Abstract
Cardiac pressure-volume loop analysis is the “gold-standard” in the assessment of load-dependent and load-independent measures of ventricular systolic and diastolic function. Measures of ventricular contractility and compliance are obtained through examination of cardiac response to changes in afterload and preload. These techniques were originally developed nearly three decades ago to measure cardiac function in large mammals and humans. The application of these analyses to small mammals, such as mice, has been accomplished through the optimization of microsurgical techniques and creation of conductance catheters. Conductance catheters allow for estimation of the blood pool by exploiting the relationship between electrical conductance and volume. When properly performed, these techniques allow for testing of cardiac function in genetic mutant mouse models or in drug treatment studies. The accuracy and precision of these studies are dependent on careful attention to the calibration of instruments, systematic conduct of hemodynamic measurements and data analyses. We will review the methods of conducting pressure-volume loop experiments using a conductance catheter in mice.
Keywords: Medicine, Issue 103, mouse, conductance, pressure-volume, cardiac, systolic, diastolic, hemodynamic, relaxation, contractility
Introduction
Cardiac pressure volume loop analysis provides detailed information of cardiac function and are the gold standard for functional assessment 1. While imaging techniques such as echocardiography or cardiac MRI provide functional measures, these measures are highly dependent on loading conditions. Load-independent measures of cardiac contractility and relaxation require dynamic measurements of the ventricular pressure and volume relation over a range of preload and afterload. This understanding of the pressure-volume relation arises from the groundbreaking work of Sagawa and colleagues 2,3. They demonstrated in ex vivo perfused canine hearts that the pressure-volume loop derived contractility measures were independent of loading conditions 4.
In vivo application of these analyses became possible with the development of conductance catheters in the 1980s. This technical advance allowed Kass and colleagues to perform pressure-volume loop analysis in humans 5,6. Miniaturization of conductance catheters and improvements in surgical techniques in the late 1990’s 7 made analysis of rodent cardiac function feasible, allowing for genetic and pharmacologic studies to be performed. This advance has since lead to the widespread use of pressure-volume loop analysis and has generated a great deal of insight into mammalian cardiac physiology.
A key concept in the use of conductance catheters and the interpretation of data obtained from it is the relationship between volume and conductance. Conductance is inversely related to voltage, which is measured using a catheter with electrodes placed proximally, usually placed below the aortic valve, and distally, at the LV apex 8. Changes in voltage or conductance are measured by changes in current flowing from proximal to distal electrode. Although the blood pool contributes significantly to conductance, the contribution of the ventricular wall, termed parallel conductance (Vp), to measured conductance must be subtracted to obtain absolute LV volume measurements.
The methods to perform this correction, called a saline calibration, are discussed in the protocol below. The mathematical relationship between conductance and volume, described by Baan and colleagues, is that volume=1/α; (ρL2)(G-Gp), where α=uniform field correction factor, ρ=blood resistivity, L=distance between the electrodes, G=conductance and Gp=non-blood conductance 9. Of note, the uniform field correction factor in mice approaches 1.0 due to small chamber volumes10. Coupled with pressure transducers, the conductance catheter provides real time simultaneous pressure and volume data.
Cardiac pressure-volume analysis presents particular advantages over other measures of cardiac function, as they allow for measurement of ventricular function independent of loading conditions and of heart rate. Specific load-independent cardiac indices of contractility include: end-systolic pressure volume relation (ESPVR), dP/dtmax–end-diastolic volume relation, maximal elastance (Emax) and preload recruitable stroke work (PRSW). A load-independent measure of diastolic function is the end-diastolic pressure volume relationship (EDPVR) 11. The following protocol describes the conduct of cardiac pressure volume loop analysis, using both a carotid and an apical approach. While the methodology to perform these studies have been described in detail previously 8,11, we will review key steps to obtain precise pressure-volume measurements, including both saline and cuvette calibration correction, and provide a visual demonstration of these procedures. Research with animals carried out for this study was handled according to approved protocols and animal welfare regulations of Duke University Medical Center’s Institutional Animal Care and Use Committee.
Protocol
1. Conductance Catheter Preparations and Pressure Calibration
Connect conductance catheter to the hemodynamic catheter module. Electronically calibrate pressure and volume measurements by recording preset pressure and volume set on the catheter module. Record a tracing of 0 mm Hg and 25 mm Hg (Figure 1A) and assign voltages to both pressure tracings (Figure 1B and 1C). Similarly, record a volume tracing of 5 RVU and 25 RVU (Figure 1D) and assign voltages to both volume tracings (1E and 1F).
