reduced motor abilities, such as traumatic brain injury or stroke patients and may also allow for a better assessment of mental speed of processing abilities between conditions where one is confounded by motor slowing and the other is not. Finally, our study did not assess University of California Performance-Based Skills Assessment-2 (UPSA-2), which links cognitive performance to everyday functioning in schizophrenia and has been associated with the MCCB composite score (Keefe et al., 2011; Shi et al., 2013).
Strengths of the CMINDS include its ease of administration and electronic data capture and scoring, which make it very amenable to large-scale studies. In addition, the CMINDS tests are highly reliable (O'Halloran et al., 2008; O'Halloran et al., 2011) and its test scores are highly correlated with the comparable paper and pencil MCCB (O'Halloran et al., 2008) as well as Alzheimer's Disease Assessment Scale: Cognitive Subscale (ADASCog) test scores (O’Halloran et al. 2011). The CMINDS also includes parallel task versions with different stimuli to counter practice effects based on repeat administration, though to our knowledge no reports using these parallel version have been published. Therefore, the susceptibility of CMINDS test to training effects remains to be fully determined. Finally, the CMINDS is compliant with computerized clinical data collection standards, including 21 CFR Part 11, HIPAA, etc. (O'Halloran et al., 2008). A drawback is that, currently, the CMINDS does not yet provide English and Spanish norms, which may make it a less attractive choice for studies that do not include a comparison group. Advantages of the MCCB are (1) the availability of Spanish and English norms in the United States (US); moreover, norms are available or at various stages of development in numerous other countries including Spain, China, Russia, India, Central and South America, and Norway (Jedrasik-Styla et al., 2012; Kern et al., 2008; Mohn et al., 2012; Rapisarda et al., 2013; Rodriguez-Jimenez et al., 2011), and (2) its FDA-approval for use in testing pro-cognitive treatments. These features, currently, may make the MCCB the most attractive choice for clinical trails that do not include a control group.
In sum, we have reported on the CMINDS neuropsychological profile, which includes 6 of the 7 cognitive domains included in the MCCB. We conclude that the CMINDS schizophrenia cognitive domain profile show the largest impairment in speed of processing, followed by attention/vigilance, working memory, verbal learning, visual learning, and reasoning/problem solving and that the battery can readily be employed in the assessment of cognitive deficits in neuropsychiatric disorders such as schizophrenia and may be particularly useful for large-scale case-control studies in which efficient electronic data capture is most beneficial.
Supplementary Material
This study presents the first CMINDS® battery schizophrenia cognitive domain profile
The schizophrenia profile shows the strongest effect size for speed of processing
Women perform better than men on attention, speed of processing, and verbal learning
Men perform better than women on reasoning
Computerized neuropsychological batteries can be used in neuropsychiatric studies
Acknowledgments
We are thankful to Mrs. Liv McMillan, BS for overall study coordination, Ms. Shichun Ling, BA for assistance with manuscript submission, Ms. Andrea Weideman for assistance with identifying similarities and differences between the CMINDS and MCCB batteries, and to the research subjects for their participation.
Funding
This work was supported by the National Center for Research Resources at the National Institutes of Health (grant numbers: NIH 1 U24 RR021992 (Function Biomedical Informatics Research Network) and NIH 1 U24 RR025736-01 (Biomedical Informatics Research Network Coordinating Center; http://www.birncommunity.org). The funding sources had no role in the study design, conduct of the study, data collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dr. Van Erp had full access to all of the data in the study, conducted the statistical analysis, and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors critically reviewed the manuscript, provided comments, and approved the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Dr. Van Erp consulted for Roche Pharmaceuticals and has a contract with Otsuka Pharmaceutical Co., Ltd. (OPCJ). Dr. Bustillo consulted with Novartis and Otsuka Pharmaceuticals. Dr. Mathalon is a consultant for Bristol-Myers Squibb and consulted for Roche Pharmaceuticals. Dr. Preda consulted for Boehringer-Ingelheim. Dr. Potkin has financial interests in Bristol-Myers Squibb, Eisai, Inc., Eli Lilly, Forest Laboratories, Genentech, Janssen Pharmaceutical, Lundbeck, Merck, Novartis, Organon, Pfizer, Roche, Sunovion, Takeda Pharmaceutical, Vanda Pharmaceutical, Novartis, Lundbeck, Merck, Sunovion and has received grant funding from Amgen, Baxter, Bristol-Myers Squibb, Cephalon, Inc., Eli Lilly, Forest Laboratories, Genentech, Janssen Pharmaceutical, Merck, Otsuka, Pfizer, Roche, Sunovion, Takeda Pharmaceutical, Vanda Pharmaceutical, NIAAA, NIBIB, NIH/NCRR, University of Southern California, UCSF, UCSD, Baylor College of Medicine. The remaining authors declare no potential conflict of interest. None of the authors of this manuscript are affiliated with or receive compensation from NeuroComp Systems, Inc., MATRICS Assessment, Inc., or Neurcog Trials, Inc.
