Abstract
Autologous platelet concentrates represent promising innovative tools in the field of regenerative medicine and have been extensively used in oral surgery. Unlike platelet rich plasma (PRP) that is a gel or a suspension, Leukocyte-Platelet Rich Fibrin (L-PRF) is a solid 3D fibrin membrane generated chair-side from whole blood containing no anti-coagulant. The membrane has a dense three dimensional fibrin matrix with enriched platelets and abundant growth factors. L-PRF is a popular adjunct in surgeries because of its superior handling characteristics as well as its suturability to the wound bed. The goal of the study is to demonstrate generation as well as provide detailed characterization of relevant properties of L-PRF that underlie its clinical success.
Keywords: Bioengineering, Issue 103, Wound Healing, Platelet Concentrates, Leukocyte-Platelet Rich Fibrin, Suture Retention Strength, Uniaxial Tensile Testing, Genipin Crosslinking
Introduction
The use of blood and blood-derived products to seal wounds and improve healing in different clinical situations started with fibrin glues, which are mainly fibrinogen concentrates. Addition of platelets to fibrin glue not only improved their strength but also promoted neoangiogenesis and regeneration. These benefits are attributed to the release of a variety of peptide growth factors from the alpha-granules of platelets upon activation1. Platelet concentrates (PC) were seen as a practical way to deliver growth factors2 and its use was driven by commercial interests rather than research characterization3. In fact, PCs are difficult to characterize unlike homogenous and defined pharmacological preparations, they are a potpourri of signaling molecules and blood cells (platelet and leukocytes) entrapped within a fibrin matrix. Different commercial and proprietary preparations yield a variety of PC that are different in cellular composition, growth factor recovery and kinetics of release4.
It is important to realize that in most oral surgeries, platelet-rich plasma (PRP) preparations are used as a gel in open surgical wounds and not as platelet suspensions. In these situations, the gelation is induced by the addition of thrombin, calcium chloride, batroxobin or other agents and directly placed in the site of injury5. Due to rapid activation, fibrinogen polymerization is often incomplete and results in friable fibrin gels with very little mechanical strength. In addition, injectable PRP gels undergo rapid fibrinolysis6,7.
In contrast, the processes of blood coagulation (fibrinogen polymerization), platelet enrichment and activation occur simultaneously in the preparation of L-PRF8. The coagulation cascade is triggered when whole blood contacts the walls of a dry glass tube and continues throughout the centrifugation process. This results in the formation of a mechanically-strong blood clot (L-PRF) that can be surgically handled and used.
Even though L-PRF has been investigated in terms of optimal methods of preparation, growth factor release and cell distribution9-11, detailed mechanical characterization of these membranes are lacking. This is significant gap in knowledge, given the popularity of these membranes in clinical practice as well as its potential to be used as a biomaterial. Current study focusses on the protocol for deriving L-PRF as well as methods that can be employed to study its mechanical properties. This data is intended to serve as baseline for ongoing studies investigating the viscoelastic properties of this interesting natural biomaterial.
Protocol
All blood-drawing procedures should be done by licensed and certified professionals. Use of human subjects for research involves approval from the Institutional Review Board or other appropriate authority. Special precautions regarding informed consent and protecting participant identification need to be followed. All experiments listed in this protocol involve handling of human blood and/or blood products and appropriate personal protective equipment need to be worn at all times. The waste should be considered as biohazard and disposed of according to regulations.
1. Venipuncture
Identify the patient/ participant and confirm with existing records. Explain the study in detail and get an informed consent.
Have a tray set up for individual patient with tubes marked on a flat stable surface.
Explain the procedure and make the patient seat comfortably with the arm supported. Inform the patient that he or she will feel a small pinch and should remain still throughout the procedure.
Attach the needle to the adapter.
Wash hands and wear gloves.
Prepare the antecubital fossa for venipuncture by cleaning with 70% isopropyl alcohol in concentric circles from center outward. Allow the site to air-dry for 30 sec.
