Abstract
Minimally invasive anal fistula treatment (MAFT) was introduced to minimize early postoperative morbidity, preserve sphincter continence, and reduce recurrence. We report our early experience with MAFT in 416 patients. Preoperative MRI was performed in 150 patients initially and subsequently thereafter. The technique involves fistuloscope-aided localization of internal fistula opening, examination and fulguration of all fistula tracks, and secure stapled closure of internal fistula opening within anal canal/rectum. MAFT was performed as day-care procedure in 391 patients (93.9 %). During surgery, internal fistula opening could not be located in 100 patients (24 %). Seven patients required readmission to hospital. Mean visual analog scale scores for pain on discharge and at 1 week were 3.1 (1–6) and 1.6 (0–3), respectively. Mean duration for return to normal activity was 3.2 days (2–11 days). Fistula recurrence was observed in 35/134 patients (26.1 %) at 1 year follow-up. MAFT may be performed as day-care procedure with benefits of less pain, absence of perianal wounds, faster recovery, and preservation of sphincter continence. However, long-term results from more centers are needed especially for recurrence.
Keywords: Minimally invasive anal fistula treatment (MAFT), Video-assisted anal fistula treatment (VAAFT), Fistuloscope, Anal fistula, Internal fistula opening, External fistula opening
Introduction
The treatment options for anal fistulae include traditional and novel techniques. The traditional methods include fistulotomy (laying open of the track) and fistulectomy (removal of the entire track) for low fistulae. For high and complex fistulae, use of seton is traditionally the favored treatment modality to minimize incontinence. More complex surgical procedures in the form of local advancement flaps have met with moderate success. The newer treatment options include use of fibrin glue, bioprosthetic plugs, and ligation of intersphincteric fistula tract (LIFT). Also, a minimally invasive technique has emerged in recent years using a specially designed fistuloscope [1].
There is lack of literature reporting on the early postoperative sequelae following both traditional and newer techniques for management of anal fistulae (postoperative pain, need for dressings, and time taken off work). Mostly, long-term outcomes in terms of recurrence and incontinence have been addressed in studies reported so far [2, 3]. Traditional techniques including fistulectomy and use of cutting seton has been associated with incontinence especially in patients who have had previous surgery [2]. Mucosal advancement flaps are technically challenging and are associated with high recurrence rates and high rates of incontinence post operatively [4–9]. LIFT procedure has been associated with good healing rates [10–13]. The video-assisted anal fistula treatment was developed by Professor P. Meinero in 2006 [1]. The technique comprises identification and secure internal closure of the internal fistula opening and visualization with cauterization of the fistulous track using a specially designed fistuloscope. We adopted this technique in an effort to reduce early postoperative morbidity and offer patients the advantages of minimally invasive surgery. We report our early experience of 416 patients with minimally invasive anal fistula treatment (MAFT) to ascertain the potential benefits of this new technique.
Materials and Methods
We are a tertiary care referral institute in a multispeciality hospital, providing a wide spectrum of minimal access surgical services for over two decades. The Division of Minimally Invasive Proctology at the institute offers management options for various anorectal pathological conditions. We adopted the MAFT technique for treatment of anal fistulae since April 2011. Preoperative magnetic resonance imaging (MRI) was obtained in initial 150 patients to aid in preoperative assessment of fistula anatomy. Subsequently, MRI was performed selectively in patients with recurrent fistulae, multiple fistulae, and suspected high fistulae. One day prior to surgery, patients were placed on a liquid diet and were prescribed oral laxatives at bedtime. An informed consent about the procedure was taken from all patients prior to surgery. The management protocol was approved by the ethics committee of the hospital.
Between April 2011 to December 2012, 580 patients with anal fistulae underwent surgical treatment. Out of these, 114 patients (19.6 %) had fistulotomy/fistulectomy for superficial/subcutaneous fistulae, 21 patients (3.6 %) had LIFT with fulguration of distal fistula track, and 29 patients (5 %) had incision and drainage of abscesses only. Of the patients, 416 (71.8 %) underwent MAFT.