- Confirm the electronic pressure calibration with a manual pressure calibration, using a mercury column sphygmomanometer. Fit the sphygmomanometer cuff port with a 3-way stopcock. Fill a 3-port hemostasis valve system, often used for coronary angioplasty, with RT water using the side port.
- Place the tip of a conductance catheter into the fluid filled hemostasis valve and gently secure without kinking catheter. Connect the hemostasis valve to the sphygmomanometer and inflate to 200 mmHg and lock 3-way stopcock. Examine whether the measured pressure corresponds with the pressure inflated on the sphygmomanometer.
Place catheter in saline heated to 37 °C that is at the level of the operating field and measure pressure. Adjust pressure control until the recorded pressures is at zero.
2. Anesthesia/Intubation
Administer ketamine/xylazine (80-100/10 mg kg-1) as an intraperitoneal injection. Note: Alternative anesthetic agents can be used. An extensive list of anesthetics are provided in previous reviews of this technique 11,12. Proper anesthetization can be confirmed by gentle tail pinch.
Once anesthetized, shave the neck and chest with hair clippers and place on a heated pad. Maintain the mouse rectal temperature at 36.5-37.5 ºC. Low body temperatures will result in depressed heart rates. Apply ointment to the eyes to prevent dryness
Make a midline incision in the neck and dissect the tracheal muscles away to expose the trachea. Place an endotracheal tube through the mouth, while visualizing the trachea to ensure intubation, and connect to the respirator.
Maintain mouse on the ventilator during the procedure. Set ventilator settings based on animal weight as previously described 11. Tidal Volume (ml)= 6.2 x (animal weight in kilograms) 1.01 and Respiratory rate= 53.5 x (animal weight in kilograms)-0.26.
3. Placement of Conductance Catheter in LV chamber
- Carotid approach
- To ensure sterility, two sets of sterile surgical instruments are used- one for initial skin incision and one to operate in the thoracotomy. Instruments should be decontaminated using a dry sterilizer between animals during an individual surgical session and autoclaved at the end of each surgical day.
- After the skin has been decontaminated with three cycles of a Chlorhexidine + alcohol skin scrub (0.5% chlorhexidine/70% alcohol solution), make an incision over the right carotid from mandible to sternum. Dissect surrounding tissue to expose the right carotid, and cut the vagus nerve which runs adjacent to the carotid.
- Place a sterile 6-0 silk suture around the distal end (away from the chest) of the carotid artery, tie and secure. Place two additional sutures beneath the carotid artery proximal (closer to the chest) to the first suture, loosely tying the middle suture. Gently pull the proximal suture and secure by clamping it to the skin. Ensure that the carotid artery has been clamped both proximally and distally before proceeding.
- Make a small incision in the R. carotid artery, proximal to the first suture, and extend longitudinally toward the chest.
- Insert the conductance catheter tip, previously soaked in warm saline for 30 min, into the vessel through the incision and secure the catheter using the middle suture.
- Gently advance the catheter in the left ventricle through the carotid, while watching the pressure-volume loop tracing to ensure correct placement. Note: Optimal placement of the catheter should yield pressure-volume loops that appear rectangular (See Figure 2). If any resistance to the passage of the catheter is encountered, gently pull back and advance again with gentle pressure. Gentle rotation of the catheter may help with placement into the left ventricle. Forcing the conductance catheter can lead to serious cardiovascular complications or damage to the catheter.
- Record baseline pressure-volume loops ~10 min after catheter placement and achieving a steady state (Figure 2).
- Apical Approach
- In an anesthetized and ventilated mouse, make an incision from the xiphoid process and cut through the chest wall laterally until the diaphragm is visible.
- Cut though the diaphragm and visualize the apex of the heart.
- Insert the conductance catheter into the apex of the left ventricle through a needle stab wound (using a 25-30 G needle), until the proximal electrode is just inside the ventricle.
- Record baseline pressure-volume loops ~10 min after catheter placement and achieving a steady state.
4. Varying Afterload Using Transient Aortic Occlusion
To perform transient aortic occlusion, make a small horizontal incision in the upper chest and dissect surrounding tissue to expose the transverse aorta.
Place a 6-0 silk ligature underneath the transverse aorta. After pressure-volume loops have returned to baseline, clasp both ends of the suture with a needle clamp, gently and slowly raise the suture over 1-2 sec, and slowly release tension.
Repeat this procedure, until three separate optimal recordings are made from the same animal. Note: Optimal recordings should have at least 5 pressure volume loop cycles and a steady rise in end systolic pressures during the application of tension on the suture (Figure 3A and 3C).
