Abstract
Flight in insects can be long-range migratory flights, intermediate-range dispersal flights, or short-range host-seeking flights. Previous studies have shown that flight mills are valuable tools for the experimental study of insect flight behavior, allowing researchers to examine how factors such as age, host plants, or population source can influence an insects' propensity to disperse. Flight mills allow researchers to measure components of flight such as speed and distance flown. Lack of detailed information about how to build such a device can make their construction appear to be prohibitively complex. We present a simple and relatively inexpensive flight mill for the study of tethered flight in insects. Experimental insects can be tethered with non-toxic adhesives and revolve around an axis by means of a very low friction magnetic bearing. The mill is designed for the study of flight in controlled conditions as it can be used inside an incubator or environmental chamber. The strongest points are the very simple electronic circuitry, the design that allows sixteen insects to fly simultaneously allowing the collection and analysis of a large number of samples in a short time and the potential to use the device in a very limited workspace. This design is extremely flexible, and we have adjusted the mill to accommodate different species of insects of various sizes.
Keywords: Neuroscience, Issue 106, Flight mill, insect, dispersal, tethered flight, flight behavior, migration
Introduction
Several laboratory techniques have been developed for the study of insect flight behavior 1,2. These range from simple static tethering 3,4 to sophisticated devices that allow greater freedom of movement for the tethered insect 5. To date flight chambers 6-9 represent the devices allowing the highest level of freedom of flight in controlled conditions. This technique has two major drawbacks: it is difficult to use for the study of large insects and the manual procedure of data collection is time consuming.
Flight mills represent one of the most common and affordable techniques for the study of insect flight under laboratory conditions 10-12. This technique is preferable to static tethering because it offers moving stimuli 13, but it differs from a free flight behavioral response 14-16. Some aspects of the flight behavior on the mill and in the wild are similar 5,17 so despite some limitations, flight mills represent a viable option to investigate questions regarding the occurrence of particular flight behavior responses, as is the case of migratory flight type. Also, flight mills are easier to realize than wind tunnels or flight chambers and the data collection can easily be automated. Thus, researchers interested in flight behavior often find that flight mills are the best choice, but should be aware of the potential limitations to the method. Here, a flexible and customizable flight mill design is presented for researchers that have chosen to utilize flight mills to investigate flight behavior.
Several authors describe alternative flight mill designs. In general the main part of the flight mill system, i.e., the pivoting mill’s arm, is quite simple to realize. Less straightforward is the electronic part of the flight mill system, which allows the recording of the data. Dealing with electronic circuits design can be challenging, especially for the entomologist or the behavioral ecologist lacking in background knowledge of electronics. Some authors describe a complicated or out of date electronic circuit component in their flight mill design 18-21, or the description of the electronic part of the flight mill is missing 22,23. Other designs describe mechanically complicated actographs, which are quite complicated to realize but can help investigators to undertake more complex behavioral observations 5.
In this paper a design for a simple to build, relatively inexpensive flight mill for the study of tethered flight in insects is described. Together with the extremely simple electronic component, the design has a number of advantages. The flight mill is designed to be used in the constrained spaces typically available in the standard insect ecology laboratory. The structure is made out of transparent acrylic plastic so that a single light source can evenly reach every individual in separate chambers of the mill. Given the transparency of the material and small size, the flight mill can be used in an incubator for standardized light and temperature conditions. Finally, the entire structure can be assembled and disassembled easily and, once disassembled, it can be stored in a small space. Another advantage to the design of the structure is that the flight mill can be modified to allow the study of insects of different size and using different revolution distances. This flight mill has been used to collect data on insects as diverse in size and shape as milkweed bugs, Oncopeltus fasciatus24, kudzu bugs, Megacopta cribraria, and burying beetles, Nicrophorus vespilloides. The flight mill design also allows for high through-put required for studies requiring large sample sizes. Data can be collected using 8 simultaneous channels for each of the data loggers used so that a high number of individuals can be analyzed simultaneously and large numbers of samples can be handled in the same day. No expensive software is needed to record and visualize the data and the custom written script for the data analysis can be modified following the specific needs of the experimental design. Flight response is highly variable in different insect species. Thus, before conducting a full flight mill experiment, preliminary tests on the flight response of the focal insect model are recommended. These will provide an understanding of the extent of the behavioral variation in flight response, which will be used to fine tune aspects of the flight analysis such as recording time or flight speed range.
Protocol
1. Construct the Flight Mill
- Construct the acrylic plastic support structure:
- Cut 3 mm thick transparent acrylic sheets into the two outside vertical walls, the one central vertical wall and the five horizontal shelves as specified by the design shown in Figure 1.
- Assemble by inserting the shelves (Figures 1 and 2; HS) into vertical walls (Figures 1 and 2; OW and CW) to form the support structure (Figure 2A).
- Strengthen the structure by inserting polystyrene columns at the external corners at the back of the device (Figure 2A and Figure 2C). If required, glue short pieces of right-angled edge-protectors along the central vertical wall junctions to provide additional support for the horizontal shelves.
