Abstract
Encephalopathy of prematurity (EoP) is a term that encompasses the central nervous system (CNS) abnormalities associated with preterm birth. To best advance translational objectives and uncover new therapeutic strategies for brain injury associated with preterm birth, preclinical models of EoP must include similar mechanisms of prenatal global injury observed in humans and involve multiple components of the maternal-placental-fetal system. Ideally, models should produce a similar spectrum of functional deficits in the mature animal and recapitulate multiple aspects of the pathophysiology. To mimic human systemic placental perfusion defects, placental underperfusion and/or chorioamnionitis associated with pathogen-induced inflammation in early preterm birth, we developed a model of prenatal transient systemic hypoxia-ischemia (TSHI) combined with intra-amniotic lipopolysaccharide (LPS). In pregnant Sprague Dawley rats, TSHI via uterine artery occlusion on embryonic day 18 (E18) induces a graded placental underperfusion defect associated with increasing CNS damage in the fetus. When combined with intra-amniotic LPS injections, placental inflammation is increased and CNS damage is compounded with associated white matter, gait and imaging abnormalities. Prenatal TSHI and TSHI+LPS prenatal insults meet several of the criteria of an EoP model including recapitulating the intrauterine insult, causing loss of neurons, oligodendrocytes and axons, loss of subplate, and functional deficits in adult animals that mimic those observed in children born extremely preterm. Moreover, this model allows for the dissection of inflammation induced by divergent injury types.
Keywords: Medicine, Issue 105, Inflammation, intra-amniotic, in utero, rat, chorioamnionitis, placenta, preterm, intrauterine
Introduction
With over 12% of infants born in the United States before 37 weeks estimated gestational age1, perinatal brain injury (PBI) from prematurity is a significant cause of permanent disability. PBI from prematurity, also termed encephalopathy of prematurity (EoP), affects the entire central nervous system (CNS). CNS injury often commences in utero, and is exacerbated by antenatal processes including chorioamnionitis and postnatal complications such as hypoxia and sepsis. PBI from systemic insults alters neurodevelopment and leads to cerebral palsy, epilepsy, cognitive delay and numerous neuropsychiatric disorders affecting emotional regulation, memory and executive function1,2. Although much progress has been made, a limited understanding remains of how the cellular and molecular consequences of CNS injury from preterm birth translate to the multitude of neurological sequelae in children who are born preterm. This lack of knowledge hinders real-time diagnosis of CNS injury severity and informed dosing of emerging interventions. Additionally, age-appropriate therapeutic strategies for this vulnerable patient population remain elusive.
Intrauterine inflammation is very common in extreme prematurity and involves a complex fetal-maternal-placental inflammatory cascade3. Intrauterine infection is often subclinical. Specific placental findings consistent with acute inflammation, or histologic chorioamnionitis, are major determinants of the fetal inflammatory response and are coincident with brain injury associated with preterm birth3-5. Indeed, the fetal inflammatory response has distinct clinical implications for long-term outcomes from preterm birth. Infants who are small for gestational age (SGA) or who experience infection are exceptionally vulnerable to neurological deficits3,4. Chorioamnionitis is a typical pathological diagnosis following preterm birth6,7, and histological examination reveals signs of inflammation in 70% of placentas from infants born very preterm4. Further, chorioamnionitis is associated with cognitive impairment at two years8. Evidence of maternal vascular underperfusion in the placenta of infants born extremely preterm is also associated with cerebral palsy in childhood9. The synergistic impact of chorioamnionitis and placental perfusion defects is well illustrated by the remarkably high risk of abnormal neurologic outcomes in this patient population at two years of age10,11.
