Abstract
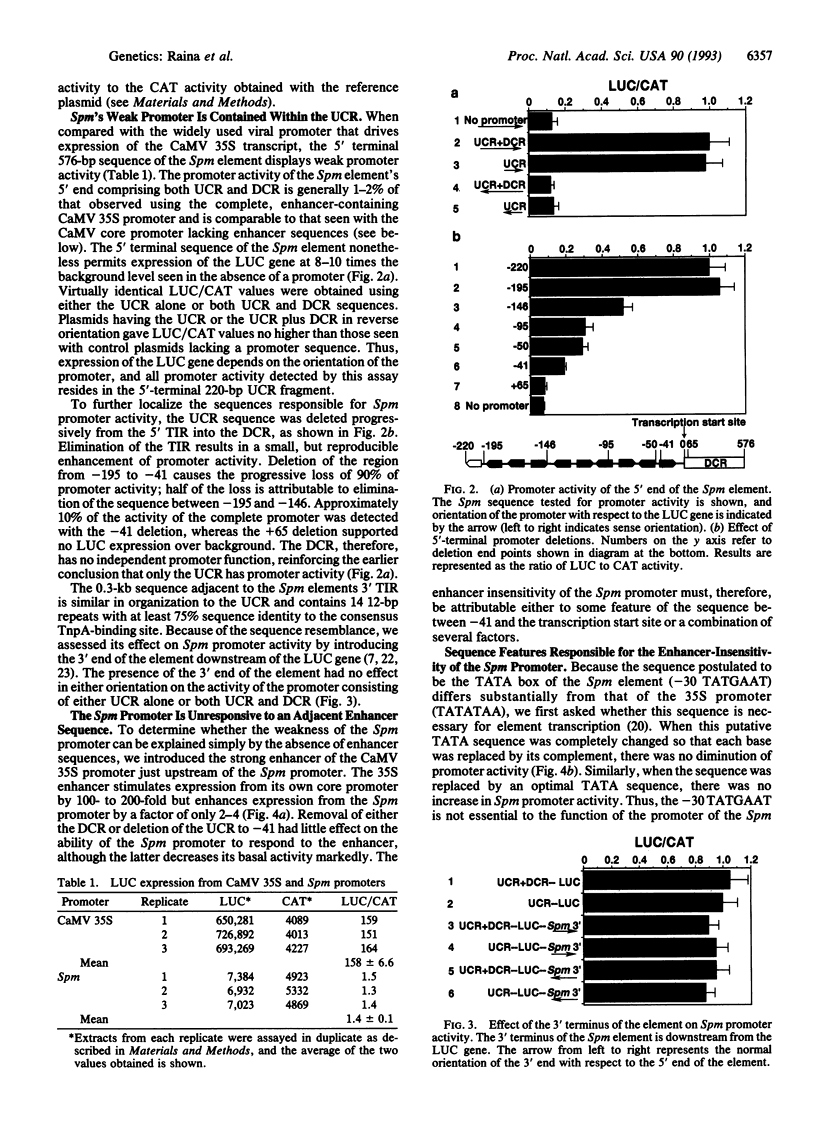
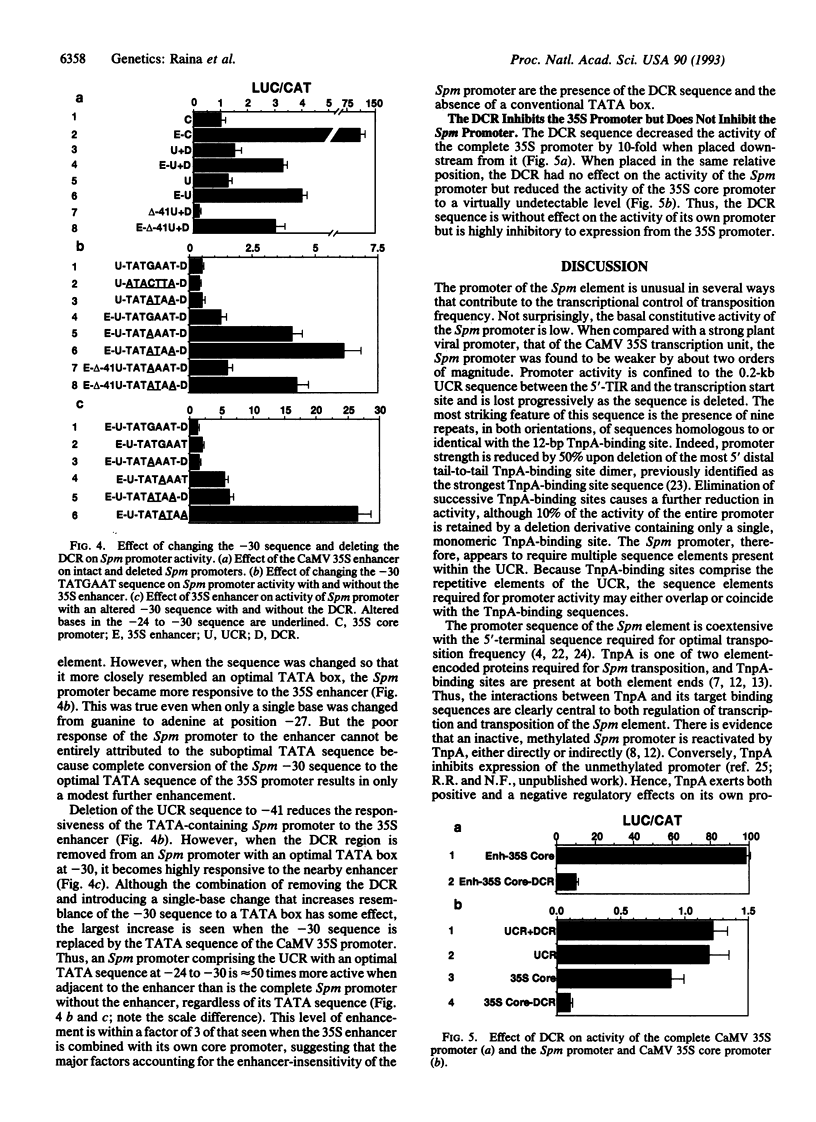
We have used a transient assay system to investigate the promoter region of the maize Suppressor-mutator (Spm) transposable element. All of the sequence required for constitutive promoter activity is confined to the 0.2-kb sequence upstream from the transcription start site of the element at nt 209 and designated the upstream control region. The element's promoter is weak, lacks a conventional TATA box, and depends on the presence of multiple, short repetitive sequence elements. The Spm promoter is quite insensitive to the enhancer sequence of the cauliflower mosaic virus 35S promoter. Enhancer sensitivity can be restored by providing a -30 TATA sequence and removing the G + C-rich sequence encoding the untranslated leader of the element, designated the downstream control region. Although the downstream control region is without effect on Spm promoter activity, it completely inhibits the 35S core promoter and markedly inhibits activity of the complete 35S promoter. The properties of the Spm element's promoter buffer it from both mutational and position-dependent changes in activity. We suggest that the inherent characteristics of the promoter are part of the genetic mechanism that controls the element's transposition frequency, ensuring it remains low and insertion-site independent.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks J. A., Masson P., Fedoroff N. Molecular mechanisms in the developmental regulation of the maize Suppressor-mutator transposable element. Genes Dev. 1988 Nov;2(11):1364–1380. doi: 10.1101/gad.2.11.1364. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Breeden L., Abraham J., Sternglanz R., Nasmyth K. Characterization of a "silencer" in yeast: a DNA sequence with properties opposite to those of a transcriptional enhancer. Cell. 1985 May;41(1):41–48. doi: 10.1016/0092-8674(85)90059-5. [DOI] [PubMed] [Google Scholar]
- Fedoroff N. V., Banks J. A. Is the Suppressor-mutator element controlled by a basic developmental regulatory mechanism? Genetics. 1988 Oct;120(2):559–577. doi: 10.1093/genetics/120.