Identify the appropriate vein by palpation.
Apply the tourniquet 3-4 in above the puncture site making sure that it is not too tight (while still feeling the radial pulse).
Perform venipuncture by inserting the bevel of the needle 15-30 degrees to the skin in one smooth motion. Push the blood collection tube through the needle and collect 9 ml of whole blood.
Remove the tourniquet. Remove the tube from the needle.
Withdraw the needle and apply the gauze square at the puncture site prior to needle removal. Dispose the needle in an appropriate biohazard container.
Ask patient to maintain pressure at the puncture site.
Label the tubes
Check the puncture site to be sure bleeding has stopped.
Apply adhesive bandage or tape over the gauze square, ask if the patient is feeling alright (no pain, swelling or light headedness)
Thank the patient/participant prior to discharge.
2. L-PRF Preparation
Immediately after venous blood collection in the red-topped dry glass tube, place it in the Centrifuge.
Centrifuge at 400 x g for 12 min at RT after placing an appropriate counter balance.
Remove the tube at the end of the cycle. Notice the three layers: platelet-poor plasma (PPP), platelet-rich fibrin (L-PRF) and RBC base (Figure 1).
Aspirate the PPP using a pipette. Using tweezers gently pull the L-PRF out and place it in a sterile, perforated metal mesh.
Using surgical scalpel, scrape the bulk of RBC layer carefully leaving the buffy coat intact.
Gently compress the L-PRF clot (using the sterile metal plate, approximate weight 225 g) for 30 sec. Platelet poor plasma will be squeezed out.
Remove the plate and gently lift the L-PRF membrane. The L-PRF membrane is ready for use in experiments12.
3. Uniaxial Tensile Testing
Place L-PRF membranes (n=6) on a filter paper for ease of handling and punch into “dog bones” using custom-made metal dies (2.75 mm wide at their narrowest point with a gauge length of 7.5 mm).
Measure the thickness of each sample at three spots and take the average.
Carefully engage the L-PRF membrane in the center of the jaw grips of the uniaxial testing system.
Carefully tear the filer paper support to expose the L-PRF membrane.
Program the instrument so that the movable head is operating at a constant rate (10.0 mm/min) and start the experiment when the L-PRF is still wet.
Record the elastic modulus, energy to break, and strain at break from the software accompanying the uniaxial testing system. These values are calculated automatically and no user defined input is required. Please see Figure 3.
4. Suture Retention Strength
Place L-PRF membranes (n=3) on a filter paper for ease of handling and cut into rectangular samples measuring (10 mm x 25 mm) using a surgical scalpel.
Measure the thickness of each sample (average of 3).
Make a pinhole in the center of the sample using the stainless steel orthodontic ligature wire (220 µm in diameter).
Pass the ligature wire through the pinhole to form a loop and fix it to the tensile testing machine. Place the edge of the L-PRF membrane to the lower jaw grip13.
Program the instrument so that the movable head is operating at a constant rate (10 mm/min) and start the experiment.
Record the elastic modulus, energy to break, and strain at break from the software accompanying the uniaxial testing system. These values are calculated automatically and no user defined input is required. Please see Figure 3.
5. Morphological Examination
Prepare the L-PRF samples for SEM examination using a 10 mm dermal biopsy punch and place them in a 24-well plate.
Wash the samples with PBS and fix with 2.5% glutaraldehyde (in PBS) for 20 min.
Dehydrate the specimens by immersion in sequentially increasing concentrations of ethanol (50%, 70%, 80%, 90% and 100%) for 5 min each.
Treat with 0.5 ml of 100% HMDS (Hexamethyldisilazane) for 5 min. Aerate O/N to remove excess HMDS14.
Mount samples on stubs using a double-sided tape, sputter-coat platinum for 70 sec and examine in a scanning electron microscope operating at an acceleration voltage of 20 kV (or appropriate setting).
