Abstract
This paper presents the pediAnklebot, an impedance-controlled low-friction, backdriveable robotic device developed at the Massachusetts Institute of Technology that trains the ankle of neurologically impaired children of ages 6-10 years old. The design attempts to overcome the known limitations of the lower extremity robotics and the unknown difficulties of what constitutes an appropriate therapeutic interaction with children. The robot's pilot clinical evaluation is on-going and it incorporates our recent findings on the ankle sensorimotor control in neurologically intact subjects, namely the speed-accuracy tradeoff, the deviation from an ideally smooth ankle trajectory, and the reaction time. We used these concepts to develop the kinematic and kinetic performance metrics that guided the ankle therapy in a similar fashion that we have done for our upper extremity devices. Here we report on the use of the device in at least 9 training sessions for 3 neurologically impaired children. Results demonstrated a statistically significant improvement in the performance metrics assessing explicit and implicit motor learning. Based on these initial results, we are confident that the device will become an effective tool that harnesses plasticity to guide habilitation during childhood.
Keywords: Robotic training, sensorimotor therapy, assist-as-needed, cerebral palsy, ankle
I. Introduction
The introduction of the concept of impedance control in 1985 [1] paved the way for a safe, gentle and effective interaction between humans and machines. This kind of interaction is essential for rehabilitation and is epitomized in the design of our manipulanda that pioneered clinical and neurological applications [2-4]. The evaluation of these and other therapeutic devices provide evidence that robots can replicate, if not augment, the sensorimotor experience as delivered by therapists [5]. This led the American Heart Association to include endorsements for upper extremity (UE) robotic therapy in its guidelines for the standard of post-stroke treatment [6]. That said, the theoretical knowledge of the underlying rehabilitation mechanisms is still lacking.
Motor learning is currently the most accurate model of sensorimotor rehabilitation [7]. Impelled by the paradigm shift on activity-dependent neural plasticity during learning [8, 9], we have introduced the assist-as-needed robotic therapy as a key mechanism for neurorehabilitation [10-12]. When the robotic assist is further guided by metrics of movement efficiency, the training can adapt to each patient's special needs and abilities to yield substantially improved outcomes [13]. Nonetheless, the optimal exercise regimen for improving motor function after a neurological disease remains to be determined.
Historically, lower extremity (LE) robotics for stroke and other neurological diseases tried to impose rhythmic patterns of whole-body movements. For example, the Lokomat (Hocoma, Zurich, Switzerland), which is the most widely used LE robot, guides the hips and knees through trajectory tracking control during partial body weight support (BWS) treadmill walking [14]. Another BWS device, the Gait Trainer I, moves the legs with footplates [15]. However, the design of mimicking the kinematics of rhythmic leg movements during BWS treadmill training had not been evaluated until recently, when surprisingly poor results were found [16, 17].
Targeting individual joints and going beyond exclusive rhythmic training is a different strategy for LE robotics that has already given promising results. We recently have introduced the MIT's Anklebot, a robotic device that follows the same guidelines of our UE designs, i.e., it is a low friction, backdriveable device with intrinsically low mechanical impedance [4]. The device allows examining separately the talocrular and the subtalar joints of the ankle and its design aligns with the current understanding that the brain can be functionally modified with practice. We targeted the ankle because it is biomechanically important in walking and balance [4, 18] and because a deficit in foot control is the most common and debilitating neurological sign of any brain lesion involving the corticospinal tract. Other actuated devices focusing on the ankle include the AAFO [19], the Rutgers ankle [20] and the robotic gait trainer [21]. Following the UE therapeutic schemes, recent studies using the Anklebot suggest that a focus on ankle sensorimotor control provides a valuable contribution to locomotor therapies [22-24].
Despite the promising results with the Anklebot, there is scarcity of studies on whether the sensorimotor control of the LE, in general, and of the ankle, in particular, resembles that of the UE. The lack of understanding or, at least, modeling the neurophysiological signature of ankle pointing movements may limit the validity of any effort to design an ideal therapeutic intervention for the ankle or evaluate its performance. To overcome this pitfall, we recently studied the sensorimotor control of ankle pointing movements at 3 modeling levels. In our first, macroscopic study, we demonstrated the adequacy of Fitts’ law to describe the mean time of major ankle pointing movements and to support the use of linear models to predict the ankle average performance in dorsal-plantar (DP) and inversion-eversion (IE) directions in healthy subjects [25]; this study verified that the central nervous system commands and controls the speed and accuracy of ankle movement in both DP and IE movements in the same way as in UE. In our second, mesoscopic study, we found a remarkable similarity between the models that described the speed profiles of unimpaired ankle pointing movements and the ones previously found for the upper extremities both during arm reaching and wrist pointing movements. [26]. In our third, microscopic study, we found that the reaction time (RT) measured in both DP and IE ankle movements increased with the number of stimuli at an equal pace, as would be predicted by Hick-Hyman law in UE [27, 28]. Interestingly enough, the intercept in the regression is significantly smaller in DP than in IE direction; this could be attributed to differences in the cognitive components known to affect RT, including motor preparation and execution [29, 30].
Herein, we describe the pediatric version of the MIT Anklebot, an impedance-controlled device that focuses on the ankle joints and aims to promote motor learning in children of ages 6-10 years old. While its concept was inherited from the adult version of the device, its hardware characteristics, the design choices for its adaptive controller and the set of serious games were expanded from the performance-based progressive robotic therapeutic scheme, used in our UE robotic devices [13]. The expansion aimed to meet the needs and the special characteristics of the LE and those of the children with neurological impairments. We also demonstrate the pediAnklebot's potential as a therapeutic device in children with LE neurological deficits by presenting evidence for motor learning in ankle movements. Motor learning, both explicit and implicit, was assessed at the 3 aforementioned modeling levels of the ankle sensorimotor control.
II. Prototype Design
A. Hardware
The pediAnklebot has inherited the same features as the adult version (low-friction and inertia, backdriveability, intrinsically low mechanical impedance) to allow normal range of motion (ROM) in all 3 degrees of freedom (DOF) of the foot relative to the shank (Fig. 1). The robot allows 25° of dorsi-flexion, 45° of plantar-flexion, 25° of inversion, 15° of eversion, and 15° of internal or external rotation. These numbers are near the ROM for normal children, which is markedly larger than that of children with CP [31] and beyond what is required for typical gait. The robot can be used both in a sitting position and during walking and gives independent, active assistance in 2 of the 3 DOF, namely DP flexion and IE, and a passive DOF for internal-external rotation following our approach to minimize the need for proper robot alignment [32]. The kinematic design consists of 2 linear actuators mounted in parallel in such a manner that if both move in the same or in opposite directions, a DP-flexion or an IE torque is applied to the ankle, respectively. Ankle angles and torque for DP and IE movement were estimated using a simple linearized mathematical model of the shank-ankle-foot system, in a straight analogy to [4]. Our main design constraints were the weight of the device and the ankle torque. An added mass of 2.5 kg does not alter the lower limb kinematics; yet, the mechanical constraint induced by the device's brace has a measurable effect on the gait of healthy children and children with CP [33]. Nonetheless, when the robot is used in a seated position, its weight is supported by the chair. The device was designed to supply torque needed to position the foot during swing phase and in the first part of stance phase (10% of peak moment). Since the peak ankle moment in the 7-8 year old group (25 Kg) is 1.19 Nm/Kg and increases with age [34], the device's maximum stall torque of 7.21Nm in DP flexion and 4.38Nm in IE is sufficient to lift approximately 25% of the entire weight of the child as well as give supplemental support to the paretic ankle plantar flexors. These torque values are within the range of resistive plantarflexion torques in children with CP, at least for speeds up to 0.5 rads/s [35]. Cueing the voluntary plantarflexor function aims to improve the dorsal-flexion, which is pathophysiologically persistent during the gait, as well as the plantar flexion, which is typically absent during the swing phase, in hemiplegic and diplegic CP children [36-39]. The device is actuated by 2 cogless, brushless DC motors (Maxon EC-powermax 22-327739) that can produce a maximum continuous torque of 52.1 mNm that is augmented by a Rohlix linear traction drive. Two sensors provide the information for motion and torque. The first is a mini-rail linear encoder (MNS9-135 length, Schneeberger) mounted in parallel with the motors and possessing a resolution of l um. The linear dimensions measured by the encoders are used to estimate ankle angle in plantar-dorsiflexion and inversion-eversion and for the robot control. The second is a Gurley rotary encoder with 40960 lines for the servo-amplifier commutation. Load cells are added at each actuator output (LSB200:00105, 25 lb, 2mV/V Futek).
