Abstract
Animal models are important tools to study the pathophysiology and pharmacology of neuropathic pain. This manuscript describes the surgical and behavioral procedures to study trigeminal neuropathic pain in rats. To meet the specificity of trigeminal neuropathic pain syndromes, the infraorbital nerve (IoN) is subjected to a chronic constriction injury (CCI) by loosely ligating the nerve. An intra-orbital approach is presented here to expose and ligate the IoN in the orbital cavity. After IoN ligation, rats exhibit changes in spontaneous behavior and in response to von Frey hair stimulation that are indicative of persistent pain and mechanical allodynia. Two phases can be defined in the development of the behavioral changes. During the first week following IoN-CCI (phase 1), rats show an increased and asymmetric face grooming activity, i.e., with face wash strokes primarily directed to the nerve-injured IoN territory. A distinction is made between face grooming behavior that is part of a more general body grooming behavior, which remains largely unaffected by IoN-CCI, and face grooming that is neither preceded nor followed by body grooming, which is significantly increased after IoN-CCI. During this period, responsiveness to mechanical stimulation of the IoN territory is reduced. This hyporesponsiveness is abruptly replaced by an extreme hyperresponsiveness whereby even very weak stimulus intensities provoke nocifensive behavior (phase 2). The phenomenological similarities between these behavioral alterations and reported signs of facial pain (i.e., responses to noxious stimulation of the face) suggest the presence of dysesthesia/paresthesia and mechanical allodynia in the ligated IoN territory.
Keywords: Medicine, Issue 103, Neuropathic pain, trigeminal Neuralgia, infraorbital nerve, rat, chronic constriction injury, face grooming, mechanical allodynia
Introduction
Damage to somatosensory nerves most often leads to loss of sensation. It is not very well understood why and how, in some cases, peripheral nerve damage leads to chronic pain. As a result, painful neuropathies remain a challenging condition to treat1 and result in substantial reductions in patients’ quality of life2-3. Animal models are an important tool to examine the efficacy of existing and newly developed drugs and to identify the pathophysiological mechanisms involved in the development and maintenance of neuropathic pain. Among the key challenges for translational pain research is identifying and measuring spontaneous pain in animals (i.e., physiological, behavioral or other manifestations that indicate the presence of pain in animals that are not being challenged with probing stimulations that are acutely superimposed on the subject’s basal condition). The overall goal of this procedure is to surgically induce an infraorbital nerve injury in a rat that leads to the development of both spontaneous and evoked nocifensive behavior that can used to study the mechanisms involved in trigeminal neuropathic pain and its treatment.
Directed, isolated face grooming is a unique measure of spontaneous neuropathic pain in rats. Following chronic constriction injury (CCI) of the infraorbital nerve (IoN), rats exhibit changes in spontaneous behavior and in response to tactile stimulation that are indicative of persistent pain and mechanical allodynia4. Following IoN-CCI, rats show an increased and asymmetric face grooming activity, i.e., with face wash strokes primarily directed to the nerve-injured IoN territory. In contrast to noxious stimulation of the face (e.g., formalin injection)5, non-painful sensory disturbances (i.e., unilateral vibrissae clipping, anesthetic infraorbital nerve blockade, application of mineral oil on vibrissae) did not or only very briefly (i.e., the first minutes after onset) induce a significant change in face grooming behavior6. It was concluded that this abnormal grooming after IoN-CCI is a behavioral manifestation of spontaneous, strongly aversive sensations in the injured nerve territory. This behavior in IoN-CCI rats bears strong resemblance to spontaneous pain observed in patients. Further studies have identified an important difference between face grooming behavior that is part of a more general body grooming behavior (i.e., “face grooming during body grooming”), which remains largely unaffected by IoN-CCI, and nocifensive face grooming that is neither preceded nor followed by body grooming (i.e., “isolated face grooming”), which is significantly increased after IoN-CCI7-9. Pharmacologically, face grooming during body grooming constitutes an excellent control measurement for non-specific drug effects (e.g., sedation) that may impair motor function. Movements of the forelimbs during the two types of face grooming are exactly the same.