6. Genipin Crosslinking of L-PRF, Trypsin Susceptibility and Ninhydrin Assay
To prepare genipin cross-linked L-PRF, rinse membranes with PBS and soak in 4 ml of 1% genipin solution (in 70% ethanol) for 48 hr. Rinse with PBS prior to experiments to remove excess genipin15, 16.
- Assess the stability of genipin crosslinking of L-PRF by its resistance to degradation by trypsin. Place L-PRF membrane (n=3) and genipin crosslinked L-PRF in 500 µl of 0.01% trypsin and incubated at 37 °C for 3 days with a daily change of trypsin.
- Weigh samples at day 1 prior to enzyme exposure and at day 3. The difference in start and end weight represents enzymatic degradation17.
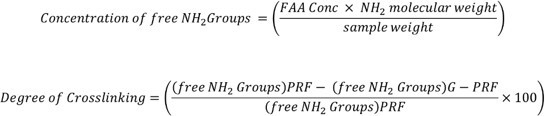
- Quantify the amount of cross-linking in genipin treated L-PRF (G-PRF) by ninhydrin assay. First prepare the standard curve using glycine (1 mM-0.031 mM) curve to establish the relationship between free amino acid concentration (FAA) and absorbance.
- Heat PRF samples with 1 ml of 2% (w/v) ninhydrin for 15 min at 100 °C.
- Allow the solution to cool to RT and add 1.5 ml of 50% ethanol.
- Analyze the absorbance at 570 nm using a suitable spectrophotometer.
- Determine cross-linking percentage using the formula below15
7. MTS Cell Proliferation Assay
Grow MC3T3 (mouse calvarial preosteoblasts) in Minimum Essential Medium –alpha modification (αMEM) in T-75 flasks until an 80% confluent monolayer is obtained.
Prepare fresh, sterile L-PRF membrane (open the L-PRF tube inside the cell culture hood) and transfer the membrane onto a new cell culture dish.
Aspirate media from the flask and rinse the monolayer with PBS, add 5 ml of 0.05% trypsin and place the flask in the 37 oC incubator for 5 min, pipette the contents of the flask into a centrifuge tube and centrifuge at 400 x g for 5 min.
Decant the supernatant, gently tap the tube to break the cell pellet and re-suspend with 4 ml of fresh α MEM, dispense a mixture of cell suspension (50 µl) and trypan blue (50 µl) into the hemocytometer and count the number of cells
Seed 4x105 cells within 10 mm glass cloning rings placed on top of L-PRF membranes to retain the cells within the membranes (rings can be removed after 24 hr).
At day 4, rinse constructs with PBS thrice for 10 min.
Add 1 ml of serum free media and 200 µl MTS reagent to each well and incubate for 2 hr at 37 °C.
Measure absorbance from 200 µl aliquots at 490 nm.
Representative Results
The scanning electron microscope image of the L-PRF clot at different sections (top, middle and bottom) layer is illustrated in Figure 2. As can be seen, the top portion is composed predominantly of fibrin network with no cells. The middle layer is enriched with platelets with evidence of their activation and degranulation. The lower layer has a mixture of leukocytes and red blood cells entrapped within a fibrin matrix.
The mechanical properties were evaluated in two modes: uniaxial tensile testing and suture retention strength test. The results demonstrate viscoelastic behavior of L-PRF. Even though the elastic modulus is low (0.47 MPa), the membrane is tough (energy to break, 5 N·mm) and is capable of undergoing significant deformation (217%, Figure 3). Data from suture retention testing, an indicator of the ability of the membrane to be sutured to the tissues, suggested a significantly tough and deformable material (modulus-0.2 MPa, strain-140% and energy to break-3.2 N.mm) in L-PRF (Figure 4).