To mimic human systemic placental perfusion defects and chorioamnionitis associated with pathogen-induced inflammation, we developed a model of prenatal transient systemic hypoxia-ischemia (TSHI) combined with intra-amniotic lipopolysaccharide (LPS) in rats. Our goal was to adapt our model of TSHI alone in rats12-16 to include intrauterine inflammation, to facilitate preclinical modeling of CNS injury associated with preterm birth. TSHI alone has revealed persistent loss of oligodendroglial lineage cells, cortical neurons, increased cell death, and elevated pro-inflammatory cytokine levels, with progressive ischemic intervals leading to a graded pattern of injury consistent with prenatal brain injury16. Modifications to the ischemic components of this model have also demonstrated deficits in memory encoding, short and long-term memory and mild musculoskeletal alterations in rats as they age17-19. Indeed, we have previously demonstrated that the combination of TSHI+LPS recapitulates the pathophysiological hallmarks of EoP, including oligodendrocyte and neuronal loss, axonal injury, cellular inflammation and functional abnormalities20.
Protocol
Institutional Care and Use Committees at both Boston Children’s Hospital and the University of New Mexico Health Sciences Center approved all experimental procedures.
NOTE: Prior to commencing the procedure, seal, sterilize and autoclave all surgical instruments and surgical drapes. Additionally, prepare post-operative medications in sterile vials including 0.125% bipivucaine and 0.1 mg/kg buprenorphine. Also prepare the lipopolysaccharide (LPS) solution sterilely: 0.04 mg/ml LPS (0111:B4) in sterile saline containing dilute Evan’s blue dye.
1. Anesthesia
Induce anesthesia in an embryonic day 18 (E18) pregnant Sprague Dawley rat with a mixture of 3% isoflurane balanced 70% nitrogen and 30% oxygen.
Remove rat from the induction chamber and place the rat supine on draped surgical circulating water blanket set at 37 °C. Transfer anesthesia to nose cone and reduce isoflurane level to 2%.
Gently apply ophthalmic ointment to each eye to prevent corneal drying. During the procedure continually monitor temperature, respiration rate and heart rate of the animal. Maternal physiology should remain stable throughout the procedure.
2. Surgical Prep and Scrub
Using small animal clippers remove all hair in the lower abdominal region. Shave in a rectangular pattern with care to avoid nicking the nipples or generating razor rash that can be irritating for future nursing of live born pups.
Prepare abdominal skin by alternating application of povidone-iodine and 70% ethanol scrub with sterile cotton swabs. Repeat the scrub such that povidone-iodine and 70% ethanol are each applied 3x in alternating fashion. Let dry.
Confirm depth of anesthesia via absence of toe-pinch reflex. In the absence of reflex and stimuli to pain, reduce isoflurane level to 1%.
Using sterile surgical towels, drape the animal. Take care to place the drapes at an appropriate angle such that they maximize the amount of irrigation fluid they absorb whilst not obstructing blood flow to the uterine horns.
3. Abdominal Laparotomy
Using a scalpel make a 3 cm midline incision in the prepared abdominal skin. Bluntly dissect the skin layer from the abdominal fascia with scissors. Using forceps and surgical scissors, elevate the abdominal fascial layer and make an incision of the avascular linea alba to gain access to the peritoneal cavity.
Place surgical gauze on the exterior of the incision and moisten with sterile saline. Using blunt forceps and external pressure on the abdomen, gently remove the uterine horns from the peritoneal cavity and arrange on the moistened gauze.
Carefully avoid entanglement and contact with intestines. Arrange fetuses using forceps by contacting only the muscular tissue in between individual amniotic sacs. Expose and isolate the 4 uterine arteries using blunt dissection. NOTE: Care must be taken to dissect the uterine arteries. Surrounding tissue and vessels themselves are extremely delicate. Damage to maternal vessels can cause bleeding, and in severe cases, fetal and maternal death.
4. Placement of Aneurysm Clips
Place a rat 30 G aneurysm clip on each uterine artery. Ensure cessation of blood flow, including proximal and distal pulses, and darkening of the uterine vessels including individual placentas. Cover the exposed horns and entire surgical field with gauze and irrigate with sterile saline. Take care to keep the field moist with irrigation approximately every 10 min.
