Abstract
Recent advances in the application of bone marrow mesenchymal stem cells (BMMSC) for the treatment of tendon and ligament injuries in the horse suggest improved outcome measures in both experimental and clinical studies. Although the BMMSC are implanted into the tendon lesion in large numbers (usually 10 - 20 million cells), only a relatively small number survive (<10%) although these can persist for up to 5 months after implantation. This appears to be a common observation in other species where BMMSC have been implanted into other tissues and it is important to understand when this loss occurs, how many survive the initial implantation process and whether the cells are cleared into other organs. Tracking the fate of the cells can be achieved by radiolabeling the BMMSC prior to implantation which allows non-invasive in vivo imaging of cell location and quantification of cell numbers.
This protocol describes a cell labeling procedure that uses Technetium-99m (Tc-99m), and tracking of these cells following implantation into injured flexor tendons in horses. Tc-99m is a short-lived (t1/2 of 6.01 hr) isotope that emits gamma rays and can be internalized by cells in the presence of the lipophilic compound hexamethylpropyleneamine oxime (HMPAO). These properties make it ideal for use in nuclear medicine clinics for the diagnosis of many different diseases. The fate of the labeled cells can be followed in the short term (up to 36 hr) by gamma scintigraphy to quantify both the number of cells retained in the lesion and distribution of the cells into lungs, thyroid and other organs. This technique is adapted from the labeling of blood leukocytes and could be utilized to image implanted BMMSC in other organs.
Keywords: Medicine, Issue 106, Tendon, tendinopathy, horse, mesenchymal stem cells, cell tracking, technetium
Introduction
Regenerative strategies for the repair of diseased or damaged tissues are based on multipotent stem cells derived from a variety of tissue and implanted into the affected area. Recent advances in the application of autologous BMMSC for the treatment of tendon and ligament injuries in the horse have shown improved outcome measures in both experimental1-5 and clinical studies6. The horse is a particularly attractive model for assessing the efficacy of stem cell related treatments because it suffers from age and overstrain related injuries to the tendons of the distal forelimb, it is an athletic animal, and it is a large, facilitating bone marrow recovery and accurate implantation. Tendon injuries heal naturally with fibrosis but the healed tendon is functionally inferior7 and has a high risk of re-injury8. The superficial digital flexor tendon (SDFT) is most commonly affected as it has evolved to act as an elastic energy store and experiences high loading stresses to achieve energy efficient and high speed locomotion. Restoring function after injury is therefore critical. These injuries are similar to those affecting the Achilles tendon in humans which performs a similar function9. There are no good treatment options to treat or achieve good repair for such injuries, therefore cell-based regenerative strategies offer an attractive opportunity to improve outcomes and to reduce re-injury.
In most studies 5 – 20 million autologous BMMSC are injected directly into the lesion which usually occur within the core of the tendon body which therefore acts as a receptacle for the cells. The fate of the cells once injected is not clear and different cell labeling methods to track the cells have been described recently. Cells labeled with a fluorescence tag were shown to survive only in relatively small numbers (<10%)10,11. Fluorescence labels necessitate tissue extraction and sectioning for histological analysis which is time consuming and does not readily facilitate temporal analysis in a large animal model or in clinical cases. In more recent work we have used the radioisotope 99mTc to label cells and follow their fate by gamma scintigraphy1. This method allows rapid comparisons to be made between different routes of cell delivery, including intralesional, intravenous via the jugular vein1 or regional perfusion via intra-arterial12 or intravenous1,12 injections. The persistence and distribution of cells can then be imaged by gamma scintigraphy of various organs. This has demonstrated that only 24% of the cells injected intralesionally remained in the lesion by 24 hr1 and this is supported by another study using experimentally created lesions and using the same radiolabel5. Furthermore, the cells show limited ability to home into tendon lesions when delivered by regional perfusion or intravenously but are dispersed into the lungs by the latter routes4.
BMMSC labeled with iron nanoparticles is an alternative method to track cells implanted into forelimb tendons13. Although iron nanoparticle labeled cells allow cell tracking in vivo by MRI, temporal studies in a large animal are limited by the number of times anesthesia can be administered at each time point for performing the MRI scans. Furthermore, iron nanoparticles are hypointense on MRI which limits the information on the migration of labeled cells into the tendon body. Other radioisotopes that can be used include Indium-111 but this suffers the disadvantage of a longer half-life than Tc-99m (2.8 days vs 6.0 hr) and higher gamma ray emission energy. In addition, cell viability has been reported to be reduced when labeled with Indium-11114. Tc-99m, on the other hand, is routinely used in both equine and human nuclear medicine to label peripheral blood mononuclear cells and follow their distribution in vivo by scintigraphy. It can be relatively easily taken up by cells using HMPAO as a linker molecule to bind the technetium, as Tc-99m-HMPAO, to cells. Tc-99m-HMPAO labeled BMMSC show good viability and can proliferate in vitro4. This protocol details the labeling and tracking of equine autologous BMMSC implanted into naturally occurring lesions in the forelimb SDFT.