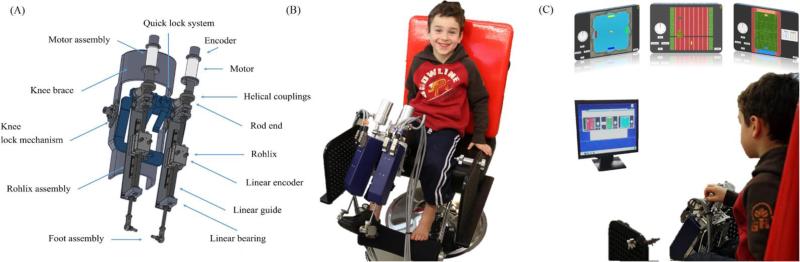
Fig. 1.
The MIT's pediatric ankle robotic system (pediAnklebot). (A) The mechanical design of the robotic prototype showing the components of the device (B) A 7-year old child wearing the pediAnklebot in seated position; the weight of the pediAnklebot is supported from the chair, through a bolt (C) playing the serious games that were developed for the device based on motor learning principles in a 20” screen positioned 1 m away from the kid (clinical setup).
B. Impedance Controller
We implemented an impedance controller with a programmable torsional stiffness and damping and a programmable reference. During therapy, the impedance controller can guide the child's ankle with a minimum-jerk speed profile from a starting to the end position. The given command force was:
| (1) |
| (2) |
where xm.j. was the controller's minimum-jerk movement reference, k was the controller stiffness, kbw was the “back-wall” stiffness, b was the controller damping, lm was the length of movement, and tm was the duration of the movement in the x direction (DP or IE). The controller provided assist-as-needed and guided the ankle of the child only when there was a lack or insufficiency of motor ability that resulted in delays with respect to the estimated minimum-jerk trajectory. As in our UE therapeutic controllers, the time allotted for the child to make the move, tm, and the primary stiffness of the impedance controller, k, could be varied based on the patient's performance and variability, whereas kbw was held constant.
C. Adaptive Serious Games
We developed a set of 3 goal-directed serious games (SGs) that were designed to be seamlessly integrated with the hardware and the controller of the pediAnklebot. The SG design principles followed the ones for UE stroke rehabilitation that include meaningful play and challenge [40]: As the players play the game and their skills and familiarity increase, the game offers a higher level of challenge to retain attention and motivation; however, if the game gets too difficult to play, the player, especially a child, may become frustrated and quit. The pediAnklebot's SGs had to address sensorimotor impairments in children including poor coordination, disorders in motor speed or accuracy, diminished strength, motor planning, and cognitive or perceptual deficiencies. Therefore, we designed the games to have a) an interesting concept, to support the level of perceptual joy throughout the therapeutic sessions; b) a simple visual interface, to communicate easily the game concept; c) easy controls, to facilitate guidance around the visual interface and focus on the game concept; and d) simple rules, to minimize the learning period [41]. These basic rules governed all our SGs, to afford consistency among the different games.
In our SGs, the kids made pointing movements with their ankle to prevent a boat from crashing into the rocks (Shipwreck), run through a race to collect animals while attempting to avoid water splashes (Noah's Ark), and play a soccer game with the computer or another child wearing a pediAnklebot as their opponent (WorldCup - Fig. 1C). To do so, they controlled a paddle representing the ship barrier, the runner, or the goalkeeper/player. DP flexion and IE controlled screen movements of the paddle in vertical and horizontal directions, respectively. Movement repetitions were displayed in a clock-style meter on the left of the game field, reflecting the number of ship bounces, animals collected, or shots towards the opponent's goalpost. The games were developed in TCL/TK and communicated with the controller coded in C, through shared memory space variables; the pediAnklebot run in a real-time Linux Ubuntu-Xenomai system.
The game parameters were modified based on the performance of the child. These parameters were the speed of the moving target (defined as the speed of falling gates in the Noah's Ark game or the speed of the boat or the ball in the other two SGs) and the size of the window (for the race game) or the paddle (for the other two games). Our parameter adaptation followed the motor learning principles embedded into the speed-accuracy trade-off (SAT), as explained in the next section.
D. Assist-as-needed: Performance-Based Adaptation
Given the importance of active participation during therapy, which focuses on tasks that trigger motor learning, we translated to the LE the concepts of an assist-as-needed robotic therapy introduced for the UE [13]. Specifically, our recent finding that the performance in visually evoked, visually guided ankle pointing movements is described by a linear function, as predicted by Fitts’ law, supported the idea that the SAT could be incorporated into an adaptive therapeutic intervention for the ankle [25]. In that sense, the SGs trained the child's ankle while challenging his/her ability to move fast and accurately: Depending on one's ability to aim, the targets became smaller or larger; depending on one's ability to move fast or slow, the speed of the game also changed.
1) Performance Metrics
To track the youngster's ability and encourage him/her to actively participate during therapy sessions, 4 performance metrics (PMs) were used, namely the ability to initiate movement (PM1), the power to move from the starting position to the target (PM2), the ability to reach the target efficiently and in a timely manner (PM3), and to reach the target accurately (PM4). PM1 recorded the times that the child self-initiated a movement. This was determined by comparing the ankle (DP flexion or IE) speed with a velocity threshold defined as the 10% of the maximum minimum-jerk speed profile, namely:
| (3) |
where lm was the distance (rads) between the initial position and the target's center and tm was the time allotted for the move in sec. PM2 was defined as the weighted sum of the assistive power (watt), PM2a, and a rotation index (rad), PM2b:
| (4) |
| (5) |
where Fx was the interaction force along the target axis, ẋ was the velocity along the target axis, x was the position along the target axis, xm.j. was the prescribed minimum jerk trajectory of the “back wall” of the impedance controller, and τ was the total time of the movement. PM2 adjusted the speed of the target (and consequently tm). As tm changed during the gameplay, Vt also changed making it harder or easier for a child to self-initiate a movement. The weights for PM2a and PM2b were empirically chosen to be 6.5 and 6, respectively, to constraint |PM2| < 1 and ensure stability (see next section). PM3 represents cognitive impairments related to pointing movements by quantifying the ability to timely and effectively point with the ankle, given the speed and accuracy limitations. It was calculated by two distinct metrics, namely PM3a that measured the dwell time, i.e., the part of the time slot that is not used for positioning the paddle into the final position (in sec) and PM3b that graded the ability to follow a trajectory of minimum length by penalizing any excessive movement (unit-less). PM3a was defined as:
| (6) |
where f, g, h were the time instances in which the center of the paddle, c, was positioned exactly on target (|c| ≤ w), near the target (w < |c| ≤ 2w) and away from the target (2w < |c| ≤ 3w), respectively, and τ was the movement time. For the above inequalities, the target's coordinate was considered as the origin. PM3b was defined as follows:
| (7) |
where r = la/lm ∋ (0,1] was the ratio of the total displacement covered by the paddle, la, to the minimum trajectory (i.e., the shortest distance) lm; d was the center of the logistic-type function and s was the steepness factor. Note that with no excessive movement, PM3b = 1 whereas for any excessive movement (e.g., oscillatory movements around the target), PM3b < 1. With a wise combination of d and s, the kid might not be penalized (i.e. PM3b = 1) until r becomes lower than a certain value; see our simulation results in [42]. The overall ability for a child to point with the ankle in a timely and efficient manner was calculated as follows:
| (8) |
where PMz was a constant that confined |PM3| < 1 to ensure stability (see next section). To better determine a timely positioning of the paddle, we empirically selected {κf = 4, κg = 2, κh = 1}; simulation results suggest that for this set of weights, PMz = 2 [42]. PM3 was used to adjust the width of the paddle. PM4 recorded the minimum distance (rads) between the final position in the movement slot and the center of the target.
2) Tracking the Child's Performance
The pediAnklebot tracked the child's speed and accuracy capabilities after a section of n repetitions, using a set of simple control laws:
| (9) |
| (10) |
where s [J ], w [J ] were the gameplay speed and paddle width during the J section, respectively, and λs > 0, λw < 0 were the gains; multiplying these gains with {|PM3|, |PM2|} < 1 ensured that the tracking system would be stable. During the initial m (out of M) repetitions of a session, the control system operated in a tracking mode, allowing both speed and accuracy to change simultaneously.