Author contributions
Dr. Van Erp conceived and designed the study. Dr. Van Erp analyzed and interpreted the data and wrote the first draft of the manuscript. All co-authors critically reviewed and revised the manuscript and provided final approval of the version to be published.
References
- Agnew-Blais J, Seidman LJ. Neurocognition in youth and young adults under age 30 at familial risk for schizophrenia: a quantitative and qualitative review. Cogn Neuropsychiatry. 2013;18(1-2):44–82. doi: 10.1080/13546805.2012.676309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N. The scale for the assessment of negative symptoms (SANS) University of Iowa, Iowa City; 1983. [Google Scholar]
- Andreasen N. The scale for the assessment of positive symp-toms (SAPS) University of Iowa, Iowa City; 1984. [Google Scholar]
- August SM, Kiwanuka JN, McMahon RP, Gold JM. The MATRICS Consensus Cognitive Battery (MCCB): clinical and cognitive correlates. Schizophr Res. 2011;134(1):76–82. doi: 10.1016/j.schres.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman P, Reichenberg A, Rice P, Woolsey M, Chaves O, Martinez D, Maples N, Velligan DI, Glahn DC. Deconstructing processing speed deficits in schizophrenia: application of a parametric digit symbol coding test. Schizophr Res. 2010;118(1-3):6–11. doi: 10.1016/j.schres.2010.02.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling for False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. J.R.statist. Soc. 1995;57(1):289–300. [Google Scholar]
- Calev A. Recall and recognition in chronic nondemented schizophrenics: use of matched tasks. J Abnorm Psychol. 1984;93(2):172–177. doi: 10.1037//0021-843x.93.2.172. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Problems in the measurement of cognitive deficit. Psychol Bull. 1973;79(6):380–385. doi: 10.1037/h0034541. [DOI] [PubMed] [Google Scholar]
- Cherkil S, Satish S, Mathew SS, Dinesh N, Kumar CT, Lombardo LE, Glahn DC, Frangou S. Cross-cultural standardization of the South Texas Assessment of Neurocognition in India. Indian J Med Res. 2012;136(2):280–288. [PMC free article] [PubMed] [Google Scholar]
- De Herdt A, Wampers M, Vancampfort D, De Hert M, Vanhees L, Demunter H, Van Bouwel L, Brunner E, Probst M. Neurocognition in clinical high risk young adults who did or did not convert to a first schizophrenic psychosis: a meta-analysis. Schizophr Res. 2013;149(1-3):48–55. doi: 10.1016/j.schres.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64(5):532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon MG, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders - Non-Patient Edition (SCID-I/NP, 11/2002 revision) New York: 2002a. [Google Scholar]
- First MB, Spitzer RL, Gibbon MG, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders - Patient Edition (SCID-I/P, 11/2002 revision) New York: 2002b. [Google Scholar]
- Fray PJ, Robbins TW, Sahakian BJ. Neuropsychiatric applications of CANTAB. Int. J. of Ger.Psych. 1996;11(4):329–336. [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, Allensworth D, Guzman-Bonilla A, Clement B, Ball MP, Kutnick J, Pender V, Martin LF, Stevens KE, Wagner BD, Zerbe GO, Soti F, Kem WR. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165(8):1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano AJ, Li H, Mesholam-Gately RI, Sorenson SM, Woodberry KA, Seidman LJ. Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Curr Pharm Des. 2012;18(4):399–415. doi: 10.2174/138161212799316019. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Almasy L, Barguil M, Hare E, Peralta JM, Kent JW, Jr, Dassori A, Contreras J, Pacheco A, Lanzagorta N, Nicolini H, Raventos H, Escamilla MA. Neurocognitive endophenotypes for bipolar disorder identified in multiplex multigenerational families. Arch Gen Psychiatry. 2010;67(2):168–177. doi: 10.1001/archgenpsychiatry.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the "right stuff"? Schizophrenia Bulletin. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004a;72(1):41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, Fenton WS, Frese F, Goldberg TE, Heaton RK, Keefe RS, Kern RS, Kraemer H, Stover E, Weinberger DR, Zalcman S, Marder SR. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004b;56(5):301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: II. The profile of schizophrenia. Neuropsychopharmacology. 2001a;25(5):777–788. doi: 10.1016/S0893-133X(01)00279-2. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001b;25(5):766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qiu H, Mentch FD, Abou-Sleiman PM, Hakonarson H, Gur RE. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26(2):251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;187(2):254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Gur RC. Gender differences in aging: cognition, emotions, and neuroimaging studies. Dialogues Clin Neurosci. 2002;4(2):197–210. doi: 10.31887/DCNS.2002.4.2/rgur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagger-Johnson G, Sabia S, Brunner EJ, Shipley M, Bobak M, Marmot M, Kivimaki M, Singh-Manoux A. Combined impact of smoking and heavy alcohol use on cognitive decline in early old age: Whitehall II prospective cohort study. Br J Psychiatry. 2013;203(2):120–125. doi: 10.1192/bjp.bp.112.122960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hollingstead AB. Four factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Holmen A, Juuhl-Langseth M, Thormodsen R, Melle I, Rund BR. Neuropsychological profile in early-onset schizophrenia-spectrum disorders: measured with the MATRICS battery. Schizophr Bull. 2009;36(4):852–859. doi: 10.1093/schbul/sbn174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Buchanan RW, Keefe RS, Kern R, McMahon RP, Green MF, Lieberman J, Goff DC, Csernansky JG, McEvoy JP, Jarskog F, Seidman LJ, Gold JM, Kimhy D, Nolan KS, Barch DS, Ball MP, Robinson J, Marder SR. Effect of the neuroprotective peptide davunetide (AL-108) on cognition and functional capacity in schizophrenia. Schizophr Res. 2012;136(1-3):25–31. doi: 10.1016/j.schres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Jedrasik-Styla M, Ciolkiewicz A, Denisiuk M, Linke M, Parnowska D, Gruszka A, Jarema M, Wichniak A. MATRICS consensus cognitive battery--standard for the assessment of cognitive functions in clinical trials in schizophrenia. Psychiatr Pol. 2012;46(2):261–271. [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry Suppl. 1989;(7):59–67. [PubMed] [Google Scholar]