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey M., Reinecke J., Grant S., Saedler H., Gierl A. Excision of the En/Spm transposable element of Zea mays requires two element-encoded proteins. EMBO J. 1990 Dec;9(12):4037–4044. doi: 10.1002/j.1460-2075.1990.tb07625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierl A., Lütticke S., Saedler H. TnpA product encoded by the transposable element En-1 of Zea mays is a DNA binding protein. EMBO J. 1988 Dec 20;7(13):4045–4053. doi: 10.1002/j.1460-2075.1988.tb03298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. A., Cone K. C., Fromm M. E. Identification of functional domains in the maize transcriptional activator C1: comparison of wild-type and dominant inhibitor proteins. Genes Dev. 1991 Feb;5(2):298–309. doi: 10.1101/gad.5.2.298. [DOI] [PubMed] [Google Scholar]
- Grant S. R., Gierl A., Saedler H. En/Spm encoded tnpA protein requires a specific target sequence for suppression. EMBO J. 1990 Jul;9(7):2029–2035. doi: 10.1002/j.1460-2075.1990.tb07369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S., Kadonaga J. T., Tjian R., Brady J. N., Merlino G. T., Pastan I. Binding of the Sp1 transcription factor by the human Harvey ras1 proto-oncogene promoter. Science. 1986 Jun 13;232(4756):1410–1413. doi: 10.1126/science.3012774. [DOI] [PubMed] [Google Scholar]
- Kaufman P. D., Rio D. C. Drosophila P-element transposase is a transcriptional repressor in vitro. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2613–2617. doi: 10.1073/pnas.88.7.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L., Holmgren-König M., Khoury G. Transcriptional "silencer" element in rat repetitive sequences associated with the rat insulin 1 gene locus. Proc Natl Acad Sci U S A. 1986 May;83(10):3151–3155. doi: 10.1073/pnas.83.10.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P., Fedoroff N. V. Mobility of the maize suppressor-mutator element in transgenic tobacco cells. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2219–2223. doi: 10.1073/pnas.86.7.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P., Rutherford G., Banks J. A., Fedoroff N. Essential large transcripts of the maize Spm transposable element are generated by alternative splicing. Cell. 1989 Aug 25;58(4):755–765. doi: 10.1016/0092-8674(89)90109-8. [DOI] [PubMed] [Google Scholar]
- Masson P., Strem M., Fedoroff N. The tnpA and tnpD gene products of the Spm element are required for transposition in tobacco. Plant Cell. 1991 Jan;3(1):73–85. doi: 10.1105/tpc.3.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P., Surosky R., Kingsbury J. A., Fedoroff N. V. Genetic and molecular analysis of the Spm-dependent a-m2 alleles of the maize a locus. Genetics. 