3) Challenging the Child's Performance
The last M-m repetitions in a therapeutic session were also grouped into sections of n repetitions but here only one of the two game parameters changed. Note that regardless of whether the speed of the game or the width of the paddle changed, the zero PM values occurred at different levels of the patient's performance. We also defined the performance level (PL) as follows:
| (11) |
The value of PL indicates whether patients perform worse (PL=−1) or better (PL=1) than their expected ability at PM=0. PL=0 denotes when patients perform approximately the same. By considering average PM values and a weighted sum of the PL values in h consecutive sections, the controller adapts to children's performance and variability, and challenges them to continue to improve. Here we introduce an integration of weighted PLs for both speed and accuracy: A window of size 3 is adjusted to each PL so that the current PL value is weighted by 4, and the previous two PL values are weighted by 2 and 1, respectively; see Fig. 5 in [42]. The proposed performance-based adaptive algorithm for the LE is stated as follows:
| (12) |
| (13) |
where
| (14) |
The desired effect of challenging patients to improve while keeping them motivated was accomplished, in part, by the asymmetry in the definition of α(PLsum). The asymmetry sought to challenge patients to improve further but made the task easier, to a lesser extent, when patient performance was worsening.
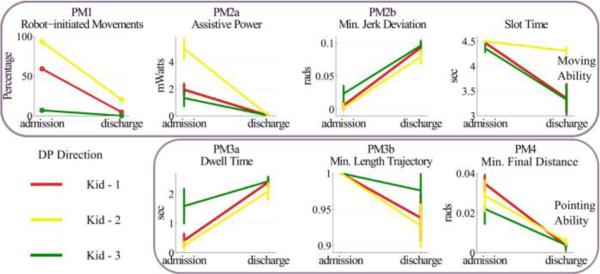
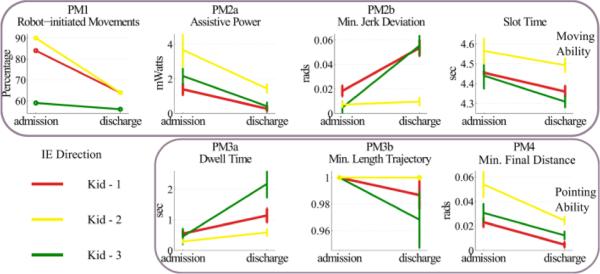
Fig. 5.
Performance metrics for assessment of moving (upper row) and pointing abilities (bottom row) for the three kids that received robotic therapy in DP direction. Metrics were estimated from the therapeutic sessions (44 movements). Error bars correspond to 95% confidence intervals.
III. Evaluation
We evaluated the pediAnklebot with two goals in mind. First, we validated how the controller tracked the individual's ability to move rapidly and accurately and how it adapted accordingly. Second, we searched for evidence of explicit and implicit motor learning after a systematic use of the robot by impaired youngsters. To address our first goal, we tested the controller on 9 healthy subjects in the lab, using the Anklebot, and 3 impaired children in the clinic, using the pediAnklebot. To address our second goal, we examined the performance of the 3 impaired children for at least 3 weeks (3 sessions per week). We then assessed their explicit learning by examining how the game parameters adapted to their performance and their implicit learning by analyzing how the distribution of their RT changed with therapy. Our evaluation was supported by our recent studies on the ankle sensorimotor control of young healthy subjects [25, 26, 29, 30].
At MIT, we recruited 9 unimpaired healthy subjects (4 females). Subjects were Caucasians post-doctoral, graduate or undergraduate students at the Massachusetts Institute of Technology. Average biometric data were 21 ± 4 years of age, 1.79 ± 0.15 m in height, and 72 ± 9 kg in mass (mean ± SD). All subjects had normal or corrected-to-normal vision and were right-foot dominant according to their preferential use of the foot during daily activities such as kicking a ball. Subjects had no reported history of traumas or neuropathies to the lower limbs. All subjects gave written informed consent according to the procedure approved by the Massachusetts Institute of Technology Committee on the Use of Humans as Experimental Subjects.
At the clinic, we recruited 3 impaired children (average age 9 years old) diagnosed with LE impairments, either of central or peripheral origin (Table I). Specifically, 2 of the children were diagnosed with CP and the other was diagnosed with a lesion of the common peroneal nerve. The children and their parents gave informed assent and consent according to the procedure approved by the Ethics and Institutional Review Board committee of “Bambino Gesù” Children's Hospital, Rome, Italy and by the Massachusetts Institute of Technology Committee on the Use of Humans as Experimental Subjects.
TABLE I.
Pediatric Patients that Received Ankle Robotic Therapy
| ID | Diagnosis | GMFM-88 | WeeFIM | TS |
|---|---|---|---|---|
| Kid - 1 | CP – Hemiplegia – Right | 93.30 | 93.65 | 15 |
| Kid - 2 | CP – Hemiplegia – Left | 95.52 | 70.47 | 9 |
| Kid - 3 | Lesion of Peroneal nerve | 99.20 | 70.63 | 9 |
GMFM-88 = Gross Motor Function Measure, WeeFIM = Pediatric Function Independence Measure, TS = Training Sessions completed with the Anklebot; A TS consisted of a set of 44 DP and 44 IE movements.
We asked the subjects to play the race game (Noah's Ark) “as quickly and accurately as possible” in DP and IE directions. We chose the race game as it gives an online visual feedback of the performance which is consistent in both directions and, therefore, ensures the same feedback resolution across both joint movements. Healthy (impaired) subjects executed 200 (44) movements per direction. This number of movements ensured that during an experiment neither central nor peripheral fatigue would affect outcomes, at least not as a sharp and persistent deviance in performance. The presentation order of the game's direction was counterbalanced and a 2-min break between playing the game in the two directions was allowed, if needed.
A. Adaptation to the Speed and Accuracy
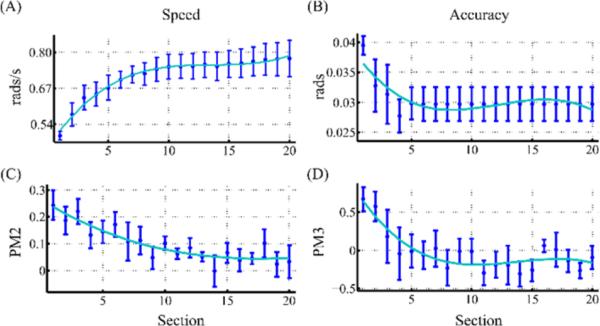
To assess how the controller adapted to the individual performance, we examined how the speed and accuracy constraints of the game changed in response to their corresponding PMs. In Fig. 2, we present the change in speed and accuracy over a single-run of the race game (M=200, 20 sections of 10 movements each), in DP movements of healthy subjects. Since the control law (12-13) essentially tries to minimize the error (here, defined as a PM), we expected to see PM2 and PM3 approaching zero as the speed and accuracy values reached their plateau level. Since the maximum accuracy constraint was faster to estimate compared to the speed, we kept the target width constant after the first 6 sections (m=60 repetitions). Note the small yet consistent positive trend at the speed of the game in the last 5 sections, despite the PM2 being close to zero; this is due to the challenging component of the controller (14). As the controller and the gameplay were symmetric with respect to the neutral position (origin), here we regarded only the DP movements. Any difference in speed and accuracy found between the DP and IE directions should be attributed to the neurophysiology and the biomechanics of the ankle joints, rather than the controller itself.
Fig. 2.
Adaptation of speed (rate of falling gates) and accuracy (target width) constraints in a single-run of Noah's Ark game. Data were averaged across 9 healthy subjects. Average (A) speed and (B) accuracy, in the ankle coordinate system and their corresponding (C) PM2 and (D) PM3. Error bars correspond to 95% confidence intervals. Cyan line corresponds to the 3rd order polynomial that best fit the average data. A single-run consisted of 20 game sections each of which had n = 10 DP movements. Gate width remained fixed after six sections. For PM3 estimation, PMz = 1.25, d = −0.25 and s = 0.4.