It is important to note that the protocol is intended only to be used as a research tool. Its use as a clinical therapeutic modality is not recommended as the effect of the radiolabel on cellular phenotype has not been fully elucidated.
Protocol
The cases described herein were performed following Ethical permission granted by the Animal Ethics and Welfare Committee of the Colegio de Veterinarios de Malaga, Spain, and the Royal Veterinary College, North Mymms, U.K. The procedures used on the horses are based on approved protocols that are used in clinic on horses receiving stem cell based therapies which includes sedation, bone-marrow aspiration, intra-tendinous injection, regional perfusion, intravenous injection, post-procedural treatment and pain management and monitoring after implantation.
1. Key Arrangements to be Made in Advance
Seek institutional ethics and animal welfare approval and adhere to local legal requirements prior to starting work.
Seek institutional approval to work with ionizing radiation as dictated by local and legal regulations including the appropriate level of protection of personnel and the environment and the disposal of contaminated waste.
Use the following inclusion criteria: horses present with a non-traumatic overstrain injury (tendinopathy or desmopathy) to the superficial flexor digital tendon of the forelimb and lesions resulting in a defect within the tendon and the defect is surrounded by intact tendon and/or paratenon. Note: The examination and diagnosis for such injuries and the clinical preparation of the horses for cell implantation has been detailed elsewhere6,15. Note: The isolation and expansion in culture of BMMSC derived from the bone marrow of horses has been detailed previously6,16. The current protocol for injecting BMMSC for SDFT injuries in the horse uses 10 million cells per ml4 of autologous bone marrow supernatant (BMS)16.
Use cells that have undergone no more than 4 passages for in vivo implantation to avoid potential cellular phenotype or genetic alterations associated with high passage number cells.
Deliver the required number of cells in the BMS (within 24 hr in a chill box at 4 – 8 oC) to the veterinary clinic where the cell implantation is to be performed. Note: The clinical use of mesenchymal stem cells in bone marrow supernatant is a patented procedure owned by ReCellerate Inc (U.S.A.) and its use may require authorization. Note: Tc-99m is a gamma emitter (140 keV) with a relatively short half-life (6.0058 hr, therefore nearly 94% of it decays to its stable form of 99Tc in 24 hr). The gamma energy makes it suitable for detection by gamma cameras (scintigraphy) and the short half-life results in very limited radiation exposure time to both the horse and the animal handling personnel. This protocol describes the on-site labeling of cells using Tc-99m-HMPAO [HMPAO labeled with Tc-99m (as sodium pertechnetate)].
Order the Tc-99m fresh from the supplier such that it is delivered and used for labeling the cells within 2 hr of elution from the Tc-99m generator. Ensure that the Tc-99m is supplied as 1 GBq in a 1 ml solution (supplier’s standard buffer).
Use all buffers at RT. Perform the labeling procedure, implantation of the cells and imaging (gamma scintigraphy) in an isotope containment room with all equipment cleaned with alcohol wipes prior to the start of work.
2. Preparation of the Horse and Ultrasonography of the Forelimb Tendon
Record ultrasound scans at first admission for injury (when bone marrow is aspirated) and then at the time point when radiolabeled cells are injected.
Perform ultrasonography and cell implantation with the horse bearing weight evenly in a stall and under standing sedation (rather than general anesthesia). Administer sedation using a mixture of detomidine HCl and butorphanol tartrate, each at 0.01 to 0.02 mg/kg IV. Note: Recovery is rapid and post-surgical treatment requires no special considerations.
After perineural anesthesia is applied (step 2.6) in the affected limb, keep the horse at the station under sedation to reduce movement of the horse during the treatment. Visually check the appropriate level of sedation judged from the lowered head position and the demeanour of the horse and that the horse is comfortable during cell injection and the movements of the gamma camera during the acquisition of scintigraphic images. Note: It is not necessary to apply vet ointment on eyes (to prevent dryness) because under sedation blinking is not affected and the horse is able to maintain hydration of the eyes. The monitoring after the implantation of the cells is non-invasive (ultrasound and gamma scintigraphy).
Clip closely the hair overlying the tendon (with horse hair clippers) then clean the clipped area using a combination of a chlorhexidine surgical scrub and finally alcohol.
Apply acoustic coupling gel to the area and scan methodically to record scans of the complete palmar metacarpal region, sequentially labeled levels 1 - 717, with a linear transducer (12 MHz or similar) to obtain serial on-incidence transverse and longitudinal images. Store the images digitally so that the cross-sectional area and the maximal injury zone4 can be obtained with the aid of image analysis software.
Prepare the area overlying the palmar nerves immediately distal to the carpus for aseptic injection of local anesthetic to ensure complete desensitization of the skin overlying the tendon and the superficial digital flexor tendon. Inject 2 ml of mepivicaine both subcutaneously and adjacent to the palmar nerves (in their sub-fascial location) between the flexor tendons and the suspensory ligament immediately distal to the carpus on both sides of the limb.