After 60 min, remove the gauze and irrigate the field. Ensure that the uterine horns and vessels are adequately moistened for successful clip removal. Gently remove each aneurysm clip using forceps. Take care not to cause trauma to the vessel, and maintain tissue integrity during removal.
Thoroughly irrigate the uterine horns and field, taking care to remove any stray threads of gauze from the amniotic sacs.
5. Injection of Lipopolysaccharide in to Amniotic Sacs
At the base of each individual amniotic sac, just anterior to the placental plate, inject 100 µl of LPS (4 µg/sac) with diluted Evan’s blue dye in to the amniotic fluid. Use blunt forceps to stabilize and rotate each amniotic sac in to an optimal position for injection. Dilute Evan’s blue dye is a contrast agent that is helpful in confirming proper syringe placement and injection. NOTE: Use only an ultra-fine 0.3 ml insulin syringe with attached 8 mm 31 G needle for the intra-amniotic injections. Using larger gauge needles will result in chronic amniotic fluid loss, fetal death and reabsorption of the pregnancy. Small amounts of amniotic fluid leakage upon removal of the syringe can be mitigated by direct pressure to the amniotic sac. Some rat fetuses may tolerate a degree of oligohydramnios. However, acute amniotic fluid loss from puncture with large gauge needles, or accidental puncture resulting in chronic fluid leakage, results in fetal loss and in severe cases, loss of neighboring pregnancies.
Irrigate the uterine horns 3x with sterile saline.
6. Closing the Laparotomy
Using forceps, carefully return the uterine horns to the peritoneal cavity. Ensuring adequate space between the amniotic sacs and the midline incision, re-approximate the musculofascial layer edges using a running 3-0 silk suture. Be aware of the placement of the amniotic sacs while closing the muscle incision. Be careful to not to suture into or through a sac.
Re-approximate the skin layer, using a running 3-0 silk suture, closing the skin layer. NOTE: The laparotomy should be closed in two layers of continuous sutures to allow for skin and muscular expansion with increasing gestation. Continuous sutures allow for evenly distributed wound tension. Interrupted sutures are less desired as multiple knots are irritating and can be easily chewed by the rat upon recovering from anesthesia. Surgical staples are not desired. Tails of the surgical knots should be cut very short (<3 mm).
Inject 1 ml of 0.125% bupivacaine subcutaneously around wound edges using a 26 G needle. Administer one dose of 0.1 mg/kg buprenorphine subcutaneously at the nape of the neck.
Turn off isoflurane and towel dry rat as necessary. Place in clean home cage and monitor recovery from anesthesia. Ensure rat does not become hypothermic.
7. Postoperative Recovery and Care
Monitor the rat every 8-12 hr for 72 hr and then daily until pups are born (approximately E22 or E23). Administer additional doses of buprenorphine q8-12 hr/72 hr or prn as dictated by the IACUC.
Monitor rats for signs of pain, discomfort, vaginal bleeding or bleeding from the surgical site. Inspect the sutures and incision and take care to ensure rat is not chewing or removing their sutures prematurely. Although exceptionally rare, rats that compromise their sutures are at risk for wound dehiscence. NOTE: Occasionally rats may ingest excessive amounts of bedding or non-food items, termed pica, as a side effect of buprenorphine administration. Although very uncommon, rats must be monitored for pica and potential subsequent bowel obstruction.
8. Tissue Processing and Cryosectioning
To prepare for Hematoxylin & Eosin (H&E) staining of the postnatal brain, remove pups from their home cage on postnatal day 2 (P2). Using surgical scissors decapitate rat pups and gently remove the brain from the skull.
Drop fix brain in a 15 ml conical tube containing 7 ml of 4% paraformaldehyde in phosphate buffered saline (PBS). Place brain at 4 °C and fix for 72 hr.
After 72 hr, transfer brains to a sterile PBS solution containing 30% sucrose (w/v) and return to 4 °C. NOTE: Once brains drop in the sucrose solution they are ready to be sectioned on a cryostat.