B. Explicit Motor-Learning in Speed and Accuracy Performance
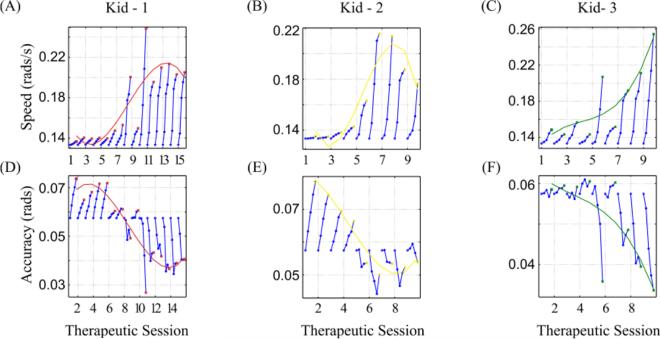
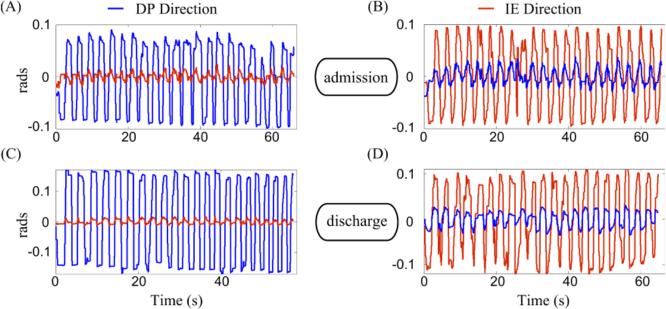
To assess how the controller adapted to the special characteristics of impaired children, we examined how the speed and accuracy constraints in the race game changed with therapy. In Fig. 3, we present how the speed and accuracy values adapted to each child's performance per therapeutic session (TS). During each TS, the speed and accuracy changed every section of n = 11 movements (circles in Fig. 3). The controller ran in tracking mode and adapted the speed and accuracy constraints. Both features reached a plateau for Kid-1 and Kid-2 near the end of the therapy (red and yellow lines in Fig. 3, respectively). Especially for the accuracy constraint, while all subjects performed poorly in the first sessions (as indicated by an increase, not a decrease, in target width from its initial value), their performance improved with time. The overall superior performance after therapy was evident even with a naked eye (Fig. 4); compared to the raw kinematics of the ankle pointing movements at admission, both DP and IE movements at discharge were less jerky and better controlled, especially when the ankle needed to be kept stable at a certain position for the race paddle to pass through the gate. In addition, at least for DP, the movement speed increased considerably (compare the time axes between Fig. 4A and Fig. 4C).
Fig. 3.
Performance curves as evidence for explicit motor learning in ankle robotic therapy. The (A, B, C) game speed and (D, E, F) accuracy constraints (target width) were adapted to each kid's performance in each of the therapeutic sessions, in DP direction. Each blue circle corresponds to the average value per section (11 movements). A 3rd order polynomial (red, yellow and green colored curve) was fit to the final value per session (indicated by colored squares).
Fig. 4.
Ankle kinematics for Kid-1 while playing the race game in (A, C) DP and (B, D) IE directions at (A, B) admission and (C, D) discharge. Blue (red) kinematics refer to DP (IE) ankle movements. For visual comparison, the amplitude of the DP movement (blue trace) in IE direction at discharge was halved. Note the decrease in game duration in the DP direction at discharge.
C. Explicit Motor Learning Assessed by the Performance Metrics
If the metrics that we used to assess performance are valid, their values should change considerably with therapy. Specifically, combining PM3 with PM4 was our best guess on estimating one's ability to aim and move fast. Therefore, if these metrics and the other PMs quantify meaningfully the ankle movement characteristics, we should expect to find substantial improvement in their values as a response to treatment. We examined how the PMs behaved at admission and discharge for both DP (Fig. 5) and IE (Fig. 6) directions. In both directions, the therapeutic intervention resulted in a statistically significant change in PMs: At discharge, all kids consistently exhibited less robot-initiated movements (PM1), less assistive power from the robot (PM2a), movements that preceded the back-wall at a greater extent (PM2b), a smaller slot time, a larger dwell time (PM3a) and more accurate final placement of the runner with respect to the center of the target (PM4). Overall, the therapeutic outcome seems more substantial in DP direction than in IE. At first look, the minimum length trajectory (PM3b) might seem inconsistent with respect to all other PMs, as it decreases with therapy, which indicates that more mechanical work was required at discharge. However, note that this metric was designed to penalize any excess movement. At admission, most of the movements were assisted by the robot; therefore, most of them followed closely the optimum trajectory of the “back-wall”. As soon as the kids started controlling their ankle better and moving it on their own, the ankle trajectories became subject to excess, redundant movements (e.g., small oscillatory movements around the target). To compensate for this, our parameter selection in (7), d = −0.25, s = 0.3 and PMz = 2, allowed the kids to be minimally penalized for this excessive movement.
Fig. 6.
Performance metrics for assessment of moving (upper row) and pointing abilities (bottom row) for the three kids that received robotic therapy in IE direction. Estimation of metrics and error bars as in Fig. 5.
D. Implicit Motor Learning Assessed by a Decrease in Average RT
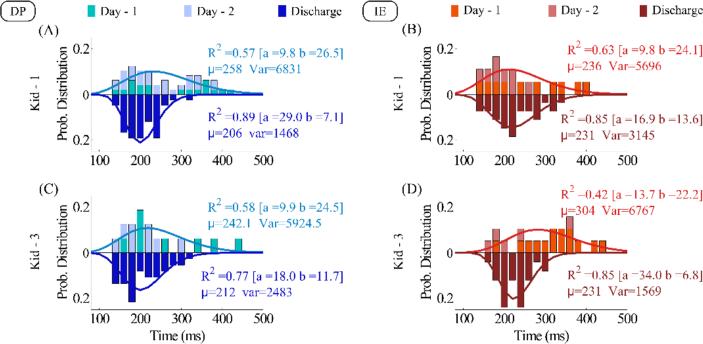
To assess implicit motor learning, we examined a metric for which we gave no formal instruction about how to improve it. Such a metric that also has the potential to become a therapeutic target is the ankle RT [29]. In Fig. 7, we present the distributions of the RT at admission and discharge. To estimate RT, we measured the temporal distance between the onset of a stimulus (new gate) and the start of the ankle movement, defined by a velocity threshold of 5% of the peak speed estimation, as described in our previous studies [29, 30]. We excluded non-terminated movements and regarded any RT that was less than 150 ms as an outlier, possibly being the result of an anticipatory movement. By employing the PM1 criterion for self-initiated movements, we also removed any movements that were robot-initiated. The latter limited the available RT data at admission, especially in IE direction. To compensate for the lack of data, we regarded as our “admission” RT the data from the first 2 days of therapy. This was possible since the PMs as well as the speed and accuracy constraints were close to each other in the first 2 days (see Fig. 3). This allowed us to analyze RT in two kids (Kid-1 and Kid-3), but not for Kid-2 that had a large number of robot-initiated movements at admission (see PM1, yellow trace in Figs. 5 and 6). By examining Fig. 7, one can make three main observations: First, the RT decreases with practice; the mean value of the best-gamma fit decreased considerably between admission and discharge. The decrease of mean RT was significant for Kid-1 in DP direction (t-test, p=4·10-4) and Kid-2 in IE direction (t-test, p=3·10-4). Second, practice seems to decrease RT variability; this is depicted by the estimated variance of the gamma functions that best fit the empirical distributions. Third, therapy seems to reestablish the order of the average RT in the ankle at least as observed in healthy young subjects: Specifically, at discharge the average RT in IE was larger than in the DP direction. This result, although not age-matched to our previous study, aligns with our speculation that the larger by 20 ms RT in IE, compared to DP direction, might be due to the direct cortical projections between the motor cortex and the tibialis anterior, the main muscle that controls dorsiflexion ankle movements [29].
Fig. 7.
Reaction Time for (A, B) Kid-1 and (C, D) Kid-3 at admission (upper distribution) and discharge (bottom distribution) in (A, C) DP and (B, D) IE directions. For admission distributions, the data were concatenated across the first 2 days of the therapy. The gamma function with the best fit on each distribution is plotted. At discharge, the fitted mean RT became smaller for the DP than for the IE direction.