One of the limitations of fibrin products in regenerative medicine is its short biological life. Made from endogenous fibrin, L-PRF is susceptible to enzyme degradation and undergoes fibrinolysis. In order to evaluate the resistance of L-PRF to enzyme-mediated degradation, fresh L-PRF was subjected to trypsin treatment (0.01%) and incubated at 37 oC. We observed complete degradation of L-PRF within three days. Genipin crosslinking of L-PRF membranes decreased degradation by almost 60% (Figure 5).
The ability of L-PRF membranes to support cell growth was evaluated by culturing mouse calvarial osteoblasts on crosslinked and uncrosslinked membranes. Uncrosslinked clots underwent degradation to various levels while the genipin crosslinked membranes retained their structure and supported cell growth (Figure 6).
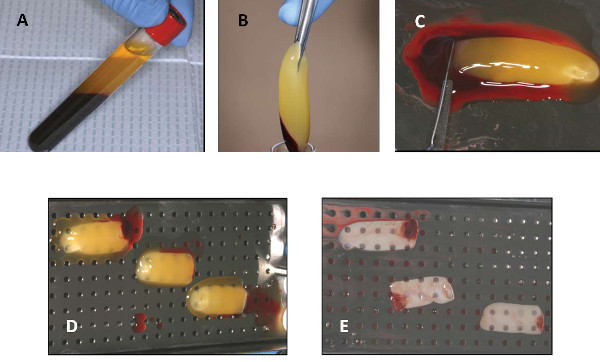 Figure 1. Steps in the generation of L-PRF. (A) After centrifugation of whole blood in a glass tube, three layers will be visible. (B) After decanting the PPP, the L-PRF is being removed using a sterile tweezers. (C) The red blood cell base is being scraped off using a scalpel and laid on a perforated metal tray (D). After gentle compression, the PPP is squeezed out and a firm L-PRF membrane is formed. Please click here to view a larger version of this figure.
Figure 1. Steps in the generation of L-PRF. (A) After centrifugation of whole blood in a glass tube, three layers will be visible. (B) After decanting the PPP, the L-PRF is being removed using a sterile tweezers. (C) The red blood cell base is being scraped off using a scalpel and laid on a perforated metal tray (D). After gentle compression, the PPP is squeezed out and a firm L-PRF membrane is formed. Please click here to view a larger version of this figure.
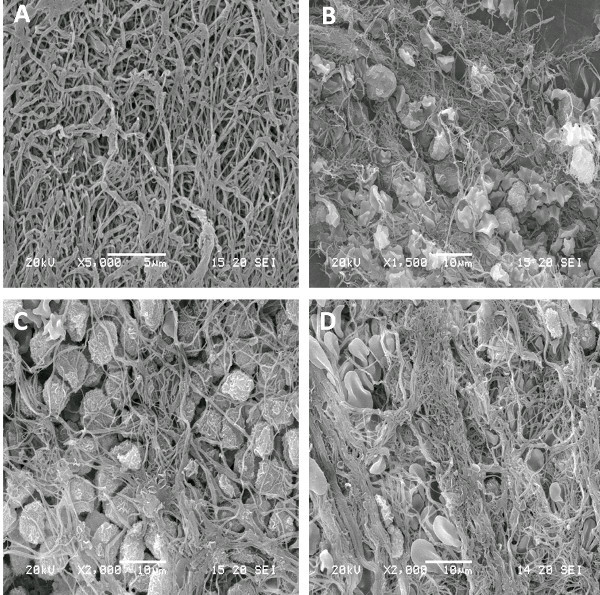 Figure 2. SEM image of different layers of fresh L-PRF. (A) represents the fibrin-rich layer; (B) is a zone of enriched platelets with various degree of activation; (C) is the buffy coat with numerous leukocytes and (D) is the red blood cell base. Please click here to view a larger version of this figure.
Figure 2. SEM image of different layers of fresh L-PRF. (A) represents the fibrin-rich layer; (B) is a zone of enriched platelets with various degree of activation; (C) is the buffy coat with numerous leukocytes and (D) is the red blood cell base. Please click here to view a larger version of this figure.