The following data of all patients was prospectively collected and maintained in a database (Microsoft Excel® Worksheet): age, sex, symptoms, previous surgery, associated comorbidities, number of fistulous openings, location of external and internal openings, discharge, associated pathology, fistula type (Park classification) [14],and surgical procedure. At follow-up, examination of the fistula, discharge, pain scores (VAS), and symptoms of the patients were recorded (at 1 week, 1 month, and 1 year). Symptoms of pain, discharge, bleeding, incontinence, and constipation was inquired from each patient at follow up.
Surgical Technique
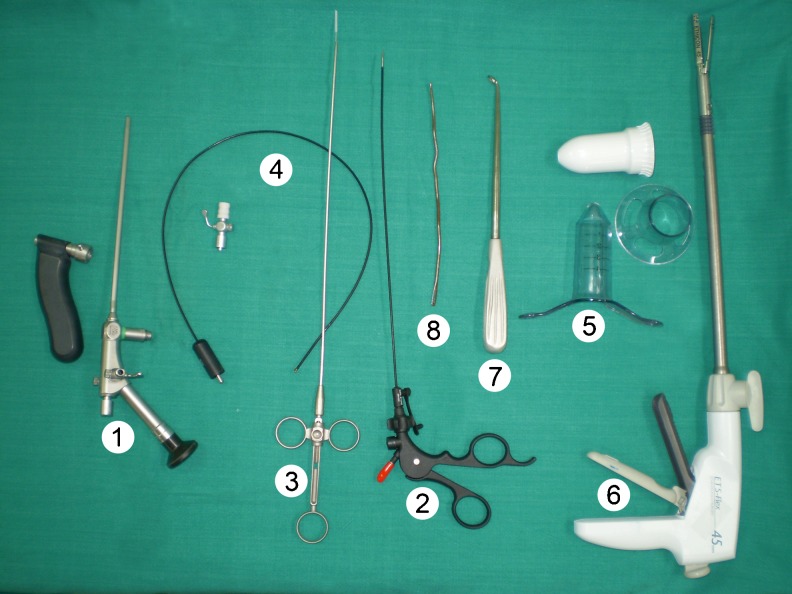
The equipment required for MAFT is manufactured and marketed by Karl Storz (Karl Storz, Tuttlingen, Germany). The instrument kit comprises (Fig. 1):
fistuloscope
3 mm forceps
Endobrush
unipolar electrode
Fig. 1.
Instrument set for minimally invasive anal fistula treatment (MAAFT). 1 Fistuloscope, 2 3-mm forceps, 3 Endobrush, 4 unipolar electrode, 5 anoscope, 6 linear Endostapler, 7 Volkmann spoon, 8 fistula probe
In addition, the surgeon also requires
anoscope
linear endostapler/semicircular stapler
glycine–mannitol 1 % solution
Volkmann spoon
fistula probe
The fistuloscope offers an 8° angled direction of view. It has a straight working channel, which is also used as an irrigation channel. The handle of the fistuloscope provides for easy handling and better maneuverability. The irrigation channel is used to irrigate the track with solution of 1 % glycine mannitol. The operative length of the fistuloscope is 18 cm and the outer diameter is 3.3 × 4.7 mm.
Operation Theater Layout
The patient is placed in a lithotomy position with 15–20° Trendlenberg tilt. The procedure is performed under spinal/general anesthesia.
Initial Examination
Initial examination includes examination of perianal area and the perineum for external fistula openings. Digital per rectal examination and proctoscopy is performed to assess anorectal pathology and to attempt to localize the site of the internal opening of the fistula.
Assembly of Instruments
Once a low fistula has been excluded, a decision to proceed with MAFT is taken. The fistuloscope is assembled with the light source, the obturator, and the bag containing glycine–mannitol 1 % solution.