1987 Sep;117(1):117–137. doi: 10.1093/genetics/117.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. W., Konecki D. S., Brennand J., Caskey C. T. Structure, expression, and mutation of the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2147–2151. doi: 10.1073/pnas.81.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menssen A., Höhmann S., Martin W., Schnable P. S., Peterson P. A., Saedler H., Gierl A. The En/Spm transposable element of Zea mays contains splice sites at the termini generating a novel intron from a dSpm element in the A2 gene. EMBO J. 1990 Oct;9(10):3051–3057. doi: 10.1002/j.1460-2075.1990.tb07501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell J. T., Nagy F., Chua N. H. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. 1985 Feb 28-Mar 6Nature. 313(6005):810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- Ogden J. E., Stanway C., Kim S., Mellor J., Kingsman A. J., Kingsman S. M. Efficient expression of the Saccharomyces cerevisiae PGK gene depends on an upstream activation sequence but does not require TATA sequences. Mol Cell Biol. 1986 Dec;6(12):4335–4343. doi: 10.1128/mcb.6.12.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow D. W., DE Wet J. R., Helinski D. R., Howell S. H., Wood K. V., Deluca M. Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science. 1986 Nov 14;234(4778):856–859. doi: 10.1126/science.234.4778.856. [DOI] [PubMed] [Google Scholar]
- Pereira A., Cuypers H., Gierl A., Schwarz-Sommer Z., Saedler H. Molecular analysis of the En/Spm transposable element system of Zea mays. EMBO J. 1986 May;5(5):835–841. doi: 10.1002/j.1460-2075.1986.tb04292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell I. J., Kössl M. Voltage responses to tones of outer hair cells in the basal turn of the guinea-pig cochlea: significance for electromotility and desensitization. Proc Biol Sci. 1992 Feb 22;247(1319):97–105. doi: 10.1098/rspb.1992.0014. [DOI] [PubMed] [Google Scholar]
- Schläppi M., Smith D., Fedoroff N. TnpA trans-activates methylated maize Suppressor-mutator transposable elements in transgenic tobacco. Genetics. 1993 Apr;133(4):1009–1021. doi: 10.1093/genetics/133.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans M. C., Maliga P., Vieira J., Messing J. The pFF plasmids: cassettes utilising CaMV sequences for expression of foreign genes in plants. J Biotechnol. 1990 Jun;14(3-4):333–344. doi: 10.1016/0168-1656(90)90117-t. [DOI] [PubMed] [Google Scholar]