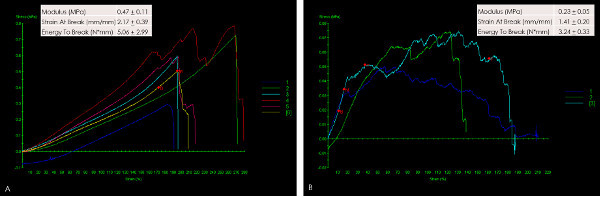 Figure 3. Stress-strain curves following mechanical loading of L-PRF in uniaxial tensile testing mode (A) and Suture retention strength (B). The loading pattern of each sample is represented in different color. The uniaxial tensile testing data (A) indicate a low modulus, a large elastic deformation and a rapid failure. Upon distension by a suture (B), L-PRF represents a membrane that is tough (area under the curve) as well as distensible. Good clustering of data suggests minimal variation between samples. Please click here to view a larger version of this figure.
Figure 3. Stress-strain curves following mechanical loading of L-PRF in uniaxial tensile testing mode (A) and Suture retention strength (B). The loading pattern of each sample is represented in different color. The uniaxial tensile testing data (A) indicate a low modulus, a large elastic deformation and a rapid failure. Upon distension by a suture (B), L-PRF represents a membrane that is tough (area under the curve) as well as distensible. Good clustering of data suggests minimal variation between samples. Please click here to view a larger version of this figure.
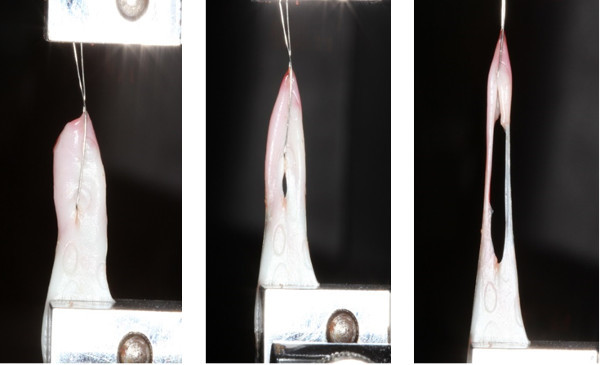 Figure 4. Photographs of actual failure in suture retention strength testing. A 220 µm thick stainless steel orthodontic ligature wire was passed through the middle of L-PRF and tied to the upper jaw member of the tensile testing machine. The other end was attached to the lower grip and stretched at a constant rate. Notice the elongation of the membrane and its resistance to tear, suggesting excellent resilience of L-PRF. Please click here to view a larger version of this figure.
Figure 4. Photographs of actual failure in suture retention strength testing. A 220 µm thick stainless steel orthodontic ligature wire was passed through the middle of L-PRF and tied to the upper jaw member of the tensile testing machine. The other end was attached to the lower grip and stretched at a constant rate. Notice the elongation of the membrane and its resistance to tear, suggesting excellent resilience of L-PRF. Please click here to view a larger version of this figure.
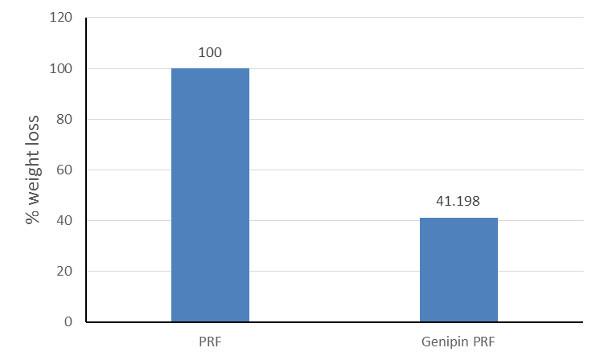 Figure 5.Degradation of L-PRF membranes following incubation in 0.01% trypsin. All L-PRF membranes disintegrated completely in trypsin within 3 days while genipin-crosslinked L-PRF were 60% more stable. This shows that chemical crosslinking can be a viable strategy to improve the longevity of L-PRF membranes when placed in vivo.