Surgical Procedure
The external opening of the fistula is dilated with the tip of a fistula probe if required. The fistuloscope, with 1 % glycine–mannitol solution running is placed just within the external opening and the solution is allowed to distend and delineate the fistula track.
Localization of Internal Opening
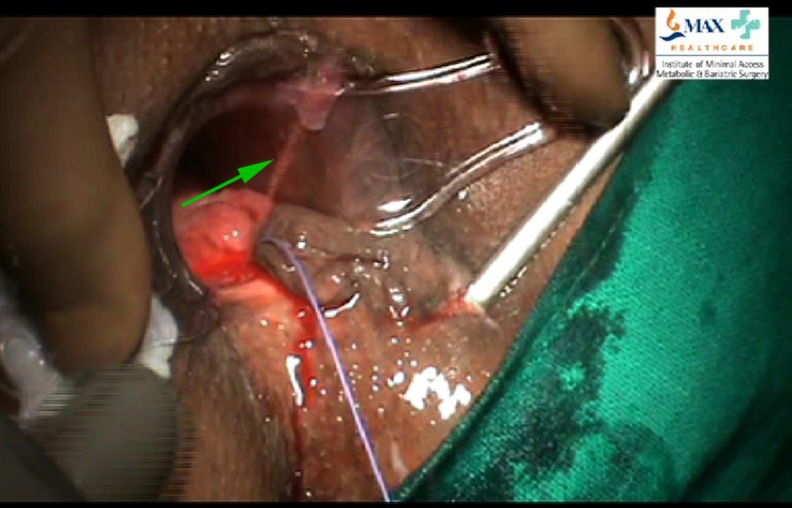
The fistuloscope is gently advanced through the fistula track, as the track distends with the irrigating solution. The fistuloscope is slowly advanced with side-to-side, rotatory, or vertical movements as may be required. The movements of the surgeon are guided from the display on the video monitor located at the head end of the patient. A typical fistula track appears as a tunnel with granulation tissue and fibrous tissue in the form of whitish flakes appearing within the fistula track. At this stage, attempt is made to locate the position of the internal fistula opening. An anal retractor is inserted to localize the position of the internal opening. In many instances, a jet of irrigating solution is seen spurting from the internal opening from within the anal canal/rectum (Fig. 2). In some patients with a well-defined fistula track and large internal opening, the fistuloscope itself may exit through the internal opening into the anal canal/rectum. In some patients, the internal opening may be narrow or concealed within a fold of mucosa. In these patients, the light of the fistuloscope shining through the bowel wall (with the O.T lights switched off) may provide a clue to the location of the internal opening.
Fig. 2.
Irrigating solution seen spurting from internal fistula opening within anal canal
Once the internal opening of the fistula has been localized on the bowel wall, it is isolated and marked with three stay sutures. The sutures are taken to include the full thickness of the rectal mucosa. The first suture is taken proximal to the opening, the second at the opening, and the third distal to the internal opening. The sutures are kept long and are held by means of an artery forceps outside the anal canal.
Examination and Fulguration of the Fistula Track
Once the internal opening has been localized and isolated with stay sutures, the entire fistula track is examined for secondary fistulous tracks and abscess cavities. The fistulous track is re-examined with the fistuloscope starting at the external opening. The fistuloscope is directed to look for presence of any secondary tracks and abscess cavities. Any abscess cavities that are identified are drained and fulguration of wall of abscess cavities is performed. Secondary tracks if present are entered with the fistuloscope and granulation tissue on the walls is fulgurated. The entire lining of the fistula tract is fulgurated. The fulguration is carried out by means of flexible monopolar electrode that passes through the working channel of the fistuloscope (Fig. 3). Debridement is completed with the help of an Endobrush (passing through the fistuloscope) or with a Volkmann spoon (after the removal of fistuloscope).