5. Varying Preload Using Transient Inferior Vena Cava Occlusion (IVC)
To perform transient inferior vena cava occlusion, make a horizontal incision below the xiphoid process, underneath the diaphragm to expose the IVC.
Place a 6-0 silk ligature underneath the IVC. After pressure-volume loops have returned to baseline, clasp both ends of the suture with a needle clamp; gently and slowly raise the suture over 1-2 sec, and slowly release tension. IVC occlusion can also be performed by the gentle application of pressure using a cotton tipped swab.
Repeat this procedure, until three separate optimal recordings are made from the same animal. Note: Optimal recordings should have at least 5 pressure volume loop cycles and a steady drop in left ventricular end-diastolic pressures during the application of tension on the suture (Figure 4A and 4B).
6. Saline Calibration
At the conclusion of the study, a parallel conductance (Vp) value can be obtain by injecting a 10 μl bolus of hypertonic saline (15%) into the animal through the jugular vein (Figure 5A and 5B). Note: This bolus will cause an apparent increase in volume with no change in pressure. This apparent change in volume is the result of a change in blood pool conductance rather than due to an actual increase in volume. A transient decline in dP/dtmax can be observed, as hypertonic saline has a negative inotropic effect 13. The calculated Vp can be entered into pressure volume loop analysis software along with the cuvette calibration parameters to and convert from RVUs in microliters.
7. Cuvette Calibration
To perform a cuvette calibration, place a cuvette with wells of known diameters provided by manufacturer on a heating pad or a water bath warmed to 37 ºC. Fill the first 4–5 holes with fresh warm heparinized blood from mice undergoing hemodynamic assessments. Note: A cuvette calibration allows for accurate assessment of left ventricular blood pool using the mouse blood and allows for conversion of volume data from RVUs to microliters.
Insert the conductance catheter into the first well, until all the electrodes are submerged. Gently move the catheter in the well, which will generate varying RVUs.
Record the conductance changes in the volume channel in RVUs. Select the highest RVU for the calibration. Note: The volume of the wells can either be calculated by 1) using the equation for the volume of a cylinder, where the radius is that of the cuvette well and the length is based on the length between the inner two sensing electrodes or 2) checking in the manufacturer's instructions. The conductance output can be correlated with the known volumes to develop a calibration equation that converts the data from RVUs into microliters 11.
8. Euthanasia
At the conclusion of the protocol, mice are euthanized using cervical dislocation while under anesthesia.
In order to ensure death, mice having undergone cervical dislocation must undergo a secondary method of euthanasia. We use exsanguination under anesthesia, with harvesting of cardiac tissue for experimentation, or bilateral thoracotomy under anesthesia.
9. Data Analysis using Pressure Volume Loop Analysis Software
- Calculate Vp from Saline Calibration
- Select pressure and volume loops obtained during the ejection of hypertonic saline (Figure 5A and 5B)
- Export loops to pressure volume analysis software. Choose option for Saline calibration (Figure 5C)
- Record the calculated Vp value (Figure 5D). Note: The Vp value is calculated by identifying the intersection of the 1) End-diastolic volume vs. End-systolic volume from the saline calibration and 2) End-diastolic volume = End-systolic volume line. The intersection of these lines provides the Vp, which is calculated by the pressure volume loop analysis software.
Enter conductance to volume (RVU) relation in volume channel options (Figure 1F)
- Measure Baseline Pressure-Volume loop relation
- Select 8-10 cardiac cycles from pressure and volume channels once steady state has been achieved (Figure 2A) and export to analysis software. Identify 5-6 end-expiratory loops (Figure 2B)
- Use the Vp value to correct for parallel conductance. Choose the “Steady State” option and generate a hemodynamic summary table (Figure 2C)
- Measure Pressure-Volume loop Relation during Aortic Constriction
- Select 8-10 cardiac cycles from pressure and volume channels that correspond to aortic constriction prior to the increase of end-diastolic pressures (Figure 3A) and export to analysis software. Identify 5-6 end-expiratory loops (Figure 3C)
- Select “Contractility” analysis (Figure 3B), which will calculate the End-Systolic Pressure Volume Relation (ESPVR)
- Measure Pressure-Volume loop Relation during IVC Constriction
- Select 8-10 cardiac cycles from pressure and volume channels that correspond to IVC constriction (Figure 4A) and export to analysis software. Identify 5-6 end-expiratory loops (Figure 4B)
- Select “Contractility” analysis (Figure 3B), which will calculate Preload Recruitable Stroke Work (PRSW) (Figure 4C), Maximum dP/dt vs EDV (Figure 4D), as well as the ESPVR and End-diastolic pressure volume relation (Figure 4E)
Representative Results
Pressure-volume loop analysis can be used to measure cardiac function in genetically modified mice 14,15 or mice undergoing drug studies16. Representative pressure volume loops are provided from previously published work 16 investigating the effect of ß-Arrestin biased AT1R ligand, TRV120023. To test whether TRV120023 affects cardiac function in vivo, pressure-volume loop analysis was performed on wild type mice receiving conventional and novel angiotensin receptor blockers. Intravenous infusion of TRV120023 increased cardiac contractility significantly (Figure 6 and Table 1, Figure modified from Kim et al. AJP 201216). Contractility measures were derived from aortic constriction.