Following IoN-CCI, rats also exhibit changes in response to tactile stimulation that are indicative of mechanical allodynia4, 10. During the first week(s) after IoN-CCI, rats no longer respond to mild or moderate tactile (i.e., von Frey) stimuli. This hyporesponsiveness is then abruptly replaced by an extreme hyperresponsiveness whereby even very weak stimulus intensities provoke nocifensive behavior. The onset of hyperresponsiveness may vary between rats, but most rats have become hyperresponsive after three to four weeks post-operative.
Protocol
Ethics statement: Animals are treated and cared for according to the guidelines of the Committee for Research and Ethical Issues of IASP11. The protocol is approved by the institutional Ethical Committee.
1. Subjects
Use male Sprague-Dawley rats (250-300 g at arrival).
House rats in solid-bottom cages in a colony room with a humidity of 40-60% and a RT of 21 ± 1 °C.
Make water and food available at libitum.
Keep rats under a reversed 12:12 hr dark/light cycle (lights on at 20 hr).
2. Surgery
Per rat, prepare two pieces of chromic gut ligature (5-0) of approximately 6 cm long and place them in sterile saline to avoid drying and becoming stiff and brittle.
Anesthetize the animal with pentobarbital (60 mg/kg, i.p.) and treat with atropine (0.1 mg/kg, i.p.). Monitor the level of anesthesia by extending one leg and pinching the web of skin between the toes with a fingernail to ensure adequate pressure is applied. Ensure that the animal does not flex its limb. If necessary, administer additional pentobarbital.
Shave the head of the rat so that a mid-line scalp incision can be made of approximately 25 mm with equal distances anterior and posterior to the center of the eyes.
Fix the rat’s head in a stereotaxic frame or otherwise fixate the rat’s head. Place the rat on a heated pad to maintain body temperature.
Apply ophthalmic ointment on both eyes to prevent damage from drying.
Scrub the shaved head area with alcohol, then betadine.
Make a mid-line scalp incision, exposing skull and nasal bone.
Use an operation microscope to perform steps 2.9 to 2.16.
Dissect the edge of the orbit free, formed by the maxillary, frontal, lacrimal and zygomatic bones, using a cotton-tipped swab and a pair of Dumont forceps. Use the swab to soak up blood from possible bleeding.
To give access to the IoN, gently deflect the orbital contents with a precision cotton swab, taking care not to overstretch the anterior ethmoidal nerve which crosses superior to the IoN. Dissect the IoN free from the surrounding connective tissue, using a precision cotton-tipped swab and a pair of Dumont forceps using a spreading motion. Use the swab to soak up blood from possible bleeding.
To perform a ligation, push one end of a single piece of chromic gut downward along the frontal bone, medial to the IoN and advance a few millimeters parallel and inferior to the IoN. The ligature is now held in place between the IoN and the frontal bone.
Slip the tip of a 45 degree angled Dumont forceps under the IoN to place it in close proximity to the ligature. Gently retract the IoN laterally to reveal the ligature and tip of the forceps. Grip the ligature with the forceps and withdraw the forceps laterally from under the IoN.
Pull out the ligature from the orbital cavity until both ends of the ligature are more or less equidistant from the IoN.
Make a “slip knot” from the two ends of the ligature to allow perfect control over the degree of constriction and slide the knot against the IoN. Slide the knot further to constrict the IoN so that the diameter of the nerve is reduced by a just noticeable amount. Make a normal knot on top of the slip knot so that the amount of constriction remains constant (i.e., to prevent the slit knot from slipping).
Cut the ligatures to leave approximately 2 mm of free ends from the knot.
Repeat the same procedure (steps 2.11 to 2.15) to make a second ligation 2 mm apart from the first.
For sham surgery, perform steps 2.2 to 2.10.
Close the scalp incision using polyester sutures (4-0) and allow the rat to recover on a heated pad. Do not leave the animal unattended until it has regained sufficient consciousness to maintain sternal recumbency and do not return the animal to the company of other animals until it is fully recovered.
