Abstract
Dysfunctional skeletal muscle mitochondria play a role in altered metabolism observed with aging, obesity and Type II diabetes. Mitochondrial respirometric assays from isolated mitochondrial preparations allow for the assessment of mitochondrial function, as well as determination of the mechanism(s) of action of drugs and proteins that modulate metabolism. Current isolation procedures often require large quantities of tissue to yield high quality mitochondria necessary for respirometric assays. The methods presented herein describe how high quality purified mitochondria (~ 450 µg) can be isolated from minimal quantities (~75-100 mg) of mouse skeletal muscle for use in high throughput respiratory measurements. We determined that our isolation method yields 92.5± 2.0% intact mitochondria by measuring citrate synthase activity spectrophotometrically. In addition, Western blot analysis in isolated mitochondria resulted in the faint expression of the cytosolic protein, GAPDH, and the robust expression of the mitochondrial protein, COXIV. The absence of a prominent GAPDH band in the isolated mitochondria is indicative of little contamination from non-mitochondrial sources during the isolation procedure. Most importantly, the measurement of O2 consumption rate with micro-plate based technology and determining the respiratory control ratio (RCR) for coupled respirometric assays shows highly coupled (RCR; >6 for all assays) and functional mitochondria. In conclusion, the addition of a separate mincing step and significantly reducing motor driven homogenization speed of a previously reported method has allowed the isolation of high quality and purified mitochondria from smaller quantities of mouse skeletal muscle that results in highly coupled mitochondria that respire with high function during microplate based respirometirc assays.
Keywords: Cellular Biology, Issue 105, Skeletal muscle, mitochondrial isolation, mouse model, metabolism, western blot, citrate synthase activity
Introduction
The primary function of mitochondria is to produce ATP from oxidative phosphorylation. However, mitochondria have many other important cellular functions including but not limited to: the production and detoxification of reactive oxygen species, the regulation of cytoplasmic and mitochondrial calcium, organelle trafficking, ionic homeostasis, and involvement in apoptosis1,2. Therefore, it is not surprising that dysfunctional mitochondria play a role in many disease pathologies, such as aging, neurodegenerative diseases, cardiovascular disease, cancer, obesity, and diabetes3,4. Importantly, skeletal muscle mitochondria specifically are involved in many of these pathologies3-5.
Mitochondrial respiration assays using isolated mitochondria allow for the assessment of electron transport chain and oxidative phosphorylation function, and the determination of mechanism(s) of action of drugs and proteins that modulate metabolism. Mitochondrial isolation procedures exist for multiple tissue and cell types for a variety of species6,7. However, these procedures often require large quantities of tissue/cells for a high quality mitochondria yield necessary for classic respirometric assays.
Microplate based respirometirc assays allow for high throughput measurements using minimal quantities of isolated mitochondria, often just several µg per well8. Therefore, we present a modification of previously published methods7 to allow for high quality mitochondria to be isolated from smaller quantities of mouse skeletal muscle for use in microplate based respirometirc assays. In addition, methods are provided to establish the quality of the mitochondrial isolation preparation and the integrity of the mitochondrial membranes. Given that skeletal muscle mitochondria are involved in many pathological conditions, the measurement of O2 consumption in mechanistically driven studies is becoming more prevalent in biomedical research9,10.
Protocol
Animal studies were performed under an approved protocol by the Institutional Animal Care and use Committee at Virginia Polytechnic Institute and State University.
1. Setup (Time: ~45 min)
Thaw frozen stores of 0.25% Trypsin, Isolation Buffer for Mitochondria (IBM) 1 and IBM2 in a 37 °C water bath.
Rinse glassware and dissecting instruments in 70% ethanol followed by high purity water.
Prepare 0.05% trypsin solution from 0.25% trypsin stock by diluting 1 part trypsin in 4 parts IBM1.
Mix protease and phosphatase inhibitor cocktail with cell lysis buffer (1:100 ratio) in a 1.5 ml microcentrifuge tube with screw cap.
Aliquot 5 ml (5 ml/mouse) of 0.05% trypsin into a 15 ml plastic tube.
Set motor driven tissue homogenizer to 80 RPM.