Once the preliminary scan has been performed prepare the implantation site by scrubbing the area with a chlorhexidine surgical scrub solution for at least 5 min and finally alcohol with soaked gauze. Perform all subsequent steps in an aseptic fashion.
3. Tc-99m-labeling of Equine Mesenchymal Stem Cells
Note: The cell labeling steps can be performed during ultrasonography of the forelimb as this will minimize isotope decay between cell labeling and implantation of labeled cells into the tendon.
Remove the BMS from the cells as it interferes with the uptake of isotope. To do this, retrieve the vial of BMMSC (10 million cells in 1 ml of BMS) from the chill box and pellet the cells in a microcentrifuge at 350 x g for 5 min at RT. Carefully remove the supernatant with an 18G or 19G needle (attached to a 2 ml syringe), leaving a small drop ( ≤0.1 ml) in the tube to aid cell resuspension. Save the supernatant (the BMS) in a sterile microcentrifuge tube and keep on ice for later re-use.
Resuspend the cells in the residual droplet of supernatant by flicking the tube gently with fingers (rather than vortex) and leave the tube in a rack at RT. Ensure the cells are completely resuspended and that there are no visible cell clumps.
Prepare the Tc-99m as follows. Transfer 1 ml of technetium pertechnetate into a vial of HMPAO using a 1 ml syringe and needle. Mix gently but thoroughly and leave for 5 min behind lead shielding. Note: This step can be done while the cells are spinning in step 2.1.
Add all of the Tc-99m-HMPAO mixture using a 1 ml syringe and needle to the cells and mix gently. Incubate for 30 min at RT behind lead shielding.
For the next steps, use a forceps (or a piece of tissue paper) to open the lid of the microcentrifuge tube to minimize isotope contamination of the (gloved) thumb and subsequently other equipment. Use a 2 ml syringe and 21 G needle to add or remove wash buffer. Note: The use of a field isotope monitor is encouraged to monitor contamination of surfaces and equipment.
Add 1 ml PBS to the Tc-99m-HMPAO-cell mixture, close the lid, mix gently and spin to pellet the cells as in step 3.1.
Carefully remove the PBS to a new tube (save this behind lead shielding and label as W1). Resuspend the cell pellet in 1 ml of fresh PBS. Centrifuge the tube and remove the supernatant as above (save this and label as W2).
Calculate the efficiency of labeling by measuring the counts in W1 and W2 tubes and in the cell pellet. Do this by placing each tubes in a Well isotope dose calibrator instrument and recording the radioactivity as counts per minute (CPM). Calculate the labeling efficiency as (Radioactivity as CPM of cells) / (Radioactivity of cells + supernatant) x 100.
Resuspend the cells thoroughly in the residual PBS and then add the BMS saved from step 3.1. The cells are ready for intralesional implantation.
4. Implantation of Tc-99m-HMPAO-labeled Cells
- Intralesional implantation into the tendon under ultrasound guidance
- Slowly introduce a 1.5 or 2 inch (50 mm) 20 G needle into the maximum injury zone of the lesion in a longitudinal orientation with the ultrasound transducer, covered in a sterile drape, in line with the needle so that the length of the needle can be identified ultrasonographically and the tip if the needle is accurately located in the center of the lesion.
- Using appropriate shielding, collect the cells into a 2 ml syringe (Luer-lock, to minimize the risk of external contamination and in a lead-shielded syringe cover) using an 20 G needle. Discard the needle being careful not to lose any fluid. Now attach the syringe with the labeled cells onto the needle pre-located in the tendon.
- Place the ultrasound probe under the needle so that the needle tract in the tissue is clearly visible. Inject the cells at a slow but steady pace into the lesion. Ensure that there is no resistance to injection. If this is encountered carefully reposition the needle under ultrasound guidance. Check that the fluid ejecting into the lesion is visible on the ultrasound image as anechoic fluid containing hyperechogenic air bubbles.
- Withdraw the needle and immediately place pressure over the needle hole to prevent loss of injected cells and to minimize the spread of isotope over the external surface of the limb. Immediately dress the limb with one layer of autoadhesive bandage. The horse is now ready for gamma scintigraphy. Perform this immediately. Note: After injecting the cells into the tendon, acquire a count of the empty syringe and needle to assess possible direct loss of cells that may remain in the syringe.
- Regional perfusion of Tc-99m-HMPAO-labeled cells
- Follow step 2.4.
- Apply a 100 mm rubber tourniquet bandage on the proximal metacarpus with two 100 mm cotton roll gauze bandage positioned medially and laterally.
- Aseptically prepare the skin over the lateral palmar digital vein at the level of the lateral proximal sesamoid bone by scrubbing the area with a chlorhexidine surgical scrub solution for at least 5 min and finally with gauze soaked in alcohol. Perform all subsequent steps in an aseptic fashion.
- Prepare a 100 mm extension set with a 21 G x ¾” (0.8 x 19 mm) butterfly needle for venipuncture by filling with heparinized sterile saline and then introduce the needle into the lateral palmar digital vein. Once the vein is entered blood will partially fill the extension set. At the Luer lock connector attach a Luer lock rubber cap for facilitating subsequent injection of labeled cells.