To avoid pre-emptive analgesic effects on the development of neuropathic pain, do not administer analgesia for post-surgical pain.
3. Behavioral Test Procedure
Allow rats to acclimate for at least 8 days to the housing conditions before pre-operative testing.
Habituate rats to the test procedure at least once daily for three days before pre-operative testing.
Conduct testing in a darkened room with light provided by a 60 W red light bulb suspended 1 m above the center of the test area lighting the center but leaving the circumference dim and the remainder of the room dark.
Make sure there is sufficient background noise to decrease interference from sudden auditory stimulation. If necessary, provide white noise.
- Observation of face grooming behavior
- Transport a single rat from the colony to the test room in a covered plastic cage without bedding. Note: Ideally, this should be a short trip with minimal external stimuli.
- Place the rat in a covered, transparent plastic cage without bedding (l x w x h: 24 cm x 14 cm x 17 cm) with a mirrored back in front of a video camera.
- Make a 10 min recording of the animal’s behavior. During recording, make sure the experimenter is not present in the test room.
- Clean the observation cage before placing the next animal.
- Make sure the recorded behavior is analyzed by an experimenter who is blind to the experimental condition of the rat.
- Make a record of each face grooming episode during the 10 min recording. Note: Face grooming is defined as movement patterns in which forepaws are brought in contact with facial areas.
- Make a distinction between two types of face grooming behavior. If a sequence is neither preceded nor followed by body grooming (i.e., grooming of a body area other than the face), categorize the episode as isolated face grooming. If body grooming is performed immediately before or after face grooming, categorize the episode as face grooming during body grooming.
- Determine the number of face grooming episodes.
- Categorize a time period between face grooming actions that does not exceed 4 sec as an intra-episode space (i.e., a pause between face grooming actions within a single episode).
- Categorize a time period between face grooming actions that exceeds 4 sec as an inter-episode space (i.e., a complete interruption of face grooming actions between two episodes).
- Mechanical stimulation testing
- Transport rats in groups of up to 6 animals from the colony to the test room in a covered cage with bedding. Ensure that this is a short trip with minimal external stimuli.
- Place the rats individually in transparent plastic cages with bedding (l x w x h: 24 cm x 14 cm x 17 cm) with a hinged lid so that it can be easily opened and closed.
- Use a graded series of five von Frey hairs. Note: The force required to bend the hairs is 0.015 g, 0.127 g, 0.217 g, 0.745 g and 2.150 g.
- Habituate the rats to the observation cage and reaching movements for 10 min. Every 30 sec, open the lid and gently touch the wall of the cage with a plastic rod.
- When the animal is in a sniffing/ no locomotion state (i.e., with four paws placed on the ground, neither moving nor freezing, but exhibiting sniffing behavior), apply the first von Frey hair within the IoN territory near the center of the vibrissal pad, on the hairy skin surrounding the mystacial vibrissae.
- Score the response of the animal to the stimulation to belong to one of the following response categories4.
- Categorize a complete lack of response as score 0.
- Categorize a stimulus detection (i.e., the rat turns the head toward the stimulating object and the stimulus object is then explored) as score 1.
- Categorize a withdrawal reaction (i.e., the rat turns the head slowly away or pulls it briskly backward when the stimulation is applied, sometimes a single face wipe ipsilateral to the stimulated area occurs) as score 2.
- Categorize an escape/attack response (i.e., the rat avoids further contact with the stimulus object, either passively by moving its body away from the stimulating object to assume a crouching position against the cage wall, or actively by attacking the stimulus object, making biting and grabbing movements) as score 3.
- Categorize asymmetric face grooming (i.e., the rat displays an uninterrupted series of at least three face-wash strokes directed toward the stimulated facial area) as score 4.
- Within each animal, apply stimuli in an ascending order of intensity. After a stimulus intensity has been applied to one side, apply it to the other side before moving on to the next stimulus intensity. Randomize the order in which the ipsilateral and contralateral sides are stimulated. Note that the animal must be in a sniffing/ no locomotion state before each stimulation.