2. Isolation of Skeletal Muscle Mitochondria (Time: ~90 min)
Euthanize a mouse by CO2 inhalation, followed by cervical dislocation.
- Remove red muscle from the quadriceps and gastrocnemius muscle, which includes the soleus (~75-100 mg total) as described in the following steps:
- Peel the skin towards the mouse.
- Remove the fat pad over the quadriceps origin point. Cut the quadriceps tendon that is attached to the patella with fine tipped scissors. Note: The quadriceps is identified by its anatomical position on the anterior portion of the proximal femur.
- Slowly snip the aponeurosis between the bone and the quadriceps, while avoiding the femoral artery, to liberate the quadriceps from the bone. Cut the tendon with fine tipped scissors at the origin point on the femur to liberate the quadriceps muscle and place the quadriceps muscle in chilled PBS.
- Remove visible adipose tissue over the quadriceps with scissors. Flip the quadriceps over so that the portion of the muscle that was overlying the femur is facing up. Open the quadriceps muscle with forceps in a fanning motion. Remove the two visible red muscle portions from each lobe with fine tipped scissors and place in a beaker containing 5 ml of chilled IBM1. Note: Quadriceps muscle appear as two lobes with a strip of red muscle located near the lateral edge of each lobe.
- Cut the skin overlying the Achilles tendon with fine tipped scissors and peel the skin toward the mouse. Cut the exposed Achilles tendon and peel the muscle towards the body of the mouse.
- Cut the tendon at the lateral and medial condyles of the femur with fine tipped scissors to liberate the gastrocnemius and place the gastrocnemius attached to its tendons in chilled PBS.
- Flip the gastrocnemius over and peel off the soleus muscle and place into the beaker containing 5 ml of chilled IBM1. Fan open the gastrocnemius. Remove the three visual red muscle portions (two lateral and one medial superficial red strips) with fine tipped scissors and transfer them to the beaker containing 5 ml of chilled IBM1.
Remove another ~75-100 mg of red muscle from the other leg of the mouse (as described in step 2.2) and place into a 1.5 ml microcentrifuge tube with protease and phosphatase inhibitor cocktail and cell lysis buffer (50 mM Tris-HCL, 1 mM EDTA, 150 mM NaCl, 1% Sodium dodecyl sulfate, 0.5% Sodium deoxycholate, 1% Polyoxyethylene (9) nonylphenylether, branched. Immediately flash-freeze this second sample in liquid nitrogen for later use in Western blotting (see step 6.1).
Place a pre-chilled, flat plastic surface on ice in a large bucket. Pour a drop of IBM1 onto the plastic surface and use tweezers to place all of the sectioned red muscle (step 2.2.4 and 2.2.7) in IBM1 droplet. Mince the muscle tissue for 2 min using 3 single edge razor blades by changing razors every 40 sec.
Transfer the minced tissue to a new beaker with 5 ml of fresh IBM1 by holding the plastic surface over the beaker and scraping the muscle into the beaker with a razor blade. Take this solution and drain it through a 100 µm cell strainer placed onto a 50 ml conical tube.
Blot the tissue with a delicate task wiper and then transfer it into 5 ml of the 0.05% trypsin solution. Use tweezers to remove the tissue from the task wiper.
Incubate the muscle tissue on ice for 30 min in 0.05% trypsin solution.
Spin the 15 ml conical tube containing trypsin/muscle mixture at 200 x g for 3 min at 4 °C.
Pour the trypsin supernatant into a waste container and resuspend the pellet with 3 ml of ice cold IBM1. Transfer the tissue to a 45 ml glass homogenizer tube. Rinse the 15 ml conical tube with another 1.5 ml IBM1 to gather any remaining sample and add this to the glass homogenizer tube.
Place the glass homogenizer tube into a beaker or plastic container filled halfway with ice so the glass homogenizer moves minimally within the beaker.
Homogenize the tissue/IMB1 mixture with the PTFE pestle attached to a motor driven tissue homogenizer with 10 passes at 80 rpm. Hold the bottom of each pass for ~2 sec.
Transfer the tissue homogenate to a new 15 ml conical tube and rinse the glass homogenizer tube with 6.5 ml of IBM1. Pour the IBM1 into the 15 ml conical tube containing tissue homogenate.