- Dilute the MSCs in 19 ml of PBS by collecting the cells in a 20 ml syringe preloaded with the PBS. Apply the cells via the rubber cup using an 18 G (1.2 x 40 mm) hypodermic needle at a slow but steady rate (30 – 40 sec). Flush the catheter with 1 ml of PBS preloaded in a syringe.
- Remove the needle and immediately apply pressure at the skin over the needle hole with a layer of five 4 x 4 cotton gauzes. Then place a light bandage with elastic adhesive over the proximal sesamoid area and leave the tourniquet in place for 30 min after injection.
- Release the tourniquet after 30 min. The horse is now ready for gamma scintigraphy. Note: After injecting the cells, acquire a count of the empty syringe and needle to assess possible direct loss of cells that may remain in the syringe.
5. Gamma Scintigraphy
- Scintigraphy images
- Acquire planar scintigraphy images with a gamma camera (set at 140 KeV photoelectric peak using a 20% symmetrical window) equipped with a low energy, parallel hole collimator.
- Obtain all images after time 0 on standing horses sedated as in step 2.2. After the time 0 scintigram, replace the light bandage over the implantation site with a padded bandage. Remove this bandage prior to each scintigraphy exam.
- Acquire static images for 60 sec each (256 by 256 matrix) using the processing software with the “acquire” – “study selection” - “bone static” options. It is essential that the horse does not move during the image acquisition period because the static mode option does not have a module for correction of movement.
- For intralesional injection, obtain lateral images from the lesion area from the carpus to the distal extremity, the equivalent area of the contralateral limb, the left lung field and the left thyroid. During the acquisition of forelimbs images, position a lead screen between the legs to avoid imaging of the limb contralateral. Acquire the images at 5 min after injection (time 0), and then at 1, 3, 6, 12, 24, 36 and 48 hr.
- For regional perfusion, obtain lateral and dorsal gamma images as for 5.1.1.3. Obtain images 5 min after tourniquet release. This will be time 0. It can be useful to image the limb immediately after injecting the cells to assess counts prior to tourniquet release. Obtain gamma images at 1, 3, 6, 12, 24, 36 and 48 hr after administration of the cells.
- After the images have been acquired, process the images manually using the “processing” option of the software with options selected for “Sokoloff” color and “Invert” color. Next select the “region of interest” (ROI) and “ellipse” as this allows reshaping of the selection mask and movement over the lesion area. Obtain the counts over the ROI by choosing the “add to statistics” option. Ensure that identically sized regions of interest (ROI) are recorded for each animal at the different time-points.
- Next, correct the counts for the predicted decay of the technetium at each time point. Use the following equation to correct for decay: A = Aoe-(0.693t/T1/2), where Ao is the initial activity of the isotope, A is the decay corrected radioactivity at time zero, t is the elapsed time and T1/2 is the half-life of the isotope. Then express the corrected counts at each time point as a percentage of the ROI at time 0 to work out the percentage of cells remaining, using the following formula:
%cells remaining (t) = 100 – {[(predicted decay(t) – decay(t))/ predicted decay(t)]} x 100
decay(t) = ROI(t) / ROI(0) x 100.
Representative Results
Tc-99m-HMPAO incorporation into BMMSs does not adversely affect their ability to adhere to tissue culture plastic and while they show proliferation ability to form monolayers (Figure 1) we have not fully determined whether proliferation rates or other cellular phenotypes are affected. Their morphology is similar to unlabeled cells with a typical spindle shape. The cellular labeling efficiency (i.e., uptake of label) typically varies from about 1.5% to 25%. The main reason for the low labeling efficiency was related to the delay in the delivery of the 99mTc to the clinic from the site where the Mo-99/Tc-99m generator has been eluted. Delays of more than 2 hr result in a major decline in labeling of the cells. However, sufficient isotope is incorporated into the cells to perform gamma scintigraphy for up to 36 hr (Figure 2). Factors that can improve labeling efficiency are detailed in the discussion.
The labeled cells can be implanted either by intralesional implantation1 (directly into the lesion) or by regional perfusion via a digital vein1 or artery12. Intralesional implantation is possible when the injury is in the core of the tendon and surrounded by intact tendon tissue that acts as a receptacle for the cells. Regional perfusion is an easier route to administer the cells as they are injected into a blood vessel but the method relies on the expectation that the MSCs are able to “home-in” to the tendon lesion. There is limited evidence that MSCs can specifically home into sites of tendon lesions but this method provides a useful experimental approach for developing applications which may increase the homing capacity of MSCs.
With the intralesional delivery route, the cells will be seen as a focal area of signal which remains relatively localized with time i.e., there is limited spread or migration of the cells into the surrounding tendon tissue. In contrast, there will be a gradual reduction of signal with time at the injection site when cells are administered by regional perfusion via the digital palmar vein (Figure 2). It is common with intralesional injection for the lung fields to be negative but in some cases at the very early time points the lung may show isotope signal which can be focal and then becomes more generalized (Figure 3). The thyroid remains negative with this administration route. Regional perfusion similarly can show focal signal in the lungs which then becomes more diffuse but the signal is significantly more intense compared to that observed with the intralesional route. The thyroid can often also show focal signal.