- Keefe RS, Fox KH, Harvey PD, Cucchiaro J, Siu C, Loebel A. Characteristics of the MATRICS Consensus Cognitive Battery in a 29-site antipsychotic schizophrenia clinical trial. Schizophr Res. 2011;125(2-3):161–168. doi: 10.1016/j.schres.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Kelleher I, Clarke MC, Rawdon C, Murphy J, Cannon M. Neurocognition in the extended psychosis phenotype: performance of a community sample of adolescents with 26 psychotic symptoms on the MATRICS neurocognitive battery. Schizophr Bull. 2012;39(5):1018–1026. doi: 10.1093/schbul/sbs086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern RS, Gold JM, Dickinson D, Green MF, Nuechterlein KH, Baade LE, Keefe RS, Mesholam-Gately RI, Seidman LJ, Lee C, Sugar CA, Marder SR. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr Res. 2011;126(1-3):124–131. doi: 10.1016/j.schres.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern RS, Green MF, Nuechterlein KH, Keefe RS. CMINDS does not have identical tests to the CATIE and MATRICS batteries. Commentary on O'Halloran et al. Schizophr Res. 2009;107(2-3):327–329. doi: 10.1016/j.schres.2008.10.015. author reply 330-321. [DOI] [PubMed] [Google Scholar]
- Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, Keefe RS, Mesholam-Gately R, Mintz J, Seidman LJ, Stover E, Marder SR. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165(2):214–220. doi: 10.1176/appi.ajp.2007.07010043. [DOI] [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ. Fundamentals of Human Neuropsychology. 3rd ed. Nwq York: W. H. Freeman and Company; 1990. [Google Scholar]
- Krysta K, Murawiec S, Klasik A, Wiglusz MS, Krupka-Matuszczyk I. Sex-specific differences in cognitive functioning among schizophrenic patients. Psychiatr Danub. 2013;25(Suppl 2):S244–S246. [PubMed] [Google Scholar]
- Levaux MN, Potvin S, Sepehry AA, Sablier J, Mendrek A, Stip E. Computerized assessment of cognition in schizophrenia: promises and pitfalls of CANTAB. Eur Psychiatry. 2007;22(2):104–115. doi: 10.1016/j.eurpsy.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. 3 ed. New York: Oxford University Press, Inc; 1995. [Google Scholar]
- Lim YY, Jaeger J, Harrington K, Ashwood T, Ellis KA, Stoffler A, Szoeke C, Lachovitzki R, Martins RN, Villemagne VL, Bush A, Masters CL, Rowe CC, Ames D, Darby D, Maruff P. Three-month stability of the CogState brief battery in healthy older adults, mild cognitive impairment, and Alzheimer's disease: results from the Australian Imaging, Biomarkers, and Lifestyle-rate of change substudy (AIBL-ROCS) Arch Clin Neuropsychol. 2013;28(4):320–330. doi: 10.1093/arclin/act021. [DOI] [PubMed] [Google Scholar]
- Marx CE, Keefe RS, Buchanan RW, Hamer RM, Kilts JD, Bradford DW, Strauss JL, Naylor JC, Payne VM, Lieberman JA, Savitz AJ, Leimone LA, Dunn L, Porcu P, Morrow AL, Shampine LJ. Proof-of-concept trial with the neurosteroid pregnenolone targeting cognitive and negative symptoms in schizophrenia. Neuropsychopharmacology. 2009;34(8):1885–1903. doi: 10.1038/npp.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Mohn C, Sundet K, Rund BR. The Norwegian standardization of the MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) Consensus Cognitive Battery. J Clin Exp Neuropsychol. 2012;34(6):667–677. doi: 10.1080/13803395.2012.667792. [DOI] [PubMed] [Google Scholar]
- Nam HJ, Kim N, Park T, Oh S, Jeon HO, Yoon SC, Lee YS, Lee WK, Ha K, Kim JH, Hong KS. Cognitive profiles of healthy siblings of schizophrenia patients: application of the cognitive domains of the MATRICS consensus battery. World J Biol Psychiatry. 2009;10(4 Pt 2):452–460. doi: 10.1080/15622970802314815. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- O'Halloran JP, Kemp AS, Gooch KN, Harvey PD, Palmer BW, Reist C, Schneider LS. Psychometric comparison of computerized and standard administration of the neurocognitive assessment instruments selected by the CATIE and MATRICS consortia among patients with schizophrenia. Schizophr Res. 2008;106(1):33–41. doi: 10.1016/j.schres.2007.11.015. [DOI] [PubMed] [Google Scholar]