Spin the 15 ml conical tube at 700 g for 10 min at 4 °C. Gently pour the supernatant into a glass high strength centrifuge tube and discard the pellet.
Spin the supernatant from the above step at 8,000 x g for 10 min at 4 °C.
Remove the IBM1 supernatant and re-suspend the pellet by slowly adding 500 µl of IBM2. Cut off the end of a pipette tip and gently homogenize the pellet in IBM2 with mixing and stirring motions. Add another 4.5 ml of IBM2 to the mixture after the pellet is fully suspended. Note: Avoid excessive trituration while breaking larger pellet pieces as this may damage the mitochondrial membranes.
Spin the IBM2/tissue homogenate at 8,000 x g for 10 min at 4 °C. Note: This step may be repeated in a clean high strength centrifuge tube for more accurate protein quantification of isolated mitochondria in Step 4 since residual BSA may contribute to total protein content.
Remove the supernatant and gently, but completely re-suspend the pellet by adding two 25 µl increments of IBM2 with a pipette tip with its point cut off. Gently stir and mix the pellet after each addition of 25 µl of IBM2. Place this mitochondrial stock on ice.
3. Homogenization of Whole Tissue Lysate (Time: ~45 min; Can Be Done During Step 7)
Remove the muscle from that was placed in the liquid nitrogen from step 2.3 and incubate at room temperature until fully thawed.
Mince the tissue for ~30 sec on ice with fine tipped scissors.
Add two 100 µl scoops of Zirconium Oxide Beads to the 1.5 ml microcentrifuge tube with screw cap from step 3.2 that contains the minced tissue. Homogenize the tissue in a bead mill tissue homogenizer using two to three 5 min cycles at a speed setting of 4-6 (medium setting).
Spin the mixture from step 3.3 at 12,000 x g for 10 min at 4 °C. Remove the supernatant using a 1,000 µl pipette tip after the spin and transfer into a 1.5 ml microcentrifuge tube. Discard the pellet and place the supernatant on ice.
4. Protein Determination (Time: ~30 min)
Determine the protein concentration of the whole tissue lysate (step 3.4) and mitochondrial stock (step 2.17) using the BCA Protein assay kit according to the manufacturer’s specifications.
5. Mitochondria Membrane Integrity; Citrate Synthase (CS) Activity (Time: ~15 min)
Make two separate 1: 20 dilutions of the mitochondrial stock in high purity water. Add 0.1% of Triton 100-X to one sample and sonicate it at a low setting (765 Watts, amplitude of 2). Leave the other dilution on ice. Return the sample to ice after sonication.
Perform a citrate synthase assay with both the non-sonicated and sonicated mitochondrial dilutions using a spectrophotometric assay as previously described11,12.
Determine the percentage of intact mitochondria membranes by using the following equations: (Non-sonicated CS activity ÷ Sonicated CS activity)* 100
100- N (percent attained from above)
6. Mitochondria Isolation Quality; Western Blotting (Time: According to Lab Protocol)
Perform Western blotting on the whole tissue lysate and isolated mitochondria as described previously12. Note: Use primary antibodies for GAPDH (1: 1,000 dilution in 5% BSA/TBS-T) and COXIV (1:1,000 dilution in 5% non-fat milk/TBS-T) followed by anti-goat, and rabbit secondary antibodies, respectively (1:10,000).
7. Respirometric Assay Run (Time: ~90 min)
Perform desired respirometric assays using microplate based O2 consumption measuring technology as previously described (see companion article and Rogers et al8).
Determine the respiratory control ratio (RCR) by dividing State 3 by State 4o (RCR) or dividing State 3u by State 4o. Use either the middle (average) point, or the highest (State 3) and lowest rates (State 4o) from the point-to-point traces when determining RCR.
Representative Results
Citrate synthase activity serves as a measure for membrane integrity since citrate synthase is located in the inner mitochondrial membrane, and thus should not be present in suspensions of mitochondria with intact membranes. Figure 1 represents citrate synthase activity in non-sonicated mitochondrial samples compared with sonicated samples from the same isolation. Sonicating the mitochondria results in a statistically significant increase in citrate synthase activity (P <0.01). Importantly, 92.5 ± 2.0% of mitochondria were intact following the isolation.