Representative Results
IoN-CCI rats show a strong post-operative increase in the amount of time spent on isolated face grooming (Figure 1). The increase peaks at the end of the first post-operative week and then declines during the following weeks. Most recent studies have found face grooming to be significantly increased for up to three weeks. Face grooming behavior in sham operated rats is more or less unaffected.
IoN-CCI rats also show an almost complete lack of responsiveness to ipsilateral mechanical stimulation of the IoN territory during the first post-operative week (Figure 2). During the next week, this hyporesponsiveness is replaced by a hyperresponsiveness. The onset of hyperresponsiveness may vary between rats, as evidenced by the increasing mean scores and diminishing variation. Hyperresponsiveness has been found to persist up to 120 days after surgery. There is also a small increase in responsiveness to contralateral mechanical stimulation in IoN-CCI rats, but it may be barely statistically significant from that in sham operated rats.
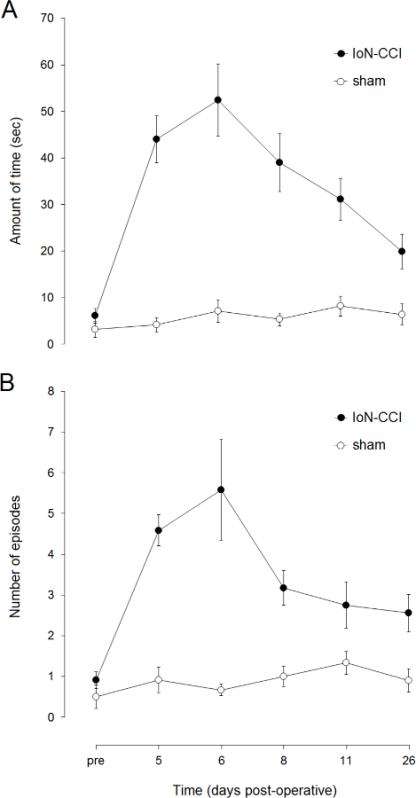 Figure 1. Post-operative changes in isolated face grooming behavior following IoN-CCI. Data points represent the mean (± SEM; n=12 per group) amount of time spent on isolated face grooming (A) and the mean (± SEM; n=12 per group) number of isolated face grooming episodes (B) one day before IoN surgery (pre) and on post-operative days 5 to 26.
Figure 1. Post-operative changes in isolated face grooming behavior following IoN-CCI. Data points represent the mean (± SEM; n=12 per group) amount of time spent on isolated face grooming (A) and the mean (± SEM; n=12 per group) number of isolated face grooming episodes (B) one day before IoN surgery (pre) and on post-operative days 5 to 26.
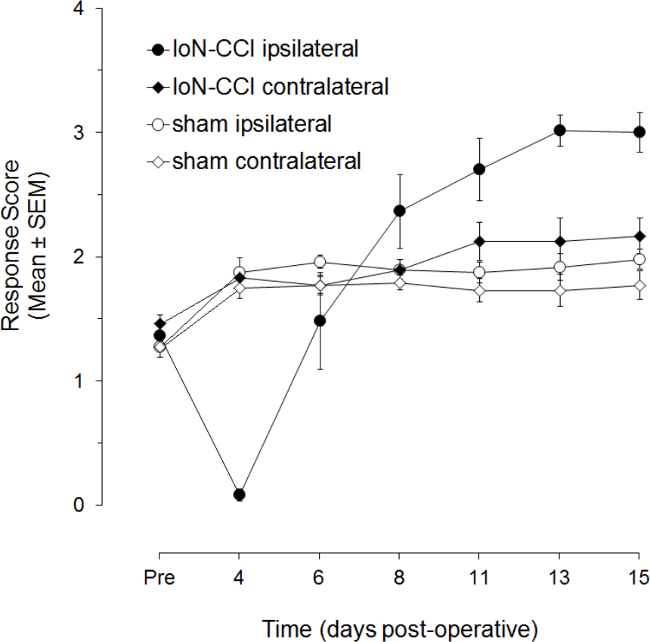 Figure 2. Post-operative changes in responsiveness to mechanical stimulation following IoN-CCI. Data points represent the mean (± S.E.M.; n=12 per group) response score to von Frey hair stimulation of the ligated IoN territory one day before IoN surgery (Pre) and on post-operative days 4 to 15 (adapted from Deseure et al, 2003).