Rapidly freeze brains and mount on cryostat pestle for acquisition of frozen coronal sections. Cut 20 µm frozen coronal sections and mount on slides. Ensure sections are collected serially.
Allow slides to dry at room temperature overnight. Store slides at -20 °C.
9. Hematoxylin & Eosin Staining
Take slide mounted frozen sections and warm to room temperature. NOTE: Prepare all solutions fresh.
Place slides on a slide warmer set to 50 °C for 2 hr.
Transfer slides to a staining rack. Dip slides 10x in double-distilled deionized water (ddH2O).
Incubate slides in 100% hematoxylin for 5 min. Time in hematoxylin can be optimized depending on degree of purple staining.
Dip slides 4x in tap H2O, and let stand in clean tap H2O for 1 min.
Dip slides 15x in acid alcohol (250 ml 70% ethanol + 1 ml concentrated hydrochloric acid).
Dip slides 4x in tap H2O, and let stand in clean tap H2O for 1 min.
Incubate in 1% lithium carbonate for 2 min.
Dip slides 4x in tap H2O, and let stand in clean tap H2O for 1 min.
Incubate in 95% ethanol for 1 min.
Dip slides 7x in 100% Eosin. Number of dips in Eosin can be modified depending on degree of pink staining.
Dip slides 5x in 95% ethanol.
Dip slide 5x in a fresh change of 95% ethanol.
Incubate slides in 100% ethanol for 1 min.
Incubate slides in a fresh change of 100% ethanol for 1 min.
Incubate slides in 100% xylene for 15 min. NOTE: Xylene steps and coverslipping should occur in a fume hood.
Incubate slides in fresh changes of xylene for 15 min.
Coverslip with Permount and let dry in fume hood.
Image slides with a light microscope.
Representative Results
Following TSHI+LPS at E18, hematoxylin and eosin staining reveals significant histopathological abnormalities in both the placenta (Figure 1) and in the brain (Figure 2). Placentas examined on E19 and E21 are grossly edematous with micro-hemorrhage, and necrosis throughout the decidua and labyrinth. Significant inflammatory infiltrate and increased vascularity is also observed. Brains examined on P2 reveal ventriculomegaly, as well as white matter and subplate neuron loss compared to shams. Previously, we have reported that TSHI+LPS induces inflammation and yields persistent white matter and axonal abnormalities concomitant with significant motor impairment in young adults20. TSHI+LPS also significantly decreases potassium chloride co-transporter 2 (KCC2) protein expression, a chloride co-transporter central to the development of γ-aminobutyric acid (GABA)ergic inhibition, in the cortex at P15 (Figure 3, *P <0.05). These data are consistent with impaired developmental upregulation of KCC2 during a critical postnatal period of circuit maturation and these findings are consistent with our prior reports of the effects of TSHI alone in the CA3 hippocampus13.
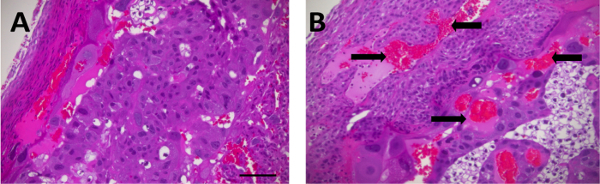 Figure 1: TSHI+LPS induces significant histological abnormalities in the placenta. Following transient in utero hypoxia-ischemia and intra-amniotic LPS administration on embryonic day 18 (E18), placentas from E19 TSHI+LPS fetuses (B) are grossly edematous with hemorrhage (arrows), necrosis and increased inflammatory infiltrate compared to sham (A, scale bar = 100 µm). Please click here to view a larger version of this figure.
Figure 1: TSHI+LPS induces significant histological abnormalities in the placenta. Following transient in utero hypoxia-ischemia and intra-amniotic LPS administration on embryonic day 18 (E18), placentas from E19 TSHI+LPS fetuses (B) are grossly edematous with hemorrhage (arrows), necrosis and increased inflammatory infiltrate compared to sham (A, scale bar = 100 µm). Please click here to view a larger version of this figure.