- O'Halloran JP, Kemp AS, Salmon DP, Tariot PN, Schneider LS. Psychometric comparison of standard and computerized administration of the Alzheimer's Disease Assessment Scale: Cognitive Subscale (ADASCog) Curr Alzheimer Res. 2011;8(3):323–328. doi: 10.2174/156720511795563692. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Olver J, Norman T, Piskulic D, Maruff P, Snyder PJ. A comparison of the CogState Schizophrenia Battery and the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Battery in assessing cognitive impairment in chronic schizophrenia. J Clin Exp Neuropsychol. 2009;31(7):848–859. doi: 10.1080/13803390802592458. [DOI] [PubMed] [Google Scholar]
- Rajji TK, Voineskos AN, Butters MA, Miranda D, Arenovich T, Menon M, Ismail Z, Kern RS, Mulsant BH. Cognitive performance of individuals with schizophrenia across seven decades: a study using the MATRICS consensus cognitive battery. Am J Geriatr Psychiatry. 2013;21(2):108–118. doi: 10.1016/j.jagp.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapisarda A, Lim TF, Lim M, Collinson SL, Kraus MS, Keefe RS. Applicability of the MATRICS Consensus Cognitive Battery in Singapore. Clin Neuropsychol. 2013;27(3):455–469. doi: 10.1080/13854046.2012.762120. [DOI] [PubMed] [Google Scholar]
- Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA, Lin DY, Duan J, Ophoff RA, Andreassen OA, Scolnick E, Cichon S, St Clair D, Corvin A, Gurling H, Werge T, Rujescu D, Blackwood DH, Pato CN, Malhotra AK, Purcell S, Dudbridge F, Neale BM, Rossin L, Visscher PM, Posthuma D, Ruderfer DM, Fanous A, Stefansson H, Steinberg S, Mowry BJ, Golimbet V, De Hert M, Jonsson EG, Bitter I, Pietilainen OP, Collier DA, Tosato S, Agartz I, Albus M, Alexander M, Amdur RL, Amin F, Bass N, Bergen SE, Black DW, Borglum AD, Brown MA, Bruggeman R, Buccola NG, Byerley WF, Cahn W, Cantor RM, Carr VJ, Catts SV, Choudhury K, Cloninger CR, Cormican P, Craddock N, Danoy PA, Datta S, de Haan L, Demontis D, Dikeos D, Djurovic S, Donnelly P, Donohoe G, Duong L, Dwyer S, Fink-Jensen A, Freedman R, Freimer NB, Friedl M, Georgieva L, Giegling I, Gill M, Glenthoj B, Godard S, Hamshere M, Hansen M, Hansen T, Hartmann AM, Henskens FA, Hougaard DM, Hultman CM, Ingason A, Jablensky AV, Jakobsen KD, Jay M, Jurgens G, Kahn RS, Keller MC, Kenis G, Kenny E, Kim Y, Kirov GK, Konnerth H, Konte B, Krabbendam L, Krasucki R, Lasseter VK, Laurent C, Lawrence J, Lencz T, Lerer FB, Liang KY, Lichtenstein P, Lieberman JA, Linszen DH, Lonnqvist J, Loughland CM, Maclean AW, Maher BS, Maier W, Mallet J, Malloy P, Mattheisen M, Mattingsdal M, McGhee KA, McGrath JJ, McIntosh A, McLean DE, McQuillin A, Melle I, Michie PT, Milanova V, Morris DW, Mors O, Mortensen PB, Moskvina V, Muglia P, Myin-Germeys I, Nertney DA, Nestadt G, Nielsen J, Nikolov I, Nordentoft M, Norton N, Nothen MM, O'Dushlaine CT, Olincy A, Olsen L, O'Neill FA, Orntoft TF, Owen MJ, Pantelis C, Papadimitriou G, Pato MT, Peltonen L, Petursson H, Pickard B, Pimm J, Pulver AE, Puri V, Quested D, Quinn EM, Rasmussen HB, Rethelyi JM, Ribble R, Rietschel M, Riley BP, Ruggeri M, Schall U, Schulze TG, Schwab SG, Scott RJ, Shi J, Sigurdsson E, Silverman JM, Spencer CC, Stefansson K, Strange A, Strengman E, Stroup TS, Suvisaari J, Terenius L, Thirumalai S, Thygesen JH, Timm S, Toncheva D, van den Oord E, van Os J, van Winkel R, Veldink J, Walsh D, Wang AG, Wiersma D, Wildenauer DB, Williams HJ, Williams NM, Wormley B, Zammit S, Sullivan PF, O'Donovan MC, Daly MJ, Gejman PV. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43(10):969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Jimenez R, Bagney A, Garcia-Navarro C, Aparicio AI, Lopez-Anton R, Moreno-Ortega M, Jimenez-Arriero MA, Santos JL, Lobo A, Kern RS, Green MF, Nuechterlein KH, Palomo T. The MATRICS consensus cognitive battery (MCCB): co-norming and standardization in Spain. Schizophr Res. 2011;134(2-3):279–284. doi: 10.1016/j.schres.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Roalf DR, Ruparel K, Erus G, Vandekar S, Gennatas ED, Elliott MA, Smith A, Hakonarson H, Verma R, Davatzikos C, Gur RE, Gur RC. Linked Sex Differences in Cognition and Functional Connectivity in Youth. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD. Neuropsychological function in schizophrenia: Selective impairment in memory and learning. Archives of General Psychiatry. 1991;48(7):618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Archives of General Psychiatry. 1994;51(2):124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Schaefer J, Giangrande E, Weinberger DR, Dickinson D. The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr Res. 2013;150(1):42–50. doi: 10.1016/j.schres.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CE, White T, Hass J, Geisler D, Wallace SR, Roessner V, Holt DJ, Calhoun VD, Gollub RL, Ehrlich S. Smoking status as a potential confounder in the study of brain structure in schizophrenia. J Psychiatr Res. 2014;50:84–91. doi: 10.1016/j.jpsychires.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsi S, Lau A, Lencz T, Burdick KE, DeRosse P, Brenner R, Lindenmayer JP, Malhotra AK. Cognitive and symptomatic predictors of functional disability in schizophrenia. Schizophr Res. 2011;126(1-3):257–264. doi: 10.1016/j.schres.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, He Y, Cheung EF, Yu X, Chan RC. An ecologically valid performance-based social functioning assessment battery for schizophrenia. Psychiatry Res. 2013;210(3):787–793. doi: 10.1016/j.psychres.2013.09.023. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Jaeger J, Donovan-Lepore AM, Wilkniss SM, Savitz A, Malinovsky I, Hawthorne D, Raines S, Carson S, Marcello S, Zukin SR, Furlong S, Dent G. A comparative study of the MATRICS and IntegNeuro cognitive assessment batteries. J Clin Exp Neuropsychol. 2010;32(9):937–952. doi: 10.1080/13803391003596496. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Stein JL, Medland SE, Hibar DP, Vasquez AA, Renteria ME, Toro R, Jahanshad N, Schumann G, Franke B, Wright MJ, Martin NG, Agartz I, Alda M, Alhusaini S, Almasy L, Almeida J, Alpert K, Andreasen NC, Andreassen OA, Apostolova LG, Appel K, Armstrong NJ, Aribisala B, Bastin ME, Bauer M, Bearden CE, Bergmann O, Binder EB, Blangero J, Bockholt HJ, Boen E, Bois C, Boomsma DI, Booth T, Bowman IJ, Bralten J, Brouwer RM, Brunner HG, Brohawn DG, Buckner RL, Buitelaar J, Bulayeva K, Bustillo JR, Calhoun VD, Cannon DM, Cantor RM, Carless MA, Caseras X, Cavalleri GL, Chakravarty MM, Chang KD, Ching CR, Christoforou A, Cichon S, Clark VP, Conrod P, Coppola G, Crespo-Facorro B, Curran JE, Czisch M, Deary IJ, de Geus EJ, den Braber A, Delvecchio G, Depondt C, de Haan L, de Zubicaray GI, Dima D, Dimitrova R, Djurovic S, Dong H, Donohoe G, Duggirala R, Dyer TD, Ehrlich