The initial total radioisotope count of the cells (time 0, pre-injection) and the counts from the initial gamma scintigram at the region of interest (time 0, post-injection) are noted and used to calculate the percentage decrease in counts at each time point. This is used to compare to the predicted decay of the Tc-99m from the initial counts (Figure 4). The calculated curve will follow the predicted curve. However, after taking the predicted decay into account, the persistence of cells remaining in the injection site after intralesional administration is about 32% (at 12 hr) and 24% (at 24 hr) (Figure 4).
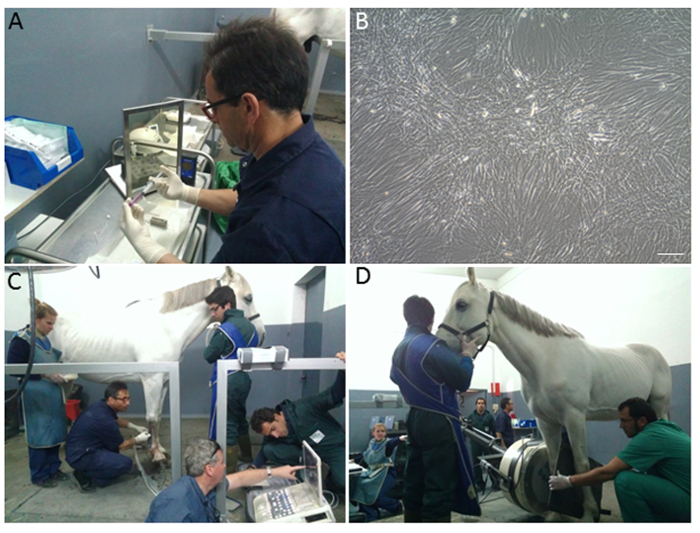 Figure 1. Cell Labeling, Injection and Gamma Scintigraphy. (A) Transfer of BMMSC into a microfuge prior to labeling with Tc-99m-HMPAO behind lead-lined shielding (screen and syringe holder) and radiation monitor and microcentrifuge; (B) An aliquot of Tc-99m-HMPAO labeled cells seeded in a tissue culture dish show good morphology, viability and proliferation; (C) Injection of Tc-99m-HMPAO labeled cells under ultrasound guidance into the injury site of the right forelimb SDFT; (D) Gamma scintigraphy of the forelimb. The gamma camera is positioned precisely using a maneuverable hydraulic arm and any potential labeled cells in the contralateral limb are eliminated by a lead shield held between the legs. The gamma camera is similarly positioned at the chest to monitor the lung field or at the neck to monitor the thyroid field. Please click here to view a larger version of this figure.
Figure 1. Cell Labeling, Injection and Gamma Scintigraphy. (A) Transfer of BMMSC into a microfuge prior to labeling with Tc-99m-HMPAO behind lead-lined shielding (screen and syringe holder) and radiation monitor and microcentrifuge; (B) An aliquot of Tc-99m-HMPAO labeled cells seeded in a tissue culture dish show good morphology, viability and proliferation; (C) Injection of Tc-99m-HMPAO labeled cells under ultrasound guidance into the injury site of the right forelimb SDFT; (D) Gamma scintigraphy of the forelimb. The gamma camera is positioned precisely using a maneuverable hydraulic arm and any potential labeled cells in the contralateral limb are eliminated by a lead shield held between the legs. The gamma camera is similarly positioned at the chest to monitor the lung field or at the neck to monitor the thyroid field. Please click here to view a larger version of this figure.
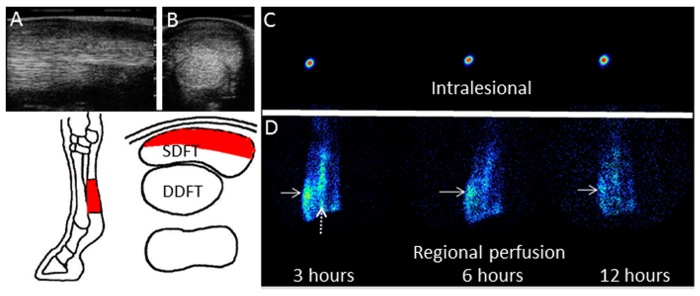 Figure 2. Ultrasound and Gamma Scintigrams of the SDFT. Typical ultrasonographs of the forelimb at the mid-metacarpal region in (A) longitudinal section and (B) cross-section. The lesion in the SDFT is clearly seen as an hypoechoic area (towards the top of the scan) compared to the uninjured (normal) tissue integrity of the deep digital flexor tendon (DDFT) adjacent to and underneath the SDFT. The area of the lesion and the relative positions of the tendons are depicted in the schematic below. Gamma scintigraphs performed at 3, 6 and 12 hr post-injection of radiolabeled cells are shown for the cells injected (C) into the lesion or (D) by intravenous regional perfusion via the digital palmar vein. In the case shown, there was uptake of radiolabeled cells in the lesion site in the SDFT (solid arrows). Persistence of uptake by the palmar digital vein is denoted by the dashed arrow. Please click here to view a larger version of this figure.