- Construct the pivoting arm assembly:
- Glue a 5 cm length of 1 cm diameter plastic tubing into the top center of each cell. Glue a 2 cm length of 1 cm diameter plastic tubing into the bottom center of each cell, making sure the top and bottom tubing in each cell is aligned. Using hot glue, affix two 10 mm x 4 mm N42 neodymium magnets to the end of each support, forming the magnetic bearing for the mill's arm.
- Insert an entomological pin into a 20 µl pipette tip and fix in place with hot glue. Position the pin such that both ends extend out of the pipette tip to form the armature of the flight mill. Note: During the flight trials, the top of the pin is held in place by the top set of magnets. The bottom set of magnets is to maintain the armature in a vertical position, allowing it to revolve around its axis.
- Cut a 24 cm length of 19 gauge non-magnetic hypodermic steel tubing. Using hot glue, affix the center point to the top of the pipette tip from step 1.2.2. Bend one end of the tubing at 2 cm from the end to an angle of 95°, leaving a long arm of 12 cm from the center point and a short arm with a 10 cm radius from the center to the bend (Figure 2B). Note: The radius length can be varied in order to accommodate different revolution distances.
- Set up the IR sensor and data logger:
- Fix the IR sensors to the eternal sides of each cell using reusable adhesive putty, allowing the sensor to extend into the cell through the openings cut into the external vertical wall supports (Figure 2C).
- Connect the IR sensors to a data loggers through a very basic electronic circuit built on a solderless breadboard (Figure 3). Connect two resistors of 180 Ω and 2.2 kΩ respectively on the input and output of the IR connection on the breadboard (Figure 3A,B). Place the resistors in alternate rows along the breadboard to minimize drops in the voltage signal during recording from multiple sensors (see Figure 3C).
2. Flight Trials
- Tether insects to the flight mill arm indirectly through an insect pin:
- Place a small foil flag at the end of the unbent end of the pivoting arm to maximize disruption of the IR beam in the sensor and to act as a counterweight.
- Depending on the insect’s size and cuticle area available for attachment, attach the experimental insect to an insect pin with reusable adhesive putty or non-toxic skin glue. If necessary, anesthetize the insect by either chilling or with CO2.
- Mould a small amount of adhesive putty around the rounded tip of an entomological pin and cover it with a drop of non-toxic skin glue. Gently apply on the pronotum area and wait 5-10s until the glue is dry. Note: The procedure in step 2.1.3 is suited for insects with hard (beetle, bugs) or soft (wasps, flies) cuticle. Insects with hairy cuticle (moths, butterflies) will need to have the hair gently removed with a very fine paintbrush before tethering.
- Insert the pin with the insect attached into the bent end of the pivoting arm assembly.
- After the flight test has ended, remove the tethering with a fine forcep. Note: Data logger set up and acquisition has been optimized as follows for the specific equipment listed in the materials table and should be adjusted for use with alternative equipment.
- Initiate a recording session with the freely available WinDAQ Lite software
- Download and install the free software WinDAQ Lite (see equipment list).
- Open the instrument hardware manager, select the data-logger from the pop-up list and press 'Start Windaq'. A new window will open and the input signal from each sensor will be shown.
- Select the desired sampling frequency at which the data-logger reads and displays the sensor’s output. Note: The sampling frequency will depend on the insect's flight speed, however sampling frequencies ranging between 30-45 Hz will be fast enough to capture the flight of small-medium sized insects.
- Press Ctrl-F4 to start a recording session. Select the destination path of the recording file in the first pop-up window. Choose the appropriate length of time to record flight for the particular insect and experiment. Define recording time in the second pop-up window. Once the recording time is elapsed press Ctrl-S to finalize the recorded file.
- Check quality of the recording.
- Open the recorded flight track and select a voltage channel. Press Ctrl-T to open a pop-up window with the voltage statistics for each channel.
- Ensure that no large drops in minimums value resulted from voltage drops across the circuit (Figure 4). Discard any channels in which the difference between the channel average and minimum voltage is greater than 0.1 V.
Save the file in a *.CSV format: Go to File > Save as and in the pop-up window select “Spreadsheet print (CSV)”. In the “Spreadsheet Comments” pop-up window select “Relative Time” and deselect all the other options. Click OK to save the file.
3. Analysis of Flight Data using Python 3.4.x
Install the latest Python 3.4.x version. Download the archive Python_scripts.zip (Supplemental Files), open it, and save standardize_peaks.py and flight_analysis.py onto the desktop.
- Standardize and select the peaks in the recorded signal as follows
- Right-click on the standardize_peaks.py icon. Select 'Open with IDLE'. Note: IDLE is the default editor for Python, but any text editor can be used for this purpose.
- In Lines 18-19, specify the threshold values around the mean voltage used to perform the standardization of the voltage signal for each channel. Note: The default values are set to deliver a fine tune signal standardization, but the user can define any desired threshold according to the value of the mean voltage for each channel. These can be found in the voltage statistics window (see step 2.3).
- In line 45, type the path to the folder in which the recorded *.CSV file is saved.
- In line 91, type the path to the folder in which you wish to record the *.TXT peak file.