IV. Discussion
In this paper, we presented the pediAnklebot, a robotic tool that promotes habilitation in children and affords quantitative measurements of kinematic and kinetic performance. We implemented an adaptive scheme that comprised a controller and a set of SGs, to employ concepts of motor learning developed for the upper extremity to the ankle of children with neurological deficits. With CP affecting 1 to 4 children out of 1,000 worldwide and both central and peripheral neurological disorders in children increasing partly due to the increased survival of pre-term babies, the development of pediatric devices targeting the LE is becoming an emergent research area in robotics. Building on our initial study on adaptive controller for the UE [13], we focused on adapting the behavioral intervention at the LE to each child's special needs and abilities.
A major design pitfall of past efforts was that relatively little effort was put into understanding how well-known motor learning strategies apply to the LE [43]. To address this limitation, we recently established the existence of SAT in goal-directed ankle pointing movements in DP and IE, in straight analogy to a linear model, widely used to quantify the UE motor system for more than half a century (Fitts’ law) [25]. This enabled the design of the control architecture on the basis of a robust adaptive approach that tracks the performance in speed and accuracy as indicators of each patient's abilities to move and point: A PM2 and PM3 decrease indicated a faster and more accurate movement (Fig. 2). The controller employs a scheduler in real time on the basis of the subject's disability level and stage of rehabilitation and provides an “assist-as-needed” therapy to the ankle. By incorporating Fitts’ law parameters into the SGs design, we developed a SAT-based therapeutic environment that not only adapts but also challenges the youngster to do his/her best in movement planning and execution [41, 42]. Given the importance of active participation during therapy [44] and the need for specificity in therapeutic tasks that resemble (if not exploit) motor learning, Fitts’ law shows a great potential to empower new therapeutic protocols that adapt to and challenge patients according to their ability to move their ankle fast and/or accurately. In that sense, our approach may serve as an effective tool for comparing motor abilities across subjects, modalities, and rehabilitation tasks and enhancing the understanding of brain plasticity and neurocognitive pathways involved in motor planning and control.
The clear changes in the kinematic profiles of ankle movements after the robotic sensorimotor therapy (Fig. 4) could reveal interesting implications for rehabilitation in people with lower limb motor disabilities. While a change in movement smoothness seems obvious, the interesting aspect is that movement speed increased simultaneously with target size decrease. Although one would expect that speeding up the exercise would decrease movement accuracy, both improve at the same time. An increase in accuracy or speed alone does not indicate improved skill; true skill acquisition requires a systematic change in the learner's SAT function [45, 46]. In addition, the somewhat smaller changes in IE direction compared to DP might suggest that a more intensive therapeutic protocol might need to be applied in that direction.
Furthermore, “an underlying, activity-dependent neural plasticity is probably a key mechanism through which robotic therapy produces clinical results” [7]. The active assist-as-needed LE robotic therapy algorithm was motivated by the working hypothesis that the processes that underlie motor habilitation are similar to the processes that underlie motor learning. A difficulty associated with quantifying motor learning is that the underlying processes of learning in the central nervous system are not easily observed or measured. Several methods exist that quantify electrical and biochemical activity in the brain as well as structural information about brain tissue, e.g., electroencephalography, magnetic resonance imaging, and positron emission tomography. Of particular interest for robotic rehabilitation is our recent fMRI study in which we examined brain activation in response to visual stimuli: We revealed the neural pathways that are associated with visual processing of the movement stimuli that are used in UE robot-mediated training as well as with the brain's ability to assimilate abstract object movements with human motor gestures [47]. Despite these achievements, the brain is extraordinarily complex and the rich data can currently be only interpreted offline. An adaptive device requires real-time guidance by measuring changes at the behavioral level [48]. Therefore, the consistent significant changes in all PMs for the sample of patients is a strong indication that the systematic use of the pediAnklebot may lead to positive clinical results.
The adaptation of the speed and accuracy was best described by a two-time scale exponential function. Specifically, motor recovery, at least as assessed by the PMs, followed an exponential progression similar to a motor learning “law of practice” which also resembled a two time scale exponential function [49, 50]. One characteristic time scale was relatively fast and captured the rapid adaptive change (warm-up) in performance at the beginning of a practice session (Fig. 3, blue circles). The other time scale was relatively slow and captured the persistent change that was more typically associated with learning [49] (Fig. 3, 3rd order polynomial fit). The second time scale was apparent when we examined the last adapted value in each session (red, yellow and green best-fit lines in Fig. 3). The two superimposed exponential functions comply with the theory of multiple time scales that is consistent with recent neurophysiological studies [51]. Keeping the number of repetitions for each session low allowed us to evaluate the device with respect to the long-term motor learning when no fatigue was present. However, as the number of repetitions affects the clinical outcome [7], we expect that increasing the number of repetitions will have a positive effect on the ankle.
The evaluation of RT changes (Fig. 7) proposes that implicit learning is possible when the pediAnklebot is systematically used. This is important as RT is a well-studied behavioral indicator of neurological integrity. Significant delays in RT measures have been found in basal ganglia disorders, such as Parkinson's disease (PD) [52-54] and Huntington's disease [55], and are commonly related to a deficit in motor planning [56, 57]. The observed decrease in the average RT is in agreement with a known phenomenon of impaired RTs that become responsive to intervention: RT has been used to quantify restoration of motor functions according to given cognitive contexts in PD patients treated with deep brain stimulation [58]; in addition, exercise and practice are found to improve simple and choice RT in both young and older adults [59, 60]. Interestingly, RT at discharge differed significantly when the ankle movement was controlled in DP rather than in IE direction, which is consistent with our recent studies on ankle RT in healthy young subjects [29, 30]. However, since the two studies are not age-matched, it seems risky to make any straight comparison between the two groups.
Some limitations of the current evaluation design need to be noted. In our evaluation, children had to meet the task objectives of the race game that imposes a block practice in a closed environment [48]. While this was useful in allowing us to gain a consistent insight into patients’ responsiveness to therapy in both DP and IE directions, it does not cover the entire spectrum of the structured practice. Employing SGs with an open game environment, such as the soccer and the shipwreck games, would allow the kids to do serial and random tasks, respectively [48]. In this context, we could use the blocked practice race game during the first (cognitive) stage of motor learning, introduce the soccer game during the associative phase and keep the last and most varying shipwreck game for the autonomous stage [61]. Varying the task demands over practice (random practice) is often associated with greater retention and transfer of skills [62]. Nonetheless, the difference in blocked versus random practice for children remains elusive; some studies have found no difference between these practice schedules for children [63, 64], whereas others have found similar results as in adults, with random practice facilitating greater motor learning [65, 66]. Since studies have already shown that people only engage in an activity if the outcome matches the effort at which they perform, we anticipate that the concepts of meaningful play and challenge, embedded in our set of SGs, would maximize engagement and sustain attention throughout a longer clinical study. This justifies our expectations for further improvement of the sensorimotor control of the ankle; whether this will translate to locomotion remains to be tested and that is on-going.
V. Conclusion
Herein we investigated the applicability and validity of a pediatric ankle robotic device that adapted the difficulty of the therapeutic exercise to each child's abilities. Performance metrics drove the controller and changed the game parameters to challenge children on improving or, at the very least, maintaining their performance. While further trials with neurologically impaired children are required to determine the therapeutic efficacy of the device, the pediAnklebot paves the way for an incorporation of behavior quantification techniques to the lower limb robotic rehabilitation. In that sense, the robot can become a platform that amalgamates behavioral psychology, sensorimotor neuroscience and physical therapy, among other disciplines. We anticipate that our endeavor will provide the necessary tools to harness plasticity and guide habilitation during this formative period.
Acknowledgments
This work was partially supported by a grant from the Cerebral Palsy International Research Foundation (CPIRF), the Niarchos Foundation and the NIH Grant R01HD069776-02; It was also partially supported by a grant from the Italian Institute of Technology (IIT) – Project Seed (“ITINERE - Interactive Technology: an Instrumented Novel Exoskeleton for Rehabilitation” 2009) and by a grant from Italian Health Ministry (Grant “Pilot study on a novel typology of medical devices: robotic exoskeletons for knee rehabilitation” 2009) to P. Cappa. K. P. Michmizos was partially supported by the Foundation for Education and European Culture. H. I. Krebs is a co-inventor in several MIT-held patents for robotic therapy. He holds equity positions in Interactive Motion Technologies, the company that manufactures this type of technology under license to MIT.