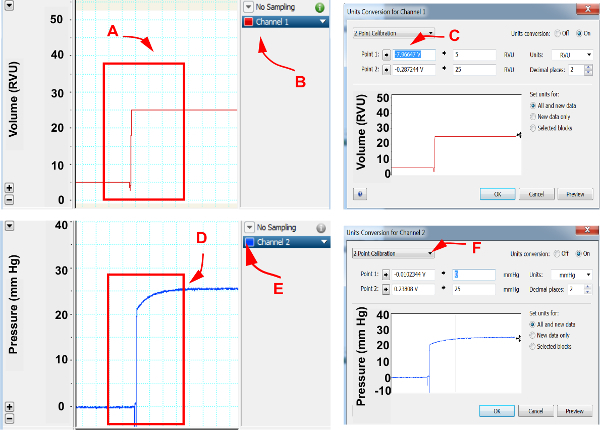 Figure 1. Cardiac pressure and volume calibration.
Please click here to view a larger version of this figure.
Figure 1. Cardiac pressure and volume calibration.
Please click here to view a larger version of this figure.
In pressure volume loop software, assign channels for pressure and volume recordings. (A) Using hemodynamic catheter module, set volume setting to 5 RVU and 25 RVU and select both volumes, (B) Open option under the channel exhibiting volume recordings, (C) Select unit conversion and open “2 point calibration”, select “point 1” and assign as 5 RVU and select “point 2” and assign as 25 RVU , (D) Set pressure to 0 mm Hg and 25 mm Hg, (E) Open options under the channel exhibiting pressure recordings, (F) Select unit conversion and open “2 point calibration”, select “point 1” and assign as 0 mm Hg and select “point 2” and assign as 25 mm Hg. Accept assignments by selecting “OK”.
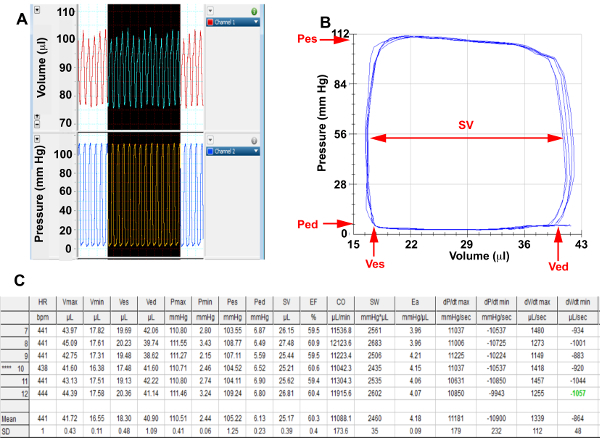 Figure 2. Baseline Hemodynamics
Please click here to view a larger version of this figure.
Figure 2. Baseline Hemodynamics
Please click here to view a larger version of this figure.
(A) Screenshots from pressure and volume channels of representative cardiac cycles in a basal state, (B) Selected end expiratory baseline pressure-volume loops that have been corrected for parallel conductance for analysis. (C) Baseline hemodynamic summary table calculated from selected loops; Pes, End systolic pressure; Ped, End diastolic pressure; Ves, End systolic volume; Ved, End diastolic volume; SV= Stroke Volume
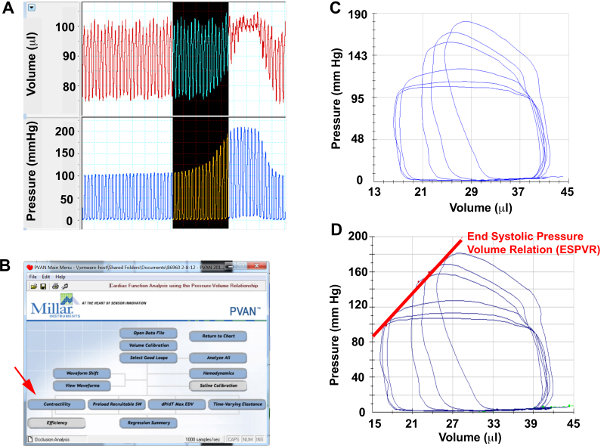 Figure 3. Aortic Constriction Hemodynamics
Please click here to view a larger version of this figure.