Figure 2 depicts GAPDH and COXIV expression in isolated mitochondria and the whole skeletal muscle tissue lysate. GAPDH is a protein located in the cytosol, but can also be found in the nucleus after translocation, while COXIV is a protein located in the inner mitochondrial membrane. Expression of GAPDH and COXIV are evident in the whole tissue lysate. In the isolated mitochondria, expression of COXIV is observed, while only faint bands of GAPDH are evident. These findings indicate good mitochondrial isolations, with little contamination from non-mitochondrial components during the isolation procedure.
Figure 3 is a representative tracing of O2 consumption rates (OCR) vs. time for a coupling assay (10 mM pyruvate/5 mM malate) performed from mitochondria isolated using this protocol and performed with microplate based O2 consumption technology. Each panel represents O2 consumption in different mitochondrial states as described by Chance and Williams13. The first panel represents basal O2 consumption, or State 2. The second panel, after injection of ADP, represents maximal coupled respiration, or State 3. The third panel, after injection of oligomycin A (an inhibitor of Complex V), represents respiration due to proton leak, or State 4o. The fourth panel, after injection of FCCP, represents maximal uncoupled respiration, or State 3u. Finally, the fifth panel, after injection of Antimycin A, represents the inhibition of oxidative respiration. The respiratory control ratio (RCR), a measure of mitochondrial function1, was calculated by dividing State 3 by State 4o. Table 4 reports the average RCR values for alternative substrate coupling assays that can be performed from the mitochondria yield from this protocol. Importantly, the O2 consumption tracing and the high RCR values represent highly functioning and coupled mitochondria. RCR values reported herein are higher or similar than previously reported using similar isolation protocols in murine skeletal muscle7,14,15, thus accentuating the high quality of the mitochondria isolated during this procedure.
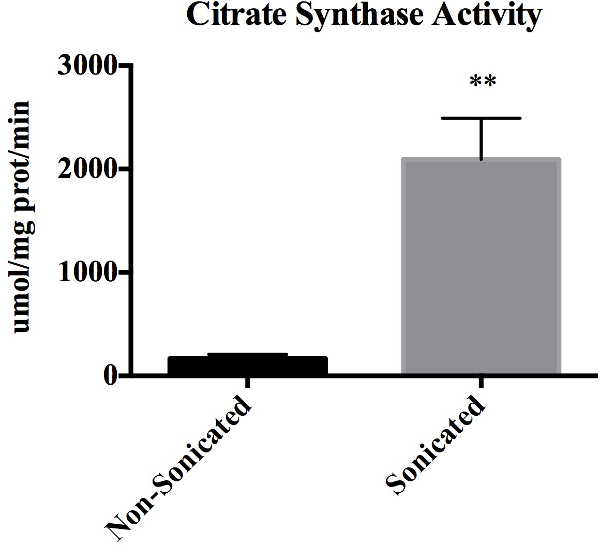
Figure 1: Citrate Synthase Activity. Citrate synthase enzyme activity as determined spectrophotometrically in non-sonicated and sonicated mitochondria preps. Values are expressed as mean ± SEM. **p <0.01. Data represents n = 3 paired biological replicates and expressed relative to mg of protein. 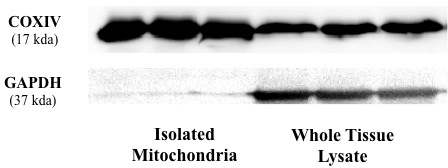
Figure 2: Cytosolic and mitochondrial protein expression in isolated mitochondria and whole tissue lysate. Both isolated mitochondria and whole tissue lysates were immunoblotted with COXIV and GAPDH antibodies. The mitochondrial protein, COXIV, was evident in both samples, however GAPDH was only faintly evident in the isolated mitochondria. The absence of a prominent GAPDH band in the isolated mitochondria is indicative of little contamination from non-mitochondrial sources during the isolation procedure.
