Abstract
Single institution and population-based studies highlight that infectious complications following transrectal ultrasound guided prostate needle biopsy (TRUS PNB) are increasing. Such infections are largely attributable to quinolone resistant microorganisms which colonize the rectal vault and are translocated into the bloodstream during the biopsy procedure. A povidone iodine rectal preparation (PIRP) at time of biopsy is a simple, reproducible method to reduce rectal microorganism colony counts and therefore resultant infections following TRUS PNB.
All patients are administered three days of oral antibiotic therapy prior to biopsy. The PIRP technique involves initially positioning the patient in the standard manner for a TRUS PNB. Following digital rectal examination, 15 ml of a 10% solution of commercially available povidone iodine is mixed with 5 ml of 1% lidocaine jelly to create slurry. A 4 cm x 4 cm sterile gauze is soaked in this slurry and then inserted into the rectal vault for 2 min after which it is removed. Thereafter, a disposable cotton gynecologic swab is used to paint both the perianal area and the rectal vault to a distance of 3 cm from the anus. The povidone iodine solution is then allowed to dry for 2 - 3 min prior to proceeding with standard transrectal ultrasonography and subsequent biopsy.
This PIRP technique has been in practice at our institution since March of 2012 with an associated reduction of post-biopsy infections from 4.3% to 0.6% (p = 0.02). The principal advantage of this prophylaxis regimen is its simplicity and reproducibility with use of an easily available, inexpensive agent to reduce infections. Furthermore, the technique avoids exposing patients to additional systemic antibiotics with potential further propagation of multi-drug resistant organisms. Usage of PIRP at TRUS PNB, however, is not applicable for patients with iodine or shellfish allergies.
Keywords: Medicine, Issue 103, Prostate needle biopsy, transrectal ultrasonography, infection, sepsis, urinary tract infection (UTI), prostate cancer
Introduction
Prostate cancer is the most common cancer in men and the second leading cause of cancer-related mortality. In 2014, approximately 233,000 cases will be diagnosed in the United States with almost 30,000 men succumbing to this malignancy.1 While the prostate specific antigen (PSA) blood test and digital rectal examination (DRE) play an essential role in prostate cancer screening, transrectal ultrasound guided prostate needle biopsy (TRUS PNB) is the most common means to obtain a histologic tissue diagnosis.
TRUS PNB involves inserting an ultrasound probe into the patient’s rectum and then generally obtaining 12-14 biopsies of the peripheral zone of the prostate located just anteriorly. Each needle biopsy requires passage of an 18 G needle through the rectal wall into the highly vascular prostate. Therefore, the theoretical risk of bleeding and infection due to bacterial translocation exists following the procedure. Nonetheless, the actuarial complication rate post-procedure has historically remained low.
Antibiotic prophylaxis prior to TRUS PNB is routinely prescribed with quinolone-based antibiotics being the most frequently used agents. Despite adequate prophylaxis, contemporary research implicates an increase in infectious complications post-biopsy.2 Furthermore, studies have attributed this rise in infectious complications following TRUS PNB to the emergence of quinolone resistant microorganisms particularly E. coli.3
This rising rate of quinolone resistance suggests the need for alternate prophylaxis regimens for TRUS PNB procedures. To this end, several different avenues are under investigation. One approach involves administration of an intravenous or intramuscular antibiotic at time of biopsy in conjunction with an oral quinolone.4 While this strategy can reduce sepsis events following TRUS PNB, a principal limitation of this approach is the potential for further developing resistant organisms to this antibiotic family. Another recently investigated methodology incorporates use of a pre-biopsy rectal swab to screen patients colonized with quinolone-resistant rectal flora.5 If such organisms are detected, a “targeted” antibiotic regimen could be employed based on the sensitivity profile of the identified resistant bacterial strain. While the methodology is elegant, the actual process of obtaining such swabs, selectively culturing on a quinolone selective medium, and tailoring antibiotics thereafter requires a clinical and laboratory infrastructure that may be lacking in many clinical practices.