Figure 3. Aortic Constriction Hemodynamics
Please click here to view a larger version of this figure.
(A) Screenshots from pressure and volume channels of representative cardiac cycles during aortic constriction, (B) Menu selection to perform contractility analysis, (C) Selected pressure-volume loops during aortic constriction for analysis. (D) ESPVR measured from aortic constriction loops.
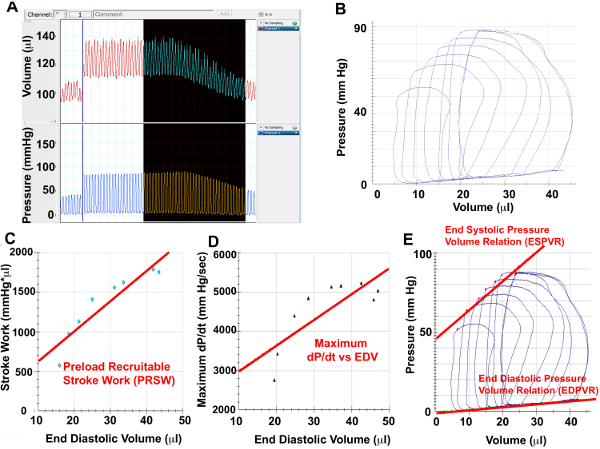 Figure 4. IVC Constriction Hemodynamics
Please click here to view a larger version of this figure.
Figure 4. IVC Constriction Hemodynamics
Please click here to view a larger version of this figure.
(A) Screenshots from pressure and volume channels of representative cardiac cycles during IVC constriction, (B) Selected pressure-volume loops during IVC constriction for analysis. Using the PV loops from IVC constriction, Preload Recruitable Stroke Work (C), Maximal dP/dt vs EDV (D) as well as ESPVR and EDPVR can be measured.
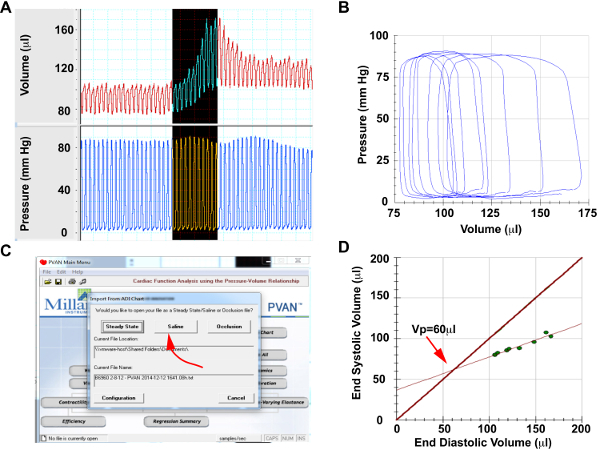 Figure 5. Saline Calibration
Please click here to view a larger version of this figure.
Figure 5. Saline Calibration
Please click here to view a larger version of this figure.
(A) Screenshots from pressure and volume channels of representative cardiac cycles during hypertonic saline injection (B) Selected saline injection pressure-volume loops for analysis. Note that pressure will remain constant, while volume will increase significantly, (C) Menu selection to perform saline calibration, (D) Generated lines of measure end-systolic vs end-diastolic volume during saline injection and end-systolic volume=end-diastolic volume. The intersection of these lines provides the parallel conductance of Vp.
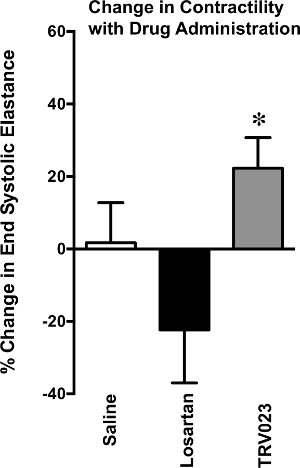 Figure 6. Change in Contractility with Drug Administration
Figure 6. Change in Contractility with Drug Administration
Change in cardiac contractility, measured by end systolic elastance, assessed in wild type mice treated with saline, Losartan 5mg/kg/hr or TRV023 100μg/kg/hr for 5 min. TRV023 treated mice developed a significant increase in end systolic elastance in comparison to Losartan treated mice. *p < 0.05 vs Losartan by 1-way ANOVA.