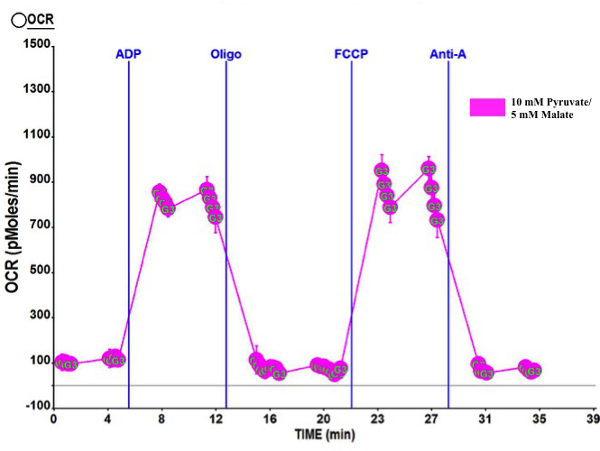 Figure 3: Representative tracing of coupling assay with mitochondria isolated from mouse skeletal muscle. 10 mM Pyruvate/5 mM malate coupled mitochondrial respiration assay tracings as determined by multi-well measurement of O2 consumption. Values are expressed as mean ± SD. Mitochondrial protein loaded per well was 2.5 µg. OCR = O2 Consumption Rate; ADP = Adenosine diphosphate; Oligo = Oligomycin A; FCCP = Carbonyl cyanide-4- (trifluoromethoxy) phenylhydrazone; Anti-A = Antimycin A.
Figure 3: Representative tracing of coupling assay with mitochondria isolated from mouse skeletal muscle. 10 mM Pyruvate/5 mM malate coupled mitochondrial respiration assay tracings as determined by multi-well measurement of O2 consumption. Values are expressed as mean ± SD. Mitochondrial protein loaded per well was 2.5 µg. OCR = O2 Consumption Rate; ADP = Adenosine diphosphate; Oligo = Oligomycin A; FCCP = Carbonyl cyanide-4- (trifluoromethoxy) phenylhydrazone; Anti-A = Antimycin A.
| Reagent | Stock Concentration | MW | Final Volume | Mass Added |
| (M) | (g/mol) | (ml) | (g) | |
| Tris/HCl, pH 7.4 | 2 | 157.56 | 250 | 78.8 |
| Tris/HCl, pH 7.4 | 1 | 157.56 | 250 | 39.39 |
| KCL | 1 | 74.55 | 500 | 37.28 |
| Tris Base | 1 | 121.14 | 100 | 12.11 |
| EDTA, pH 8 | 0.5 | 416.2 | 100 ml (in 1 M Tris Base) | 20.81 |
| EGTA, pH 7.2 | 0.1 | 380.35 | 100 (in 1 M Tris Base) | 3.801 |
Table 1: Preparation of Stock Solutions.
| Reagent | Stock Concentration | Mass Added | Final Molarity/Percent |
| (M) | (g or ml) | ||
| Sucrose | - | 11.47 g | 67 mM |
| Tris/Hcl, pH 7.4 | 2 | 12.5 ml | 50 mM |
| KCL | 1 | 25 ml | 50 mM |
| EDTA/Tris Base, pH 8 | 0.5 | 10 ml | 10 mM |
| Essentially FA Free BSA | - | 1 g | 0.20% |
| pH 7.4, 500 ml: Aliquot 50 ml and freeze |
Table 2: Preparation of Isolation Buffer for Mitochondria 1 (IBM1).
| Reagent | Stock Concentration | Mass Added | Final Molarity/Percent |
| (M) | (g or ml) | ||
| D-Mannitol | - | 10.9 g | 200 mM |
| Sucrose | - | 7.188 g | 70 mM |
| EGTA/Tris Base | 0.1 | 15 ml | 5 mM |
| Tris-HCl, pH 7.4 | 1 | 3 ml | 10 mM |
| pH 7.4, 300 ml: Aliquot 15 ml and freeze |
Table 3: Preparation of Isolation Buffer for Mitochondria 2 (IBM2).
| Substrate Medium | Final Concentration | RCR |
| Pyruvate/Malate | 10 mM/5 mM | 16.2 ± 4.6 |
| Succinate/Rotenone | 10 mM/2 μM | 10.6 ± 3.8 |
| Palmitoyl L-Carnitine/Malate | 40 μM/1 mM Malate | 6.7 ± 0.6 |
| Glutamate/Malate | 10 mM/10 mM | 8.6 ± 0.4 |
Table 4: Respiratory Control Ratios for Coupled Mitochondrial Respiration Assays. Respiratory control ratios as determined by first measuring O2 consumption rates with microplate based respirometric assays, followed by dividing maximal coupled respiration (ADP Stimulated State 3) by respiration due to proton leak (Oligomycin A response State 4o). Values are expressed as mean ± SE. Mitochondrial protein loaded per well was 2.5 µg for the pyruvate/malate and succinate/rotenone assays, and 3.5 µg of mitochondrial protein per well for the palmitoyl L-carnitine/malate and glutamate/malate assays.