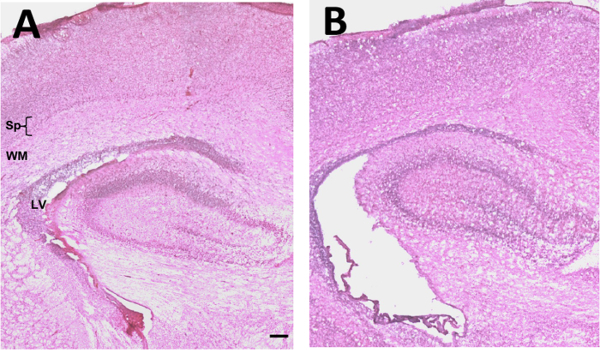 Figure 2: TSHI+LPS induces significant histological abnormalities in the brain. Following transient in utero hypoxia-ischemia and intra-amniotic LPS administration on embryonic day 18 (E18), postnatal ventriculomegaly, subplate neuron and white matter loss is observed in pups subjected to dual TSHI+LPS (B) compared to sham (A) acutely at P2. (Scale bar = 100 µm; Sp = subplate; WM = white matter; LV = lateral ventricle) Please click here to view a larger version of this figure.
Figure 2: TSHI+LPS induces significant histological abnormalities in the brain. Following transient in utero hypoxia-ischemia and intra-amniotic LPS administration on embryonic day 18 (E18), postnatal ventriculomegaly, subplate neuron and white matter loss is observed in pups subjected to dual TSHI+LPS (B) compared to sham (A) acutely at P2. (Scale bar = 100 µm; Sp = subplate; WM = white matter; LV = lateral ventricle) Please click here to view a larger version of this figure.
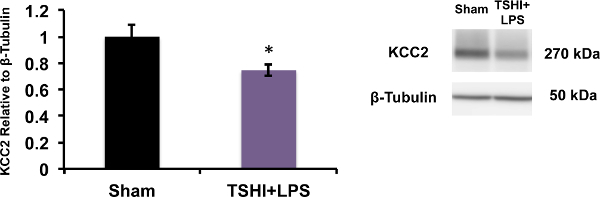 Figure 3: TSHI+LPS reduces KCC2 expression. Western blots performed from membrane preparations of micro-dissected cortical tissue, show in utero transient systemic hypoxia-ischemia and intra-amniotic LPS administration on embryonic day 18 (E18) significantly reduces expression of KCC2, a neuron-specific potassium chloride co-transporter central to the development of integrated cerebral circuits and inhibition, on postnatal day 15. (n = 6-10, mean ± SEM, Student’s t-test,*P <0.05).
Figure 3: TSHI+LPS reduces KCC2 expression. Western blots performed from membrane preparations of micro-dissected cortical tissue, show in utero transient systemic hypoxia-ischemia and intra-amniotic LPS administration on embryonic day 18 (E18) significantly reduces expression of KCC2, a neuron-specific potassium chloride co-transporter central to the development of integrated cerebral circuits and inhibition, on postnatal day 15. (n = 6-10, mean ± SEM, Student’s t-test,*P <0.05).
Discussion
Encephalopathy of prematurity is difficult to model in animals because of the complex interaction of etiologies, neurodevelopmental time course, intricacy of human cerebral network formation, overlapping mechanisms of injury, and the diverse phenotypes of CNS insults manifest in human preterm infants. EoP is associated with specific cell-type vulnerabilities (i.e. immature oligodendrocytes)21, as well as diverse developmentally-regulated pathways (i.e. subplate, membrane transporters and receptor subunits)12,13,22. However, significant progress can be made when animal models replicate the human condition as closely as possible. Here, we developed a model a prenatal insult that incorporates the heterogeneity of mechanisms of CNS injury observed in the preterm infant, allowing for subsequent evaluation of both gray and white matter damage and recovery. In humans, ascending bacterial infections weaken the amnion and precipitate premature rupture of membranes. Additionally, placental perfusion defects stress the placental interface and disrupt placental homeostasis. Thus, placental underperfusion compounds CNS injury from an intrauterine infection. Undeniably, it is challenging to model the common clinical scenario of ascending bacterial infections that precede chorioamnionitis in rodents as they have a duplex uterus. Each uterine horn has its own cervix, and multiple pregnancies are carried at once. Despite these challenges, preclinical models have been adapted to involve multiple components of the maternal-placental-fetal unit and incorporate in utero inflammation to various degrees. While no individual preclinical model is ideal to test every specific hypothesis, the model described herein incorporates the cellular and molecular abnormalities, behavioral and functional impairment, maternal-placental-fetal system, and the intrauterine infection and placental inflammation component common to so many preterm births20,23.