Administration of a topic antiseptic to reduce rectal vault microorganism colony counts prior to biopsy may present an alternative strategy to limit TRUS PNB infections. Povidone iodone is an inexpensive, readily available agent that is documented to reduced bacterial counts when applied to surgical sites. Applications in both colorectal and gynecologic surgeries are well known. Therefore, use of povidine iodine as a rectal preparation would present a producible, simple, and cost-effective method to reduced TRUS PNB infections without need for additional preparation prior to biopsy. With respect to the urologic literature, previously work has noted a reduction in clinical infections following TRUS PNB.6-9 This video demonstrates the technique of PIRP to highlight the ease and simplicity to integrate into clinical practices.
All patients undergoing TRUS PNB were initially seen in a specialty urology clinic for referral or initial evaluation for prostate cancer screening. This evaluation entailed a review of the patient’s serum prostate specific antigen (PSA) and performance of a digital rectal examination (DRE). Abnormality of either the PSA or DRE prompted recommendation of a TRUS PNB.
The TRUS PNB procedure was discussed with all patients and associated risks including infection, bleeding, and urinary retention were clarified. All patients received three days of oral antibiotic prophylaxis with either a quinolone-based medication (i.e., Ciprofloxacin) or trimethoprim-sulfamethoxazole (Bactrim) prior to biopsy.
Protocol
This patient related study was approved by the institutional review board (IRB) for human research.
1. Patient Preparation and Positioning
Bring the patient to the procedure suite and place them on their right or left side with knees flexed to the chest.
Perform a surgical timeout whereby the patient, surgical procedure, and indication are reviewed and confirmed.
When indicated, administer intravenous sedation via the anesthesia service.
2. Perform a Digital Rectal Examination (DRE)
Place size appropriate gloves onto both hands.
Insert gloved finger after lubrication into rectal vault and examine prostate to feel for potential suspicious areas in need of focused attention during transrectal ultrasonography.10
3. Creation of Povidone Iodine Rectal Preparation (PIRP)
Pour 15 ml of a 10% commercially available povidone iodine solution into a sterile basin.
Add 5 ml of a commercially available 1% lidocaine gel to this basin.
Mix the contents for 15 sec to create a povidone iodine slurry.
4. Primary Administration of PIRP
Use a sterile rectal swab to culture the rectal vault.
Open up a 4 cm x 4 cm sterile gauze, soak in the povidone iodine slurry, and then insert into the rectal vault. Only a small tail of gauze is left emanating from the anus.
Leave the gauze in place for 2 min after which it is removed and discarded.
5. Secondary Administration of PIRP
Use a disposable gynecologic swab to paint the perianal area with the PIRP solution.
Insert this gynecologic swab into the rectal vault to a distance of 3 cm from the anus.
To further maximize the exposure of the rectal mucosa to PIRP, swab the rectal vault in a similar manner at least three times.
Allow the povidone iodine solution to dry for 2 - 3 min prior to proceeding with standard transrectal ultrasonography and subsequent biopsy.11 This is essential as the bactericidal effects of the PIRP require the solution to dry on the exposed mucosa.
Use a sterile rectal swab to culture the rectal vault.
6. Transrectal Ultrasonography and Biopsy
Insert the ultrasound probe via the rectum.
Perform measurements of the prostate and subsequent biopsy in the standard manner consistent with all urologic practices.11,12
Representative Results
This PIRP technique has been in practice since January of 2012 (until present) with 165 patients having been enrolled in this quality improvement measure. In this time, only one patient (0.6%) has experienced an infectious complication in the form of systemic sepsis. This patient was colonized with a multi-drug resistant E. coli strain. Rectal swabs following administration of PIRP in this patient noted that the rectal vault was still colonized with 2.1 x 105 CFU/ml bacteria thereby questioning whether the PIRP adequately treated the rectal vault.
A contemporary population undergoing TRUS PNB between January 2008 and January 2012 who only received oral antibiotic therapy served as a comparative control population. This cohort experienced a 4.3% infection rate (22 of 515 patients) including 17 patients with urinary tract infections (UTI) and 5 with clinical sepsis defined as fever with positive blood culture. All 5 patients with sepsis had multi-drug resistant E. coli whilst the UTI cases were attributable to several different organisms.
Overall, integration of the PIRP technique into clinical practice was associated with a reduction in post-biopsy infections from 4.3% to 0.6% (p = 0.02); Figure 1.