Figure 5.Degradation of L-PRF membranes following incubation in 0.01% trypsin. All L-PRF membranes disintegrated completely in trypsin within 3 days while genipin-crosslinked L-PRF were 60% more stable. This shows that chemical crosslinking can be a viable strategy to improve the longevity of L-PRF membranes when placed in vivo.
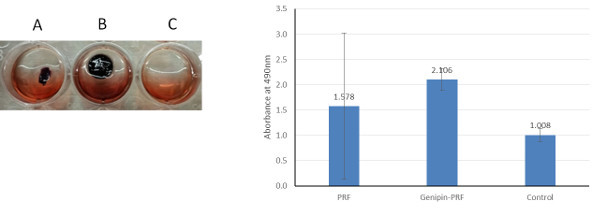 Figure 6. Effect of L-PRF crosslinking on cell viability. Representative images of 4 day culture of MC3T3 cells on uncrosslinked L-PRF (A), genipin-crosslinked L-PRF (B) and tissue culture plastic (C). Uncrosslinked L-PRF degraded in culture to variable extent and showed cell activity similar to plastic. Genipin crosslinked L-PRF maintained their structure and supported robust cell survival. To the right is quantified data (+SD) from independent experiments with three replicates. Please click here to view a larger version of this figure.
Figure 6. Effect of L-PRF crosslinking on cell viability. Representative images of 4 day culture of MC3T3 cells on uncrosslinked L-PRF (A), genipin-crosslinked L-PRF (B) and tissue culture plastic (C). Uncrosslinked L-PRF degraded in culture to variable extent and showed cell activity similar to plastic. Genipin crosslinked L-PRF maintained their structure and supported robust cell survival. To the right is quantified data (+SD) from independent experiments with three replicates. Please click here to view a larger version of this figure.
Discussion
Autologous platelet concentrates are promising in the field of regenerative medicine18 because of the abundance of growth factors. However, these preparations often lacked a defined structure that makes surgical manipulation very difficult. Many times, the suspensions and gels are not retained effectively at the site of delivery, resulting in unpredictable outcomes. L-PRF represents a huge advance in the evolution of platelet concentrates in that it is essentially a firm fibrin membrane with entrapped platelets. These solid membranes possess excellent handling characteristics, and can be securely sutured at an anatomically desired location during open surgeries. However, its physical and biological properties are relatively unknown.
The L-PRF will form consistently when steps described above are strictly adhered to (Figure 1). One of the important considerations in generating a good L-PRF membrane is the time delay between blood draw and centrifugation. The success of L-PRF technique entirely depends on the speed of blood collection and immediate transfer to the centrifuge19, usually within a minute. It is impossible to generate a well-structured L-PRF clot (with its specific cell content, matrix architecture and growth factor release profile), if blood harvesting is prolonged and not homogenous; a small incoherent, friable mass of fibrin with unknown content is formed instead.
It has been accepted that mechanobiological interactions between cells and extracellular matrix (ECM) have a critical influence in all aspects of cell behavior including migration, proliferation and differentiation20,21. L-PRF, a unique type of blood clot, is formed under specific circumstances and is comprised of complex, branched network of fibrin. L-PRF functions as a provisional ECM that is turned over into functional tissue during healing. Being subjected to mechanical forces, successful healing outcomes are dependent on the structural integrity of L-PRF and hence elucidating their physical properties is important. We performed uniaxial tensile testing (to identify the intrinsic material properties) and suture retention testing (to identify the failure characteristics) on fresh L-PRF. Unlike PRP gel or clotted blood that does not have a defined structures, L-PRF resembles dense connective tissue with superior handling characteristics. We report an elastic modulus of 0.470 MPa (SD=0.107) for L-PRF membranes and stretch twice its initial length before failure (strain of 215%). These data match with published literature22,23 who reported low stiffness (1-10 MPa) and high strain (up to 150%) before breaking. The difference in the values can be due to the use of fibrin network compared to the use of AFM analysis of single fibrin fiber in the above mentioned studies.