The choice of species used to model EoP impacts the interpretation of experimental data in the context of the inherent limitations posed by the species. In the simplest terms, birth does not equate to similar points of CNS development across all animals24. The model described here can be performed in both pregnant mice and rats, although pup survival in mice is significantly decreased in inexperienced or stressed dams. Consistent with our prior reports, fetal loss in rats at birth (P0) is increased in TSHI+LPS animals (approximately 40%) compared to sham, LPS and TSHI alone, but surviving pups do not exhibit significant weight differences through P2820. Similar to differences among species, the timing of injury during gestation has a crucial role in the neurodevelopmental trajectory of the offspring. The spatiotemporal regulation of neural cell developmental stages of proliferation, migration and differentiation differs amongst various mammals24-26. These cell-specific developmental programs influence the vulnerability to injury. For example, the overlap of the timing of oligodendrocyte lineage and GABAergic neuronal development with the timing of preterm birth makes these cells particularly susceptible to perinatal insults27-29. Thus, this model was developed in E18 rats (and successfully translated to E17 mice on a C57BL/6 background) as this timing corresponds to the intrauterine global prenatal insult that occurs in human infants prior to extremely preterm birth at 23-25 weeks gestation20. We previously showed O4-immunoreactive immature oligodendrocytes are most affected at this stage of development16. Their loss correlates with decreased survival and maturation14, with the most notable reductions in O4+ and O1+ stages of the lineage16, consistent with previous reports by other investigators30. Moreover, we have demonstrated premature loss of the subplate, reduced KCC2 expression, lower seizure threshold and impaired gait12,13,15 consistent with dysregulated GABAergic signaling in preterm infants31.
The model described here offers numerous benefits over previous models in rodents used to study perinatal brain injury from preterm birth23. It incorporates the entire maternal-placental-fetal system and causes both brain and placental injury. We have previously published comparisons between sham, TSHI alone, LPS alone and TSHI+LPS and differences in functional outcomes and biochemistry20, and differences with graded TSHI16. While prior investigations of unilateral carotid ligations and systemic hypoxia in neonatal rats have shed mechanistic insight into numerous pathophysiological processes (i.e. the susceptibility of immature oligodendrocytes to ischemia), direct translational and clinical relevance for such models is less robust. In addition to the applications described, the model described here may be an informative tool for investigation of other organ systems impacted by prematurity, including necrotizing enterocolitis (NEC), heart, lung, renal and hypothalamic-pituitary axis dysfunction. Owing to the complexities of LPS pharmacology and differences in maternal and fetal pharmacodynamics, intraperitoneal LPS injections in dams are less likely to produce the same fetal inflammatory response shown here. Further, LPS does not cross the placenta reliably20,32. Previously, we attempted direct cervical application of LPS and intrauterine injection similar to what has been described in other mouse models33. However, we found that mortality and inconsistency of CNS injury was significantly increased among pups within the same litter. Here, the dose of 4 µg/sac was optimized using dose-response experiments. Increasing doses of LPS administered to the amniotic compartment results in increased fetal mortality. LPS has the advantage over direct infection with typical intrauterine gram-negative bacteria in that it activates inflammatory signaling through toll-like receptor 4 without causing active bacterial infection and the associated risk of pathogen spread. However, this model could be modified to include common pathogens and organisms isolated from human placentas, including group B streptococcus, which causes placental and neuropathological abnormalities, and autistic-like behavior in rats34. Similarly, Ureaplasma lipoprotein multiple-banded antigen can simulate Ureaplasma species infection. Since Ureaplasma is the most common cause of human chorioamnionitis35, this could also be an avenue for future investigation. As more inactivated-infectious agents become available, it will be informative to determine how they differentially impact neurodevelopment and the efficacy of neuro-reparative interventions.