- In line 61 and line 72, specify the number of channels needed. Add or delete channels by deleting the # at the beginning of line 61-63 and 72-74 up to a maximum of 8 channels.
- Save the file and launch the script by pressing F5.
- Enter the name of the *.CSV file (with any additional sub-folders) in to the pop-up window and press return to save a new *.TXT file with the standardized signals in the specified folder. Note: Depending on the number of channels used n, this file contains n+1 columns: the first column is the relative time of the sampling event, the other n columns represent the base and peaks events from the n channels used for the recording. A value of 0 represents the base voltage, while a value of 1 represents a peak derived from the passage of the flag through the IR sensor.
- Analyze the flight track using the standardized file: Edit the flight_analysis.py script to accommodate the user experimental conditions:
- Right click on the flight_analysis.py icon. Select 'Open with IDLE'.
- In line 39 and line 80 adjust the length of the circular flight path according to the arm radius.
- If required, activate an optional speed correction loop by deleting the # in lines 50-52. Change speed value accordingly.
- In line 77 and line 85, edit the speed threshold and the time gap values to correct for false speed readings in the flight track and account for very short time gaps occurring between two consecutive long uninterrupted flying bouts.
- In line 198, specify the total recording time in seconds. Change the value ranges in the output lines from line 287 onwards. Note: The default ranges can be modified according to the user experimental requirements. In order to do so, all the numerical values inside the function (included the ones in the variable name, for example in the variable “flight_300_900”) need to be changed to the desired value.
- In line 248 type the path to the folder in which the *.txt standardized file is saved.
- Specify the number of channels. Add or delete channels by adding or deleting a # at the beginning of lines 257-259, lines 270-272 and lines 279-281 up to a maximum of 8 channels.
- In line 304 type the path to the folder in which you wish to save the output files.
- Once all the user settings are specified, save the file and launch the script by pressing F5.
- Enter the name of the *.TXT file to analyze (with any additional sub-folders) in the pop-up window and press return.
Representative Results
Figure 5 shows representative examples of the type of graphs that can be obtained using the scripts described in the previous section. Flight data were obtained from experimental work conducted in the Department of Zoology at the University of Cambridge using the burying beetle Nicrophorus vespilloides as model (Attisano, unpublished data). Two young unmated males of about 20 days of age were tethered to the flight mills and placed in controlled environmental conditions of 14:10 L:D and 21 °C. The beetles were left in the flight mill for 8 consecutive hours and the flight activity was recorded. The on screen analysis and the graphic output make it possible to resolve individual differences in flight activity patterns. For example, the first male (Figure 5A) showed a strong flight activity within the first hour of recording, characterized by high speed and continuous flight that lasted about three hours. This prolonged activity phase is characterized by a gradual decrease in speed from about 1.6 m/s to about 1 m/s which. After the initial flying bout, the individual showed an almost periodical pattern of relatively short flight bouts about 10-15 minutes duration each. The second male showed a very different flight pattern with flying bouts that never exceeded the duration of 15-20 minutes (Figure 5B). In this individual the flight activity is characterized by a wide spread of flying bouts in the first 4 hours of recording, after which its activity becomes almost periodical. This individual also presented very low flying speed that only occasionally exceeded 0.4 m/s.
Another representative example was obtained using a different insect model, the milkweed bug Oncopeltus fasciatus. Data were collected during a study on the migratory behavior and physiological response to food stress in milkweed bug females24. In this study the recording time was set to one hour in order to characterize females as migrants or residents. These behavioral types are characterized by an “all or nothing” response. Migratory females engage in sustained and continuous flights usually lasting for few hours, while resident females never show flight activity longer than few minutes. Thus, a migrant female will show a flight pattern like in Figure 6A, while a resident female will be characterized by a movement pattern like the one in Figure 6B.
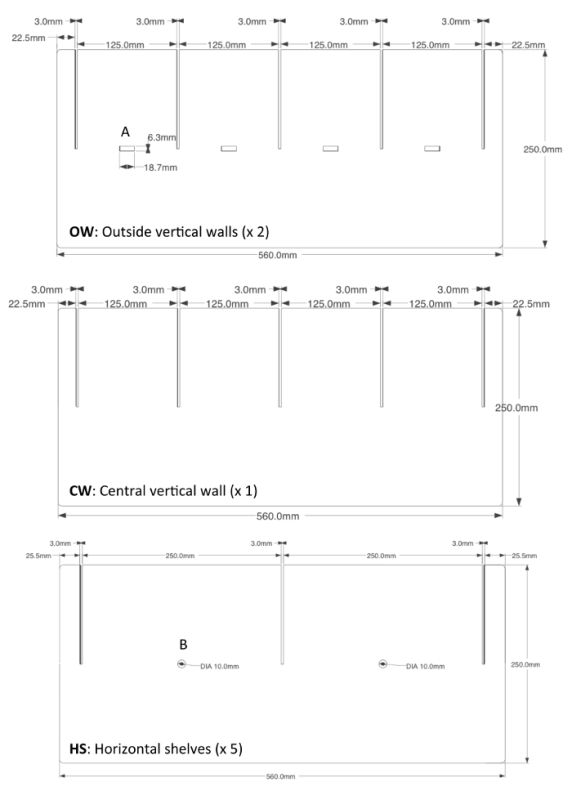 Figure 1. Design configuration for acrylic plastic support structure. The acrylic plastic support structure for the flight mills is constructed from three different components. There are two outside vertical walls (OW) containing both slots for the shelves and an opening to accommodate the IR sensors (A). There is a single central vertical wall (CW) with slots for the shelves. And there are 5 horizontal shelves (HS) with slots for the walls. The magnetic pivot is glued to the horizontal shelves at position B. Please click here to view a larger version of this figure.