Suture retention strength is a surgically important parameter of graft materials and it is defined as the force necessary to pull a suture from the graft or cause the wall of the graft to fail. Our experiments used straight-across procedure (as defined by ANSI24). The force required to the pull the ligature wire through the L-PRF of 3.23 N.mm (SD=0.329). Overall, we found the L-PRF to be mechanically tough, capable of supporting loads and the ability to stretch twice as much on tension and retains sutures quite well (deforms significantly before tearing).
The lack of stability and structural integrity of L-PRF in biological environments is a major limitation in its use in tissue engineering. We sought to address this issue by chemically crosslinking L-PRF using genipin. Unlike gluteraldehyde which is associated with toxicity, genipin is a naturally occurring biodegradable molecule with low cytotoxicity. After genipin treatment, the membranes were significantly stable in trypsin and supported cell proliferation over 4 days. However, only 20% of L-PRF was crosslinked with genipin (determined by ninhydrin assay). This data suggests that while chemical crosslinking is a viable strategy, other alternatives need to be explored.
Based on these findings, it is clear that L-PRF is a novel biomaterial with unique attributes: predictable preparation from autologous blood, simplicity of protocol, defined architecture, impressive mechanical properties and abundance of growth factors from activated platelets. The blood is allowed to clot under physiological conditions with no exposure to anti-coagulants, exogenous thrombin and calcium chloride. All of these characteristics make L-PRF promising biomaterial for applications in regenerative medicine.
One of the clinical issues to deal with in the application of L-PRF is the heterogeneity in the quality of platelets and blood components. At present, very little is understood about L-PRF generated from patients with coagulation disorders or patients on medications that affect blood clotting (heparin, warfarin or platelet inhibitors). Answers to these questions will undoubtedly improve our understanding of healing as well as contribute to advance the field of personalized medicine.
Disclosures
The authors have nothing to disclose and confirm that there are no known conflicts of interest associated with this publication.
Acknowledgments
The project was supported by CTSA (UL1TR000058) from the National Center for Advancing Translational Sciences) and the CCTR Endowment Fund of Virginia Commonwealth University. The contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
References
- Marx RE, et al. Platelet-rich plasma Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638–646. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez IA, Growney Kalaf EA, Bowlin GL, Sell SA. Platelet-rich plasma in bone regeneration: Engineering the delivery for improved clinical efficacy. BioMed Res In. 2014;2014 doi: 10.1155/2014/392398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Corso M, et al. Current Knowledge and Perspectives for the Use of Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in Oral and Maxillofacial Surgery Part 1: Periodontal and Dentoalveolar Surgery. Curr Pharm Biotechno. 2012;13(7):1207–1230. doi: 10.2174/138920112800624391. [DOI] [PubMed] [Google Scholar]
- Mazzucco L, Balbo V, Cattana E, Guaschino R, Borzini P. Not every PRP-gel is born equal Evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet, RegenPRP-Kit, Plateltex and one manual procedure. Vox San. 2009;97(2):110–118. doi: 10.1111/j.1423-0410.2009.01188.x. [DOI] [PubMed] [Google Scholar]
- Fernández-Barbero JE, et al. Flow cytometric and morphological characterization of platelet-rich plasma gel. Clin Oral Implants Re. 2006;17(6):687–693. doi: 10.1111/j.1600-0501.2006.01179.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Guan J. Hydrogels for cardiac tissue engineering. Polymer. 2011;3(2):740–761. [Google Scholar]