Rectal cultures were performed in the 165 patients before and after PIRP to document changes in rectal vault microorganism colony count attributable to the treatment. Briefly, cultures were obtained by use of sterile culture swabs. The swabs were then immersed in PBS, vortexed to release the bacteria, and serially diluted on Mueller-Hinton agar plates. Bacteria were allowed to grow for 36 - 48 hr and counted. All morphological varieties of bacterial colonies arising on the plate were included to provide a total count. Overall, a 97.2% reduction in microorganism colonies was noted after PIRP administration (mean 2.3 x 105 CFU/ml vs. 5.8 x 103 CFU/ml, p < 0.001)
No adverse effects of the PIRP were reported by patients either at time of administration or 7-days post-procedure.
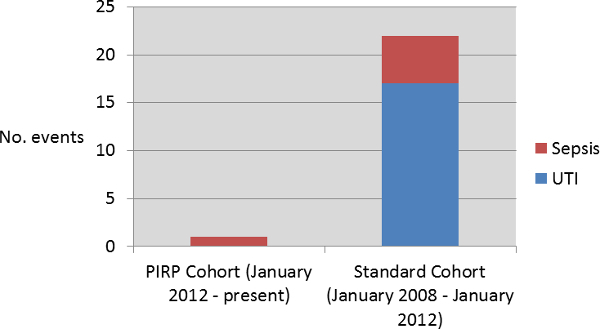 Figure 1. Urinary Tract Infection (UTI) and Sepsis Events Occurring in the Povidone Iodine Rectal Preparation (PIRP) and Standard Cohort.
Figure 1. Urinary Tract Infection (UTI) and Sepsis Events Occurring in the Povidone Iodine Rectal Preparation (PIRP) and Standard Cohort.
Discussion
Infections following TRUS PNB are secondary to translocation of rectal vault bacteria into the highly vascular prostate. Based on this principle, we have explored the simple method of using a topic antiseptic such as povidone iodine to reduce microorganism colony counts prior to prostate needle biopsy. Our experience highlights a reduction from 4.3% to 0.6% (p=0.02) with use of this PIRP regimen at time of biopsy. Furthermore, we hypothesize that the 97% reduction in rectal vault microorganisms is the principle factor underlying this decrease in systemic infections.
Our results with topical povidone are similar to that presented by other groups. Specifically, Park et al.7 evaluated 121 patients who received antibiotic prophylaxis consisting of a single intravenous injection of a 3rd generation cephalosporin and 5 days of cefixime 100 mg BID versus 360 patients who received the same antibiotic regimen in addition to a povidone iodine suppository just prior to biopsy. This study noted an infectious complication in 8 patients (6.6%) in the control group compared to 1 (0.3%) in their study cohort. Recently, AbuGhosh et al. prospectively randomized 865 men receiving oral ciprofloxacin therapy to rectal cleaning with povidone iodine or no rectal cleaning before TRUS PNB.8 This study observed infectious complications in 11 (2.6%) of the rectal preparation group and in 20 (4.5%) of the control group, and sepsis was seen in 4 (1.0%) of the treated group and in 7 (1.6%) of the control group.
The novelty and attractiveness of our PIRP technique as presented in this video is several fold. First, the PIRP requires no additional preparation or systemic antibiotic therapy beyond the standard oral prophylaxis agent. Second, the PIRP technique is cheap with minimal associated cost for practices to purchase commercially available povidone iodine. Third, the PIRP is simple with the ability of any urologist to perform within current office infrastructure while adding only 5 min to a biopsy procedure. Finally, the side effect profile of PIRP therapy is low with no patients in our current series presenting with adverse events.
There are several key steps in this protocol that warrant further discussion. It is important to first insert the povidone iodine soaked gauze into the rectal vault for 2 min and then remove it allowing for the solution to dry. This process is essential as the bactericidal effect of povidone iodine is predicated on the solution drying on a mucosal surface. A second key protocol step involves painting the perianal area with povidone iodine thereby preventing translocation of bacteria in the perianal area into the rectal vault at the time of biopsy.