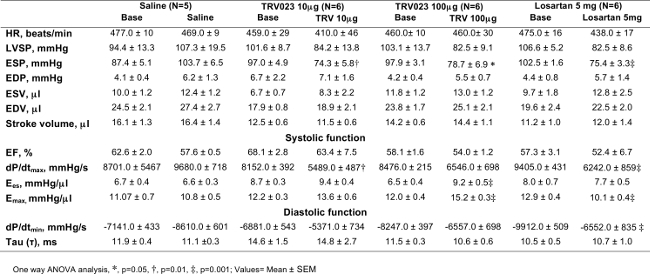 Table 1. Wild type Mice; Hemodynamic Profile in Response of
β-arrestin 2 Biased AT1R Agonist
Table 1. Wild type Mice; Hemodynamic Profile in Response of
β-arrestin 2 Biased AT1R Agonist
End-systolic pressure (ESP) was decreased significantly after TRV120023 (TRV) and losartan infusion. Cardiac contractility, Ees and Emax was increased significantly in TRV120023 100 μg · kg-1 · min-1 infusion group. (*p < 0.01; †p<0.05; ‡p< 0.001; n=5–6/group). p values reflect comparisons with basal condition within the same treatment group with the use of 1-way ANOVA. Cardiac contractility parameters were derived using an aortic constriction protocol. AT1R, ANG II type 1 receptor; HR, heart rate; EDP, end-diastolic pressure; ESV, end-systolic volume; EDV, end-diastolic volume; Ees, end-systolic elastance; EF, ejection fraction; Emax, maximal elastance; dP/dtmax and dP/dtmin, maximum and minimum rate of pressure change in the ventricle, respectively; τ, isovolumic relaxation constant. Please click here to view a larger version of this table.
Discussion
We describe a method for perfoming pressure-volume loop analysis using a conductance catheter in mice, to derive comprehensive analyses of both cardiac contractility and relaxation. Suga, Sagawa and colleagues utilized pressure-volume loops to define measures of cardiac contractility, specifically the slope of the ESPVR, or the end-systolic elastance (Ees), and Emax. Elastance, defined by the ratio of pressure to volume (P/V), varies over the duration of systole. During each systole, the instantaneous elastance is dependent on heart rate and cardiac contractility, but is largely independent of preload or afterload 3,17. Thus, the peak elastance or Emax is used to define cardiac contractility that is mostly independent of loading conditions from individual cardiac cycles18. A closely related contractility term, Ees is defined by the slope of the ESPVR over a series of cardiac cycles in a stable condition. While Ees appears linear over a limited range of loads, Ees can be curvilinear and curvilinearity is correlated with contractile state19. An increase in Ees or Emax indicates increased contractility and a decrease denotes diminished contractility. In addition to Ees or Emax, pressure-volume loop data can be used to derive alternative contractility indices such as: dP/dtmax-EDV relation 20, preload-recruitable stroke work (PRSW) 21 or maximal power-EDV relations 22. These alternative parameters examine the cardiac response over a range of preloads and can be obtained with IVC constriction. It is worth noting that while ESPVR is relatively load-independent, this is not absolute. There are differences in ESPVRs derived from aortic or IVC constriction 23, with aortic constriction having a larger impact on the duration of systole and the extent of shortening 8.
Similar to the ESPVR, the end-diastolic pressure-volume relation (EDPVR) provides a load independent measure of cardiac compliance. This relation is derived by identifying end-diastolic pressures over a range of loading conditions, which is then fit to either an exponential model defined as P=α(eßV-1) +P0 (α is a stiffness and scaling coefficient,ß=chamber stiffness coefficient and P0 = pressure at a 0 volume)8 or a linear model (shown in Figure 4C). Pressure-volume loop analysis can provide additional information about diastolic function. A measure of active relaxation is derived from the decline of ventricular pressure during isovolumic relaxation. The monoexponential decay from the peak rate of relaxation to the onset of LV filling is expressed as time constant t8.
The measurement of parallel conductance is critical to the assessment of cardiac volume. While we have described the use of saline calibration to assess parallel conductance, a growing body of literature has identified alternative methods to assess parallel conductance. Saline calibration utilizes Baan’s equation 9, in which α is uniform field correction factor. However, the moving heart wall changes the electrical field thus raising the issue of a dynamic α constant 24. Moreover, the contribution of the ventricular wall to conductance varies during systole and diastole, however the saline calibration uses a fixed value for parallel conductance 24. To address this, the concepts of time varying field correction, termed Wei’s equation, and instantaneous parallel conductance, termed “admittance”, have been devised 25. Newer micro-catheters that measure admittance have been created and successfully utilized in cardiac injury models 1. These technologies represent a significant advance in the assessment of pressure-volume loop analysis.