- Zhu J, Cai B, Ma Q, Chen F, Wu W. Cell bricks-enriched platelet-rich plasma gel for injectable cartilage engineering – an in vivo experiment in nude mice. J Tissue Eng Regen Med. 2013;7(10):819–830. doi: 10.1002/term.1475. [DOI] [PubMed] [Google Scholar]
- Dohan DM, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e37–e44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Dohan DM, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e45–e50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Choukroun J, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):299–303. doi: 10.1016/j.tripleo.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Dohan Ehrenfest DM, Bielecki T, et al. Do the Fibrin Architecture and Leukocyte Content Influence the Growth Factor Release of Platelet Concentrates? An Evidence-based Answer Comparing a Pure Platelet-Rich Plasma (P-PRP) Gel and a Leukocyte- and Platelet-Rich Fibrin (L-PRF) Curr Pharma Biotechno. 2012;13(7):1145–1152. doi: 10.2174/138920112800624382. [DOI] [PubMed] [Google Scholar]
- Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechno. 2009;27(3):158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Mine Y, et al. Suture Retention Strength of Expanded Polytetrafluoroethylene (ePTFE) Graft. Acta Med Okayam. 2010;64(2):121–128. doi: 10.18926/AMO/32846. [DOI] [PubMed] [Google Scholar]
- Braet F, De Zanger R, Wisse E. Drying cells for SEM , AFM and TEM by hexamethyldisilazane: a study on hepatic endothelial cells. J Micros. 1997;186(1):84–87. doi: 10.1046/j.1365-2818.1997.1940755.x. [DOI] [PubMed] [Google Scholar]
- Yuan Y, et al. The effect of cross-linking of chitosan microspheres with genipin on protein release. Carbohydr Poly. 2007;68(3):561–567. [Google Scholar]
- Sell SA, et al. Cross-linking methods of electrospun fibrinogen scaffolds for tissue engineering applications. Biomed Mater. 2008;3(4) doi: 10.1088/1748-6041/3/4/045001. [DOI] [PubMed] [Google Scholar]
- Gorczyca G, et al. Preparation and characterization of genipin cross-linked porous chitosan-collagen-gelatin scaffolds using chitosan-CO2 solution. Carbohydr Poly. 2014;102:901–911. doi: 10.1016/j.carbpol.2013.10.060. [DOI] [PubMed] [Google Scholar]
- Rozman P, Semenic D. Chapter 15. The Role of Platelet Gel in Regenerative Medicine. In: Wislet-Gendebien Sabine., editor. Advances In Regenerative Medicine. In Tech; 2011. pp. 319–349. [Google Scholar]
- Dohan Ehrenfest , Lemo DM, Jimbo N. Selecting a relevant animal model for testing the in vivo effects of Choukroun’s platelet-rich fibrin (PRF): Rabbit tricks and traps. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(4):413–416. doi: 10.1016/j.tripleo.2010.05.057. [DOI] [PubMed] [Google Scholar]
- Guilak F, Baaijens FP. Functional tissue engineering: Ten more years of progress. J Biomec. 2014;47(9):1931–1932. doi: 10.1016/j.jbiomech.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Guilak F, Butler DL, Goldstein SA, Baaijens FP. Biomechanics and mechanobiology in functional tissue engineering. J Biomec. 2014;47(9):1933–1940. doi: 10.1016/j.jbiomech.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet JP, Shuman H, Ledger RE, Lee S. The elasticity of an individual fibrin fiber in a clot. Proc Natl Acad Sci. 2005;102(26):9133–9137. doi: 10.1073/pnas.0504120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, et al. Fibrin fibers have extraordinary extensibility and elasticity. Science. 2006;313(5787):634. doi: 10.1126/science.1127317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American National Standard. Association for the Advancement of Medica lnstrumentation Guidance document: Cardiovascular Implants - Tubular vascular prostheses. ANSI/AAMI/ISO; 2001. Chapter 8: Test methods for vascular prostheses; pp. 33–34. [Google Scholar]