Figure 2. Ultrasound and Gamma Scintigrams of the SDFT. Typical ultrasonographs of the forelimb at the mid-metacarpal region in (A) longitudinal section and (B) cross-section. The lesion in the SDFT is clearly seen as an hypoechoic area (towards the top of the scan) compared to the uninjured (normal) tissue integrity of the deep digital flexor tendon (DDFT) adjacent to and underneath the SDFT. The area of the lesion and the relative positions of the tendons are depicted in the schematic below. Gamma scintigraphs performed at 3, 6 and 12 hr post-injection of radiolabeled cells are shown for the cells injected (C) into the lesion or (D) by intravenous regional perfusion via the digital palmar vein. In the case shown, there was uptake of radiolabeled cells in the lesion site in the SDFT (solid arrows). Persistence of uptake by the palmar digital vein is denoted by the dashed arrow. Please click here to view a larger version of this figure.
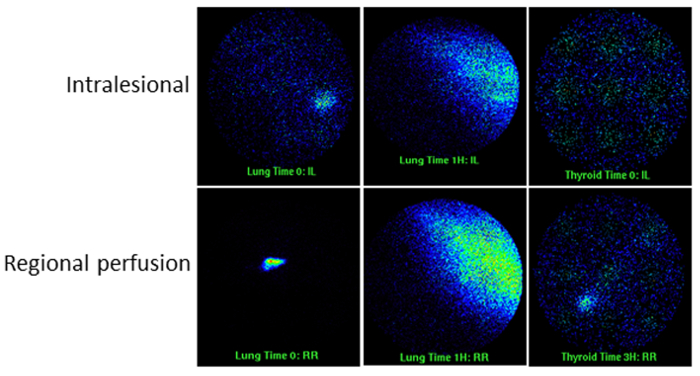 Figure 3. Gamma Scintigrams of the Lung and Thyroid. Typical scans from early time points are shown for both intralesional injection and regional perfusion of labeled cells. Note the focal and diffuse signal in the lung and thyroid fields. Please click here to view a larger version of this figure.
Figure 3. Gamma Scintigrams of the Lung and Thyroid. Typical scans from early time points are shown for both intralesional injection and regional perfusion of labeled cells. Note the focal and diffuse signal in the lung and thyroid fields. Please click here to view a larger version of this figure.
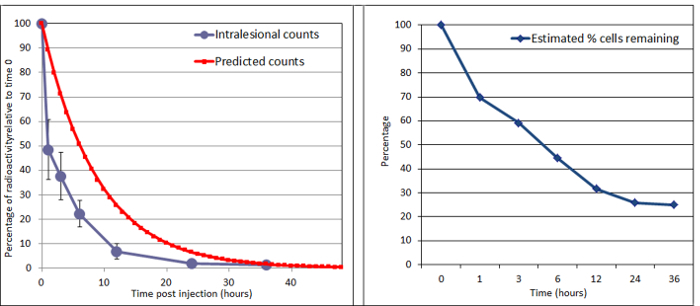 Figure 4. Decay of Radioactivity Recorded from the Regions of Interest after Intralesional Injection. In this horse, counts were measured from gamma scintigrams of the SDFT over 36 hr after injection into the lesion. Left panel: the radioactivity at each time point was calculated against the time 0 value. The natural decay in radioactivity of 99mTc is shown for comparison. Right panel: the estimated percentage of cells remaining. This is calculated from the radioactivity level from the regions of interest. In this case the remaining percentage of cells at 24 hr was about 24%. We have performed this protocol (labeling of MSC and implantation) on 21 horses. Please click here to view a larger version of this figure.
Figure 4. Decay of Radioactivity Recorded from the Regions of Interest after Intralesional Injection. In this horse, counts were measured from gamma scintigrams of the SDFT over 36 hr after injection into the lesion. Left panel: the radioactivity at each time point was calculated against the time 0 value. The natural decay in radioactivity of 99mTc is shown for comparison. Right panel: the estimated percentage of cells remaining. This is calculated from the radioactivity level from the regions of interest. In this case the remaining percentage of cells at 24 hr was about 24%. We have performed this protocol (labeling of MSC and implantation) on 21 horses. Please click here to view a larger version of this figure.
Discussion
In addition to bone marrow, stem cells isolated from sources such as adipose tissue are suitable for labeling with this protocol. Furthermore, cells from a frozen state may be revived and expanded in culture to the desired numbers for labeling studies12.