The protocol typically does not require modifications owing to its simplicity in delivery. Certain scenarios such as those patients with rectal fissures or exposed hemorrhoids may experience local discomfort and irritation from the povidone iodine solution. Therefore, this therapy is withheld in such patients. Additionally, patients with an iodine or shellfish allergy are at risk for an allergic reaction (rarely anaphylaxis) and therapy should not be used in such patients. Finally, approximately 10% of prostate biopsies at our institution are done in an office setting while the remaining cases are completed under intravenous sedation. Patients undergoing local anesthesia experienced no adverse reactions to the PIRP although larger studies are necessary to validate these observations.
Limitations of this approach involve the delivery system of the iodine into the rectal vault. While the current rectal vault counts following PIRP are 97% below baseline, there still remain some organisms that are persistent which could theoretically be a source of infection. Therefore, the potential exists to improve the delivery system of the PIRP to further reduce rectal microorganism counts. Additionally, the data presented within this study are done in the context of a prospective, albeit non-randomized design. We acknowledge that randomization of patients would have provided the most objective means to assess treatment efficacy.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors acknowledge the Carefusion Foundation for support via a clinical excellence grant of this work titled “Prospective evaluation of procedural povidone iodine rectal preparation to reduce infectious complications following ultrasound guided prostate needle biopsy.”
References
- American Cancer Society. 2015. Available from: http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics.
- Nam RK, et al. Increasing hospital admission rates for complications after transrectal ultrasound guided prostate biopsy. J Urol. 2013;189(1):S12–S17. doi: 10.1016/j.juro.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Feliciano J, et al. The incidence of fluoroquinolone resistant infections after prostate biopsy are fluoroquinolones still effective prophylaxis. J Urol. 2008;179(3):952–955. doi: 10.1016/j.juro.2007.10.071. [DOI] [PubMed] [Google Scholar]
- Kehinde O, Al-Maghrebi M, Sheikh M, Anim JT. Combined ciprofloxacin and amikacin prophylaxis in the prevention of septicemia after transrectal ultrasound guided biopsy of the prostate. J Urol. 2013;189(3):911–915. doi: 10.1016/j.juro.2012.08.237. [DOI] [PubMed] [Google Scholar]
- Taylor AK, et al. Targeted antimicrobial prophylaxis using rectal swab cultures in men undergoing transrectal ultrasound guided prostate biopsy is associated with reduced incidence of postoperative infectious complications and cost of care. J Urol. 2012;187(4):1275–1279. doi: 10.1016/j.juro.2011.11.115. [DOI] [PubMed] [Google Scholar]
- Gyorfi JR, et al. Peri procedural povidone iodine rectal preparation reduces microorganism counts and infectious complications following ultrasound-guided needle biopsy of the prostate. World J Urol. 2014;32(4):905–909. doi: 10.1007/s00345-014-1291-8. [DOI] [PubMed] [Google Scholar]
- Park DS, et al. Control of infective complications of transrectal prostate biopsy. Surg Infect Larchmt. 2014;15(4):431–436. doi: 10.1089/sur.2013.138. [DOI] [PubMed] [Google Scholar]
- Park DS, Oh JJ, Lee JH, Jang WK, Hong YK, Hong SK. Simple use of the suppository type povidone iodine can prevent infectious complications in transrectal ultrasound guided prostate biopsy. Adv Urol. 2009. [DOI] [PMC free article] [PubMed]
- AbuGhosh Z, et al. A prospective randomized trial of povidone iodine prophylactic cleansing of the rectum before transrectal ultrasound guided prostate biopsy. J Urol. 2013;189(4):1326–1331. doi: 10.1016/j.juro.2012.09.121. [DOI] [PubMed] [Google Scholar]
- Gerber GS, Brendler CB. Evaluation of the Urological Patient: History, Physical Exam, and Urinalysis. Campbell Walsh Urology. 10th ed. 2015. Available from: http://www.campbellsurology.com.
- Hodge KK, McNeal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989;142(1):71–74. doi: 10.1016/s0022-5347(17)38664-0. [DOI] [PubMed] [Google Scholar]
- Donoghue PM, McSweeney SE, Jhaveri K. Genitourinary imaging: current and emerging applications. J Postgrad Med. 2010;56(2):131–139. doi: 10.4103/0022-3859.65291. [DOI] [PubMed] [Google Scholar]