Discussion
The methods presented herein provide a detailed description of a mitochondrial isolation procedure from minimal quantities (~75-100 mg) of mouse skeletal muscle. This isolation procedure is able to yield high functioning, pure mitochondria (~450 µg) as evidenced by O2 consumption rates, RCR values, maximal citrate synthase activity and protein expression from immunoblotting. Importantly, the mitochondria isolated from this procedure can be used for multiple respirometirc assays with microplate based O2 consumption technology that allows for high throughput.
The isolation of skeletal muscle mitochondria often requires harsh methods to liberate mitochondria from surrounding connective tissue and structural proteins7,16. Consequently, mitochondrial membranes can become disrupted, thus impairing the activity (and thus the accuracy) of mitochondrial O2 consumption during subsequent respirometric assays. By including an additional step of tissue mincing using razor blades after tissue excision to a previously described protocol7, a significantly reduced rotor speed for motor driven homogenization was possible (80 rpm vs. 1,600 rpm). These gentler homogenization and resuspension techniques leads to a high percentage of mitochondria with intact membranes (92.5± 2.0%), as assessed from citrate synthase activity following the isolation procedure. Our findings are consistent with Asmann et al.17, who report between 90-95% intact mitochondrial preparations following isolation from human skeletal muscle. It is important to note that measuring citrate synthase activity is better suited for measuring physical integrity of the mitochondrial membranes and should not be used to assess functional integrity of the isolated mitochondria. On the other hand, O2 consumption assays and determination of RCR from these assays should be used to measure the function of isolated mitochondria1. In addition, RCR can also be used as an indicator of coupled mitochondria. Therefore, the OCR and mitochondrial states within a typical range from the pyruvate/malate assay8, and the high RCR values obtained from a variety of respirometric assays indicates that the mitochondria derived from this protocol are tightly coupled and highly functional.
Isolation of intact and pure mitochondria has important implications for respirometic assays. The contamination of non-mitochondrial proteins in the final mitochondrial preparation can result in loading unequal quantities of mitochondrial protein to each well during O2 consumption measurements, thus decreasing the accuracy of any comparison among experiments performed using different preparations of mitochondria. Furthermore, contamination of the mitochondrial preparation with other respiring organelles (e.g., peroxisomes) can further decrease the accuracy of O2 consumption measurements. The presence of the inner mitochondrial protein, COXIV, and the absence of a prominent band of the cytosolic (and potentially nuclear) protein, GAPDH, in our isolated mitochondrial preparation following immunoblotting is indicative of little contamination from non-mitochondrial sources during the isolation procedure.
It is important to note that although the isolation of a purified mitochondrial preparation is important with respect to the above mentioned, the maintenance of mitochondrial integrity is of utmost importance in respiration assays. The outer mitochondrial membrane can be easily damaged during the isolation procedure, leading to the escape of cytochrome c into the isolation buffer and thus becoming rate limiting during O2 consumption and ATP synthesis18. Therefore, the integrity of isolated mitochondria can be further assessed by quantifying cytochrome c protein expression in the supernatant of the mitochondrial preparation (step 2.15, prior to resuspension) and in the isolated mitochondria with Western blotting. Cytochrome c should not be present in the mitochondrial preparation supernatant if the mitochondrial membranes are intact following the isolation procedures.
All steps of this protocol are important, however there are three critical steps to this protocol. First, the mincing of red skeletal muscle with three different razor blades (step 2.4) is critical because this step allows for slower rotor speeds during the homogenization step (step 2.11). Second, the performance of many steps on ice or in chilled buffers is critical to maintain the mitochondria in a resting state prior to the microplate based respirometric assays. Finally, the gentle resuspension of the mitochondria pellet into IBM2 is of utmost importance. Gentle mixing maintains mitochondrial integrity, which is crucial for accurate respirometic assays.