Fig. 3.
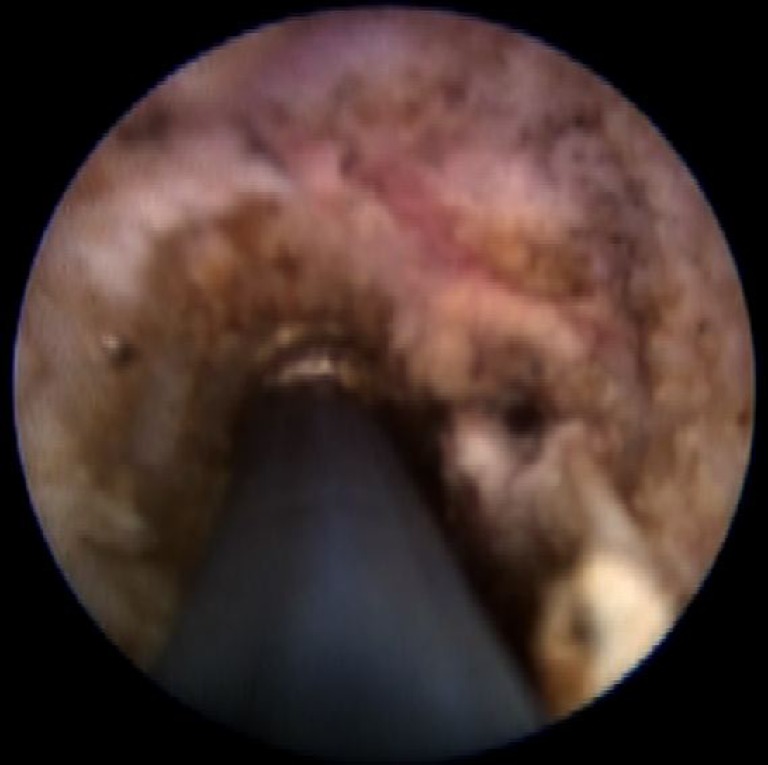
Fulguration of fistula track during fistuloscopy
Closure of the Internal Opening
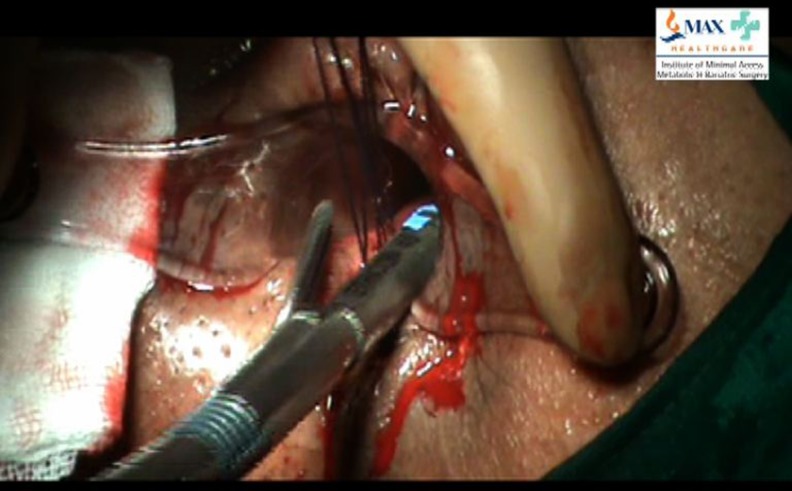
The anal retractor is re-inserted so that a good view of the internal opening with stay sutures is obtained. Traction is applied on stay sutures perpendicular to the bowel wall and a linear/semicircular endostapler is applied at the base. This ensures a secure stapled closure of internal opening (Fig. 4). The staple line is inspected for hemostasis. A temporary dressing is applied at the external opening.
Fig. 4.
Stapled closure of anal mucosa at the site of internal fistula opening
Analgesia protocol is followed for all patients postoperatively which consists of oral Diclofenac sodium 75 mg BD for 2 days and Tab Tramadol 50 mg SOS for breakthrough pain.