Figure 2. Post-operative changes in responsiveness to mechanical stimulation following IoN-CCI. Data points represent the mean (± S.E.M.; n=12 per group) response score to von Frey hair stimulation of the ligated IoN territory one day before IoN surgery (Pre) and on post-operative days 4 to 15 (adapted from Deseure et al, 2003).
Discussion
The most interesting aspect of the IoN-CCI model is that it provides a unique measure of spontaneous, non-evoked (neuropathic) pain. Most animal studies of neuropathic pain, using spinal nerve models, exclusively address nocifensive behaviors (e.g., vocalization and withdrawal responses), reflecting hypersensitivity to mechanical or thermal stimulations12. However, the chief clinical complaint in patients is ongoing pain, not hyperalgesia or allodynia13.
The present article describes two measures of neuropathic pain following IoN-CCI, isolated face grooming (spontaneous pain) and responsiveness to von Frey hair stimulation (mechanical allodynia). Other measures of neuropathic pain behavior following IoN-CCI have also been described elsewhere, i.e., thermal hyperalgesia14 and cold allodynia15. Furthermore, newly developed methods of orofacial pain assessment such as the Orofacial Pain Assessment Device (OPAD)16-17 or meal duration as a measure of orofacial nociceptive responses18, may prove to be valuable measures of pain after IoN-CCI.
The present article describes an intra-orbital approach to the IoN. Note that two other approaches, i.e., a peripheral approach via an incision into the hairy skin caudal to the vibrissal pad and an intra-oral approach, are also possible. Although the peripheral approach is by far the easiest technique, the intra-orbital approach has the clear advantage of leaving the fine musculature that controls vibrissal movements intact and maybe even more importantly, it avoids an incision wound in the territory of the injured nerve that may seriously hamper sensory testing in that region. Other authors have successfully applied the intra-oral approach14. However, the close contact between the ventral side of the IoN and a bony ridge at the ventral end of the maxillary bone makes the intra-oral approach seem more complicated to us and surgically challenging than the intra-orbital approach.
Considering the close proximity of the surgical field to the mouth of the rat, anesthesia via i.p. injection (i.c. pentobarbital) was chosen over inhalation anesthesia (e.g., isoflurane). However, given the disadvantages of pentobarbital such as risk of overdose, respiratory depression, buildup of fluids in the upper respiratory tract, other anesthetic drugs or even inhalation anesthesia via a gas anesthesia mask for stereotaxic frames, may be considered. In our experience, careful dosing, timely administration of atropine as soon as the animal becomes anesthetized (even partially), proper maintenance of body temperature, and aspiration of fluids from the upper respiratory tract minimizes the risks associated with pentobarbital.
Variability between IoN-CCI rats in the amount of face grooming behavior may be quite high. Recently, it has been shown that part of this variability can be eliminated by observing rats more frequently9. Twice daily observations significantly increased the statistical power, reducing the number of animals needed. Responses to von Frey hair stimulation of the IoN territory are categorized according to a series of rank-ordered descriptive response categories (cf. supra). It may be considered a drawback that the data should be analyzed nonparametrically and represented by nonparametric measures of central tendency (e.g., median) and of variation (e.g., interquartiles). It has been argued that the results of parametric analysis show a very good accordance with those of nonparametric analysis and that median scores fail to appropriately visualize the effects of experimental conditions on response scores10.
The main applications of the IoN-CCI model are in the pharmacological testing of existing or newly developed analgesic drugs, the development of new therapeutic strategies and the study of the pathophysiological mechanisms involved in the development and maintenance of (trigeminal) neuropathic pain.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors acknowledge the work that was done by the first author of the IoN-CCI model, Bart Vos†.