Limitations of this model include amniotic fluid loss from the intra-amniotic injections. Although no effect is noted with intra-amniotic injections of sterile saline, the gauge of the needle used to perform the injections is a crucial technical element. Care must be taken not to use needles larger than 31 G. Surgical complications in the mother rat related to the laparotomy are extremely rare, including wound dehiscence, bowel obstruction, peritonitis and complete loss of the pregnancy, with maternal mortality less than 5%.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors are grateful to Dan Firl, Chris Corbett and Jesse Denson, PhD. Funding was provided by NIH NINDS R01 NS060765 to SR, the P30 CoBRE Pilot Program to LJ and the Child Health Signature Program to LJ at the University of New Mexico.
References
- Blencowe H, et al. Preterm birth associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr Res. 2013;74(1):17–34. doi: 10.1038/pr.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long term neurodevelopmental outcomes after intrauterine and neonatal insults a systematic review. Lancet. 2012;379:445–452. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res. 2014;75:376–380. doi: 10.1038/pr.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leviton A, et al. Microbiologic and histologic characteristics of the extremely preterm infant's placenta predict white matter damage and later cerebral palsy. the ELGAN study Pediatr Res. 2010;67:95–101. doi: 10.1203/PDR.0b013e3181bf5fab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med. 2006;11:296–301. doi: 10.1016/j.siny.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Lee J, et al. Chronic chorioamnionitis is the most common placental lesion in late preterm birth. Placenta. 2013;34:681–689. doi: 10.1016/j.placenta.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Lee SM, et al. Acute histologic chorioamnionitis is a risk factor for adverse neonatal outcome in late preterm birth after preterm premature rupture of membranes. PloS one. 2013;8:e79941. doi: 10.1371/journal.pone.0079941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas A, et al. Chorioamnionitis and early childhood outcomes among extremely low gestational age neonates. JAMA Peds. 2014;168:137–147. doi: 10.1001/jamapediatrics.2013.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard R, Arends LR, Steegers EA, Hofman A, Jaddoe VW. Second and third trimester placental hemodynamics and the risks of pregnancy complications the Generation R Study. Am J Epidemiol. 2013;177:743–754. doi: 10.1093/aje/kws296. [DOI] [PubMed] [Google Scholar]
- Trivedi S, et al. Fetal placental inflammation but not adrenal activation is associated with extreme preterm delivery. Am J Obstet Gynecol. 2012;206:236. doi: 10.1016/j.ajog.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanowitz TD, et al. Hemodynamic disturbances in premature infants born after chorioamnionitis association with cord blood cytokine concentrations. Pediatr Res. 2002;51:310–316. doi: 10.1203/00006450-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Jantzie LL, Corbett CJ, Firl DJ, Robinson S. Postnatal Erythropoietin Mitigates Impaired Cerebral Cortical Development Following Subplate Loss from Prenatal Hypoxia Ischemia. Cereb Cortex. 2014. [DOI] [PMC free article] [PubMed]
- Jantzie LL, et al. Erythropoietin attenuates loss of potassium chloride co transporters following prenatal brain injury. Mol Cell Neurosci. 2014;61:152–162. doi: 10.1016/j.mcn.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, Miller RH, Robinson S. Erythropoietin signaling promotes oligodendrocyte development following prenatal systemic hypoxic ischemic brain injury. Pediatric Res. 2013;74:658–667. doi: 10.1038/pr.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur M, Miller RH, Robinson S. Postnatal erythropoietin treatment mitigates neural cell loss after systemic prenatal hypoxic ischemic injury. J Neurosurg Peds. 2010;6:206–221. doi: 10.3171/2010.5.PEDS1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, et al. Developmental changes induced by graded prenatal systemic hypoxic ischemic insults in rats. Neurobiol Dis. 2005;18:568–581. doi: 10.1016/j.nbd.