S, Ekman CJ, Elvsashagen T, Emsell L, Erk S, Espeseth T, Fagerness J, Fears S, Fedko I, Fernandez G, Fisher SE, Foroud T, Fox PT, Francks C, Frangou S, Frey EM, Frodl T, Frouin V, Garavan H, Giddaluru S, Glahn DC, Godlewska B, Goldstein RZ, Gollub RL, Grabe HJ, Grimm O, Gruber O, Guadalupe T, Gur RE, Gur RC, Goring HH, Hagenaars S, Hajek T, Hall GB, Hall J, Hardy J, Hartman CA, Hass J, Hatton SN, Haukvik UK, Hegenscheid K, Heinz A, Hickie IB, Ho BC, Hoehn D, Hoekstra PJ, Hollinshead M, Holmes AJ, Homuth G, Hoogman M, Hong LE, Hosten N, Hottenga JJ, Hulshoff Pol HE, Hwang KS, Jack CR, Jr, Jenkinson M, Johnston C, Jonsson EG, Kahn RS, Kasperaviciute D, Kelly S, Kim S, Kochunov P, Koenders L, Kramer B, Kwok JB, Lagopoulos J, Laje G, Landen M, Landman BA, Lauriello J, Lawrie SM, Lee PH, Le Hellard S, Lemaitre H, Leonardo CD, Li CS, Liberg B, Liewald DC, Liu X, Lopez LM, Loth E, Lourdusamy A, Luciano M, Macciardi F, Machielsen MW, Macqueen GM, Malt UF, Mandl R, Manoach DS, Martinot JL, Matarin M, Mather KA, Mattheisen M, Mattingsdal M, Meyer-Lindenberg A, McDonald C, McIntosh AM, McMahon FJ, McMahon KL, Meisenzahl E, Melle I, Milaneschi Y, Mohnke S, Montgomery GW, Morris DW, Moses EK, Mueller BA, Munoz Maniega S, Muhleisen TW, Muller-Myhsok B, Mwangi B, Nauck M, Nho K, Nichols TE, Nilsson LG, Nugent AC, Nyberg L, Olvera RL, Oosterlaan J, Ophoff RA, Pandolfo M, Papalampropoulou-Tsiridou M, Papmeyer M, Paus T, Pausova Z, Pearlson GD, Penninx BW, Peterson CP, Pfennig A, Phillips M, Pike GB, Poline JB, Potkin SG, Putz B, Ramasamy A, Rasmussen J, Rietschel M, Rijpkema M, Risacher SL, Roffman JL, Roiz-Santianez R, Romanczuk-Seiferth N, Rose EJ, Royle NA, Rujescu D, Ryten M, Sachdev PS, Salami A, Satterthwaite TD, Savitz J, Saykin AJ, Scanlon C, Schmaal L, Schnack HG, Schork AJ, Schulz SC, Schur R, Seidman L, Shen L, Shoemaker JM, Simmons A, Sisodiya SM, Smith C, Smoller JW, Soares JC, Sponheim SR, Sprooten E, Starr JM, Steen VM, Strakowski S, Strike L, Sussmann J, Samann PG, Teumer A, Toga AW, Tordesillas-Gutierrez D, Trabzuni D, Trost S, Turner J, Van den Heuvel M, van der Wee NJ, van Eijk K, van Erp TG, van Haren NE, van 't Ent D, van Tol MJ, Valdes Hernandez MC, Veltman DJ, Versace A, Volzke H, Walker R, Walter H, Wang L, Wardlaw JM, Weale ME, Weiner MW, Wen W, Westlye LT, Whalley HC, Whelan CD, White T, Winkler AM, Wittfeld K, Woldehawariat G, Wolf C, Zilles D, Zwiers MP, Thalamuthu A, Schofield PR, Freimer NB, Lawrence NS, Drevets W. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014 doi: 10.1007/s11682-013-9269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttl B. North American Adult Reading Test: age norms, reliability, and validity. J Clin Exp Neuropsychol. 2002;24(8):1123–1137. doi: 10.1076/jcen.24.8.1123.8375. [DOI] [PubMed] [Google Scholar]
- Van Haren NE, Cahn W, Hulshoff Pol HE, Kahn RS. Confounders of excessive brain volume loss in schizophrenia. Neurosci Biobehav Rev. 2013;37(10 Pt 1):2418–2423. doi: 10.1016/j.neubiorev.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Wesnes KA, McKeith IG, Ferrara R, Emre M, Del Ser T, Spano PF, Cicin-Sain A, Anand R, Spiegel R. Effects of rivastigmine on cognitive function in dementia with lewy bodies: a randomised placebo-controlled international study using the cognitive drug research computerised assessment system. Dement Geriatr Cogn Disord. 2002;13(3):183–192. doi: 10.1159/000048651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.