References
- Jensen TS, Gottrup H, Sindrup SH, Bach FW. The clinical picture of neuropathic pain. Eur J Pharmacol. 2001;429(1-3):1–11. doi: 10.1016/s0014-2999(01)01302-4. [DOI] [PubMed] [Google Scholar]
- Meyer Rosberg K, et al. Neuropathic pain a multidimensional burden for patients. Eur J Pain. 2001;5(4):379–389. doi: 10.1053/eujp.2001.0259. [DOI] [PubMed] [Google Scholar]
- Meyer Rosberg K, et al. Comparison of the SF 36 and Nottingham Health Profile in patients with chronic neuropathic pain. Eur J Pain. 2001;5(4):391–403. doi: 10.1053/eujp.2001.0260. [DOI] [PubMed] [Google Scholar]
- Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rats infraorbital nerve. J Neurosci. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavelou P, Pajot J, Dallel R, Raboisson P. Application of the formalin test to the study of orofacial pain. Neurosci Lett. 1989;103:349–353. doi: 10.1016/0304-3940(89)90125-0. [DOI] [PubMed] [Google Scholar]
- Vos BP, Hans G, Adriaensen H. Behavioral assessment of facial pain in rats face grooming patterns after painful and non-painful sensory disturbances in the territory of the rats infraorbital nerve. Pain. 1998;76(1-2):173–178. doi: 10.1016/s0304-3959(98)00039-6. [DOI] [PubMed] [Google Scholar]
- Deseure KR, Adriaensen HF. Comparison between two types of behavioral variables of non evoked facial pain after chronic constriction injury to the rat infraorbital nerve. Comp Med. 2002;52(1):44–49. [PubMed] [Google Scholar]
- Deseure K, Adriaensen H. Nonevoked facial pain in rats following infraorbital nerve injury a parametric analysis. Physiol Behav. 2004;81(4):595–604. doi: 10.1016/j.physbeh.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Deseure K, Hans G. Behavioral study of non evoked orofacial pain following different types of infraorbital nerve injury in rats. Physiol Behav. 2015;138:292–296. doi: 10.1016/j.physbeh.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Deseure K, Koek W, Adriaensen H, Colpaert FC. Continuous administration of the 5 hydroxytryptamine1A agonist (3-Chloro-4-fluoro-phenyl)-[4-fluoro-4-[[(5-methyl-pyridin-2-ylmethyl) -amino]-methyl]piperidin-1-yl]-methadone (F 13640) attenuates allodynia like behavior in a rat model of trigeminal neuropathic pain. J Pharmacol Exp Ther. 2003;306(2):505–514. doi: 10.1124/jpet.103.050286. [DOI] [PubMed] [Google Scholar]
- Zimmerman M. Ethical Guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals. Pain. 2004;112(1-2):12–15. doi: 10.1016/j.pain.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Backonja MM, Stacey B. Neuropathic pain symptoms relative to overall pain rating. J Pain. 2004;5(9):491–497. doi: 10.1016/j.jpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Kawamoto H, Nakanishi O. Characterization of heat hyperalgesia in an experimental trigeminal neuropathy in rats. Exp Brain Res. 1997;116:97–103. doi: 10.1007/pl00005748. [DOI] [PubMed] [Google Scholar]
- Chichorro JG, Zampronio AR, Souza GE, Rae GA. Orofacial cold hyperalgesia due to infraorbital nerve constriction injury in rats reversal by endothelin receptor antagonists but not non steroidal anti inflammatory drugs. Pain. 2006;123(1-2):64–74. doi: 10.1016/j.pain.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Kramer PR, Bellinger LL. Meal Duration as a Measure of Orofacial Nociceptive Responses in Rodents. J Vis Exp. 2014. p. e50745. [DOI] [PMC free article] [PubMed]
- Rossi HL, et al. Characterization of bilateral trigeminal constriction injury using an operant facial pain assay. Neuroscience. 2012;224:294–306. doi: 10.1016/j.neuroscience.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EM, et al. Use of the Operant Orofacial Pain Assessment Device (OPAD) to Measure Changes in Nociceptive Behavior. J Vis Exp. 2013. p. 50336. [DOI] [PMC free article] [PubMed]