Figure 1. Design configuration for acrylic plastic support structure. The acrylic plastic support structure for the flight mills is constructed from three different components. There are two outside vertical walls (OW) containing both slots for the shelves and an opening to accommodate the IR sensors (A). There is a single central vertical wall (CW) with slots for the shelves. And there are 5 horizontal shelves (HS) with slots for the walls. The magnetic pivot is glued to the horizontal shelves at position B. Please click here to view a larger version of this figure.
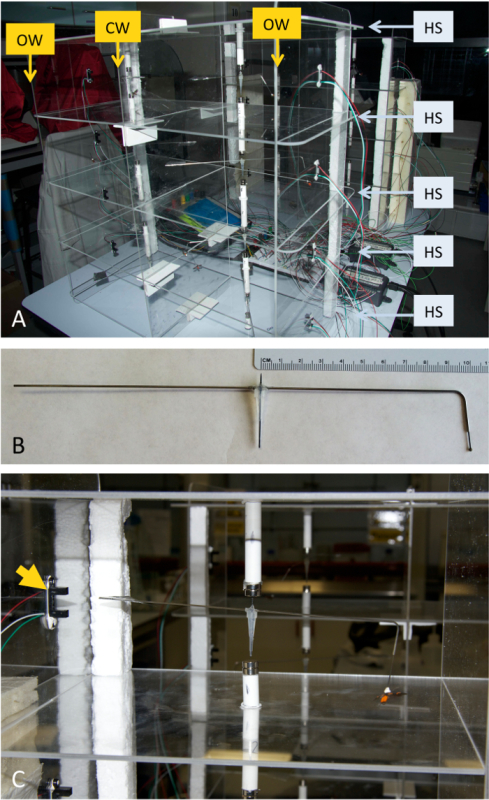 Figure 2. Assembled flight mill. (A) The acrylic plastic support structure is assembled by sliding the five horizontal shelves (HS) into the slots in the two outside walls (OW) and the central wall (CW), resulting in a structure with 8 individual cells each containing a magnetic pivot and an IR sensor, allowing for 8 individuals to be flown at the same time. (B) The pivot arm to which the insects are tethered can be constructed to accommodate a variety of sizes and morphologies of insects. (C) As the tethered insect moves the pivot arm suspended between the magnets, the foil flag at the other end of the arm activates the IR sensor (arrow). Please click here to view a larger version of this figure.
Figure 2. Assembled flight mill. (A) The acrylic plastic support structure is assembled by sliding the five horizontal shelves (HS) into the slots in the two outside walls (OW) and the central wall (CW), resulting in a structure with 8 individual cells each containing a magnetic pivot and an IR sensor, allowing for 8 individuals to be flown at the same time. (B) The pivot arm to which the insects are tethered can be constructed to accommodate a variety of sizes and morphologies of insects. (C) As the tethered insect moves the pivot arm suspended between the magnets, the foil flag at the other end of the arm activates the IR sensor (arrow). Please click here to view a larger version of this figure.
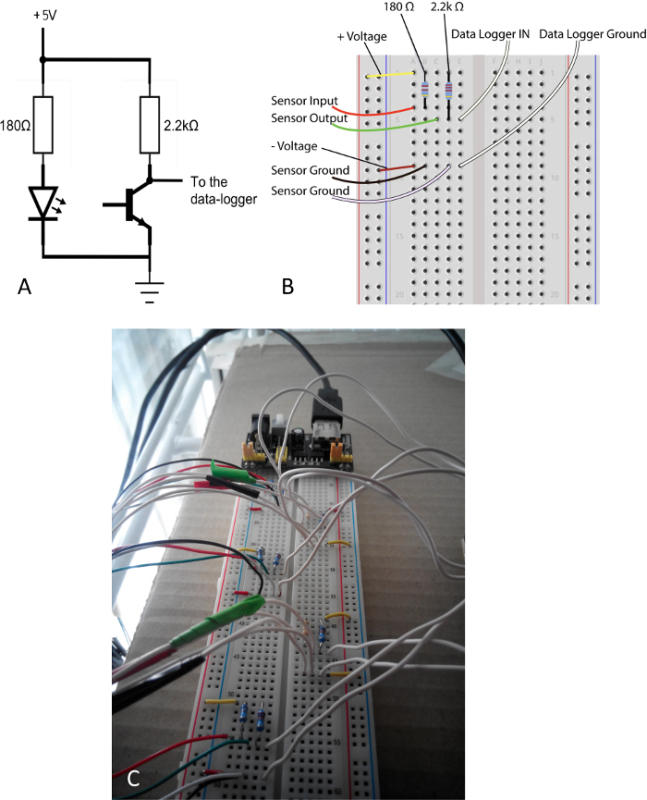 Figure 3. Design of the circuit connecting the IR sensors to the data logger. (A) A simple circuit connects input from the IR sensor to the data logger. (B) Each data logger can be powered and connected to the data logger via a solderless breadboard using the diagram. (C) Multiple sensors can be connected to the single data logger using the same breadboard. Please click here to view a larger version of this figure.