Results
Out of 580 patients operated for anal fistula, MAFT was performed in 416 patients (71.7 %). Of the patients, 391 (93.9 %) underwent MAFT as a day-care procedure. Seven patients required readmission to hospital for in-patient care (five patients for bleeding per rectum and two patients for excessive bloody discharge from fistula track).
The types of fistulae were recorded according to Park’s classification [14]. The fistulae were classified on the basis of preoperative MRI (when performed) and intraoperative findings. The commonest type of fistula was intersphincteric (41 %), followed by transsphincteric (34 %), and suprasphincteric (22 %). The extrasphincteric fistula was the least common type (3 %). The external opening of fistula was located anteriorly in 112 patients (27 %) and posteriorly in 304 patients (73 %). During surgery, the internal opening of the fistula could not be located in 101 patients (24.2 %; Table 1). In five patients, strands of hair were identified at the base of the fistula track which did not have an internal communication with the bowel. In five patients, the internal opening of anal fistula was located high in the rectum (beyond the reach of anoscope). Perineal edema from extravasation of irrigation fluid during fistuloscopy as a result of creation of a false passage was observed in 29 patients. The operative time reduced to a mean of 38 min (22–67 min) in the last 219 patient, as compared to 63 min (36–94 min) of the first 197 patients.
Table 1.
Internal opening of fistula (n = 416 patients)
| Site | Patients (%) |
|---|---|
| At/below dentate line | 290 (69.8) |
| Rectal wall | 20 (4.8) |
| High in rectum/colon | 5 (1.2) |
| Not located | 101 (24.2) |
The VAS scores for pain were recorded on discharge and on the first postoperative follow up at 1 week. The VAS scale was calibrated from 0 to 10, 10 denoting worst possible pain and 0 no pain at all. The mean VAS score for pain at discharge was 3.1 (1–6) and was 1.6 (0–3) at 1 week postoperative. Information on VAS scores and analgesia requirements was available for 350 patients (84.1 %). Fifty patients (12 %) required additional analgesia for breakthrough pain. Three patients had acute retention of urine in postoperative period which required an indwelling urinary catheter.
Patients were followed up postoperatively at 1 week, 1 month, and 1 year. At 1 week, 362 patients (87 %) had discharge from external fistula openings which was intermittently bloody. The mean duration for return to normal activity was 3.2 days (2–11 days). At 1 month, 175 patients (42 %) had discharge from fistula which was predominantly serous in nature. Of the patients, 172 completed 1 year after surgery, of whom 134 patients were available for a follow-up examination at 1 year (follow-up rate, 77.9 % at 1 year). Out of these, primary healing of fistula was observed in 99 patients (73.8 %). Thirty-five patients had recurrence (26.1 %; 20 patients with serous discharge, 9 patients with pus discharge, and 6 patients with bloody discharge). Thirty patients with recurrence were advised MAFT and 18 patients underwent the procedure again.
Anal continence was not formally evaluated by scoring before and after surgery. However, none of the patients reported worsening of symptoms of incontinence at 1 year follow up.
Tissue obtained after debridement of fistula track was submitted for histopathological examination in all patients. Tuberculous granulomas were identified in one patient who was subsequently placed on an antitubercular regimen.
Discussion
The present study was conducted to report our early experience with MAFT, a new modality for management of anal fistulae. Traditional surgical procedures for anal fistulae lead to creation of peri-anal wounds with the need for regular dressings and long follow-up. Pain, discomfort, and prolonged time off work are natural sequelae of these surgical procedures. Fecal incontinence is a significant complication in these patients, especially in patients with complex and recurrent fistulae [1]. This results from division and injury to the musculature that constitutes the sphincter mechanism of the anal canal.