Biographies
 Konstantinos P. Michmizos (S’09 – M’12) received the 5-year Diploma (with distinction) in computer engineering and informatics from the University of Patras, Greece, in 2004, the M.Eng. degree in biomedical engineering from McGill University, Montreal, Canada, in 2006, and the Ph.D. degree in electrical and computer engineering from the National Technical University of Athens, Athens, Greece in 2011.
Konstantinos P. Michmizos (S’09 – M’12) received the 5-year Diploma (with distinction) in computer engineering and informatics from the University of Patras, Greece, in 2004, the M.Eng. degree in biomedical engineering from McGill University, Montreal, Canada, in 2006, and the Ph.D. degree in electrical and computer engineering from the National Technical University of Athens, Athens, Greece in 2011.
From 2011 to 2013, he was a Postdoctoral Research Associate with the Newman Laboratory for Biomechanics and Human Rehabilitation, Department of Mechanical Engineering, Massachusetts Institute of Technology. He is currently with the Martinos Center for Biomedical Imaging, Department of Neurology, Harvard Medical School, Boston, MA and the McGovern Institute for Brain Research, Massachusetts Institute of Technology, Cambridge, MA. His research tries to couple sensorimotor neuroscience with rehabilitation engineering and physiological modeling with robotics.
 Stefano Rossi received the 5-year Laurea degree cum laude in mechanical engineering from the Sapienza University of Rome, Italy, in 2004 and the Ph.D in mechanical and thermal measurements with specialization in measurements in biomechanics from the University of Padua, Italy, in 2008. He currently is Assistant Professor at the Department of Economics and Management - Industrial Engineering, University of Tuscia, Viterbo, Italy. His research interests include biomechanics, design of robotic devices for rehabilitation robots and analysis of performances of measurement systems for the analysis of human movements.
Stefano Rossi received the 5-year Laurea degree cum laude in mechanical engineering from the Sapienza University of Rome, Italy, in 2004 and the Ph.D in mechanical and thermal measurements with specialization in measurements in biomechanics from the University of Padua, Italy, in 2008. He currently is Assistant Professor at the Department of Economics and Management - Industrial Engineering, University of Tuscia, Viterbo, Italy. His research interests include biomechanics, design of robotic devices for rehabilitation robots and analysis of performances of measurement systems for the analysis of human movements.
 Enrico Castelli graduated in Medicine and Surgery at the University of Milan, Italy in 1983, where he also specialized in Rehabilitation (1986) and in Neurology (1991). He has been the Head of the three Pediatric Neuro-Rehabilitation Units of the “Bambino Gesù” Children's Hospital in Rome (Italy) since 2005. From 1999 to 2005 he was the Head of the Pediatric Acquired Brain Injury Unit of the Eugenio Medea Scientific Institute in Lecco (Italy).
Enrico Castelli graduated in Medicine and Surgery at the University of Milan, Italy in 1983, where he also specialized in Rehabilitation (1986) and in Neurology (1991). He has been the Head of the three Pediatric Neuro-Rehabilitation Units of the “Bambino Gesù” Children's Hospital in Rome (Italy) since 2005. From 1999 to 2005 he was the Head of the Pediatric Acquired Brain Injury Unit of the Eugenio Medea Scientific Institute in Lecco (Italy).
Dr. Castelli was the President elect from 2010 to 2012 of the European Academy of Childhood Disability (EACD), Board of the Governors member of the International Brain Injury Association (IBIA), member of the Italian Society of Physical and Rehabilitation Medicine (SIMFER) and National Coordinator of the Pediatric Rehabilitation Group of this Scientific Society.
 Paolo Cappa received the 5-year Laurea degree cum laude in mechanical engineering from the Sapienza University, Rome in 1980. He is currently a Full Professor with the Department of Mechanical and Aerospace Engineering, Sapienza University of Rome, Rome, Italy. He also holds affiliate positions as an Adjunct Professor at the Department of Mechanical and Aerospace Engineering, New York University, NY, USA, and as researcher at the Movement Analysis and Robotics Laboratory, “Bambino Gesù” Children's Hospital, Rome, Italy. His current research interests include development of novel transducers in mechanical measurements and Biomechanics, innovative rehabilitation robots.
Paolo Cappa received the 5-year Laurea degree cum laude in mechanical engineering from the Sapienza University, Rome in 1980. He is currently a Full Professor with the Department of Mechanical and Aerospace Engineering, Sapienza University of Rome, Rome, Italy. He also holds affiliate positions as an Adjunct Professor at the Department of Mechanical and Aerospace Engineering, New York University, NY, USA, and as researcher at the Movement Analysis and Robotics Laboratory, “Bambino Gesù” Children's Hospital, Rome, Italy. His current research interests include development of novel transducers in mechanical measurements and Biomechanics, innovative rehabilitation robots.
 Hermano Igo Krebs (SM’04, F'13) joined MIT's Mechanical Engineering Department in 1997 where he is a Principal Research Scientist and Lecturer – Newman Laboratory for Biomechanics and Human Rehabilitation. He also holds an affiliate position as an Adjunct Professor at University of Maryland School of Medicine, Department of Neurology, and as a Visiting Professor at Fujita Health University, Department of Physical Medicine and Rehabilitation and at University of Newcastle, Institute of Neuroscience. He is one of the founders and member of the Board of Directors of Interactive Motion Technologies, a Massachusetts-based company commercializing robot technology for rehabilitation. He is a Fellow of the IEEE (Institute of Electrical and Electronics Engineers). Dr. Krebs was nominated by two of IEEE societies: IEEE-EMBS (Engineering in Medicine & Biology Society) and IEEE-RAS (Robotics and Automation Society) to this distinguished engineering status “for contributions to rehabilitation robotics and the understanding of neurorehabilitation.”
Hermano Igo Krebs (SM’04, F'13) joined MIT's Mechanical Engineering Department in 1997 where he is a Principal Research Scientist and Lecturer – Newman Laboratory for Biomechanics and Human Rehabilitation. He also holds an affiliate position as an Adjunct Professor at University of Maryland School of Medicine, Department of Neurology, and as a Visiting Professor at Fujita Health University, Department of Physical Medicine and Rehabilitation and at University of Newcastle, Institute of Neuroscience. He is one of the founders and member of the Board of Directors of Interactive Motion Technologies, a Massachusetts-based company commercializing robot technology for rehabilitation. He is a Fellow of the IEEE (Institute of Electrical and Electronics Engineers). Dr. Krebs was nominated by two of IEEE societies: IEEE-EMBS (Engineering in Medicine & Biology Society) and IEEE-RAS (Robotics and Automation Society) to this distinguished engineering status “for contributions to rehabilitation robotics and the understanding of neurorehabilitation.”
Dr. Krebs has published and presented extensively on rehabilitation robotics, particularly applied to stroke and neuro-recovery. His work goes beyond Stroke and has been extended to Cerebral Palsy for which he received “The 2009 Isabelle and Leonard H. Goldenson Technology and Rehabilitation Award,” from the Cerebral Palsy International Research Foundation (CPIRF). In 2015, he received the prestigious IEEE-INABA Technical Award for Innovation leading to Production “for contributions to medical technology innovation and translation into commercial applications for Rehabilitation Robotics.” His goal is to revolutionize the way rehabilitation medicine is practiced today by applying robotics and information technology to assist, enhance, and quantify rehabilitation.
Contributor Information
Konstantinos P. Michmizos, Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, MA, 02115, USA; the Department of Neurology, Harvard Medical School, Boston, MA, 02115, USA; and the McGovern Institute for Brain Research, Massachusetts Institute of Technology, Cambridge, MA, 02139, USA. He previously was with the Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA, 02139, USA (konmic@mit.edu)..
Stefano Rossi, Department of Economics and Management, Industrial Engineering, University of Tuscia, Viterbo, Italy..
Enrico Castelli, “Bambino Gesù” Children's Hospital, Pediatric Neurorehabilitation Division and the Department of Neuroscience and Neurorehabilitation, Rome, Italy..
Paolo Cappa, “Sapienza” University of Rome, Department of Mechanical and Aerospace Engineering, 00184 Rome, Italy; with the NYU, Department of Mechanical and Aerospace Engineering, Brooklyn, NY 11201, USA; and with the “Bambino Gesù” Children's Hospital, 00050 Fiumicino (Roma), Italy..