Figure 3. Design of the circuit connecting the IR sensors to the data logger. (A) A simple circuit connects input from the IR sensor to the data logger. (B) Each data logger can be powered and connected to the data logger via a solderless breadboard using the diagram. (C) Multiple sensors can be connected to the single data logger using the same breadboard. Please click here to view a larger version of this figure.
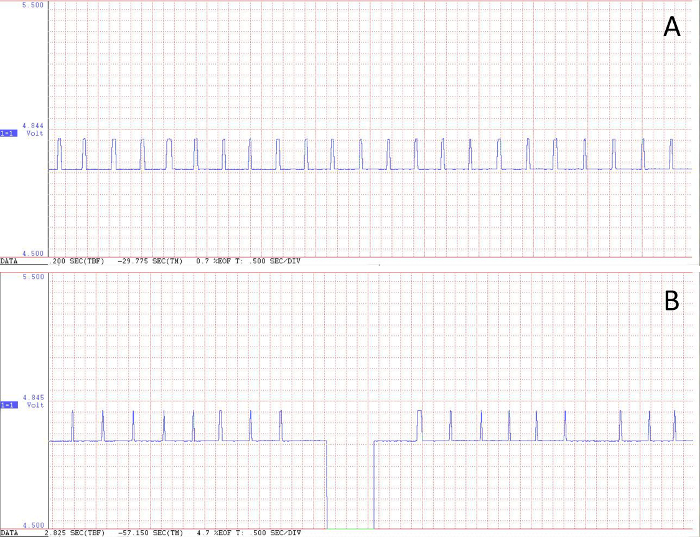 Figure 4. Examples of recorded flight events. Voltage peaks represent complete revolutions of the flight mill’s arm. (A) A high quality recording of a flight event with no voltage drops in the recorded signal. (B) A flight event with a voltage drop in the recorded signal. Please click here to view a larger version of this figure.
Figure 4. Examples of recorded flight events. Voltage peaks represent complete revolutions of the flight mill’s arm. (A) A high quality recording of a flight event with no voltage drops in the recorded signal. (B) A flight event with a voltage drop in the recorded signal. Please click here to view a larger version of this figure.
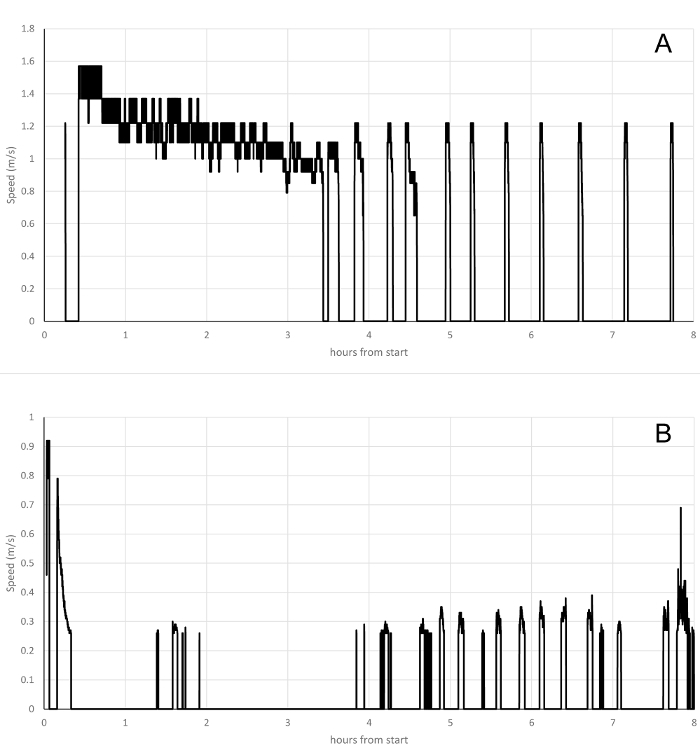 Figure 5. Representative flight data from the burying beetle Nicrophorus vespilloides. Individual variation in flight behavior is easily recognized in the flight recordings. (A) One individual flew continuously for about three hours after the start of the trial and then flew periodically at high speed throughout the rest of the trial. (B) The behavior of the individual is different in that this beetle flew only sporadically throughout the trial and never flew at the high speeds seen in the individual in panel A (note the difference in scale on the Y axis). Please click here to view a larger version of this figure.
Figure 5. Representative flight data from the burying beetle Nicrophorus vespilloides. Individual variation in flight behavior is easily recognized in the flight recordings. (A) One individual flew continuously for about three hours after the start of the trial and then flew periodically at high speed throughout the rest of the trial. (B) The behavior of the individual is different in that this beetle flew only sporadically throughout the trial and never flew at the high speeds seen in the individual in panel A (note the difference in scale on the Y axis). Please click here to view a larger version of this figure.