A critical factor that determines the efficiency of labeling the BMMSC is the time between elution of the Tc-99m from the molybdenum generator at the radiopharmacy, preparation of Tc-99m-HMPAO and use of the radiopharmaceutical in the clinic. There is an inverse relationship between labeling efficiency and time after Tc-99m elution from the generator such that delays beyond this period can significantly impair the efficiency of the cell labeling. Therefore, close co-ordination is required between delivery of the cells from the laboratory and the delivery of the Tc-99m to the clinic on the day that the cells will be radiolabeled and implanted. The transport time to the clinic is therefore an important factor. The supplier’s data sheet specify that the decay of the Tc-99m reduces the purity of the radiochemical after 2 h post-elution which can interfere with the binding of the HMPAO to the Tc-99m and subsequently the amount of Tc-99m-HMPAO taken up by the cells. We have also noted that labeling of cultured cells in a laboratory environment gave more efficient labeling than in the clinic. This has also reported by another study in horses5 where the cells were labeled in a laboratory environment. In addition, in our experience, label is taken up more efficiently by cells that are labeled immediately following preparation from blood or tissue samples compared to cells prepared from culture. However, even with low efficiency of labeling there is sufficient label bound to the cells to perform scintigraphy over 36 hr.
Labeling efficiencies of 40% – 80% can be achieved for leucocytes if the Tc-99m is used from a fresh generator eluate within 30 min and the reaction performed in a small volume (1 – 0.5 ml)18. Labeling efficiencies of 47% – 71% have been achieved for equine MSCs19 demonstrating that it is possible to increase the labeling efficiency of MSCs under ideal conditions.
We routinely implant 20 million cells for the treatment of SDFT core lesions although up to 40 million cells have been implanted for the largest lesions. A similar number of cells could be implanted via regional perfusion although this method is not commonly used because of the more complex procedure. However, it may be useful for lesions which extend to the periphery of the tendon surface with the expectation that sufficient cells home into the lesion. Tracking of the cells by gamma scintigraphy can provide useful information on the movement of cells administered by different routes. In this protocol, the cells implanted by the intralesional route are mostly retained at the injection site by the intralesional route. Radioisotope signal may be observed in the lung fields in some cases but it appears in general that there is little migration of cells away from the lesion site. Delivery of BMMSC by regional perfusion suggests that there may be retention of cells at the injury site, but when delivered by intravenous injection4 we have not observed homing of the cells. The Tc-99m-HMPAO label does not affect the cells migration ability20 and, as the Tc-99m-HMPAO is internalized by the cells and retained in the cytoplasm, it is unlikely to interfere with adhesion properties.
The calculation of signal from regions of interest may be an underestimate as it is possible that that there may be significant dissociation of the label from cells (released from dead cells or other mechanisms of release from live cells). The thyroid captures free isotope (Tc-99m) released from dead or dying cells, and Tc-99m-HMPAO may be eliminated by the kidney and gut. These tissues could be monitored by gamma scintigraphy to assess potential loss of label.
The cell labeling procedure can be adapted for testing cell retention and uptake at various sites (organs and injury) using different delivery routes. The focal nature of the signal in the lungs may be indicative of cell aggregates which presumably break up with time or enter cell lysis. The thyroid, which captures free isotope, presumably shows an increase in uptake following regional perfusion because the injected cells and resulting debris from dead cells have a more accessible route to the thyroid via the circulation. Although only about 24% of cells remain after 24 hr, the intralesional route into the tendon is the most effective delivery route as most cases showed no free Tc-99m in the thyroid. Information gained from utilizing such protocols can inform future design and development of stem cell applications for more effective clinical therapies.
The cell labeling protocol was adapted for use in the horse from Tc-99m-HMPAO labeling of leucocytes in human medical applications. Its use in this protocol demonstrates applicability in a large animal in which tendon disease occurs spontaneously for investigations of cell-based therapies. The recruitment of clinical cases can help to offset the higher costs associated with working with a larger animal. The labeling procedure for use as an imaging modality has been described in small animal models21 and we believe that the protocol can similarly be utilized in larger animals such as the sheep for investigations of other tendon diseases.
Disclosures
JD and RKS are Scientific Advisory Board members to ReCellerate Inc.
Acknowledgments
The authors would like to acknowledge funding from the Horserace Betting Levy Board U.K. (grant number 721) and VetCell BioScience Ltd, U.K. and by Consejerìa de Innovaciòn, Ciencia y Empresa, Junta de Andalucìa, Spain.