The systolic and diastolic parameters obtained from pressure-volume loop analysis provides a comprehensive evaluation of cardiac function. The accuracy and precision of these analyses depend on attention to experimental detail. To obtain good quality cardiac pressure-volume loops in mice, a skillful operator is crucial. Additionally, care must be taken in the selection of anesthesia, proper ventilation, body temperature, and positioning of the catheter into the LV. The correct analysis of obtained data will depend on consistent instrument, saline and cuvette calibration. These aspects are highlighted in these written methods and the accompanying video should provide a framework upon which to embark on these cardiac physiology studies.
Trouble Shooting
1. Hypotension or bradycardia in a basal state: Normal mouse blood pressure and heart rate have been summarized by in previous reviews of this topic 11.
a) Ensure that body temperature is above 36 ºC using a rectal thermometer. If below 36 ºC, a heating pad or heating lamps can be used to raise the mouse body temperature.
b) Assess for bleeding during the surgical procedure. Hemostasis can be achieved with manual pressure or with cautery. Significant volume loss can be treated with saline fluid boluses.
c) Assess whether the mouse is over-anesthetized. If this is suspected, saline fluid boluses can be used to treat hypotension. Of note, ketamine/xylazine, which is used for the demonstrated experiments, can be cardiodepressive. Alternatively, inhaled anesthesia using isoflurane (Induction 3-4%, maintenance 1.5% mixed with 95% oxygen & 5% CO2) can be substituted. Alternative anesthetic agents such as urethane (800 mg kg-1)/etomidate (5-10 mg kg-1)/ morphine (2 mg kg-1) or pentobarbital sodium (40-80 mg kg-1) can be administered intraperitoneally11.
2. Noise in the pressure or volume channels: This may result from electromagnetic interference or a broken/dirty conductance catheter. Make a careful assessment of electronic devices that may contribute to interference. If noise persists, examine the conductance catheter under a microscope to assess for adherent material or damage to the catheter tip. If available, try a fresh conductance catheter to see if it remedies the problem.
3. Volume recording drifts over time: This may be due to an inadequate warm-up time for the hemodynamic module. Allow the module to warm up for 30 min before calibration steps and analysis.
5. Volume reading is larger than expected: This can occur if the measured volume has not been corrected for parallel conductance. Perform the saline calibration outlined in protocol step 6 to obtain the parallel conductance.
6. Poor quality pressure volume loops: The ideal pressure-volume loop has a square or rectangular appearance (see Figure 2 for example). If the shape of the loop is irregular, gently manipulate or twist the conductance catheter in the LV cavity to see if it changes the shape of the loop. Our preferred approach to accessing the LV is through the carotid artery (Protocol step 3.1) as this does not require opening the chest, which can affect hemodynamics. However, the carotid approach can be prone to catheter artifact an irregular loops. Thus, an apical approach (Protocol step 3.2) can be used to address this issue.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work is supported by the American Heart Association 14FTF20370058 (DMA) and NIH T32 HL007101-35 (DMA).
References
- Clark JE, Marber MS. Advancements in pressure-volume catheter technology - stress remodelling after infarction. Exp Physiol. 2013;98(3):614–621. doi: 10.1113/expphysiol.2012.064733. [DOI] [PubMed] [Google Scholar]
- Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol. 1983;245(Pt 1):H773–H780. doi: 10.1152/ajpheart.1983.245.5.H773. [DOI] [PubMed] [Google Scholar]
- Suga H, Sagawa K, Demer L. Determinants of instantaneous pressure in canine left ventricle. Time and volume specification. Circ Res. 1980;46(2):256–263. doi: 10.1161/01.res.46.2.256. [DOI] [PubMed] [Google Scholar]
- Suga H, Sagawa K, Shoukas AA. Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ Res. 1973;32(3):314–322. doi: 10.1161/01.res.32.3.314. [DOI] [PubMed] [Google Scholar]
- Kass DA, et al. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999;99(12):1567–1573. doi: 10.1161/01.cir.99.12.1567. [DOI] [PubMed] [Google Scholar]