MAFT qualifies as a true minimal invasive surgical procedure. There are no iatrogenic incisions for access on the patient. Surgical access is obtained from a pre-existing pathological opening of the fistula. The essential features of the MAFT technique include fistuloscopic exploration of the fistula track, early identification of the internal fistula opening, identification of secondary fistula tracks and abscess cavities, fulguration, and destruction of the fistula track under direct vision and secure stapled closure of the internal fistula opening. An angled telescope aids in detection of secondary tracks and abscess cavities, thereby lowering chances of recurrence. Destruction of the fistula track is performed by fulguration under direct vision and with the use of the flexible fistula brush (for a curved track) and Volkmann’s spoon (for straight track).
A stapled closure of the internal fistula opening with anoscopic access ensures sealing of the internal fistula opening which is a very important step of the surgical procedure. A secure sealed closure of the internal fistula opening is not possible with sutures and moreover the use of sutures may be contraindicated in this contaminated surgical field. Patients experience only mild discomfort from the staples in the early post operative period. This is because the staple line is longitudinal and not circular as in stapled haemorrhoidectomy. Fistuloscopy with irrigation facilitates accurate identification and localization of internal fistula opening in the anal canal. In difficult circumstances, digital probing of the anal canal to locate the tip of the fistuloscope is helpful. Also, the light from the fistuloscope shining through the bowel wall may aid in localization of the internal fistula opening. However, in 101 patients (24.2 %), we could not identify the internal fistula opening. In these patients, no attempt was made to create an internal opening and only fulguration of fistula tracks was performed. Any associated abscess cavities were drained under direct vision. It is likely that patients in whom the internal fistula opening is not identified are at a higher risk of recurrence.
MAFT in the management of anal fistulae is a new and evolving technique. As with many other new surgical techniques, there is likely to be an associated learning curve. In our experience, in five patients, the internal opening of the anal fistula was very high (beyond the reach of anoscope). This precludes the possibility of a secure stapled closure of the internal opening as was performed in other patients. The definitive surgical management of such patients remains unresolved at present. Furthermore, it is likely that early in the experience of a surgeon with MAFT, the internal fistula opening may not be identified. Also, secondary fistula tracks may be missed. These factors may lead to a higher recurrence rate in patients in the initial learning phase of the surgeon. However, the resultant morbidity to the patient is limited as there are no perianal wounds and the musculature of the anal sphincter remains intact. MAFT can be easily performed again for recurrent fistulae and this is acceptable to patients in view of low morbidity and rapid recovery.
Our early results indicate that the MAFT procedure confers several advantages to patients in the post operative period. In our experience, MAFT was performed as a day-care procedure in 159 patients (94 %) with a very low rate of readmission. Early and late postoperative pain in these patients was minimal, as measured by VAS scores and analgesia requirements in the postoperative period. In patients undergoing MAFT, there were no postoperative perianal wounds and hence no requirement for major postoperative dressings. Only three patients had acute retention of urine postoperatively which may be a reflection of less postoperative pain and discomfort. The mean duration for return to normal activity was 3.2 days (2–11 days).
The minimally invasive anal fistula treatment was initially described by Meinero [1]. In the study conducted by Meinero, 136 patients underwent video-assisted anal fistula treatment technique between May 2006 and May 2011. Primary healing was achieved in 72 patients (73.5 %) within 2–3 months of surgery. The longest time off work reported was 3 days. In the study, internal fistula opening could be located in 81 patients (82.6 %) [1].
The present study is a single-center prospective study using a standardized operative protocol. The obvious drawback of this study is the absence of medium- and long-term follow-up for recurrence rates. In this study, only 172 patients had completed 1 year follow up. A formal assessment and examination for incontinence was not performed in these patients. The data provided on incontinence was obtained only on the basis of symptoms of the patient. It is likely that as more experience is gained with MAFT, we may identify a subset of patients with anal fistulae who are most likely to benefit from this new technique. In our experience, MAFT should not be performed in patients with low fistulae (subcutaneous fistulae), high fistulae (with internal openings above the levator ani musculature), and in acute (abscess) stage. MAFT should also be avoided in patients in whom there has been no fistula discharge for a period of 6 months or more.