Hermano Igo Krebs, Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA, 02139, USA; the Department of Neurology, University of Maryland, School of Medicine, Baltimore, MD, 21201, USA; the Department of Physical Medicine and Rehabilitation, Fujita Health University, Nagoya, Japan; and the Institute of Neuroscience, Newcastle University, Newcastle upon Tyne, UK (hikrebs@mit.edu)..
REFERENCES
- 1.Hogan N. Impedance control: An approach to manipulation: Part II— Implementation. Journal of dynamic systems, measurement, and control. 1985;107:8–16. [Google Scholar]
- 2.Krebs HI, Hogan N, Aisen ML, Volpe BT. Robot-aided neurorehabilitation. Rehabilitation Engineering, IEEE Transactions on. 1998;6:75–87. doi: 10.1109/86.662623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krebs HI, Volpe BT, Williams D, Celestino J, Charles SK, Lynch D, et al. Robot-aided neurorehabilitation: a robot for wrist rehabilitation. Neural Systems and Rehabilitation Engineering, IEEE Transactions on. 2007;15:327–335. doi: 10.1109/TNSRE.2007.903899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy A, Krebs HI, Williams DJ, Bever CT, Forrester LW, Macko RM, et al. Robot-aided neurorehabilitation: a novel robot for ankle rehabilitation. Robotics, IEEE Transactions on. 2009;25:569–582. [Google Scholar]
- 5.Kwakkel G, Kollen BJ, Krebs HI. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil Neural Repair. 2008 Mar-Apr;22:111–21. doi: 10.1177/1545968307305457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller EL, Murray L, Richards L, Zorowitz RD, Bakas T, Clark P, et al. Comprehensive Overview of Nursing and Interdisciplinary Rehabilitation Care of the Stroke Patient A Scientific Statement From the American Heart Association. Stroke. 2010;41:2402–2448. doi: 10.1161/STR.0b013e3181e7512b. [DOI] [PubMed] [Google Scholar]
- 7.Hogan N, Krebs HI, Rohrer B, Palazzolo JJ, Dipietro L, Fasoli SE, et al. Motions or muscles? Some behavioral factors underlying robotic assistance of motor recovery. Journal of rehabilitation research and development. 2006;43:605. doi: 10.1682/jrrd.2005.06.0103. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins WM, Merzenich MM. Reorganization of neocortical representations after brain injury: a neurophysiological model of the bases of recovery from stroke. Prog Brain Res. 1987;71:249–66. doi: 10.1016/s0079-6123(08)61829-4. [DOI] [PubMed] [Google Scholar]
- 9.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 10.Patton JL, Mussa-Ivaldi FA. Robot-assisted adaptive training: custom force fields for teaching movement patterns. Biomedical Engineering, IEEE Transactions on. 2004;51:636–646. doi: 10.1109/TBME.2003.821035. [DOI] [PubMed] [Google Scholar]
- 11.Patton JL, Stoykov ME, Kovic M, Mussa-Ivaldi FA. Evaluation of robotic training forces that either enhance or reduce error in chronic hemiparetic stroke survivors. Experimental Brain Research. 2006;168:368–383. doi: 10.1007/s00221-005-0097-8. [DOI] [PubMed] [Google Scholar]
- 12.Volpe B, Krebs H, Hogan N, Edelstein L, Diels C, Aisen M. A novel approach to stroke rehabilitation Robot-aided sensorimotor stimulation. Neurology. 2000;54:1938–1944. doi: 10.1212/wnl.54.10.1938. [DOI] [PubMed] [Google Scholar]
- 13.Krebs HI, Palazzolo JJ, Dipietro L, Ferraro M, Krol J, Rannekleiv K, et al. Rehabilitation Robotics: Performance-Based Progressive Robot-Assisted Therapy. Autonomous Robots. 2003;15:7–20. 2003/07/01. [Google Scholar]
- 14.Colombo G, Joerg M, Schreier R, Dietz V. Treadmill training of paraplegic patients using a robotic orthosis. Journal of rehabilitation research and development. 2000;37:693–700. [PubMed] [Google Scholar]
- 15.Hesse S, Uhlenbrock D. A mechanized gait trainer for restoration of gait. Journal of rehabilitation research and development. 2000;37:701–708. [PubMed] [Google Scholar]
- 16.Dobkin BH, Duncan PW. Should Body Weight–Supported Treadmill Training and Robotic-Assistive Steppers for Locomotor Training Trot Back to the Starting Gate? Neurorehabilitation and neural repair. 2012;26:308–317. doi: 10.1177/1545968312439687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Body-weight–supported treadmill rehabilitation after stroke. New England Journal of Medicine. 2011;364:2026–2036. doi: 10.1056/NEJMoa1010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry J. Gait analysis: normal and pathological function. Slack; Thorofare, NJ: 1992. [Google Scholar]
- 19.Blaya JA, Herr H. Adaptive control of a variable-impedance ankle-foot orthosis to assist drop-foot gait. Neural Systems and Rehabilitation Engineering, IEEE Transactions on. 2004;12:24–31. doi: 10.1109/TNSRE.2003.823266. [DOI] [PubMed] [Google Scholar]
- 20.Girone M, Burdea G, Bouzit M, Popescu V, Deutsch JE. A Stewart platform-based system for ankle telerehabilitation. Autonomous robots. 2001;10:203–212. [Google Scholar]
- 21.Bharadwaj K, Sugar TG, Koeneman JB, Koeneman EJ. Design of a robotic gait trainer using spring over muscle actuators for ankle stroke rehabilitation. Journal of biomechanical engineering. 2005;127:1009–1013. doi: 10.1115/1.2049333. [DOI] [PubMed] [Google Scholar]
- 22.Forrester LW, Roy A, Goodman RN, Rietschel J, Barton JE, Krebs HI, et al. Clinical application of a modular ankle robot for stroke rehabilitation. NeuroRehabilitation. 2013;33:85–97. doi: 10.3233/NRE-130931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forrester LW, Roy A, Krebs HI, Macko RF. Ankle training with a robotic device improves hemiparetic gait after a stroke. Neurorehabilitation and neural repair. 2011;25:369–377. doi: 10.1177/1545968310388291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forrester LW, Roy A, Krywonis A, Kehs G, Krebs HI, Macko RF. Modular Ankle Robotics Training in Early Subacute Stroke A Randomized Controlled Pilot Study. Neurorehabilitation and neural repair. 2014:1545968314521004. doi: 10.1177/1545968314521004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michmizos KP, Krebs HI. Pointing with the Ankle: the Speed-Accuracy Tradeoff. Experimental Brain Research. 2014;232:647–57. doi: 10.1007/s00221-013-3773-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michmizos KP, Vaisman L, Krebs HI. A Comparative Analysis of Speed Profile Models for Ankle Pointing Movements: Evidence that Lower and Upper Extremity Discrete Movements are controlled by a Single Invariant Strategy. Frontiers in Human Neuroscience. 2014;8:962. doi: 10.3389/fnhum.2014.00962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyman R. Stimulus information as a determinant of reaction time. Journal of Experimental Psychology. 1953;45:188–196. doi: 10.1037/h0056940. [DOI] [PubMed] [Google Scholar]
- 28.Hick WE. On the rate of gain of information. Quarterly Journal of Experimental Psychology. 1952;4:11–26. 1952/03/01. [Google Scholar]
- 29.Michmizos KP, Krebs HI. Reaction time in ankle movements: a diffusion model analysis. Exp Brain Res. 2014 doi: 10.1007/s00221-014-4032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michmizos KP, Krebs HI. Modeling Reaction Time in the Ankle. presented at the Biomedical Robotics and Biomechatronics (BioRob), 2014 5th IEEE RAS & EMBS International Conference on; Sao Paulo, Brazil: IEEE; 2014. [Google Scholar]
- 31.Peng Q, Park H-S, Shah P, Wilson N, Ren Y, Wu Y-N, et al. Quantitative evaluations of ankle spasticity and stiffness in neurological disorders using manual spasticity evaluator. Journal of rehabilitation research and development. 2011;48:473. doi: 10.1682/jrrd.2010.04.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krebs HI, Rossi S, Kim S, Artemiadis PK, Williams D, Castelli E, et al. Pediatric anklebot,” in Rehabilitation Robotics (ICORR) IEEE International Conference on. 2011:1–5. doi: 10.1109/ICORR.2011.5975410. 2011. [DOI] [PubMed] [Google Scholar]