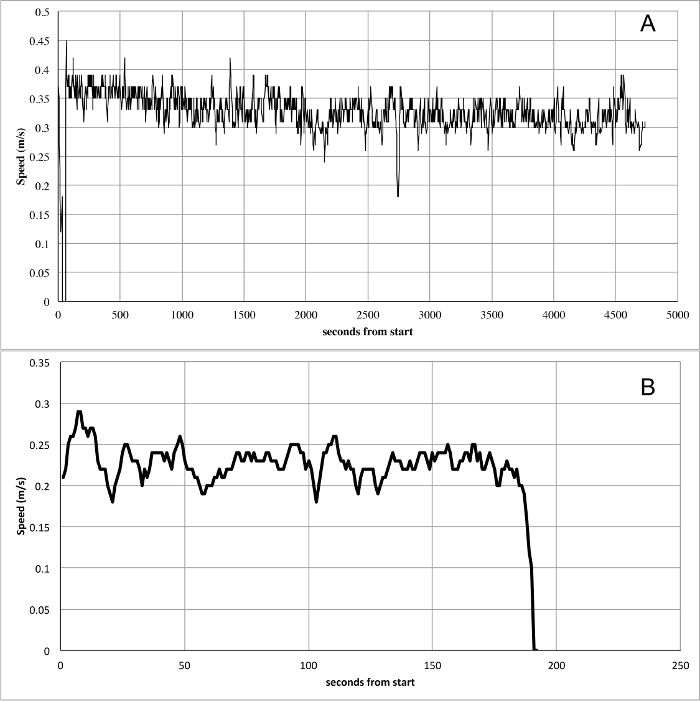 Figure 6. Representative flight data from the milkweed bug Oncopeltus fasciatus. Two different patterns of behavior are clearly observed between the flight data recordings. (A) This recording is typical of the type of flight behavior seen in migratory individuals. Migratory individuals fly at a relatively steady speed over long periods of time. (B) The behavior in Panel A is contrasted with the typical flight behavior of a resident individual. Residents fly at lower speeds and flight bouts only last a short time (note the difference in scale on the X axis for A and B). Please click here to view a larger version of this figure.
Figure 6. Representative flight data from the milkweed bug Oncopeltus fasciatus. Two different patterns of behavior are clearly observed between the flight data recordings. (A) This recording is typical of the type of flight behavior seen in migratory individuals. Migratory individuals fly at a relatively steady speed over long periods of time. (B) The behavior in Panel A is contrasted with the typical flight behavior of a resident individual. Residents fly at lower speeds and flight bouts only last a short time (note the difference in scale on the X axis for A and B). Please click here to view a larger version of this figure.
Discussion
An affordable, flexible, and adjustable flight mill design.
Insect flight behavior is of interest to a range of scientists, from those interested in the basic behavior of insects under variable environments to specialists in biocontrol who needing to understand how conditions influence the propensity of a pest species to disperse. Flight behavior can be studied by various methods that range from flight 'treadmills' and wind tunnels that approximate field conditions to static tethered flight devices. Tethered flight mills, like the one presented here, are limited in that certain aspects of flight, such as changes in altitude, cannot be measured14. However, tethered flight mills do allow insects to fly uninterrupted and thus allow researchers to quantify parameters such as speed, distance and periodicity of flight and correlate these parameters with environmental conditions, physiology, and morphology.
The flight mill presented here was designed to allow researchers without specialized knowledge of electronics to build and use a tethered flight mill in order to study flight behavior in insects. One advantage of this design is that the overall cost of the flight mill is low compared to other designs. The overall cost can be maintained well below 300 US dollars. The plastic acrylic sheets are the most costly item. The second advantage is that the flight mill is adaptable for the limited controlled condition workspaces available in many laboratories, as opposed to a specialized wind tunnel. The use of 3 mm thick transparent acrylic plastic sheets means that the structure is both transparent, to allow easy observation of the insects, and also light weight, enabling the flight mill to be moved to the appropriate location for flight trials. The stacked configuration of the flight mill cells maximizes the number of samples run while minimizing the footprint of the device. Further, the device can be easily disassembled for storage. Additionally, the flight mill was designed to allow for large numbers of individuals to be sampled relatively easily. Each flight mill contains 8 cells, enabling researchers to record flight activity of multiple individuals simultaneously. Attaching insects indirectly to the pivoting arm through an insect pin allows for individual insects to be placed in and removed from the flight mill rapidly. Finally, the data recording electronics is simple and easy to use, with freely available software for data analysis. Once assembled, the flight mill uses simple IR sensors to record flight activity. The passage of the foil flag at the end of the arm through the infrared beam allows each revolution of the arm to be recorded. The rate of revolution allows data like speed, distance traveled, total flight time and patterns of flight to be recorded as input into a data-logger.