Several innovative surgical techniques have been proposed in an effort to avoid fecal incontinence, reduce perianal wounds, and decrease postoperative morbidity. These include Surgisis Anal fistula plug (Cook Biotech, West Lafayett, IN, USA) [15–17], fibrin sealant [18, 19], endoanal advancement flaps [20–22], and LIFT [23].
MAFT involves initial expenditure for equipment. However, the fistuloscope and ancillary equipment are reusable and the initial costs are likely to be recovered soon. This technique provides significant advantages to patients in terms of less pain, minimal morbidity, and earlier return to normal activities. The global socioeconomic costs of this procedure are therefore likely to be low.
MAFT is safe and feasible and can be mostly performed as a day-care procedure. There are distinct advantages to patients in terms of lesser pain, absence of perianal wounds, faster recovery, and earlier return to work. However, applicability of MAFT in very high extrasphincteric fistulae remains unclear at present. Long-term results from more centers are awaited especially for fistula recurrence rates.
Acknowledgments
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Meinero P, Mori L. Video-assisted anal fistula treatment (VAAFT): a novel sphincter-saving procedure for treating complex anal fistulas. Tech Coloproctol. 2011;15:417–422. doi: 10.1007/s10151-011-0769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joy HA, Williams JG. The outcomes of surgery for complex anal fistula. Colorect Dis. 2002;4:254–261. doi: 10.1046/j.1463-1318.2002.00357.x. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Aguilar J, Belmonte C, Wong WD, Goldberg SM, Madoff RD. Anal fistula surgery—factors associated with recurrence and incontinence. Dis Colon Rectum. 1996;39:723–729. doi: 10.1007/BF02054434. [DOI] [PubMed] [Google Scholar]
- 4.Ritchie RD, Sackier JM, Hodde JP. Incontinence rates after cutting seton treatment for anal fistula. Color Dis. 2009;11:564–571. doi: 10.1111/j.1463-1318.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 5.Aguilar PS, Plasencia G, Hardy TG, Jr, Hartmann RF, Stewart WR. Mucosal advancement in the treatment of anal fistula. Dis Colon Rectum. 1985;28:496–498. doi: 10.1007/BF02554093. [DOI] [PubMed] [Google Scholar]
- 6.Ozuner G, Hull TL, Cartmill J, Fazio VW. Long-term analysis of the use of transanal rectal advancement flaps for complicated anorectal/vaginal fistulas. Dis Colon Rectum. 1998;39:10–14. doi: 10.1007/BF02048261. [DOI] [PubMed] [Google Scholar]
- 7.Schouten WR, Zimmermann DD, Briel JW. Transanal advancement flap repair of transsphincteric fistulas. Dis Colon Rectum. 1999;42:1419–1423. doi: 10.1007/BF02235039. [DOI] [PubMed] [Google Scholar]
- 8.Mizrahi N, Wexner SD, Zmora O, et al. Endorectal advancement flap: are there predictors of failure? Dis Colon Rectum. 2002;45:1616–1621. doi: 10.1007/s10350-004-7248-z. [DOI] [PubMed] [Google Scholar]
- 9.Sonoda T, Hull T, Piedmonte MR, Fazio VW. Outcomes of primary repair of anorectal and rectovaginal fistulas using the endorectal advancement flap. Dis Colon Rectum. 2002;45:1622–1628. doi: 10.1007/s10350-004-7249-y. [DOI] [PubMed] [Google Scholar]