Some limitations of this technique are worth mentioning. First, the equipment needed (e.g., ultracentrifuge, motor driven homogenizer, etc.) to perform the isolation procedure can be expensive. In addition, multiple quality control methods are needed to ensure clean and intact mitochondria. However, once the researcher is proficient in the isolation procedure, high quality mitochondria are yielded that can be used in a multitude of measurements including, but not limited to: Western blots, enzymatic assays, RT-PCR, PCR, H2O2 assays, and high throughput microplate respiratory measurements. It is important to note that this isolation procedure has been optimized and modified for small quantities of mouse skeletal muscle and caution should be taken when trying to apply isolation techniques from a given tissue from the same species (and even to the same tissue from different species) to other tissues/species. For optimal results, searching the literature will be the best option when trying to find the best isolation method for the intended tissue/species.
Disclosures
George Rogers is an employee of Seahorse Bioscience that produces the instrument for which this protocol was modified.
Acknowledgments
The Fralin Life Science Research Institute and The Metabolic Phenotyping Core at Virginia Tech supported this work.
References
- Brand M, Nicholls D. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Ferguson S. Bioenergetics. Academic Press; 2013. [Google Scholar]
- Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri A, Pompilio G, Capogrossi MC. The mitochondrial genome in aging and senescence. Ageing Res. Rev. 2014;18:1–15. doi: 10.1016/j.arr.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Dube JJ, et al. Effects of acute lipid overload on skeletal muscle insulin resistance, metabolic flexibility, and mitochondrial performance. Am. J. Physiol. Endocrinol. Metab. 2014;12:1117–1124. doi: 10.1152/ajpendo.00257.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Vizarra E, Lopez-Perez MJ, Enriquez JA. Isolation of biogenetically competent mitochondria from mammalian tissues and cultured cells. Methods (San Diego, Calif) 2002;26:292–297. doi: 10.1016/S1046-2023(02)00034-8. [DOI] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat. Protoc. 2007;2:287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- Rogers GW, et al. High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PloS one. 2011;6:e21746. doi: 10.1371/journal.pone.0021746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino RD, et al. Method for determining oxygen consumption rates of static cultures from microplate measurements of pericellular dissolved oxygen concentration. Biotechnol. and Bioeng. 2004;86:775–787. doi: 10.1002/bit.20072. [DOI] [PubMed] [Google Scholar]
- Will Y, Hynes J, Ogurtsov VI, Papkovsky DB. Analysis of mitochondrial function using phosphorescent oxygen-sensitive probes. Nat. Protoc. 2007;1:2563–2572. doi: 10.1038/nprot.2006.351. [DOI] [PubMed] [Google Scholar]
- Hulver MW, et al. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005;2:251–261. doi: 10.1016/j.cmet.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisard MI, et al. Toll-like receptor 4 modulates skeletal muscle substrate metabolism. Am. J. Physiol. Endocrinol. Metab. 2010;298:e988–e998. doi: 10.1152/ajpendo.00307.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Williams GR. The respiratory chain and oxidative phosphorylation. Adv. Enzymol. Relat. Subjects of Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Garcia-Cazarin ML, Snider NN, Andrade FH. Mitochondrial isolation from skeletal muscle. JoVE. 2011. [DOI] [PMC free article] [PubMed]
- Gross VS, et al. Isolation of functional mitochondria from rat kidney and skeletal muscle without manual homogenization. Anal. Biochem. 2011;418:213–223. doi: 10.1016/j.ab.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger DA, Tate CA, McMillin-Wood J, Booth FW. Populations of rat skeletal muscle mitochondria after exercise and immobilization. J. Appl. Physiol.: Respir., Envir. and Ex. Physiol. 1980;48:23–28. doi: 10.1152/jappl.1980.48.1.23. [DOI] [PubMed] [Google Scholar]
- Asmann YW, et al. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes. 2006;55:3309–3319. doi: 10.2337/db05-1230. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Nair KS. Functional assessment of isolated mitochondria in vitro. Methods Enzymol. 2009;457:349–372. doi: 10.1016/S0076-6879(09)05020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