The flight mill is able to be adapted for a number of different types of insects. The use of hypodermic steel tubing for the pivoting arm is more effective than other options, such as wooden sticks or drinking straws because, even though heavier, the drag produced is reduced by the narrow diameter, allowing even small insects to be flight-tested. Recently, small pieces of optic fiber have been used in a flight mill for small insects25. The bent ending of the arm can be glued to the armature at different angles relative to the support axis in order to position the experimental insect in its natural flight orientation. In the design presented, in which the radius is 10 cm in length, the entire distance traveled in one revolution is 62.8 cm. Removing the central vertical wall will allow an alternative configuration of the flight mill in which the arm radius can be doubled in length to accommodate larger insects and revolution distances up to 1.20 m. In this case, stronger magnets are recommended to accommodate and stabilize longer mill’s arm.
As stated throughout, the flight mill design is flexible and adaptable for the insect species of interest and researchers are able to customize it for their particular needs. This includes not only the physical needs of the insect, including parameters such as size, power, structure of the cuticle, but also biological differences among species. One potential drawback to all flight mills is that the lack of tarsal support 'forces' the insects to fly, perhaps to exhaustion. While this is true in some species, for example, we observed the automatic flight response with our milkweed bug trials, it is not true for all the insects we have tested (for example N. vespilloides). However, even with the automatic response, we never observed insects flying to exhaustion or death, in part because of the recording time we chose to accommodate the biology of the insects. Thus, it is important to do preliminary observations on the insect of interest to understand its behavior in the flight mill in order to optimize data collection. An additional, well-known issue with flight mills, is that inertia can maintain motion even after the insect has stopped actively flying. The script provided accounts for the misreadings due to inertia of the flight mill, characterized by rapid decrease in flight speed and increasing distances between peaks. The script 'flight_analysis.py' discards these 'false peaks' and constructs a new signal for analysis. The user can choose the speed threshold for correction, as explained in the notes provided in the script.
A 5 V power source is enough to obtain a readable voltage signal, however a power unit with variable output voltage can be used as power source to allow the power input to be varied and thus optimize working voltage for each sensor. Such a solution can also help to increase the visualization quality of peak signals in the software's recording interface. The sensor’s output is shown in the software interface as formed by a base and peak voltages where the base voltage represents the lowest output voltage from the sensor at rest (when the IR beam is not interrupted) while the peak voltage is the rise from base voltage that occurs when the IR beam is interrupted as the arm travels through the beam. An input voltage of 5 V provides a rise of around 100 mV while increasing the input to 7 V increases the peak’s rise to 300 mV allowing for a clearer discrimination of base and peak voltages. The size of the chosen solderless breadboard determines how many flight cells can be accommodated. In order to minimize drops in the voltage signal during recording from multiple sensors, it is recommended to place the resistors in alternate rows along the breadboard (see Figure 3C).
Customizable signal standardization and analysis scripts written for the open access programming language Python.
The standardization and analysis of the voltage signal are conducted by using custom written scripts in Python, which is a free, widely used general-purpose and high-level programming language. The end user can easily customize the scripts to work with own specified settings. The customization is achieved by simply changing numerical values or variable names. Notes on how to customize the parameters can be found within the scripts themselves. The default values in the scripts are set to deliver a fine tune signal standardization, but the user can define any desired threshold according to the value of the mean voltage for each channel. In the flight analysis script, the function flying_bouts from line 105 calculates the duration in seconds of longest and shortest flying bouts, the percentage of time spent in flight over the total recording time and the number of flying bout events of a specified duration range. The ranges can be modified according to the user experimental requirements. In order to do so, all the numerical values inside the function (included the ones in the variable name, for example in the variable “flight_300_900”) need to be changed to the desired value. The number of ranges and their duration simply depends on the user’s specification. The script will print on screen the results of the analysis for each channel. These include: average flying speed, total flight time, distance travelled, shortest and longest flying bouts and flight composition. Additionally, the script returns a *.DAT file for each channel and saves it in the output folder specified by the user. Each *.DAT file contains two columns: the first one represents the relative time of the peak event, the second is the detailed speed variation between two successive peak events. This file can be imported in Excel or R to produce a graph of the speed variation over time and visualize the flight activity patterns.
In conclusion, these results demonstrate that this flight mill design can be easily and successfully implemented to gather data for behavioral studies looking at flying activity patterns in different insect models. Such data can be used to investigate individual variation in movement patterns as dependent for example on physiology and morphology. This can offer great insights into the underlying physiological and morphological traits determining individual variation in movement patterns like foraging or migratory activity, which ultimately affects population as a whole. The detailed speed variation over time can be used in combination with detailed physiological and morphological measurements, offering a tool to study patterns of resource consumption or effects of variation in body part morphology on the flight activity.
Disclosures
The authors have nothing to disclose.
Acknowledgments
Alfredo Attisano was supported by a European Social Fund studentship. James T. Murphy is supported by USDA-NIFA Award 2013-34103-21437.