- 33.Rossi S, Colazza A, Petrarca M, Castelli E, Cappa P, Krebs HI. Feasibility study of a wearable exoskeleton for children: is the gait altered by adding masses on lower limbs? PloS one. 2013;8:e73139. doi: 10.1371/journal.pone.0073139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chester VL, Tingley M, Biden EN. A comparison of kinetic gait parameters for 3–13 year olds. Clinical Biomechanics. 2006;21:726–732. doi: 10.1016/j.clinbiomech.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Engsberg JR, Ross SA, Olree KS, Park TS. Ankle spasticity and strength in children with spastic diplegic cerebral palsy. Developmental Medicine & Child Neurology. 2000;42:42–47. doi: 10.1017/s0012162200000086. [DOI] [PubMed] [Google Scholar]
- 36.O'Byrne JM, Jenkinson A, O'brien T. Quantitative analysis and classification of gait patterns in cerebral palsy using a three-dimensional motion analyzer. Journal of child neurology. 1998;13:101–108. doi: 10.1177/088307389801300302. [DOI] [PubMed] [Google Scholar]
- 37.Rodda J, Graham H. Classification of gait patterns in spastic hemiplegia and spastic diplegia: a basis for a management algorithm. European Journal of Neurology. 2001;8:98–108. doi: 10.1046/j.1468-1331.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 38.Sutherland DH, Davids JR. Common gait abnormalities of the knee in cerebral palsy. Clinical orthopaedics and related research. 1993;288:139–147. [PubMed] [Google Scholar]
- 39.Winters T, Gage J, Hicks R. Gait patterns in spastic hemiplegia in children and young adults. J Bone Joint Surg Am. 1987;69:437–441. [PubMed] [Google Scholar]
- 40.Burke JW, McNeill M, Charles DK, Morrow PJ, Crosbie JH, McDonough SM. Optimising engagement for stroke rehabilitation using serious games. The Visual Computer. 2009;25:1085–1099. [Google Scholar]
- 41.Michmizos KP, Krebs HI. Serious Games for the Pediatric Anklebot. Biomedical Robotics and Biomechatronics (BioRob), 2012 4th IEEE RAS & EMBS International Conference on; 2012.pp. 1710–1714. [Google Scholar]
- 42.Michmizos KP, Krebs HI. Assist-as-needed in lower extremity robotic therapy for children with cerebral palsy. Biomedical Robotics and Biomechatronics (BioRob), 2012 4th IEEE RAS & EMBS International Conference on; 2012.pp. 1081–1086. [Google Scholar]
- 43.Andrew Sawers C, Hahn ME, Kelly VE, Czerniecki JM. Beyond componentry: How principles of motor learning can enhance locomotor rehabilitation of individuals with lower limb loss—A review. 2012 doi: 10.1682/jrrd.2011.12.0235. [DOI] [PubMed] [Google Scholar]
- 44.Ferraro M, Palazzolo J, Krol J, Krebs H, Hogan N, Volpe B. Robot-aided sensorimotor arm training improves outcome in patients with chronic stroke. Neurology. 2003;61:1604–1607. doi: 10.1212/01.wnl.0000095963.00970.68. [DOI] [PubMed] [Google Scholar]
- 45.Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proceedings of the National Academy of Sciences. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shmuelof L, Krakauer JW, Mazzoni P. How is a motor skill learned? Change and invariance at the levels of task success and trajectory control. Journal of neurophysiology. 2012;108:578–594. doi: 10.1152/jn.00856.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nocchi F, Gazzellini S, Grisolia C, Petrarca M, Cannat V, Cappa P, et al. Brain network involved in visual processing of movement stimuli used in upper limb robotic training: an fMRI study. J Neuroeng Rehabil. 2012;9:49. doi: 10.1186/1743-0003-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt RA, Lee T. Motor Control and Learning, 5E. Human kinetics; 1988. [Google Scholar]
- 49.Mayer-Kress G, Liu Y-T, Newell KM. Applications of Nonlinear Dynamics. Springer; 2009. Time Scales of Performance Levels During Training of Complex Motor Tasks; pp. 445–448. [Google Scholar]
- 50.Newell KM, Liu Y-T, Mayer-Kress G. Progress in Motor Control. Springer; 2009. Time scales, difficulty/skill duality, and the dynamics of motor learning; pp. 457–476. [DOI] [PubMed] [Google Scholar]
- 51.Bernacchia A, Seo H, Lee D, Wang X-J. A reservoir of time constants for memory traces in cortical neurons. Nature neuroscience. 2011;14:366–372. doi: 10.1038/nn.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown RG, Jahanshahi M, Marsden CD. Response choice in Parkinson's disease. The effects of uncertainty and stimulus-response compatibility. Brain. 1993 Aug;116(Pt 4):869–85. doi: 10.1093/brain/116.4.869. [DOI] [PubMed] [Google Scholar]
- 53.Evarts EV, Teravainen H, Calne DB. Reaction time in Parkinson's disease. Brain. 1981 Mar;104:167–86. doi: 10.1093/brain/104.1.167. [DOI] [PubMed] [Google Scholar]
- 54.Goodrich S, Henderson L, Kennard C. On the existence of an attention-demanding process peculiar to simple reaction time: Converging evidence from Parkinson's disease. Cognitive Neuropsychology. 1989;6:309–331. 1989/05/01. [Google Scholar]
- 55.Jahanshahi M, Brown RG, Marsden CD. A comparative study of simple and choice reaction time in Parkinson's, Huntington's and cerebellar disease. J Neurol Neurosurg Psychiatry. 1993;56:1169–77. doi: 10.1136/jnnp.56.11.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marsden CD. The mysterious motor function of the basal ganglia: the Robert Wartenberg Lecture. Neurology. 1982 May;32:514–39. doi: 10.1212/wnl.32.5.514. [DOI] [PubMed] [Google Scholar]
- 57.Rogers MW, Chan CW. Motor planning is impaired in Parkinson's disease. Brain research. 1988;438:271–276. doi: 10.1016/0006-8993(88)91346-7. [DOI] [PubMed] [Google Scholar]
- 58.Mirabella G, Iaconelli S, Modugno N, Giannini G, Lena F, Cantore G. Stimulation of subthalamic nuclei restores a near normal planning strategy in Parkinson's patients. PLoS One. 2013;8:e62793. doi: 10.1371/journal.pone.0062793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baird BJ, Tombaugh TN, Francis M. The effects of practice on speed of information processing using the Adjusting-Paced Serial Addition Test (Adjusting-PSAT) and the Computerized Tests of Information Processing (CTIP) Appl Neuropsychol. 2007;14:88–100. doi: 10.1080/09084280701319912. [DOI] [PubMed] [Google Scholar]
- 60.Light KE, Reilly MA, Behrman AL, Spirduso WW. Reaction times and movement times: Benefits of practice to younger and older adults. Journal of Aging and Physical Activity. 1996;4:27–41. [Google Scholar]
- 61.Fitts PM, Posner MI. Human Performance. Brooks. Cole; Belmont, CA: 1967. [Google Scholar]
- 62.Lee TD, Swanson LR, Hall AL. What is repeated in a repetition? Effects of practice conditions on motor skill acquisition. Physical therapy. 1991;71:150–156. doi: 10.1093/ptj/71.2.150. [DOI] [PubMed] [Google Scholar]
- 63.Pollock BJ, Lee TD. Dissociated contextual interference effects in children and adults. Perceptual and motor skills. 1997;84:851–858. doi: 10.2466/pms.1997.84.3.851. [DOI] [PubMed] [Google Scholar]
- 64.Wegman E. Contextual interference effects on the acquisition and retention of fundamental motor skills. Perceptual and Motor Skills. 1999;88:182–187. doi: 10.2466/pms.1999.88.1.182. [DOI] [PubMed] [Google Scholar]
- 65.Ste-Marie DM, Clark SE, Findlay LC, Latimer AE. High levels of contextual interference enhance handwriting skill acquisition. Journal of motor behavior. 2004;36:115–126. doi: 10.3200/JMBR.36.1.115-126. [DOI] [PubMed] [Google Scholar]
- 66.Vera JG, Montilla MM. Practice schedule and acquisition, retention, and transfer of a throwing task in 6-yr.-old children. Perceptual and motor skills. 2003;96:1015–1024. doi: 10.2466/pms.2003.96.3.1015. [DOI] [PubMed] [Google Scholar]