- 10.Rojanasakul A. LIFT procedure: a simplified technique for fistula-in-ano. Tech Coloproctol. 2009;13:237–240. doi: 10.1007/s10151-009-0522-2. [DOI] [PubMed] [Google Scholar]
- 11.Rojanasakul A, Pattanaarun J, Sahakitrungruang C, Tantiphlachiva K. Total anal sphincter saving technique for fistula-in-ano: the ligation of intersphinteric fistula tract. J Med Asso Thai. 2007;90:581–586. [PubMed] [Google Scholar]
- 12.Shanwani A, Nor AM, Amri N. Ligation of the intersphincteric fistula tract (LIFT): a sphincter-saving technique for fistula-in-ano. Dis Colon Rectum. 2010;53:39–42. doi: 10.1007/DCR.0b013e3181c160c4. [DOI] [PubMed] [Google Scholar]
- 13.Bleier JI, Moloo H, Goldberg SM. Ligation of the intersphincteric fistula tract: an effective new technique for complex fistulas. Dis Colon Rectum. 2010;53:43–46. doi: 10.1007/DCR.0b013e3181bb869f. [DOI] [PubMed] [Google Scholar]
- 14.Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1–12. doi: 10.1002/bjs.1800630102. [DOI] [PubMed] [Google Scholar]
- 15.Schwandner T, Roblick MH, Kierer W, Brom A, Padberg W, Hirschburger M. Surgical treatment of complex anal fistulas with the anal fistula plug: a prospective, multicenter study. Dis Colon Rectum. 2009;52:1578–1583. doi: 10.1007/DCR.0b013e3181a8fbb7. [DOI] [PubMed] [Google Scholar]
- 16.Zubaidi A, Al-Obeed O. Anal fistula plug in high fistula-in-ano: an early Saudi experience. Dis Colon Rectum. 2009;52:1584–1588. doi: 10.1007/DCR.0b013e3181a90b65. [DOI] [PubMed] [Google Scholar]
- 17.McGee MF, Champagne BJ, Stulberg JJ, Reynolds H, Marderstein E, Delaney CP. Tract length predicts successful closure with anal fistula plug in cryptoglandular fistulas. Dis Colon Rectum. 2010;53:1116–1120. doi: 10.1007/DCR.0b013e3181d972a9. [DOI] [PubMed] [Google Scholar]
- 18.Tyler KM, Aarons CB, Sentovich SM. Successful sphincter-sparing surgery for all anal fistulas. Dis Colon Rectum. 2007;50:1535–1539. doi: 10.1007/s10350-007-9002-9. [DOI] [PubMed] [Google Scholar]
- 19.Loungnarath R, Dietz DW, Mutch MG, Birnbaum EH, Kodner IJ, Fleshman JW. Fibrin glue treatment of complex anal fistulas has low success rate. Dis Colon Rectum. 2004;47:432–436. doi: 10.1007/s10350-003-0076-8. [DOI] [PubMed] [Google Scholar]
- 20.Hyman N. Endoanal advancement flap repair for complex anorectal fistulas. Am J Surg. 1999;178:337–340. doi: 10.1016/S0002-9610(99)00180-4. [DOI] [PubMed] [Google Scholar]
- 21.Nelson RL, Cintron J, Abcarian H. Dermal island-flap anoplasty for transsphincteric fistula-in-ano: assessment of treatment failures. Dis Colon Rectum. 2000;43:681–684. doi: 10.1007/BF02235588. [DOI] [PubMed] [Google Scholar]
- 22.Soltani A, Kaiser AM. Endorectal advancement flap for cryptoglandular or Crohn’s fistula-in-ano. Dis Colon Rectum. 2010;53:486–495. doi: 10.1007/DCR.0b013e3181ce8b01. [DOI] [PubMed] [Google Scholar]
- 23.Rojanasakul A, Pattanaarun J, Sahakitrungruang C, Tantiphlachiva K. Total anal sphincter saving technique for fistula-in-ano; the ligation of intersphincteric fistula tract. J Med Assoc Thai. 2007;90:581–586. [PubMed] [Google Scholar]