References
- Hardie J. Flight Behavior in Migrating Insects. J. Agric. Entomol. 1993;10:239–245. [Google Scholar]
- Reynolds D, Riley J. Remote-sensing, telemetric and computer-based technologies for investigating insect movement: a survey of existing and potential techniques. Comput. Electron. in Agric. 2002;35:271–307. [Google Scholar]
- Davis MA. Geographic patterns in the flight ability of a monophagous beetle. Oecologia. 1986;69:407–412. doi: 10.1007/BF00377063. [DOI] [PubMed] [Google Scholar]
- Dingle H, Blakley NR, Miller ER. Variation in body size and flight performance in milkweed bugs (Oncopeltus) Evolution. 1980. pp. 371–385. [DOI] [PubMed]
- Gatehouse A, Hackett D. A technique for studying flight behaviour of tethered Spodoptera exempta moths. Physiol. Entomol. 1980;5:215–222. [Google Scholar]
- Grace B, Shipp J. A laboratory technique for examining the flight activity of insects under controlled environment conditions. Inter. J Biometeorol. 1988;32:65–69. [Google Scholar]
- Kennedy J, Booth C. Free flight of aphids in the laboratory. J. Exp. Biol. 1963;40:67–85. [Google Scholar]
- Kennedy J, Ludlow A. Co-ordination of two kinds of flight activity in an aphid. J. Exp. Biol. 1974;61:173–196. doi: 10.1242/jeb.61.1.173. [DOI] [PubMed] [Google Scholar]
- Laughlin R. A modified Kennedy flight chamber. Aust. J. Entomol. 1974;13:151–153. [Google Scholar]
- Krell RK, Wilson TA, Pedigo LP, Rice ME. Characterization of bean leaf beetle (Coleoptera: Chrysomelidae) flight capacity. J. Kansas Entomol Soc. 2003. pp. 406–416.
- Liu Z, Wyckhuys KA, Wu K. Migratory adaptations in Chrysoperla sinica (Neuroptera: Chrysopidae) Environ. Entomol. 2011;40:449–454. [Google Scholar]
- Wang XG, Johnson MW, Daane KM, Opp S. Combined effects of heat stress and food supply on flight performance of olive fruit fly (Diptera: Tephritidae) Ann. Entomol. Soc. Am. 2009;102:727–734. [Google Scholar]
- Dingle H. Migration: the biology of life on the move. Oxford University Press; 2014. [Google Scholar]
- Blackmer JL, Naranjo SE, Williams LH. Tethered and untethered flight by Lygus hesperus and Lygus lineolaris (Heteroptera: Miridae) Environ. Entomol. 2004;33:1389–1400. [Google Scholar]
- Riley J, Downham M, Cooter R. Comparison of the performance of Cicadulina leafhoppers on flight mills with that to be expected in free flight. Entomol. Exp. App. 1997;83:317–322. [Google Scholar]
- Taylor R, Bauer LS, Poland TM, Windell KN. Flight performance of Agrilus planipennis (Coleoptera: Buprestidae) on a flight mill and in free flight. J. Insect Behav. 2010;23:128–148. [Google Scholar]
- Cooter R, Armes N. Tethered flight technique for monitoring the flight performance of Helicoverpa armigera (Lepidoptera: Noctuidae) Environ. Entomol. 1993;22:339–345. [Google Scholar]
- Chambers D, Sharp J, Ashley T. Tethered insect flight: A system for automated data processing of behavioral events. Behav. Res. Meth. Instr. 1976;8:352–356. [Google Scholar]
- Clarke J, Rowley W, Christiansen S, Jacobson D. Microcomputer-based monitoring and data acquisition system for a mosquito flight. Ann. Entomol. Soc. Am. 1984;77:119–122. [Google Scholar]
- Resurreccion A, Showers W, Rowley W. Microcomputer-interfaced flight mill system for large moths such as black cutworm (Lepidoptera: Noctuidae) Ann. Entomol. Soc. Am. 1988;81:286–291. [Google Scholar]
- Taylor R, Nault L, Styer W, Cheng Z-B. Computer-monitored, 16-channel flight mill for recording the flight of leafhoppers (Homoptera: Auchenorrhyncha) Ann. Entomol. Soc. Am. 1992;85:627–632. [Google Scholar]
- Bruzzone OA, Villacide JM, Bernstein C, Corley JC. Flight variability in the woodwasp Sirex noctilio (Hymenoptera: Siricidae): an analysis of flight data using wavelets. J. Exp. Biol. 2009;212:731–737. doi: 10.1242/jeb.022517. [DOI] [PubMed] [Google Scholar]
- Schumacher P, Weyeneth A, Weber DC, Dorn S. Long flights in Cydia pomonella L. (Lepidoptera: Tortricidae) measured by a flight mill: influence of sex, mated status and age. Physiol. Entomol. 1997;22:149–160. [Google Scholar]
- Attisano A, Tregenza T, Moore AJ, Moore PJ. Oosorption and migratory strategy of the milkweed bug, Oncopeltus fasciatus. An. Behav. 2013;86:651–657. [Google Scholar]
- Martini X, Hoyte A, Stelinski LL. Abdominal color of the Asian citrus psyllid (Hemiptera: Liviidae) is associated with flight capabilities. Ann. Entomol. Soc. Am. 2014;107:627–632. [Google Scholar]