References
- Becerra P, et al. Distribution of injected technetium(99m)-labeled mesenchymal stem cells in horses with naturally occurring tendinopathy. Journal of Orthopaedic Research. 2013;31:1096–1102. doi: 10.1002/jor.22338. [DOI] [PubMed] [Google Scholar]
- Nixon AJ, Dahlgren LA, Haupt JL, Yeager AE, Ward DL. Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. American Journal of Veterinary Research. 2008;69:928–937. doi: 10.2460/ajvr.69.7.928. [DOI] [PubMed] [Google Scholar]
- Schnabel LV, et al. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. Journal of Orthopaedic Research. 2009;27:1392–1398. doi: 10.1002/jor.20887. [DOI] [PubMed] [Google Scholar]
- Smith RK, et al. Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PloS one. 2013;8:e75697. doi: 10.1371/journal.pone.0075697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole A, et al. Distribution and persistence of technetium-99 hexamethyl propylene amine oxime-labelled bone marrow-derived mesenchymal stem cells in experimentally induced tendon lesions after intratendinous injection and regional perfusion of the equine distal limb. Equine Veterinary Journal. 2013;45:726–731. doi: 10.1111/evj.12063. [DOI] [PubMed] [Google Scholar]
- Godwin EE, Young NJ, Dudhia J, Beamish IC, Smith RK. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Veterinary Journal. 2012;44:25–32. doi: 10.1111/j.2042-3306.2011.00363.x. [DOI] [PubMed] [Google Scholar]
- Crevier-Denoix N, et al. Mechanical properties of pathological equine superficial digital flexor tendons. Equine Veterinary Journal. 1997;29(S23):23–26. doi: 10.1111/j.2042-3306.1997.tb05046.x. [DOI] [PubMed] [Google Scholar]
- O'Meara B, Bladon B, Parkin TD, Fraser B, Lischer CJ. An investigation of the relationship between race performance and superficial digital flexor tendonitis in the Thoroughbred racehorse. Equine Veterinary Journal. 2010;42:322–326. doi: 10.1111/j.2042-3306.2009.00021.x. [DOI] [PubMed] [Google Scholar]
- Alexander RM. Energy-saving mechanisms in walking and running. The Journal of Experimental Biology. 1991;160:55–69. doi: 10.1242/jeb.160.1.55. [DOI] [PubMed] [Google Scholar]
- Guest DJ, Smith MR, Allen WR. Monitoring the fate of autologous and allogeneic mesenchymal progenitor cells injected into the superficial digital flexor tendon of horses: preliminary study. Equine Veterinary Journal. 2008;40:178–181. doi: 10.2746/042516408X276942. [DOI] [PubMed] [Google Scholar]
- Guest DJ, Smith MR, Allen WR. Equine embryonic stem-like cells and mesenchymal stromal cells have different survival rates and migration patterns following their injection into damaged superficial digital flexor tendon. Equine Veterinary Journal. 2010;42:636–642. doi: 10.1111/j.2042-3306.2010.00112.x. [DOI] [PubMed] [Google Scholar]
- Sole A, et al. Scintigraphic evaluation of intra-arterial and intravenous regional limb perfusion of allogeneic bone marrow-derived mesenchymal stem cells in the normal equine distal limb using (99m) Tc-HMPAO. Equine Veterinary Journal. 2012;44:594–599. doi: 10.1111/j.2042-3306.2011.00530.x. [DOI] [PubMed] [Google Scholar]
- Carvalho AM, et al. Evaluation of mesenchymal stem cell migration after equine tendonitis therapy. Equine Veterinary Journal. 2014;46:635–638. doi: 10.1111/evj.12173. [DOI] [PubMed] [Google Scholar]
- Welling MM, Duijvestein M, Signore A, van der Weerd L. In vivo biodistribution of stem cells using molecular nuclear medicine imaging. Journal of Cellular Physiology. 2011;226:1444–1452. doi: 10.1002/jcp.22539. [DOI] [PubMed] [Google Scholar]
- Dowling BA, Dart AJ, Hodgson DR, Smith RK. Superficial digital flexor tendonitis in the horse. Equine Veterinary Journal. 2000;32:369–378. doi: 10.2746/042516400777591138. [DOI] [PubMed] [Google Scholar]
- Kasashima Y, Ueno T, Tomita A, Goodship AE, Smith RK. Optimisation of bone marrow aspiration from the equine sternum for the safe recovery of mesenchymal stem cells. Equine Veterinary Journal. 2011;43:288–294. doi: 10.1111/j.2042-3306.2010.00215.x. [DOI] [PubMed] [Google Scholar]
- Avella CS, et al. Ultrasonographic assessment of the superficial digital flexor tendons of National Hunt racehorses in training over two racing seasons. Equine Veterinary Journal. 2009;41:449–454. doi: 10.2746/042516409x391042. [DOI] [PubMed] [Google Scholar]
- de Vries EF, Roca M, Jamar F, Israel O, Signore A. Guidelines for the labelling of leucocytes with (99m)Tc-HMPAO. Inflammation/Infection Taskgroup of the European Association of Nuclear Medicine. European Journal of Nuclear Medicine and Molecular Imaging. 2010;37:842–848. doi: 10.1007/s00259-010-1394-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trela JM, et al. Scintigraphic comparison of intra-arterial injection and distal intravenous regional limb perfusion for administration of mesenchymal stem cells to the equine foot. Equine Veterinary Journal. 2014;46:479–483. doi: 10.1111/evj.12137. [DOI] [PubMed] [Google Scholar]
- Barbash IM, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- Heckl S. Future contrast agents for molecular imaging in stroke. Current Medicinal Chemistry. 2007;14:1713–1728. doi: 10.2174/092986707781058896. [DOI] [PubMed] [Google Scholar]