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Delcour M, et al. Neuroanatomical sensorimotor and cognitive deficits in adult rats with white matter injury following prenatal ischemia. Brain Pathol. 2012;22:1–16. doi: 10.1111/j.1750-3639.2011.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcour M, et al. Impact of prenatal ischemia on behavior cognitive abilities and neuroanatomy in adult rats with white matter damage. Behav Brain Res. 2012;232:233–244. doi: 10.1016/j.bbr.2012.03.029. [DOI] [PubMed] [Google Scholar]
- Delcour M, et al. Mild musculoskeletal and locomotor alterations in adult rats with white matter injury following prenatal ischemia. Intl J Devel Neurosci. 2011;29:593–607. doi: 10.1016/j.ijdevneu.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Jantzie LL, et al. Complex pattern of interaction between in utero hypoxia ischemia and intra amniotic inflammation disrupts brain development and motor function. J Neuroinflam. 2014;11:131. doi: 10.1186/1742-2094-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, et al. Developmental Expression of N Methyl d Aspartate (NMDA) Receptor Subunits in Human White and Gray Matter Potential Mechanism of Increased Vulnerability in the Immature Brain. Cereb Cortex. 2015;25:482–495. doi: 10.1093/cercor/bht246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, Robinson S. Preclinical Models of Encephalopathy of Prematurity. Devel Neurosci. 2015. [DOI] [PMC free article] [PubMed]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci. 2013;33:7368–7383. doi: 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Exper Neurol. 2004;190:S8–S21. doi: 10.1016/j.expneurol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Kelsom C, Lu W. Development and specification of GABAergic cortical interneurons. Cell Biosci. 2013;3:19. doi: 10.1186/2045-3701-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney H, Back S. Human oligodendroglial development Relationship to periventricualr leukomalacia. Semin Pediatr Neuro. 1998;5:180–189. doi: 10.1016/s1071-9091(98)80033-8. [DOI] [PubMed] [Google Scholar]
- Robinson S, Li Q, DeChant A, Cohen M. Neonatal loss of gamma amino butyric acid pathway expression after human perinatal brain injury. J Neurosurg Peds. 2006;104:396–408. doi: 10.3171/ped.2006.104.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, et al. Late development of the GABAergic system in the human cerebral cortex and white matter. J Neuropathol Exp Neurol. 2011;70:841–858. doi: 10.1097/NEN.0b013e31822f471c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia KN, et al. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 2008;63:520–530. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Li Q, Dechant A, Cohen ML. Neonatal loss of gamma aminobutyric acid pathway expression after human perinatal brain injury. J Neurosurg. 2006;104:396–408. doi: 10.3171/ped.2006.104.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksa P. Effects of prenatal infection on brain development and behavior a review of findings from animal models. Brain Behav Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Burd I, Brown A, Gonzalez JM, Chai J, Elovitz MA. A mouse model of term chorioamnionitis unraveling causes of adverse neurological outcomes. Repro Sci. 2011;18:900–907. doi: 10.1177/1933719111398498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron JD, et al. White matter injury and autistic like behavior predominantly affecting male rat offspring exposed to group B streptococcal maternal inflammation. Devel Neurosci. 2013;35:504–515. doi: 10.1159/000355656. [DOI] [PubMed] [Google Scholar]
- Uchida K, et al. Effects of Ureaplasma parvum lipoprotein multiple banded antigen on pregnancy outcome in mice. J Reprod Immunol. 100:118–127. doi: 10.1016/j.jri.2013.10.001. [DOI] [PubMed] [Google Scholar]