- Kass DA, et al. Diastolic Compliance of Hypertrophied Ventricle Is Not Acutely Altered by Pharmacological Agents Influencing Active Processes. Annals of Internal Medicine. 1993;119(6):466–473. doi: 10.7326/0003-4819-119-6-199309150-00004. [DOI] [PubMed] [Google Scholar]
- Georgakopoulos D, et al. In vivo murine left ventricular pressure-volume relations by miniaturized conductance micromanometry. Am J Physiol. 1998;274(4 pt 2):H1416–H1422. doi: 10.1152/ajpheart.1998.274.4.H1416. [DOI] [PubMed] [Google Scholar]
- Cingolani OH, Kass DA. Pressure-volume relation analysis of mouse ventricular function. Am J Physiol Heart Circ Physiol. 2011;301(6):H2198–H2206. doi: 10.1152/ajpheart.00781.2011. [DOI] [PubMed] [Google Scholar]
- Baan J, et al. Continuous Measurement of Left-Ventricular Volume in Animals and Humans by Conductance Catheter. Circulation. 1984;70(5):812–823. doi: 10.1161/01.cir.70.5.812. [DOI] [PubMed] [Google Scholar]
- Pearce JA, Porterfield JE, Larson ER, Valvano JW, Feldman MD. Accuracy considerations in catheter based estimation of left ventricular volume. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:3556–3558. doi: 10.1109/IEMBS.2010.5627712. [DOI] [PubMed] [Google Scholar]
- Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nature Protocols. 1038;3(9):1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanusch C, Hoeger S, Beck GC. Anaesthesia of small rodents during magnetic resonance imaging. Methods. 2007;43(1):68–78. doi: 10.1016/j.ymeth.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Georgakopoulos D, Kass DA. Estimation of parallel conductance by dual-frequency conductance catheter in mice. Am J Physiol Heart Circ Physiol. 2000;279(1):H443–H450. doi: 10.1152/ajpheart.2000.279.1.H443. [DOI] [PubMed] [Google Scholar]
- Esposito G, et al. Increased myocardial contractility and enhanced exercise function in transgenic mice overexpressing either adenylyl cyclase 5 or 8. Basic Res Cardiol. 2008;103(1):22–30. doi: 10.1007/s00395-007-0688-6. [DOI] [PubMed] [Google Scholar]
- Kohout TA, et al. Augmentation of cardiac contractility mediated by the human beta(3)-adrenergic receptor overexpressed in the hearts of transgenic mice. Circulation. 2001;104(20):2485–2491. doi: 10.1161/hc4501.098933. [DOI] [PubMed] [Google Scholar]
- Kim KS, et al. beta-Arrestin-biased AT1R stimulation promotes cell survival during acute cardiac injury. Am J Physiol Heart Circ Physiol. 2012;303(8):H1001–H1010. doi: 10.1152/ajpheart.00475.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga H, Sagawa K. Mathematical Interrelationship between Instantaneous Ventricular Pressure-Volume Ratio and Myocardial Force-Velocity Relation. Annals of Biomedical Engineering. 1972;1(2):160–181. doi: 10.1007/BF02584205. [DOI] [PubMed] [Google Scholar]
- Suga H. Ventricular energetics. Physiol Rev. 1990;70(2):247–277. doi: 10.1152/physrev.1990.70.2.247. [DOI] [PubMed] [Google Scholar]
- Kass DA, et al. Influence of contractile state on curvilinearity of in situ end-systolic pressure-volume relations. Circulation. 1989;79(1):167–178. doi: 10.1161/01.cir.79.1.167. [DOI] [PubMed] [Google Scholar]
- Little WC. The left ventricular dP/dtmax-end-diastolic volume relation in closed-chest dogs. Circ Res. 1985;56(6):808–815. doi: 10.1161/01.res.56.6.808. [DOI] [PubMed] [Google Scholar]
- Glower DD, et al. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation. 1985;71(5):994–1009. doi: 10.1161/01.cir.71.5.994. [DOI] [PubMed] [Google Scholar]
- Sharir T, et al. Ventricular systolic assessment in patients with dilated cardiomyopathy by preload-adjusted maximal power. Validation and noninvasive application. Circulation. 1994;89(5):2045–2053. doi: 10.1161/01.cir.89.5.2045. [DOI] [PubMed] [Google Scholar]
- Baan J, Van der Velde ET. Sensitivity of left ventricular end-systolic pressure-volume relation to type of loading intervention in dogs. Circ Res. 1988;62(6):1247–1258. doi: 10.1161/01.res.62.6.1247. [DOI] [PubMed] [Google Scholar]
- Wei CL, et al. Volume catheter parallel conductance varies between end-systole and end-diastole. IEEE Trans Biomed Eng. 2007;54(8):1480–1489. doi: 10.1109/TBME.2007.890732. [DOI] [PubMed] [Google Scholar]
- Porterfield JE, et al. Dynamic correction for parallel conductance, GP, and gain factor, alpha, in invasive murine left ventricular volume measurements. J Appl Physiol (1985) 2009;107(6)(1985):1693–1703. doi: 10.1152/japplphysiol.91322.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


